Abstract
Hantavirus pulmonary syndrome (HPS) is a highly pathogenic disease (40% case fatality rate) carried by rodents chronically infected with certain viruses within the genus Hantavirus of the family Bunyaviridae. The primary mode of transmission to humans is thought to be inhalation of excreta from infected rodents; however, ingestion of contaminated material and rodent bites are also possible modes of transmission. Person-to-person transmission of HPS caused by one species of hantavirus, Andes virus (ANDV), has been reported. Previously, we reported that ANDV injected intramuscularly causes a disease in Syrian hamsters that closely resembles HPS in humans. Here we tested whether ANDV was lethal in hamsters when it was administered by routes that more accurately model the most common routes of human infection, i.e., the subcutaneous, intranasal, and intragastric routes. We discovered that ANDV was lethal by all three routes. Remarkably, even at very low doses, ANDV was highly pathogenic when it was introduced by the mucosal routes (50% lethal dose [LD50], ∼100 PFU). We performed passive transfer experiments to test the capacity of neutralizing antibodies to protect against lethal intranasal challenge. The neutralizing antibodies used in these experiments were produced in rabbits vaccinated by electroporation with a previously described ANDV M gene-based DNA vaccine, pWRG/AND-M. Hamsters that were administered immune serum on days −1 and +5 relative to challenge were protected against intranasal challenge (21 LD50). These findings demonstrate the utility of using the ANDV hamster model to study transmission across mucosal barriers and provide evidence that neutralizing antibodies produced by DNA vaccine technology can be used to protect against challenge by the respiratory route.
Hantaviruses are rodent-borne enveloped viruses that have a trisegmented, negative-sense, single-stranded RNA genome and are members of the family Bunyaviridae (20, 24, 28). Pathogenic hantaviruses found in Europe and Asia cause a vascular leak disease known as hemorrhagic fever with renal syndrome. In 1993, pathogenic hantaviruses were discovered in the Americas (reviewed in references 14 and 21). The “New World” hantaviruses cause a vascular leak syndrome characterized by massive pulmonary edema followed by shock. Hantavirus pulmonary syndrome (HPS), also known as hantavirus cardiopulmonary syndrome, is highly lethal (30 to 50% case fatality rate), and at least one of the HPS-associated hantaviruses, Andes virus (ANDV), can spread from person to person (7, 19, 25, 27).
ANDV is the only pathogenic hantavirus for which there is an animal model that closely resembles human disease. When ANDV is injected into Syrian hamsters, the animals develop a disease that closely mimics human HPS (12). Similarities include the incubation time, rapid disease onset, infected endothelial cells, pulmonary edema, pleural effusion, thrombocytopenia, neutrophilia, and shock (4, 12). This model has been used to test candidate medical countermeasures to prevent and treat HPS, including an immunotherapeutic approach. Serum containing neutralizing antibodies protected hamsters from lethal HPS when it was administered before or up to 5 days after challenge with 250 50% lethal doses (LD50) of ANDV (6).
In all previous studies involving the ANDV hamster model, the hamsters were infected by intramuscular (i.m.) injection of virus. Here we investigated the possibility that ANDV can be transmitted to hamsters by routes that more closely resemble possible modes by which humans are infected, i.e., subcutaneous (s.c.) injection, modeling an animal bite; intranasal (i.n.) injection, modeling exposure to the respiratory mucosa; and intragastric (i.g.) injection, modeling ingestion of infectious virus. We found that ANDV caused a lethal disease by all routes tested. Next, we produced high-titer neutralizing antibody, using a molecular vaccine, and tested the capacity of this laboratory-produced antibody to protect against a respiratory (i.n.) challenge with ANDV. Antibody administered before and/or after i.n. challenge protected hamsters against lethal HPS. These studies demonstrate the utility of using the ANDV hamster model to study transmission across mucosal barriers and provide evidence that neutralizing antibodies produced using DNA vaccine technology can be used to protect against challenge by the respiratory route.
MATERIALS AND METHODS
Viruses and cells.
ANDV strain Chile-9717869 (12), Black Creek Canal virus (22), Sin Nombre virus (SNV) strain CC107 (23), and Hantaan virus (HTNV) strain 76-118 (16) were propagated in Vero E6 cells (Vero C1008; ATCC CRL 1586). Cells were maintained in Eagle's minimal essential medium with Earle's salts containing 10% fetal bovine serum, 10 mM HEPES, pH 7.4, and antibiotics (penicillin [100 U/ml], streptomycin [100 μg/ml], and gentamicin sulfate [50 μg/ml]) at 37°C in a 5% CO2 incubator.
