Abstract
Recently, Misinzo et al. (G. Misinzo, P. Meerts, M. Bublot, J. Mast, H. M. Weingartl, and H. J. Nauwynck, J. Gen. Virol. 86:2057-2068, 2005) reported that inhibiting endosome-lysosome system acidification reduced porcine circovirus 2 (PCV2) infection of monocytic 3D4/31 cells. The present study examined the effect of inhibiting endosome-lysosome system acidification in epithelial cells, since epithelial cells support PCV2 infection in vivo and are used in culturing PCV2 in vitro. Ammonium chloride (NH4Cl), chloroquine diphosphate (CQ), and monensin were used to inhibit endosome-lysosome system acidification. NH4Cl, CQ, or monensin increased PCV2 (Stoon-1010) infection by 726% ± 110%, 1,212% ± 34%, and 1,100% ± 179%, respectively, in porcine kidney (PK-15) cells; by 128% ± 7%, 158% ± 3%, and 142% ± 11% in swine kidney cells; by 160% ± 28%, 446% ± 50%, and 162% ± 56% in swine testicle (ST) cells; and by 313% ± 25%, 611% ± 86%, and 352% ± 44% in primary kidney epithelial cells. Similarly, increased PCV2 infection was observed with six other PCV2 strains in PK-15 cells treated with endosome-lysosome system acidification inhibitors. The mechanism behind increased PCV2 infection was further investigated in PK-15 cells using CQ. PCV2 infection of PK-15 cells was increased only when CQ was added early during PCV2 infection. CQ did not affect PCV2 virus-like particle (VLP) attachment to PK-15 cells but increased the disassembly of internalized PCV2 VLPs. In untreated PK-15 cells, internalized PCV2 VLPs localized within the endosome-lysosome system. PCV2 infection of untreated 3D4/31 and PK-15 cells and CQ-treated PK-15 cells was blocked by a serine protease inhibitor [4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride] but not by aspartyl protease (pepstatin A), cysteine protease (E-64), and metalloprotease (phosphoramidon) inhibitors. These results suggest that serine protease-mediated PCV2 disassembly is enhanced in porcine epithelial cells but inhibited in monocytic cells after inhibition of endosome-lysosome system acidification.
Porcine circovirus 2 (PCV2) is a member of the family Circoviridae. PCV2 has a circular single-stranded DNA genome of 1,768 bases in which open reading frame 1 (ORF1) encodes replication-associated proteins (Rep and Rep′), ORF2 encodes a capsid protein, and ORF3 encodes a 105-amino-acid protein (9, 34, 52, 54). PCV2 infection is associated with postweaning multisystemic wasting syndrome (PMWS), a multifactorial disease in weaned pigs characterized by severe growth retardation, rapid weight loss, and high mortality rates in affected pigs (13, 20, 52). High PCV2 replication has been found to be associated with the development of PMWS and has thus been included as a criterion in the case definition of PMWS (61). In PCV2-positive swine herds and experimentally PCV2-inoculated pigs, only a small proportion of pigs experience high PCV2 replication and subsequently develop severe clinical signs or PMWS (33, 55, 59). The identification of factors that predispose individual pigs to high PCV2 replication compared to other pigs within the same herd is important in understanding the pathogenesis of PMWS. Some host-specific factors that influence the level of PCV2 replication in pigs have already been identified. The absence of PCV2-neutralizing antibodies (44); a general stimulation of the immune system arising from concurrent infections, vaccination, or concanavalin A treatment (3, 31, 32, 43, 56); and/or immunosuppression (30, 47) may predispose pigs to persistent high PCV2 replication and development of PMWS.
The susceptibilities of different cell types to PCV2 infection depend on the age of the fetus or the piglet (59). The differential susceptibilities of cells have been linked to mitosis, since PCV2 does not encode its own DNA polymerase and requires active cellular polymerases for viral replication (59). Treatment of cells in vitro with either glucosamine or gamma interferon increases PCV1 and PCV2 infection, respectively (46, 66). However, another factor(s) or mechanism(s) that explains the specific tropisms and differential susceptibilities of target cells to PCV2 infections may be involved. Misinzo et al. (51) showed that the susceptibility of the 3D4/31 monocytic-cell line to PCV2 infection was reduced following inhibition of endosome-lysosome system acidification, suggesting that an acidic environment is necessary for PCV2 infection. Generalization of this finding to other cell types is not possible. For instance, inhibition of endosome-lysosome system acidification inhibits human immunodeficiency virus type 1 (HIV-1) replication in primary T cells and monocytes, as well as T-cell and monocytic-cell lines (62), whereas it increases HIV-1 infectivity in human 293T and HeLa Magi cells (16).
Epithelial cells support PCV2 replication in pigs experimentally infected with PCV2, as well as in pigs with naturally occurring PCV2-associated PMWS (23, 57). In addition, PCV2 replicates and is cultivated in vitro in porcine kidney (PK-15) (2, 42) and swine kidney (SK) epithelial-cell lines (58). Until now, no study has investigated the effect of inhibiting endosome-lysosome system acidification on PCV2 infection of PCV2 target cells other than the monocytic-cell line 3D4/31. The aim of this study was to investigate the importance of endosome-lysosome system acidification on PCV2 infection of epithelial cells.
MATERIALS AND METHODS
Cells, virus, and PCV2 virus-like particles (VLP).
PK-15, SK, and swine testicle (ST) epithelial-cell lines and a 3D4/31 monocytic-cell line free from porcine circoviruses were used. Cells were seeded at 2 × 105/ml and maintained in culture medium containing 10% fetal bovine serum (FBS) (Invitrogen, Grand Island, MI), 0.3 mg/ml l-glutamine (BDH Chemicals, Ltd., Poole, England), 1% nonessential amino acids (100×; Invitrogen), 1 mM sodium pyruvate (Invitrogen), 100 U/ml penicillin, 0.1 mg/ml streptomycin, and 0.1 mg/ml kanamycin in RPMI 1640 (Invitrogen).
Primary porcine kidney epithelial cells were obtained by trypsinization of the kidney cortices from three 3-week-old PCV2-negative conventional piglets. The kidney cortices were dissected from the medullas after the removal of the kidney capsule, minced, and incubated in phosphate-buffered saline (PBS) containing 2.5 mg/ml trypsin (Sigma, Bornem, Belgium) at 37°C for 10 min. The trypsinization of the minced kidney cortices was done twice. After each trypsinization, primary kidney cells were collected by sieving them through three-layered cotton gauze. The cells were pelleted by centrifugation and resuspended at 2 × 105/ml in Dulbecco's modified Eagle medium (Invitrogen) containing 10% FBS, 0.3 mg/ml l-glutamine, and antibiotics.
The seeded cells were maintained at 37°C in a humidified 5% CO2 incubator. All experiments were carried out 24 h postseeding, when the cells reached approximately 50% confluence.
Different PCV2 strains passaged in PK-15 cells, previously described by Meerts et al. (45), were included in this study. The cells were inoculated with PCV2 strains Stoon-1010, 1121, 1103, 48285, VC2002, 1206, and 1147 at a multiplicity of infection of 0.3 for 1 h at 37°C.
Recombinant PCV2 VLP were used for analysis of PCV2 binding, intracellular localization, and disassembly in PK-15 cells as previously described (50, 51).
Effects of lysosomotropic agents on PCV2 infection of epithelial cells.