Injection of hamsters with virus.
Six- to 8-week-old Syrian hamsters (Harlan, Indianapolis, IN) were anesthetized by i.m. injection with approximately 0.1 ml/100 g of body weight of a ketamine-acepromazine-xylazine mixture. Female hamsters were used except where indicated. Once anesthetized, hamsters were injected with virus diluted in sterile phosphate-buffered saline (PBS), pH 7.4. i.m. (caudal thigh) injections consisted of 0.2 ml delivered with a 1-ml syringe with a 25-gauge, 5/8-in. needle. i.g. injections consisted of 0.1 ml delivered with a 1-ml syringe with a 2-inch, 18-gauge gavage needle. s.c. injections (scruff of neck) consisted of 0.2 ml delivered with a 1-ml syringe with a 25-gauge, 5/8-in. needle. i.n. injections consisted of 50 μl delivered as 25 μl per naris with a plastic pipette tip. All work involving hamsters infected with ANDV was performed in a biosafety level 4 (BSL-4) laboratory. Research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adhered to the principles stated in the Guide for the Care and Use of Laboratory Animals (18a). The facility where this research was conducted is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Electroporation of rabbits.
Two rabbits were vaccinated four times each (weeks 0, 4, 8, and 11) with a previously described ANDV M gene-based DNA vaccine, pWRG/AND-M(x). Acclimated female New Zealand White rabbits aged 11 weeks and weighing between 1.8 and 2.2 kg at the study commencement received 400 μg of plasmid DNA on days 0, 28, 56, and 77. The DNA (1 mg/ml in PBS) was administered bilaterally as 200-μl doses to the gluteus superficialis muscles of the hind limbs. An Inovio (San Diego, CA) Twin Injector electroporation device (a prototype of the Elgen system) was used in all instances. Briefly, 100 μl of buffered DNA was injected into the muscle through each needle (21 gauge) of the electrode pair, followed by six pulses at 250 mA, and the injection was repeated on the other limb. The rabbits were bled for serum from the central ear artery on days 0, 42, 56, 77, and 91, followed by cardiac puncture and termination on day 105. Blood was allowed to clot for 20 to 30 min at room temperature, and then serum was collected by centrifugation at 1,150 × g for 10 min and stored at −20°C. All procedures were undertaken using an anesthetic cocktail consisting of ketamine (28 mg/kg) and xylazine (4 mg/kg).
PRNT.
Plaque reduction neutralization tests (PRNT) were performed as previously described (6). Sera were heat inactivated (56°C for 30 min), and 5% guinea pig complement (Accurate Chemical and Scientific Corp., Westbury, NY) was included in assays. Sera obtained from hantavirus-infected hamsters were gamma irradiated on dry ice (3 million rad from a 60C source) to eliminate infectious virus before use in assays.
Injection of hamsters with antibody.
Hamsters were anesthetized as described above, and 0.5 ml of heat-inactivated rabbit serum, undiluted or diluted in sterile PBS, pH 7.4, was injected s.c. into the scruff of the neck with a 1-ml syringe with a 25-gauge, 5/8-in. tuberculin needle.
ELISA.
Antibodies to ANDV nucleocapsid cross-react with Puumala virus nucleocapsid. This cross-reactivity made it possible to use a Puumala virus nucleocapsid-based enzyme-linked immunosorbent assay (ELISA) to detect antibodies to ANDV nucleocapsid. Antinucleocapsid ELISAs were performed as previously described (11, 12). The plasmid pPUUSXdelta (kindly provided by F. Elgh) was expressed in Escherichia coli BL21(DE3) cells (Novagen, Madison, WI) to generate a histidine-tagged truncated Puumala virus nucleocapsid fusion protein. The fusion protein was subsequently affinity purified with Ni-nitrilotriacetic acid columns (Qiagen, Valencia, CA). Hamster sera were gamma irradiated on dry ice (3 million rad from a 60C source) and heat inactivated before being tested in ELISA.
RESULTS
ANDV is highly lethal in both male and female hamsters.
Previously, we demonstrated that ANDV is lethal in female Syrian hamsters when it is administered i.m. (12). To test the unlikely possibility that the sex of the animal might impact virulence, we injected eight male hamsters with ANDV i.m. All eight animals died, with kinetics essentially identical to those previously observed for the female hamsters. The mean time to death was 13 days, with a range of 12 to 16 days (data not shown). Thus, the virulence of ANDV in hamsters does not depend on the sex of the animal. Animals in subsequent experiments were females.