Endosome-lysosome system acidification was inhibited using lysosomotropic weak bases (ammonium chloride [NH4Cl] and chloroquine diphosphate [CQ]) and the carboxylic ionophore monensin. NH4Cl, CQ, and monensin are lysosomotropic agents because they selectively accumulate in lysosomes (12). Apart from inhibiting the acidification of the endosome-lysosome system, NH4Cl, CQ, and monensin decrease intralysosomal proteolysis and cause intracellular vesicular swelling (10, 14). Therefore, other lysosomotropic agents without an effect on endosome-lysosome system acidification but that (i) decrease intralysosomal proteolysis and cause intracellular vesicular swelling (suramin) (1, 26, 27) or (ii) have no effect on intralysosomal proteolysis and cause intracellular vesicular swelling (polyvinylpyrrolidone [PVP]) (10, 17, 27) were included. All chemical compounds were purchased from Sigma.
The highest concentrations for each of the lysosomotropic agents that did not affect PK-15 cell viability after a 24-h incubation period were used: 25 mM, 125 μM, 6 μM, 1 mg/ml, and 1 mg/ml of NH4Cl, CQ, monensin, suramin, and PVP, respectively. PK-15, ST, SK, and primary porcine kidney epithelial cells were washed once and pretreated or not with lysosomotropic agents for 1 h. The cells were inoculated with equal doses of PCV2 in the presence or absence of the lysosomotropic agents. The viral inoculum was washed off, and the cells were further incubated in culture medium with or without lysosomotropic agents (i) for 24 h to assess the effect on PCV2 infection or (ii) for different durations to determine the time points at which the lysosomotropic agents had their effects. Subsequently, culture medium with or without lysosomotropic agents was replaced with culture medium without lysosomotropic agents. After the first cycle of PCV2 replication, at 36 h postinoculation (p.i.), the cells were fixed with methanol at −20°C for 10 min.
For epithelial-cell lines, PCV2-infected cells were stained using an immunoperoxidase monolayer assay (IPMA) as previously described (50). PCV2-infected cells were counted by examination under an Olympus light microscope (Olympus Optical Co., Hamburg, Germany). The number of infected cells per well in untreated cells was used as a reference, and all results were expressed as a percentage of this reference.
For primary porcine kidney epithelial cells, a double immunofluorescence staining was performed to identify epithelial cells and PCV2-infected cells in primary kidney cell cultures. Kidney epithelial cells were identified with mouse anti-human cytokeratin monoclonal antibody (clone MNF116; DakoCytomation, Glostrup, Denmark), followed by incubation with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin G (IgG) (Invitrogen). PCV2-infected cells in kidney cell cultures were identified by incubation with biotinylated anti-PCV2 swine antibodies (59), followed by streptavidin-conjugated Texas Red (Invitrogen). Each of the staining incubations was carried out for 1 h at 37°C. Cell nuclei were stained by incubating the cells for 10 min at room temperature with Hoechst 33342 (Invitrogen) at a concentration of 10 μg/ml. After each of the incubations, the cells were washed with PBS. Finally, the stained cells were mounted, and analysis of the number of PCV2-infected kidney epithelial cells per 104 cells was done by using a Leica DM/RBE fluorescence microscope. The number of infected cells among the untreated cells was used as a reference, and all results were expressed as a percentage of this reference.
All experiments were performed three times, and each condition in an experiment was performed in quadruplicate.
Effects of inhibiting endosome-lysosome system acidification on PCV2 VLP attachment and disassembly of internalized PCV2 VLP.
PK-15 cells were chilled on ice and washed with ice-cold RPMI 1640. PCV2 VLP were then added at 4°C for 1 h in the presence or absence of 125 μM CQ to allow binding to PK-15 cells. Unbound PCV2 VLP were washed off, and the cells were refreshed with either prewarmed medium without FBS or similar culture medium containing 125 μM CQ. The PK-15 cells were then incubated at 37°C in a humidified 5% CO2 incubator. At 0 and 3 h after the 37°C shift, the cells were fixed in 3% (wt/vol) paraformaldehyde in PBS containing Ca2+ and Mg2+ (PBS+). A double immunofluorescence staining was performed in order to distinguish bound and internalized PCV2 VLP. Bound PCV2 VLP were stained using the anti-PCV2 capsid-specific monoclonal antibody F190 (40), followed by FITC-conjugated goat anti-mouse IgG. The cells were subsequently washed with PBS+ and permeabilized with Triton X-100 (0.1% in PBS+) for 2 min at room temperature. After permeabilization, all PCV2 VLP were identified using the same monoclonal antibody F190, followed by Texas Red-conjugated goat anti-mouse IgG (Invitrogen). Finally, the cells were mounted and analyzed using a Leica TCS SP2 laser scanning spectral confocal system linked to a Leica DM/IRB inverted microscope. The total fluorescence area of attached PCV2 VLP in cells treated or not with CQ was estimated using the image analysis software SigmaScan Pro 5.0 as previously described (50, 51).
Intracellular localization of internalized PCV2 VLP in PK-15 cells.
To identify the intracellular organelle(s) in which PCV2 VLP localizes, PK-15 cells were chilled on ice, washed with ice-cold RPMI 1640, and incubated with PCV2 VLP for 1 h at 4°C. Unbound PCV2 VLP were washed off, and the cells were refreshed with prewarmed medium without FBS. The PK-15 cells were then incubated at 37°C in a humidified 5% CO2 incubator for 3 h, after which they were fixed in 3% (wt/vol) paraformaldehyde in PBS+. The cells were subsequently washed and permeabilized with Triton X-100 (0.1% in PBS+) for 2 min at room temperature. Intracellular localization of PCV2 VLP was determined by a double immunofluorescence labeling of internalized PCV2 VLP and early endosomes, lysosomes, or Golgi apparatus and/or the endoplasmic reticulum. Early endosomes were labeled using goat polyclonal anti-early endosome antigen 1 (EEA-1) IgG (Santa Cruz Biotechnology, Santa Cruz, CA), the lysosomes were labeled with goat polyclonal anti-cathepsin D IgG (Santa Cruz Biotechnology), the Golgi apparatus was labeled using rabbit polyclonal anti-giantin IgG (Eurogentec, Seraing, Belgium), and the endoplasmic reticulum was labeled using goat polyclonal anti-calnexin IgG (Santa Cruz Biotechnology). The cells were then incubated with Alexa Fluor 594-conjugated rabbit anti-goat (Invitrogen) to recognize goat polyclonal IgG or Texas Red-conjugated goat anti-rabbit (Invitrogen) to recognize rabbit polyclonal IgG. PCV2 VLP were labeled using the anti-PCV2 capsid-specific monoclonal antibody F190, followed by FITC-conjugated goat anti-mouse IgG. The cells were mounted, and images were acquired by confocal microscopy.
Effects of protease inhibitors on PCV2 infection of CQ-treated PK-15 cells.
The protease inhibitors 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride (AEBSF) (which inhibits serine proteases), pepstatin A (which inhibits aspartyl proteases), trans-epoxysuccinyl-l-leucylamido-(4-guanidino) butane (E-64) (which inhibits cysteine proteases), and phosphoramidon (which inhibits metalloproteases) were used to analyze the potential involvement of proteases during PCV2 infection and to identify the class of cellular protease potentially responsible for PCV2 capsid disassembly. All protease inhibitors were from Sigma. PK-15 and 3D4/31 cells were preincubated with or without 0.5 mM or 0.25 mM AEBSF, 1 μg/ml pepstatin A, 100 μM E-64, or 45 μM phosphoramidon for 1 h at 37°C. After 1 h, PK-15 and 3D4/31 cells were inoculated with PCV2 (Stoon-1010) for 1 h at 37°C in the presence or absence of protease inhibitors. The cells were then washed and incubated for 24 h in culture medium with or without protease inhibitors containing 125 μM CQ for PK-15 cells and 0 μM CQ for 3D4/31 cells. At 24 h p.i., the culture medium with or without protease inhibitors and CQ was replaced with fresh culture medium. The cells were fixed at 36 h p.i., and an IPMA staining was performed to identify PCV2-infected cells. The number of PCV2-infected PK-15 cells among cells treated with a combination of a protease inhibitor and CQ was expressed as a percentage of the number of PCV2-infected cells among cells treated with CQ alone. The number of PCV2-infected 3D4/31 cells among cells treated with a protease inhibitor was expressed as a percentage of the number of PCV2-infected cells among untreated cells.