ANDV is highly pathogenic in hamsters when it is administered by the s.c., i.n., and i.g. routes.
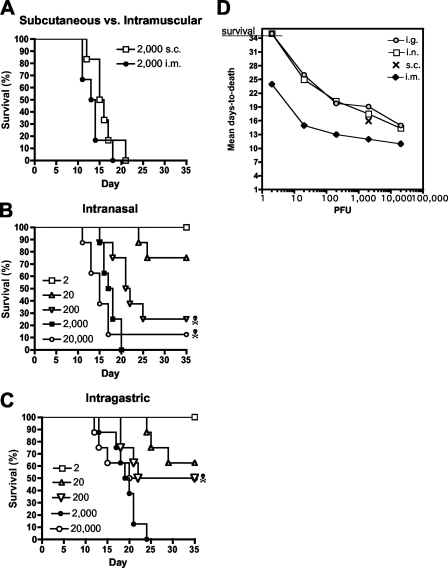
The i.m. injections performed in previous studies modeled exposure by animal bite; however, other routes of exposure, including those where the virus must penetrate the mucosa, were not tested. We performed a series of experiments testing the lethality of ANDV delivered via the s.c., i.n., and i.g. routes. To test lethality via the s.c. route, a single dose of 2,000 PFU of ANDV was injected s.c. into six hamsters. As positive controls for lethality, six hamsters were injected with the same dose by the i.m. route. Within 3 weeks, 100% of the hamsters in both groups had developed lethal HPS. The mean time to death was 16 days for the s.c. group and 13.5 days for the i.m. group (Fig. 1A). Next, we performed two LD50 experiments to determine if ANDV was lethal when administered i.n. or i.g. Groups of eight hamsters were injected with 10-fold dilutions of ANDV ranging from 2 to 20,000 PFU. Doses as low as 20 PFU were lethal when administered by the i.n. or i.g. route (Fig. 1B and C). The LD50 by the i.n. route was only 95 PFU, and the LD50 by the i.g. route was only 225 PFU. There was no significant difference between these LD50 values (P = 0.5522).
FIG. 1.
ANDV is lethal by the s.c., i.n., and i.g. routes. (A) ANDV is lethal by s.c. infection. Groups of six hamsters were injected s.c. or i.m. (as a positive control for lethality) with ANDV at a dose of 2,000 PFU. Survival curves are shown. (B) ANDV is lethal by i.n. infection. Groups of eight hamsters were exposed to serial 10-fold dilutions of ANDV by the i.n. route. Survival curves are shown. (C) ANDV is lethal by i.g. infection. A gavage needle was used to administer serial 10-fold dilutions of ANDV to groups of eight hamsters per dose. Survival curves are shown. The skull-and-crossbones symbol indicates a hamster in the group that was infected but survived. (D) Mean day to death increases as challenge dose decreases. Mean day-to-death data from i.n., i.g., and i.m. LD50 experiments were plotted versus challenge doses. The i.m. LD50 was reported previously. The mean day to death for the 2,000-PFU s.c. challenge group was also plotted. Experiments were terminated on day 35.
Hamsters injected i.n. or i.g. with ANDV displayed the same signs of disease (e.g., dyspnea and lethargy) as hamsters injected i.m. Thus, although necropsies were not performed, it is likely that the animals developed severe pulmonary edema and shock, as reported previously after i.m. injection. Animals injected with low doses of virus survived longer than animals injected with high doses. A graph showing the inverse relationship between challenge dose and time to death is shown in Fig. 1D. These experiments were terminated 5 weeks after challenge, so lethal disease beyond 35 days would not have been detected. Evidence of infection in three of the survivors is described below.
Hamsters that received the lowest dose, 2 PFU, by the i.n. or i.g. route survived and were not infected, as measured by the presence of antinucleocapsid antibodies in their day 35 sera (data not shown). Three hamsters that were challenged either i.n. or i.g. were infected but survived until day 35 (terminal bleed date) (Fig. 1B and C). The antinucleocapsid antibody titers of the infected survivors ranged between 200 and 800 (data not shown). Combining the infected survivors with the fatalities, the doses of ANDV that infected 50% of the hamsters by the i.n. and i.g. routes were calculated to be 48 and 162 PFU, respectively. There was no significant difference between these 50% infectious doses (P = 0.4463).