Effects of protease inhibitors on disassembly of internalized PCV2 VLP in CQ-treated PK-15 cells.
PK-15 cells were preincubated with or without 0.5 mM AEBSF for 1 h at 37°C. Afterwards, the PK-15 cells were chilled on ice and washed with ice-cold RPMI 1640. PCV2 VLP were then added at 4°C for 1 h in the presence of 125 μM CQ to allow binding to the PK-15 cells. Unbound PCV2 VLP were washed off, and the cells were refreshed with either prewarmed medium without FBS or similar culture medium containing 125 μM CQ with or without 0.5 mM AEBSF. The PK-15 cells were then incubated at 37°C in a humidified 5% CO2 incubator. At 0 and 3 h after the 37°C shift, the cells were fixed in 3% (wt/vol) paraformaldehyde in PBS+. The double immunofluorescence staining to distinguish bound and internalized PCV2 VLP, confocal microscopy, and analysis were performed as described above for the investigation of the effect of inhibiting endosome-lysosome system acidification on disassembly of internalized PCV2 VLP.
RESULTS
Inhibiting endosome-lysosome system acidification enhances the number of PCV2-infected cells among porcine epithelial cells.
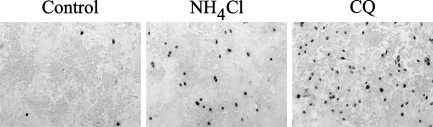
The endosome-lysosome system is characterized by gradual acidification of its vesicles as they mature from early endosomes (pH ∼6.0 to 6.8) into lysosomes (pH ∼5.0) (48). Endosome-lysosome system acidification can be inhibited by some lysosomotropic agents. Epithelial-cell lines (PK-15, SK, and ST) and primary porcine kidney epithelial cells were treated with lysosomotropic agents to investigate the effect of inhibiting endosome-lysosome system acidification on PCV2 infection. The effects of different lysosomotropic agents on PCV2 infection of epithelial cells inoculated with the prototype PCV2 strain Stoon-1010 are shown in Fig. 1 and 2 and Tables 1 and 2. In general, greater increases in the numbers of PCV2-infected cells were observed in PK-15 and primary porcine kidney epithelial cells than in SK and ST cells treated with NH4Cl, CQ, monensin, and suramin (Table 2). Treatment of cells with PVP did not increase the number of PCV2-infected cells in any epithelial-cell type.
FIG. 1.
Effects of lysosomotropic weak bases on PCV2 Stoon-1010 infection of PK-15 cells. PK-15 cells were treated with RPMI 1640 (control), 25 mM NH4Cl, or 125 μM CQ from 1 h before virus inoculation until 24 h after virus inoculation. The cells were inoculated with the same dose of PCV2 (Stoon-1010) for 1 h. At 36 h after virus inoculation, the cells were fixed and stained for PCV2 antigens by IPMA. Magnification, ×50.
FIG. 2.
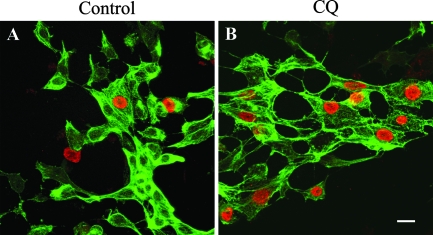
PCV2 infection of primary kidney epithelial cells. Primary kidney cell cultures were obtained by trypsinization of kidney cortices. After 24 h in culture, the kidney cells were inoculated with PCV2 (Stoon-1010) and further cultured in cell culture medium (A) or cell culture medium containing 125 μM CQ (B). The cells were fixed 36 h after virus inoculation and analyzed by fluorescence confocal microscopy after a double immunofluorescence staining was performed to visualize epithelial cells (cytokeratin; green fluorescence) and PCV2 antigens (red fluorescence). The susceptibility of primary kidney epithelial cells to PCV2 infection increased following treatment with CQ (B). Each panel represents an overlay of confocal images taken from the apex to the base of the cell. Bar, 20 μm.
TABLE 1.
Effects of lysosomotropic agents on PCV2 (Stoon-1010) infection of PK-15 cells
| Lysosomotropic agent | Concn | % PCV2-infected cellsa |
|---|---|---|
| NH4Cl | 25.0 mM | 726 ± 110 |
| 6.25 mM | 540 ± 96 | |
| 1.56 mM | 252 ± 79 | |
| CQ | 125.0 μM | 1,212 ± 34 |
| 31.25 μM | 834 ± 169 | |
| 7.81 μM | 501 ± 121 | |
| Monensin | 6 μM | 1,100 ± 179 |
| 1.5 μM | 859 ± 134 | |
| 0.375 μM | 574 ± 24 | |
| Suramin | 1,000 μg/ml | 237 ± 29 |
| 250 μg/ml | 184 ± 23 | |
| 62.5 μg/ml | 133 ± 28 | |
| PVP | 1,000 μg/ml | 73 ± 6 |
| 250 μg/ml | 91 ± 3 | |
| 62.5 μg/ml | 95 ± 10 |
The percentages of PCV2-infected PK-15 cells following treatment with lysosomotropic agents are expressed relative to the number of PCV2-infected cells among untreated PK-15 cells. The data represent means ± standard deviations from three experiments, with each experiment performed in quadruplicate.
TABLE 2.
Effects of lysosomotropic agents on infection of the prototype PCV2 strain Stoon-1010 in PK-15, SK, ST, and PPKE cells
| Cell type | % PCV2-infected cells treated witha:
|
||||
|---|---|---|---|---|---|
| NH4Cl | CQ | Monensin | Suramin | PVP | |
| PK-15 | 726 ± 110 | 1,212 ± 34 | 1,100 ± 179 | 237 ± 29 | 73 ± 6 |
| SK | 128 ± 7 | 158 ± 3 | 142 ± 11 | 118 ± 3 | 80 ± 27 |
| ST | 160 ± 28 | 446 ± 50 | 162 ± 56 | 117 ± 5 | 86 ± 17 |
| PPKEb | 313 ± 25 | 611 ± 86 | 352 ± 44 | 109 ± 15 | 90 ± 19 |
The numbers of PCV2-infected cells following treatment with lysosomotropic agents are expressed as percentages of the numbers of PCV2-infected cells among the respective untreated cells. The data represent means ± standard deviations of three experiments, with each experiment performed in quadruplicate.
PPKE, primary porcine kidney epithelial.
Different PCV2 strains were used to investigate if the enhancement of PCV2 infection by inhibitors of endosome-lysosome system acidification is general in other PCV2 strains. PCV2 infection was increased in PK-15 cells with all strains following treatment with NH4Cl, CQ, monensin, and suramin (Table 3). The magnitudes of the increases of PCV2 infection following inhibition of endosome-lysosome system acidification were different between strains, with PCV2 strain 1103 showing the least increase in the number of infected cells. No significant increase in the number of PCV2-infected PK-15 cells was found in cells treated with PVP.
TABLE 3.
Effects of lysosomotropic agents on infection of different PCV2 strains of PK-15 cells
| PCV2 strain | % PCV2-infected cells treated witha:
|
||||
|---|---|---|---|---|---|
| NH4Cl | CQ | Monensin | Suramin | PVP | |
| Stoon-1010 | 726 ± 110 | 1,212 ± 34 | 1,100 ± 179 | 237 ± 29 | 73 ± 6 |
| 1121 | 1,041 ± 277 | 1,452 ± 62 | 752 ± 148 | 206 ± 24 | 60 ± 10 |
| 1103 | 138 ± 86 | 183 ± 151 | 115 ± 12 | 128 ± 10 | 115 ± 9 |
| 48285 | 345 ± 147 | 856 ± 76 | 994 ± 119 | 434 ± 71 | 102 ± 9 |
| VC2002 | 340 ± 92 | 905 ± 150 | 963 ± 246 | 271 ± 67 | 94 ± 20 |
| 1206 | 235 ± 88 | 446 ± 87 | 792 ± 206 | 344 ± 109 | 95 ± 2 |
| 1147 | 234 ± 88 | 530 ± 185 | 457 ± 42 | 201 ± 46 | 108 ± 2 |
The percentages of PK-15 PCV2-infected cells following treatment with lysosomotropic agents are expressed relative to the number of PK-15 PCV2-infected cells among untreated cells. The data represent means ± standard deviations of results from three experiments, with each experimental condition performed in quadruplicate.
CQ treatment of PK-15 cells increases the disassembly of internalized PCV2 VLP.
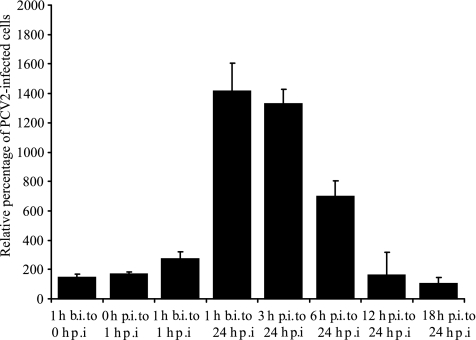
In order to examine the stage during the PCV2 replication cycle in which lysosomotropic agents exert their effects, CQ was added at different time points before, during, or after PCV2 inoculation, as shown in Fig. 3. Addition of CQ for 1 h before inoculation, for 1 h during inoculation, or from 12 h p.i. onward did not increase the number of PCV2-infected PK-15 cells significantly. However, addition of CQ during the first 6 hours of PCV2 infection increased the number of PCV2-infected PK-15 cells significantly (Fig. 3). Thus, inhibiting endosome-lysosome system acidification increases PCV2 infection by affecting an early stage(s) of PCV2 infection.
FIG. 3.
Effects of CQ at different time points and durations throughout the PCV2 infection cycle. PK-15 cells were inoculated with the same dose of PCV2 Stoon-1010 for 1 h (0 to 1 h p.i.), after which the viral inoculum was washed off. CQ (0 or 125 μM) was added at different times before (b.i.) or after (p.i.) virus inoculation and left with the cells for different durations, as indicated. After 36 h p.i., the cells were fixed and stained for PCV2 antigens. The percentages of PCV2-infected PK-15 cells following CQ treatment are expressed relative to the number of PCV2-infected PK-15 cells among untreated cells. The data represent means plus standard deviations of results from three experiments, with each experimental condition performed in quadruplicate.
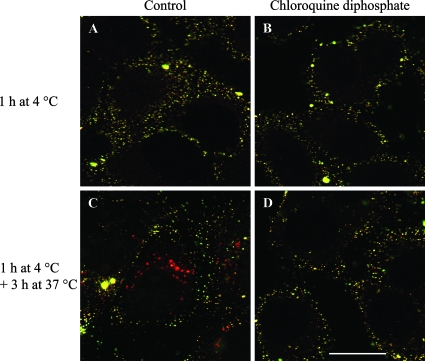
The effects of CQ on early stages of PCV2 entry, such as attachment and disassembly, were further investigated. To examine attachment, PCV2 VLP were allowed to bind to PK-15 cells for 1 h at 4°C in the absence or presence of CQ. The relative total fluorescences of bound PCV2 VLP in PK-15 cells treated or not with CQ were 107 ± 22 and 100 ± 20, respectively. In order to examine the effects of endosome-lysosome system acidification inhibition on PCV2 internalization and disassembly, cells were incubated with PCV2 VLP for 3 h at 37°C in the absence or presence of CQ. PCV2 VLP were visible within the cells after 3 h of incubation in untreated cells, but not in CQ-treated cells (Fig. 4). This observation indicates that under normal circumstances PCV2 is slowly disassembled within the cell and that inhibiting endosome-lysosome system acidification provides an optimal environment that enhances PCV2 disassembly.
FIG. 4.
Effects of CQ treatment on PCV2 attachment and internalization. PCV2 VLP were either allowed to bind to PK-15 cells for 1 h at 4°C or further incubated for 3 h at 37°C in the absence or presence of 125 μM CQ and then fixed with 3% paraformaldehyde. PCV2 VLP were stained with F190 monoclonal antibody and FITC-conjugated goat anti-mouse, followed by permeabilization of the cells. PCV2 VLP were stained again with F190 and Texas Red-conjugated goat anti-mouse antibodies. (A to D) Bound PCV2 VLP showed both green and red fluorescence (yellow), while internalized PCV2 VLP showed only red fluorescence in merged confocal images of single z sections. Internalized PCV2 VLP were visible in untreated cells (C), while no PCV2 VLP were visible within the cell in CQ-treated cells (D), most probably due to increased disassembly of internalized PCV2 VLP. Bar, 20 μm.
Internalized PCV2 VLP localize within endosome-lysosome system compartments of PK-15 cells.
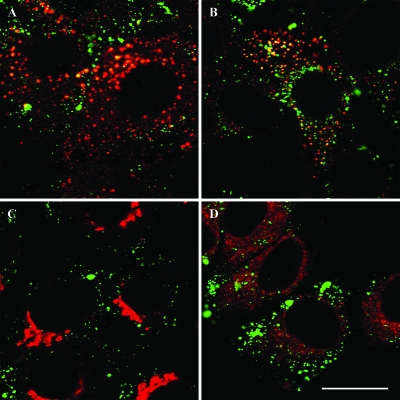
To allow a better understanding of the mechanism by which CQ enhances PCV2 infection, identification of the intracellular compartment in which PCV2 is transported following internalization was performed. Intracellular localization of PCV2 VLP was determined using double immunofluorescent labeling for PCV2 VLP and markers of early endosomes, lysosomes, the Golgi apparatus, and the endoplasmic reticulum. Immunofluorescent labeling of PCV2 VLP prior to shifting the cells to 37°C demonstrated no colocalization of PCV2 VLP with markers for early endosome, lysosome, Golgi apparatus, and endoplasmic reticulum. PCV2 VLP within the cell, 3 h after internalization (shift to 37°C), colocalized with early endosomes and lysosomes, but not with the Golgi apparatus and the endoplasmic reticulum (Fig. 5).
FIG. 5.
Colocalization of internalized PCV2 VLP with cellular organelles. PCV2 VLP were allowed to bind to PK-15 cells at 4°C for 1 h, after which the cells were shifted to 37°C to initiate PCV2 VLP internalization. After 3 h of internalization, the cells were fixed and stained for PCV2 VLP (green fluorescence) and one of the cellular organelles (red fluorescence). Representative merged images of PCV2 VLP and early endosomes (A), lysosomes (B), the Golgi apparatus (C), and the endoplasmic reticulum (D) after 3 h of internalization are shown. Bar, 20 μm.
Proteolytic cleavage of PCV2 capsid is mediated by a cellular serine protease.