Production of high-titer hantavirus neutralizing antibodies in rabbits, using a DNA vaccine delivered by electroporation.
We were interested in testing the possibility that passive transfer of neutralizing antibodies could protect against a challenge with ANDV administered to the mucosa. In earlier passive transfer studies, we tested sera from monkeys vaccinated with an ANDV M gene-based DNA vaccine or convalescent-phase sera from HPS patients for the capacity to protect hamsters against an i.m. challenge with a dose of 250 LD50 of ANDV. In those studies, passive transfer afforded protection even 5 days after challenge (6). Here we used an alternative approach to rapidly generate sera containing high-titer ANDV neutralizing antibodies. Rabbits were vaccinated by muscle electroporation with an ANDV M gene-based DNA vaccine plasmid, pWRG/AND-M (6). Sera were collected and tested for neutralizing antibodies by PRNT (Fig. 2). Both rabbits vaccinated with the ANDV M gene-based DNA vaccine produced antibodies that neutralized ANDV. The neutralizing antibody (50% PRNT titer = 2,560) was detected in the first bleed collected postvaccination (after two vaccinations). Subsequent vaccinations maintained the level of neutralizing antibody but did not increase the titer above a maximum 50% PRNT titer of 5,120.
FIG. 2.
High-titer neutralizing antibodies produced in rabbits vaccinated by electroporation. Rabbits 1334 (squares) and 1335 (triangles) were vaccinated with an ANDV M gene-based DNA vaccine, pWRG/AND-M, by muscle electroporation on days 0, 28, 56, and 77 (arrowheads). Sera were collected prevaccination and again on days 42, 56, 77, 91, and 102. Sera were heat inactivated and then evaluated for neutralizing antibodies by ANDV PRNT (filled symbols) and HTNV PRNT (open symbols). The lowest dilution tested was 1:20 (dashed line).
We tested the capacity of the rabbit sera to cross-neutralize heterologous hantaviruses. The sera did not cross-neutralize HTNV, Black Creek Canal virus, or SNV (data not shown). This finding was in contrast to our previous findings that nonhuman primates vaccinated with pWRG/AND-M or a plasmid containing both the HTNV and ANDV M genes cross-neutralized heterologous hantaviruses (6, 9).
Passive transfer of neutralizing antibodies protects against respiratory challenge.
Three concurrent experiments were performed to test the capacity of passively transferred ANDV neutralizing antibodies to protect hamsters against a challenge by the respiratory route. The source of the antibodies was vaccine-produced immune serum from rabbit 1334 on day 102, which had a 50% PRNT titer of 2,560 (Fig. 2). The challenge was 2,000 PFU of ANDV (21 LD50) injected i.n. on day 0. In the first experiment, groups of six hamsters were injected s.c. with undiluted immune serum, diluted immune serum (1:4 and 1:16), or normal rabbit serum 1 day before challenge (Fig. 3A). A control group that received no antibody was also included. Five of six hamsters injected with undiluted immune serum survived the challenge. Similarly, four of five hamsters injected with immune serum diluted 1:4 survived. When the immune serum was diluted 1:16, there were no survivors. There were two survivors in the normal serum control group and one survivor in the no-antibody group. The second experiment was identical to the first, except that serum was administered on day 5 after challenge (Fig. 3B). All six of the hamsters injected with the undiluted serum survived challenge, and four of six hamsters injected with the 1:4 dilution survived. When the immune serum was diluted 1:16, it did not protect five of six animals. There were two survivors in the normal serum control group. In the third experiment, the passive transfer of serum was done on days −1 and +5 (Fig. 3C). All 12 of the hamsters that were injected with either the undiluted immune serum or the 1:4 dilution of immune serum survived. When the immune serum was diluted 1:16, half of the animals survived. There was one survivor in the normal serum control group.
FIG. 3.
Evaluation of the capacity of ANDV neutralizing antibodies to protect in a respiratory challenge model. Hamsters were challenged i.n. with 2,000 PFU of ANDV on day 0 and monitored for 35 days. (A) Passive transfer of serum on day −1. (B) Passive transfer of serum on day +5. (C) Passive transfer of serum on days −1 and +5. NAb, rabbit 1334 day 102 serum containing ANDV neutralizing antibodies with a 50% PRNT titer of 2,560. Serum was administered undiluted, diluted 1:4, or diluted 1:16. Arrows indicate the days on which antibody was injected s.c.