Internalized PCV2 VLP disassembled within endosome-lysosome system compartments in CQ-treated PK-15 cells. Because disassembly of viruses within the endosome-lysosome system can be mediated by proteases, the roles of cellular proteases in PCV2 infection were investigated. Cellular proteases are classified into serine, cysteine, and aspartic proteases and metalloproteases (4). PCV2 infection was completely blocked when CQ-treated PK-15 cells were incubated with 0.5 mM AEBSF. Treatment of non-CQ-treated PK-15 cells with 0.5 mM AEBSF also completely blocked PCV2 infection. The relative numbers of PCV2-infected cells among CQ-treated PK-15 cells were 30% ± 14%, 82% ± 2%, 84% ± 3%, and 82% ± 14% when they were incubated with 0.25 mM AEBSF, 1 μg/ml pepstatin A, 100 μM E-64, and 45 μM phosphoramidon, respectively. To investigate if the reductions of infection resulted from an effect on PCV2 disassembly, the effect of AEBSF on the uncoating of PCV2 VLP was analyzed. The disassembly of PCV2 VLP observed in CQ-treated PK-15 cells was inhibited by AEBSF. The accumulation of internalized PCV2 VLP in CQ-treated PK-15 cells treated with AEBSF indicated that the absence of PCV2 VLP within the cell in CQ-treated cells was not a result of CQ interference with internalization but rather on accelerated disassembly of internalized PCV2 VLP. The effect of inhibiting cellular protease on PCV2 infection was also investigated in 3D4/31 cells, in which inhibiting endosome-lysosome system acidification in monocytic cells reduced PCV2 infection, in contrast to epithelial cells. PCV2 infection of 3D4/31 cells was also completely blocked by treatment of the cells with 0.5 mM AEBSF. The relative numbers of PCV2-infected cells among 3D4/31 cells were 36% ± 14%, 128% ± 26%, 70% ± 10%, and 93% ± 10% when 3D4/31 cells were incubated with 0.25 mM AEBSF, 1 μg/ml pepstatin A, 100 μM E-64, and 45 μM phosphoramidon, respectively.
DISCUSSION
In the present study, inhibition of endosome-lysosome system acidification enhanced PCV2 infection of porcine epithelial cell lines, as well as of primary porcine kidney epithelial cells. Increased PCV2 infection occurred when endosome-lysosome system acidification was inhibited during early stages of PCV2 infection. The effects of inhibitors of endosome-lysosome system acidification were not at the level of PCV2 capsid attachment but at the level of PCV2 capsid disassembly of internalized PCV2. The disassembly of PCV2 capsid was mediated by a serine protease.
To our knowledge, the present study is the first to show enhanced infection of a single-stranded DNA nonenveloped virus as a result of elevating the pH of the endosome-lysosome system. The fact that neutralizing the acidic endosome-lysosome system enhances PCV2 infection of epithelial cells is in contrast with numerous studies that have shown that neutralizing the acidic endosome-lysosome system inhibits the infection of pH-dependent nonenveloped viruses (7, 8, 18, 35, 36, 63) and enveloped viruses (21, 38, 64, 65, 70). Inhibiting endosome-lysosome system acidification also inhibits PCV2 infection of 3D4/31 monocytic cells (51), further contrasting the results presented in this study for epithelial cells. In vitro, inhibition of endosome-lysosome system acidification has been shown to increase the infection of enveloped HIV-1 (16, 69). Most HIV-1 particles are endocytosed (37, 60) and do not result in productive infection because an acidic environment within the endosome-lysosome system leads to virus degradation in lysosomes (15, 37, 60). Inhibition of endosome-lysosome system acidification has been suggested to slow down the proteolytic degradation of endocytosed infectious HIV-1 particles, thus providing sufficient time for HIV-1 to fuse with endocytic membranes and deliver virion cores to the cytoplasm (16). PCV2 is a nonenveloped virus and does not uncoat by fusion with endocytic membranes. Instead, this study shows that a serine protease-mediated PCV2 capsid disassembly is essential for PCV2 uncoating. Several possible scenarios may explain the enhanced PCV2 infection of epithelial cells resulting from elevated pH within the endosome-lysosome system. First, inhibiting endosome-lysosome system acidification may provide sufficient time and optimum pH for a serine protease to disassemble the PCV2 capsid. Inhibiting endosome-lysosome system acidification causes all compartments of the endosome-lysosome system to have an “endosome-like” pH. This “endosome-like” pH is assumed to be optimal for serine proteases that cleave the PCV2 capsid. The inhibitors of endosome-lysosome system acidification also inhibit the maturation of endosomes to lysosomes, further restricting the localization of internalized PCV2 within endosomes, where optimum PCV2 capsid cleavage seems to occur. Second, inhibitors of endosome-lysosome system acidification may directly activate certain proteases within the endosome-lysosome system (5, 28, 29), which may be responsible for the PCV2 capsid dissasembly. In this way, PCV2 capsid disassembly may be increased. Third, inhibition of endosome-lysosome system acidification alters the sorting of certain proteins (including proteases) that are normally destined to be transported to the plasma membrane to be directed toward endosome-lysosome system compartments (24, 25). If these proteins happen to be proteases that cleave PCV2 capsid, enhanced PCV2 disassembly may occur, explaining the observed increase in PCV2 infection. Fourth, lysosomotropic agents that inhibit endosome-lysosome system acidification can also cause intracellular vesicular swelling (10, 14, 49), which may result in increased release of endocytosed cargo into the cytoplasm. This is unlikely for endocytosed PCV2, since PVP, a lysosomotropic agent with no effect on endosome-lysosome system acidification but which causes osmosis-related intracellular vesicular swelling (10, 17, 27), did not affect PCV2 infection. Surprisingly, suramin, which causes intracellular vesicular swelling and decreases intralysosomal proteolysis (7, 8, 26, 27, 29), slightly increased PCV2 infection of epithelial cells. Another suramin activity may be responsible for the enhanced PCV2 infection, such as the activation of DNA synthesis (53, 71), since PCV2 replication is cell cycle dependent and the stimulation of cellular proliferation by suramin may help PCV2 to replicate.
PCV2 infects PK-15 and SK porcine epithelial cell lines (2, 40, 42, 58). In this study, a testis-derived epithelial-cell line (ST) was susceptible to PCV2 infection, showing that epithelial cells from other organs may also be susceptible to PCV2 infection. PCV2 infects epithelial cells in pigs experimentally infected with PCV2 (23), in PMWS pigs (13, 57), and in primary kidney cultures (22), based on histological and morphological identification. This study confirmed the susceptibility of primary kidney epithelial cells to PCV2 infection using double immunofluorescence labeling of PCV2 antigens and cytokeratin, an epithelial-cell-specific intermediate filament. The susceptibility of primary kidney epithelial cells was equal to that observed in PK-15 cells, suggesting that the latter provide a good in vitro model for studying PCV2 interactions with epithelial cells. This observation is supported by previous studies that have reported that PK-15 cells display cellular growth characteristics similar to those of primary epithelial cells in culture (11).
This study investigated the effects of inhibiting endosome-lysosome system acidification on the infection of different PCV2 strains isolated from cases of PMWS (13, 41, 47), porcine dermatitis and nephropathy syndrome (41), or reproductive failure (41). All PCV2 strains (with the exception of the 1103 strain) showed increased infection upon inhibition of endosome-lysosome system acidification; however, differences in magnitude were observed. This indicates that, although a common PCV2 infection mechanism at the level of disassembly in epithelial cells is shared by PMWS-, porcine dermatitis and nephropathy syndrome-, and reproductive-failure-associated PCV2 strains, there are subtle differences that differentiate them. These might arise from differences in amino acid sequence at a protease cleavage site(s) that may cause the different susceptibilities of PCV2 capsids to cleavage by a cellular serine protease(s). At this point, these differences cannot be determined, because the serine protease(s) responsible for PCV2 capsid disassembly is unknown. Other studies have also reported differences in the replication kinetics of different PCV2 strains isolated from distinct PCV2-related diseases (45). Why the replication of strain 1103 could not be influenced by the inhibitors of endosome-lysosome system acidification is not clear. It is possible that the serine protease cleavage site is absent in this strain.
PCV2 is internalized via clathrin-mediated endocytosis in monocytic (51) and dendritic (67) cells. Although the mechanism of PCV2 entry in epithelial cells was not investigated, the localization of PCV2 in early endosomes in epithelial cells suggests that PCV2 becomes internalized via a pathway whose vesicles bud on the early endosome. Proteins endocytosed into early endosomes can be recycled to the cell surface, transported to the trans-Golgi network, or routed to late endosomes and lysosomes (19). Internalized PCV2 was localized in lysosomes but not in the trans-Golgi network, indicating that PCV2 was transported from endosomes onto lysosomes. The exact internalization pathway for PCV2 in epithelial cells remains to be determined.
The present study showed that a serine protease is also important in the disassembly of PCV2 in monocytic 3D4/31 cells. Because PCV2 disassembly requires a low pH in 3D4/31 cells and a neutral pH in epithelial cells, it is reasonable to suggest that two different serine proteases in these cell types are involved in PCV2 disassembly. Further studies are required to identify these serine proteases.
PCV2 had been circulating in the pig population for years before the emergence of PMWS (61). The reason why PMWS emerged suddenly is still not clear. One may speculate that drugs that inhibit endosome-lysosome system acidification may predispose pigs to high PCV2 replication and PMWS. The carboxylic ionophore monensin has been used for the prevention or treatment of certain porcine diseases, and another carboxylic ionophore, salinomycin, has been used as a growth promoter in pigs (6). Whether PCV2 infection can be enhanced in pigs, which may result in PMWS, following administration of endosome-lysosome system acidification inhibitors in PCV2-infected pigs remains to be investigated. Further, different human pathophysiological conditions characterized by failure of endosome-lysosome system acidification, such as hereditary and acquired forms of the Fanconi syndrome, have been reported (39, 68). It is not known if such conditions exist in pigs or, if they exist, whether they predispose pigs to PCV2-induced PMWS.
Acknowledgments
The skillful technical assistance of Carine Boone, Chris Bracke, Chantal Vanmaercke, Nele Dennequin, and Lieve Sys is appreciated. PCV2 VLP were a kind gift from Michel Bublot (Merial SAS, Biological Research, Lyon, France). We also acknowledge Francis McNeilly and Gordon Allan (Department of Agriculture and Rural Development for Northern Ireland, Veterinary Sciences Division, United Kingdom) for kindly providing the PCV2-specific monoclonal antibody F190.
This work was funded by the European Union (Sixth Framework Programme, Project no. 513928, coordinated by G. Allan). G.M. was supported by a scholarship for candidates from developing countries from the Special Research Fund (BOF) of Ghent University. P.L.D was supported by a fellowship (B/06524) from BOF of Ghent University.
Footnotes
Published ahead of print on 21 November 2007.
REFERENCES
- 1.Akanji, M. A. 1988. Rat kidney lysosomal membrane damage induced by suramin in vitro and in vivo. Pharmacol. Toxicol. 62318-321. [DOI] [PubMed] [Google Scholar]
- 2.Allan, G. M., F. McNeilly, B. M. Meehan, S. Kennedy, D. P. Mackie, J. A. Ellis, E. G. Clark, E. Espuna, N. Saubi, P. Riera, A. Botner, and C. E. Charreyre. 1999. Isolation and characterisation of circoviruses from pigs with wasting syndromes in Spain, Denmark and Northern Ireland. Vet. Microbiol. 66115-123. [DOI] [PubMed] [Google Scholar]
- 3.Allan, G. M., F. McNeilly, J. Ellis, S. Krakowka, B. Meehan, I. McNair, I. Walker, and S. Kennedy. 2000. Experimental infection of colostrum deprived piglets with porcine circovirus 2 (PCV2) and porcine reproductive and respiratory syndrome virus (PRRSV) potentiates PCV2 replication. Arch. Virol. 1452421-2429. [DOI] [PubMed] [Google Scholar]
- 4.Barrett, A. J. 1994. Classification of peptidases. Methods Enzymol. 2441-15. [DOI] [PubMed] [Google Scholar]
- 5.Bednarski, E., and G. Lynch. 1998. Selective suppression of cathepsin L results from elevations in lysosomal pH and is followed by proteolysis of tau protein. Neuroreport 92089-2094. [DOI] [PubMed] [Google Scholar]
- 6.Butaye, P., L. A. Devriese, and F. Haesebrouck. 2003. Antimicrobial growth promoters used in animal feed: effects of less well known antibiotics on gram-positive bacteria. Clin. Microbiol. Rev. 16175-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canning, W. M., and B. N. Fields. 1983. Ammonium chloride prevents lytic growth of reovirus and helps to establish persistent infection in mouse L cells. Science 219987-988. [DOI] [PubMed] [Google Scholar]
- 8.Carrillo, E. C., C. Giachetti, and R. H. Campos. 1984. Effect of lysosomotropic agents on the foot-and-mouth disease virus replication. Virology 135542-545. [DOI] [PubMed] [Google Scholar]
- 9.Cheung, A. K. 2003. Transcriptional analysis of porcine circovirus type 2. Virology 305168-180. [DOI] [PubMed] [Google Scholar]
- 10.Ciftci, K., and R. J. Levy. 2001. Enhanced plasmid DNA transfection with lysosomotropic agents in cultured fibroblasts. Int. J. Pharm. 21881-92. [DOI] [PubMed] [Google Scholar]
- 11.Connolly, J. A., V. I. Kalnins, and B. H. Barber. 1981. Microtubules and microfilaments during cell spreading and colony formation in PK 15 epithelial cells. Proc. Natl. Acad. Sci. USA 786922-6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Duve, C., T. de Barsy, B. Poole, A. Trouet, P. Tulkens, and F. Van Hoof. 1974. Lysosomotropic agents. Biochem. Pharmacol. 232495-2531. [DOI] [PubMed] [Google Scholar]
- 13.Ellis, J., L. Hassard, E. Clark, J. Harding, G. Allan, P. Willson, J. Strokappe, K. Martin, F. McNeilly, B. Meehan, D. Todd, and D. Haines. 1998. Isolation of circovirus from lesions of pigs with postweaning multisystemic wasting syndrome. Can. Vet. J. 3944-51. [PMC free article] [PubMed] [Google Scholar]
- 14.Erbacher, P., A. C. Roche, M. Monsigny, and P. Midoux. 1996. Putative role of chloroquine in gene transfer into a human hepatoma cell line by DNA/lactosylated polylysine complexes. Exp. Cell Res. 225186-194. [DOI] [PubMed] [Google Scholar]
- 15.Fackler, O. T., and B. M. Peterlin. 2000. Endocytic entry of HIV-1. Curr. Biol. 101005-1008. [DOI] [PubMed] [Google Scholar]
- 16.Fredericksen, B. L., B. L. Wei, J. Yao, T. Luo, and J. V. Garcia. 2002. Inhibition of endosomal/lysosomal degradation increases the infectivity of human immunodeficiency virus. J. Virol. 7611440-11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gavrilova, N. I., A. B. Putyshev, and T. A. Korolenko. 1982. Lysosomotropic properties of polyvinylpyrrolidone (an experimental study). Biull. Eksp. Biol. Med. 9458-60. [PubMed] [Google Scholar]
- 18.Greber, U. F., M. Willetts, P. Webster, and A. Helenius. 1993. Stepwise dismantling of adenovirus 2 during entry into cells. Cell 75477-486. [DOI] [PubMed] [Google Scholar]
- 19.Gruenberg, J. 2001. The endocytic pathway: a mosaic of domains. Nat. Rev. Mol. Cell Biol. 2721-730. [DOI] [PubMed] [Google Scholar]
- 20.Harding, J. C. S. 1996. Postweaning multisystemic wasting syndrome (PMWS): preliminary epidemiology and clinical presentation, p. 21. Proc. Western Canadian Assoc. Swine Practitioners.