A statistical analysis was performed on the data generated in the aforementioned passive transfer experiments. When undiluted immune serum or a 1:4 dilution of immune serum was injected on days −1 and +5, there was a significant level of protection relative to that of the normal serum group (P = 0.0076) or the no-antibody group (P = 0.0379). In the first two passive transfer experiments, there were two survivors in the normal serum groups. These survivors, coupled with the small number of animals, dictated that the level of protection in those groups was not statistically significant. However, when survival data from the i.n. LD50 experiment (2,000 PFU by the i.n. route) were included in the analysis, it became evident that the level of protection when antibody was passively transferred only on day −1 or +5 was significant relative to that of untreated animals (Table 1).
TABLE 1.
Statistical analysis of survival ratesa
| Group | Antibody treatmentb | No. of survivors | No. of animals that died | % Survival | Adjusted P value for comparison to normal serum treatmentc | Adjusted P value for comparison to no serum treatmentc |
|---|---|---|---|---|---|---|
| 1 | ANDV NAb, undiluted, day −1 | 5 | 1 | 83 | 0.6061 | 0.0154 |
| 2 | ANDV NAb, diluted 1:4, day −1 | 4 | 1 | 67 | 0.7013 | 0.0366 |
| 3 | ANDV NAb, diluted 1:16, day −1 | 0 | 6 | 0 | 1.0000 | 1.0000 |
| 4 | Normal rabbit serum, undiluted, day −1 | 2 | 4 | 33 | 0.7013 | |
| 5 | ANDV NAb, undiluted, day +5 | 6 | 0 | 100 | 0.1515 | 0.0013 |
| 6 | ANDV NAb, diluted 1:4, day +5 | 4 | 2 | 67 | 0.8506 | 0.0836 |
| 7 | ANDV NAb, diluted 1:16, day +5 | 1 | 5 | 17 | 1.0000 | 1.0000 |
| 8 | Normal rabbit serum, undiluted, day +5 | 2 | 4 | 33 | 0.8070 | |
| 9 | ANDV NAb, undiluted, days −1 and +5 | 6 | 0 | 100 | 0.0054 | 0.0013 |
| 10 | ANDV NAb, diluted 1:4, days −1 and +5 | 6 | 0 | 100 | 0.0054 | 0.0013 |
| 11 | ANDV NAb, diluted 1:16, days −1 and +5 | 3 | 3 | 50 | 0.1827 | 0.1827 |
| 12 | Normal rabbit serum, undiluted, days −1 and +5 | 0 | 6 | 0 | 1.0000 | |
| 13 | No serum | 1 | 13d | 7 |
Survival rates were analyzed with Fisher exact tests with stepdown Bonferroni adjustment.
ANDV NAb, rabbit serum containing ANDV neutralizing antibodies.
Values in bold are significant.
The no-serum group included eight historical controls injected i.n. with 2,000 PFU (see Fig. 1B).
DISCUSSION
In this report, we demonstrated that ANDV is highly pathogenic in Syrian hamsters when it is administered by the respiratory or gastric route. All previous studies had involved i.m. challenges. It is not completely surprising that a virus thought to be transmissible from rodent to rodent by saliva or from rodents to humans by inhalation of contaminated rodent excreta would be transmissible across the mucosa in an animal model. Nevertheless, it is remarkable that as little as 20 PFU of ANDV administered i.n. or i.g. was sufficient to penetrate all host physical and immunological barriers and cause lethal disease in hamsters. The efficient transmission (LD50 of ∼100 PFU) of ANDV implies that the virus is not completely inactivated by secretions in the airways and/or gut (e.g., saliva, mucus, and gastric juices). This could be due to an inherent stability of the virions, rapid adherence and uptake of the virions by cells lining the luminal space, or a combination of both inherent stability and rapid uptake.
The data in Fig. 1 indicate that, regardless of the route of injection, as the dose of virus increases, the incubation period decreases. Longer incubation periods after i.n. and i.g. exposure could reflect either a decrease in the number of infectious particles that gain access to replication-competent cells (i.e., a lower effective dose), a delay in infection resulting from the added time required to penetrate barriers, or a combination of both. The similarity in the shapes of the curves in Fig. 1D and the ∼1-log difference in LD50 values suggest that lower effective doses rather than a delay in penetration for the i.n. and i.g. groups likely explain the extended incubation periods relative to that for the i.m. group.
The findings from this study have implications for hantavirus vaccine development. This is the first report of a candidate M gene-based hantavirus DNA vaccine (i.e., pWRG/AND-M) being delivered by electroporation. Previous studies involved delivery of hantavirus DNA vaccines by particle-medicated epidermal delivery (gene gun) or i.m. injection (6, 10, 11, 13). The use of electroporation technology coupled with M gene-based DNA vaccines to elicit hantavirus neutralizing antibodies adds an alternative means for producing molecular hantavirus vaccines. In addition, it is now possible to test vaccines against HPS by using a respiratory challenge model (i.e., the i.n. route). To date, animal models used to evaluate hantavirus vaccines have involved challenges by parenteral routes, including the i.m., s.c., and intraperitoneal routes (13).
Data reported here and by others suggest that it might be possible to use an immunotherapeutic approach to prevent and treat HPS. For humans, higher neutralizing antibody titers at the time of hospital admission correlated with a favorable clinical course of HPS caused by SNV, suggesting that neutralizing antibody may mitigate disease (2). We reported earlier that passive transfer of immune serum could protect hamsters against a lethal i.m. challenge when the serum was administered up to 5 days after challenge (6). Here we demonstrated for the first time that the s.c. administration of antibodies postexposure protected hamsters against a lethal respiratory challenge with ANDV.
ANDV causes severe HPS in Argentina and Chile (17, 18). Clustered cases of HPS have been documented in both northern and southern Argentina (25) and in adjacent regions of Chile (15, 26, 27). The average interval between index cases and subsequent cases within clusters was ∼23 days, which is within the estimated incubation time of HPS caused by ANDV, i.e., 7 to 40 days (3, 24). The modes of rodent-to-person transmission of HPS are indistinguishable from those of hemorrhagic fever with renal syndrome (e.g., inhalation, ingestion, or animal bite); however, the mode of person-to-person transmission remains unknown. Epidemiology indicates that the disease involves close and prolonged contact (7). For example, a recent prospective evaluation of household contacts revealed that almost 18% of sex partners of HPS patients developed HPS (8). There has been at least one instance where a medical worker (physician) treating an HPS patient contracted lethal HPS (20, 27). These epidemiological findings suggest that it might be beneficial to treat contacts of HPS patients, or other persons potentially exposed to the virus, with neutralizing antibodies. This would be especially applicable in regions where ANDV is endemic because of the potential for person-to-person transmission.
One source of neutralizing antibodies is the plasmas or sera of patients who survived HPS. This material can be obtained from willing donors, stored, and used on patients having the same blood type. This approach has been used successfully to combat other acute viral diseases, such as Argentine hemorrhagic fever (1). Another possibility would be to isolate protective monoclonal antibodies, as has been done for respiratory syncytial virus (5). Alternatively, it might be possible to use a DNA vaccine approach (e.g., electroporation of pWRG/AND-M) to vaccinate animals capable of producing antibodies that are safe for human use. This approach does not require human donors, does not involve the use of infectious or inactivated virus, and does not require the production and purification of recombinant protein. We have successfully produced high-titer hantavirus-neutralizing antibodies in nonhuman primates and rabbits, using the gene gun technique and electroporation, respectively. We are currently exploring the possibility of using our DNA vaccine to vaccinate other species, including transgenic species engineered to produce “human” antibodies, to produce a product suitable for use in humans.
Here we report the development of a respiratory/i.g. model of HPS. Low doses of virus administered i.n. or directly into the gut by use of a gavage needle resulted in lethal HPS in hamsters. The mean time to death ranged from 14 to 26 days, depending on the dose of virus. From a biodefense viewpoint, data demonstrating that any highly lethal human pathogen (e.g., ANDV) is infectious by the respiratory or i.g. route at low doses are a concern. However, this concern is tempered by our finding that HPS caused by i.n. challenge can be prevented by the presence of neutralizing antibodies. It is likely that neutralizing antibodies prevent the dissemination of virus to endothelial cells rather than blocking infection at the mucosa. Thus, vaccines and immunotherapeutics that prevent the dissemination of virus will likely prevent the morbidity and mortality associated with these highly pathogenic viruses. The development of HPS vaccines and postexposure prophylactics and therapeutics will be facilitated by the availability of this respiratory/i.g. model of HPS.
Acknowledgments
We thank the team at Aldevron, and John Ballantyne in particular, for performing the rabbit vaccinations and for providing the sera used in this study. We thank Michael Zimmerman for expert assistance with the BSL-4 animal experiments and Sarah Norris for providing statistical analyses. All experiments involving the use of ANDV in animals were performed in USAMRIID's BSL-4 laboratory.