- 21.Helenius, A., M. Marsh, and J. White. 1982. Inhibition of Semliki forest virus penetration by lysosomotropic weak bases. J. Gen. Virol. 5847-61. [DOI] [PubMed] [Google Scholar]
- 22.Hirai, T., T. Nunoya, T. Ihara, T. Saitoh, K. Shibuya, and K. Nakamura. 2006. Infectivity of porcine circovirus 1 and circovirus 2 in primary porcine hepatocyte and kidney cell cultures. J. Vet. Med. Sci. 68179-182. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy, S., D. Moffett, F. McNeilly, B. Meehan, J. Ellis, S. Krakowka, and G. M. Allan. 2000. Reproduction of lesions of postweaning multisystemic wasting syndrome by infection of conventional pigs with porcine circovirus type 2 alone or in combination with porcine parvovirus. J. Comp. Pathol. 1229-24. [DOI] [PubMed] [Google Scholar]
- 24.Klionsky, D. J., H. Nelson, and N. Nelson. 1992. Compartment acidification is required for efficient sorting of proteins to the vacuole in Saccharomyces cerevisiae. J. Biol. Chem. 2673416-3422. [PubMed] [Google Scholar]
- 25.Klionsky, D. J., H. Nelson, N. Nelson, and D. S. Yaver. 1992. Mutations in the yeast vacuolar ATPase result in the mislocalization of vacuolar proteins. J. Exp. Biol. 17283-92. [DOI] [PubMed] [Google Scholar]
- 26.Korolenko, T. A., A. B. Pupyshev, and A. E. Malygin. 1981. Heterophagic function and rate of intralysosomal proteolysis during lysosomotropic agents administration. Acta Biol. Med. Ger. 401613-1617. [PubMed] [Google Scholar]
- 27.Korolenko, T. A., A. B. Pupyshev, A. E. Malygin, N. I. Gavrilova, and N. G. Kurysheva. 1985. Facilitation of the structural and functional disorders of liver lysosomes in toxic hepatitis due to the suppression of intralysosomal proteolysis. Biull. Eksp. Biol. Med. 100169-172. [PubMed] [Google Scholar]
- 28.Korolenko, T. A., E. V. Rukavishnikova, and A. B. Pupyshev. 1990. The effect of single and repeated administration of chloroquine on the activity of lysosomal proteinases in rat liver cells. Vopr. Med. Khim. 3620-23. [PubMed] [Google Scholar]
- 29.Korolenko, T. A., E. V. Rukavishnikova, A. F. Safina, G. I. Mynkina, and E. I. Vereshchagin. 1991. Effects of chloroquine on lysosomes and endocytosis by liver cells in vivo. Acta Biol. Hung. 42301-309. [PubMed] [Google Scholar]
- 30.Krakowka, S., J. A. Ellis, F. McNeilly, D. Gilpin, B. Meehan, K. McCullough, and G. Allan. 2002. Immunologic features of porcine circovirus type 2 infection. Viral Immunol. 15567-582. [DOI] [PubMed] [Google Scholar]
- 31.Krakowka, S., J. A. Ellis, F. McNeilly, S. Ringler, D. M. Rings, and G. Allan. 2001. Activation of the immune system is the pivotal event in the production of wasting disease in pigs infected with porcine circovirus-2 (PCV-2). Vet. Pathol. 3831-42. [DOI] [PubMed] [Google Scholar]
- 32.Kyriakis, S. C., K. Saoulidis, S. Lekkas, C. Miliotis, P. A. Papoutsis, and S. Kennedy. 2002. The effects of immuno-modulation on the clinical and pathological expression of postweaning multisystemic wasting syndrome. J. Comp. Pathol. 12638-46. [DOI] [PubMed] [Google Scholar]
- 33.Ladekjaer-Mikkelsen, A. S., J. Nielsen, T. Stadejek, T. Storgaard, S. Krakowka, J. Ellis, F. McNeilly, G. Allan, and A. Botner. 2002. Reproduction of postweaning multisystemic wasting syndrome (PMWS) in immunostimulated and nonimmunostimulated 3-week-old piglets experimentally infected with porcine circovirus type 2 (PCV2). Vet. Microbiol. 8997-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu, J., I. Chen, and J. Kwang. 2005. Characterization of a previously unidentified viral protein in porcine circovirus type 2-infected cells and its role in virus-induced apoptosis. J. Virol. 798262-8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madshus, I. H., S. Olsnes, and K. Sandvig. 1984. Different pH requirements for entry of the two picornaviruses, human rhinovirus 2 and murine encephalomyocarditis virus. Virology 139346-357. [DOI] [PubMed] [Google Scholar]
- 36.Madshus, I. H., S. Olsnes, and K. Sandvig. 1984. Mechanism of entry into the cytosol of poliovirus type 1: requirement for low pH. J. Cell Biol. 981194-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marechal, V., F. Clavel, J. M. Heard, and O. Schwartz. 1998. Cytosolic Gag p24 as an index of productive entry of human immunodeficiency virus type 1. J. Virol. 722208-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marsh, M., J. Wellsteed, H. Kern, E. Harms, and A. Helenius. 1982. Monensin inhibits Semliki Forest virus penetration into culture cells. Proc. Natl. Acad. Sci. USA 795297-5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marshansky, V., D. A. Ausiello, and D. Brown. 2002. Physiological importance of endosomal acidification: potential role in proximal tubulopathies. Curr. Opin. Nephrol. Hypertens. 11527-537. [DOI] [PubMed] [Google Scholar]
- 40.McNeilly, F., I. McNair, D. P. Mackie, B. M. Meehan, S. Kennedy, D. Moffett, J. Ellis, S. Krakowka, and G. M. Allan. 2001. Production, characterisation and applications of monoclonal antibodies to porcine circovirus 2. Arch. Virol. 146909-922. [DOI] [PubMed] [Google Scholar]
- 41.Meehan, B. M., F. McNeilly, I. McNair, I. Walker, J. A. Ellis, S. Krakowka, and G. M. Allan. 2001. Isolation and characterization of porcine circovirus 2 from cases of sow abortion and porcine dermatitis and nephropathy syndrome. Arch. Virol. 146835-842. [DOI] [PubMed] [Google Scholar]
- 42.Meehan, B. M., F. McNeilly, D. Todd, S. Kennedy, V. A. Jewhurst, J. A. Ellis, L. E. Hassard, E. G. Clark, D. M. Haines, and G. M. Allan. 1998. Characterization of novel circovirus DNAs associated with wasting syndromes in pigs. J. Gen. Virol. 792171-2179. [DOI] [PubMed] [Google Scholar]
- 43.Meerts, P. 2005. The complex interaction between porcine circovirus type 2 and the pig's immune system. Ph.D.thesis. Ghent University, Merelbeke, Belgium.
- 44.Meerts, P., G. Misinzo, D. Lefebvre, J. Nielsen, A. Botner, C. S. Kristensen, and H. J. Nauwynck. 2006. Correlation between the presence of neutralizing antibodies against porcine circovirus 2 (PCV2) and protection against replication of the virus and development of PCV2-associated disease. BMC Vet. Res. 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meerts, P., G. Misinzo, F. McNeilly, and H. J. Nauwynck. 2005. Replication kinetics of different porcine circovirus 2 strains in PK-15 cells, fetal cardiomyocytes and macrophages. Arch. Virol. 150427-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meerts, P., G. Misinzo, and H. J. Nauwynck. 2005. Enhancement of porcine circovirus 2 replication in porcine cell lines by IFN-gamma before and after treatment and by IFN-alpha after treatment. J. Interferon Cytokine Res. 25684-693. [DOI] [PubMed] [Google Scholar]
- 47.Meerts, P., H. Nauwynck, R. Sanchez, B. Mateusen, and M. Pensaert. 2004. Prevalence of porcine circovirus 2 (PCV2)-related wasting on Belgian farms with or without a history of postweaning multisystemic wasting syndrome. Flem. Vet. J. 7331-38. [Google Scholar]
- 48.Mellman, I. 1996. Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol. 12575-625. [DOI] [PubMed] [Google Scholar]
- 49.Mellman, I., R. Fuchs, and A. Helenius. 1986. Acidification of the endocytic and exocytic pathways. Annu. Rev. Biochem. 55663-700. [DOI] [PubMed] [Google Scholar]
- 50.Misinzo, G., P. L. Delputte, P. Meerts, D. J. Lefebvre, and H. J. Nauwynck. 2006. Porcine circovirus 2 uses heparan sulfate and chondroitin sulfate B glycosaminoglycans as receptors for its attachment to host cells. J. Virol. 803487-3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Misinzo, G., P. Meerts, M. Bublot, J. Mast, H. M. Weingartl, and H. J. Nauwynck. 2005. Binding and entry characteristics of porcine circovirus 2 in cells of the porcine monocytic line 3D4/31. J. Gen. Virol. 862057-2068. [DOI] [PubMed] [Google Scholar]
- 52.Morozov, I., T. Sirinarumitr, S. D. Sorden, P. G. Halbur, M. K. Morgan, K. J. Yoon, and P. S. Paul. 1998. Detection of a novel strain of porcine circovirus in pigs with postweaning multisystemic wasting syndrome. J. Clin. Microbiol. 362535-2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakata, H. 2004. Stimulation of extracellular signal-regulated kinase pathway by suramin with concomitant activation of DNA synthesis in cultured cells. J. Pharmacol. Exp. Ther. 308744-753. [DOI] [PubMed] [Google Scholar]
- 54.Nawagitgul, P., I. Morozov, S. R. Bolin, P. A. Harms, S. D. Sorden, and P. S. Paul. 2000. Open reading frame 2 of porcine circovirus type 2 encodes a major capsid protein. J. Gen. Virol. 812281-2287. [DOI] [PubMed] [Google Scholar]
- 55.Olvera, A., M. Sibila, M. Calsamiglia, J. Segales, and M. Domingo. 2004. Comparison of porcine circovirus type 2 load in serum quantified by a real time PCR in postweaning multisystemic wasting syndrome and porcine dermatitis and nephropathy syndrome naturally affected pigs. J. Virol. Methods 11775-80. [DOI] [PubMed] [Google Scholar]
- 56.Opriessnig, T., E. L. Thacker, S. Yu, M. Fenaux, X. J. Meng, and P. G. Halbur. 2004. Experimental reproduction of postweaning multisystemic wasting syndrome in pigs by dual infection with Mycoplasma hyopneumoniae and porcine circovirus type 2. Vet. Pathol. 41624-640. [DOI] [PubMed] [Google Scholar]
- 57.Rosell, C., J. Segales, J. Plana-Duran, M. Balasch, G. M. Rodriguez-Arrioja, S. Kennedy, G. M. Allan, F. McNeilly, K. S. Latimer, and M. Domingo. 1999. Pathological, immunohistochemical, and in-situ hybridization studies of natural cases of postweaning multisystemic wasting syndrome (PMWS) in pigs. J. Comp. Pathol. 12059-78. [DOI] [PubMed] [Google Scholar]
- 58.Rovira, A., M. Balasch, J. Segales, L. Garcia, J. Plana-Duran, C. Rosell, H. Ellerbrok, A. Mankertz, and M. Domingo. 2002. Experimental inoculation of conventional pigs with porcine reproductive and respiratory syndrome virus and porcine circovirus 2. J. Virol. 763232-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sanchez, R. E., Jr., P. Meerts, H. J. Nauwynck, and M. B. Pensaert. 2003. Change of porcine circovirus 2 target cells in pigs during development from fetal to early postnatal life. Vet. Microbiol. 9515-25. [DOI] [PubMed] [Google Scholar]
- 60.Schaeffer, E., R. Geleziunas, and W. C. Greene. 2001. Human immunodeficiency virus type 1 Nef functions at the level of virus entry by enhancing cytoplasmic delivery of virions. J. Virol. 752993-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Segales, J., and M. Domingo. 2002. Postweaning multisystemic wasting syndrome (PMWS) in pigs. Vet. Q. 24109-124. [DOI] [PubMed] [Google Scholar]
- 62.Sperber, K., T. H. Kalb, V. J. Stecher, R. Banerjee, and L. Mayer. 1993. Inhibition of human immunodeficiency virus type 1 replication by hydroxychloroquine in T cells and monocytes. AIDS Res. Hum. Retrovir. 991-98. [DOI] [PubMed] [Google Scholar]
- 63.Sturzenbecker, L. J., M. Nibert, D. Furlong, and B. N. Fields. 1987. Intracellular digestion of reovirus particles requires a low pH and is an essential step in the viral infectious cycle. J. Virol. 612351-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Superti, F., L. Seganti, F. M. Ruggeri, A. Tinari, G. Donelli, and N. Orsi. 1987. Entry pathway of vesicular stomatitis virus into different host cells. J. Gen. Virol. 68387-399. [DOI] [PubMed] [Google Scholar]
- 65.Talbot, P. J., and D. E. Vance. 1982. Biochemical studies on the entry of Sindbis virus into BHK-21 cells and the effect of NH4Cl. Virology 118451-455. [DOI] [PubMed] [Google Scholar]
- 66.Tischer, I., D. Peters, R. Rasch, and S. Pociuli. 1987. Replication of porcine circovirus: induction by glucosamine and cell cycle dependence. Arch. Virol. 9639-57. [DOI] [PubMed] [Google Scholar]
- 67.Vincent, I. E., C. P. Carrasco, L. Guzylack-Piriou, B. Herrmann, F. McNeilly, G. M. Allan, A. Summerfield, and K. C. McCullough. 2005. Subset-dependent modulation of dendritic cell activity by circovirus type 2. Immunology 115388-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wagner, C. A., K. E. Finberg, S. Breton, V. Marshansky, D. Brown, and J. P. Geibel. 2004. Renal vacuolar H+-ATPase. Physiol. Rev. 841263-1314. [DOI] [PubMed] [Google Scholar]
- 69.Wei, B. L., P. W. Denton, E. O'Neill, T. Luo, J. L. Foster, and J. V. Garcia. 2005. Inhibition of lysosome and proteasome function enhances human immunodeficiency virus type 1 infection. J. Virol. 795705-5712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.White, J., K. Matlin, and A. Helenius. 1981. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J. Cell Biol. 89674-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhuang, S., and R. G. Schnellmann. 2005. Suramin promotes proliferation and scattering of renal epithelial cells. J. Pharmacol. Exp. Ther. 314383-390. [DOI] [PubMed] [Google Scholar]