The research described herein was sponsored by the Military Infectious Disease Research Program, U.S. Army Medical Research and Materiel Command, under project no. T0020_06_RD.
Opinions, interpretations, conclusions, and recommendations in this study are those of the authors and are not necessarily endorsed by the U.S. Army or the Department of Defense.
Footnotes
Published ahead of print on 21 November 2007.
REFERENCES
- 1.Avila, M. M., S. R. Samoilovich, R. P. Laguens, M. S. Merani, and M. C. Weissenbacher. 1987. Protection of Junin virus-infected marmosets by passive administration of immune serum: association with late neurologic signs. J. Med. Virol. 2167-74. [DOI] [PubMed] [Google Scholar]
- 2.Bharadwaj, M., R. Nofchissey, D. Goade, F. Koster, and B. Hjelle. 2000. Humoral immune responses in the hantavirus cardiopulmonary syndrome. J. Infect. Dis. 18243-48. [DOI] [PubMed] [Google Scholar]
- 3.Butler, J. C., and C. J. Peters. 1994. Hantaviruses and hantavirus pulmonary syndrome. Clin. Infect. Dis. 19387-394. [DOI] [PubMed] [Google Scholar]
- 4.Campen, M. J., M. L. Milazzo, C. F. Fulhorst, C. J. Obot Akata, and F. Koster. 2006. Characterization of shock in a hamster model of hantavirus infection. Virology 35645-49. [DOI] [PubMed] [Google Scholar]
- 5.Crowe, J. E., Jr., B. R. Murphy, R. M. Chanock, R. A. Williamson, C. F. Barbas III, and D. R. Burton. 1994. Recombinant human respiratory syncytial virus (RSV) monoclonal antibody Fab is effective therapeutically when introduced directly into the lungs of RSV-infected mice. Proc. Natl. Acad. Sci. USA 911386-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Custer, D. M., E. Thompson, C. S. Schmaljohn, T. G. Ksiazek, and J. W. Hooper. 2003. Active and passive vaccination against hantavirus pulmonary syndrome with Andes virus M genome segment-based DNA vaccine. J. Virol. 779894-9905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enria, D., P. Padula, E. L. Segura, N. Pini, A. Edelstein, C. R. Posse, and M. C. Weissenbacher. 1996. Hantavirus pulmonary syndrome in Argentina. Possibility of person to person transmission. Medicina (Buenos Aires) 56709-711. [PubMed] [Google Scholar]
- 8.Ferres, M., P. Vial, C. Marco, L. Yanez, P. Godoy, C. Castillo, B. Hjelle, I. Delgado, S. J. Lee, and G. J. Mertz. 2007. Prospective evaluation of household contacts of persons with hantavirus cardiopulmonary syndrome in Chile. J. Infect. Dis. 1951563-1571. [DOI] [PubMed] [Google Scholar]
- 9.Hooper, J. W., D. M. Custer, J. Smith, and V. Wahl-Jensen. 2006. Hantaan/Andes virus DNA vaccine elicits a broadly cross-reactive neutralizing antibody response in nonhuman primates. Virology 347208-216. [DOI] [PubMed] [Google Scholar]
- 10.Hooper, J. W., D. M. Custer, E. Thompson, and C. S. Schmaljohn. 2001. DNA vaccination with the Hantaan virus M gene protects hamsters against three of four HFRS hantaviruses and elicits a high-titer neutralizing antibody response in rhesus monkeys. J. Virol. 758469-8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hooper, J. W., K. I. Kamrud, F. Elgh, D. Custer, and C. S. Schmaljohn. 1999. DNA vaccination with hantavirus M segment elicits neutralizing antibodies and protects against Seoul virus infection. Virology 255269-278. [DOI] [PubMed] [Google Scholar]
- 12.Hooper, J. W., T. Larsen, D. M. Custer, and C. S. Schmaljohn. 2001. A lethal disease model for hantavirus pulmonary syndrome. Virology 2896-14. [DOI] [PubMed] [Google Scholar]
- 13.Hooper, J. W., and D. Li. 2001. Vaccines against hantaviruses. Curr. Top. Microbiol. Immunol. 256171-191. [DOI] [PubMed] [Google Scholar]
- 14.Khan, A. S., T. G. Ksiazek, and C. J. Peters. 1996. Hantavirus pulmonary syndrome. Lancet 347739-741. [DOI] [PubMed] [Google Scholar]
- 15.Lazaro, M. E., G. E. Cantoni, L. M. Calanni, A. J. Resa, E. R. Herrero, M. A. Iacono, D. A. Enria, and S. M. Gonzalez Cappa. 2007. Clusters of hantavirus infection, southern Argentina. Emerg. Infect. Dis. 13104-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee, H. W., P. W. Lee, and K. M. Johnson. 1978. Isolation of the etiologic agent of Korean hemorrhagic fever. J. Infect. Dis. 137298-308. [DOI] [PubMed] [Google Scholar]
- 17.Lopez, N., P. Padula, C. Rossi, M. E. Lazaro, and M. T. Franze-Fernandez. 1996. Genetic identification of a new hantavirus causing severe pulmonary syndrome in Argentina. Virology 220223-226. [DOI] [PubMed] [Google Scholar]
- 18.Lopez, N., P. Padula, C. Rossi, S. Miguel, A. Edelstein, E. Ramirez, and M. T. Franze-Fernandez. 1997. Genetic characterization and phylogeny of Andes virus and variants from Argentina and Chile. Virus Res. 5077-84. [DOI] [PubMed] [Google Scholar]
- 18a.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC.
- 19.Padula, P. J., A. Edelstein, S. D. Miguel, N. M. Lopez, C. M. Rossi, and R. D. Rabinovich. 1998. Epidemic outbreak of hantavirus pulmonary syndrome in Argentina. Molecular evidence of person to person transmission of Andes virus. Medicina (Buenos Aires) 58(Suppl. 1)27-36. [PubMed] [Google Scholar]
- 20.Padula, P. J., A. Edelstein, S. D. Miguel, N. M. Lopez, C. M. Rossi, and R. D. Rabinovich. 1998. Hantavirus pulmonary syndrome outbreak in Argentina: molecular evidence for person-to-person transmission of Andes virus. Virology 241323-330. [DOI] [PubMed] [Google Scholar]
- 21.Peters, C. J., and A. S. Khan. 2002. Hantavirus pulmonary syndrome: the new American hemorrhagic fever. Clin. Infect. Dis. 341224-1231. [DOI] [PubMed] [Google Scholar]
- 22.Rollin, P. E., T. G. Ksiazek, L. H. Elliott, E. V. Ravkov, M. L. Martin, S. Morzunov, W. Livingstone, M. Monroe, G. Glass, S. Ruo, et al. 1995. Isolation of black creek canal virus, a new hantavirus from Sigmodon hispidus in Florida. J. Med. Virol. 4635-39. [DOI] [PubMed] [Google Scholar]
- 23.Schmaljohn, A. L., D. Li, D. L. Negley, D. S. Bressler, M. J. Turell, G. W. Korch, M. S. Ascher, and C. S. Schmaljohn. 1995. Isolation and initial characterization of a newfound hantavirus from California. Virology 206963-972. [DOI] [PubMed] [Google Scholar]
- 24.St. Jeor, S. C. 2004. Three-week incubation period for hantavirus infection. Pediatr. Infect. Dis. J. 23974-975. [DOI] [PubMed] [Google Scholar]
- 25.Toro, J., J. D. Vega, A. S. Khan, J. N. Mills, P. Padula, W. Terry, Z. Yadon, R. Valderrama, B. A. Ellis, C. Pavletic, R. Cerda, S. Zaki, W. J. Shieh, R. Meyer, M. Tapia, C. Mansilla, M. Baro, J. A. Vergara, M. Concha, G. Calderon, D. Enria, C. J. Peters, and T. G. Ksiazek. 1998. An outbreak of hantavirus pulmonary syndrome, Chile, 1997. Emerg. Infect. Dis. 4687-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vial, P. A., F. Valdivieso, G. Mertz, C. Castillo, E. Belmar, I. Delgado, M. Tapia, and M. Ferres. 2006. Incubation period of hantavirus cardiopulmonary syndrome. Emerg. Infect. Dis. 121271-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wells, R. M., S. Sosa Estani, Z. E. Yadon, D. Enria, P. Padula, N. Pini, J. N. Mills, C. J. Peters, and E. L. Segura. 1997. An unusual hantavirus outbreak in southern Argentina: person-to-person transmission? Emerg. Infect. Dis. 3171-174. [DOI] [PMC free article] [PubMed] [Google Scholar]