Abstract
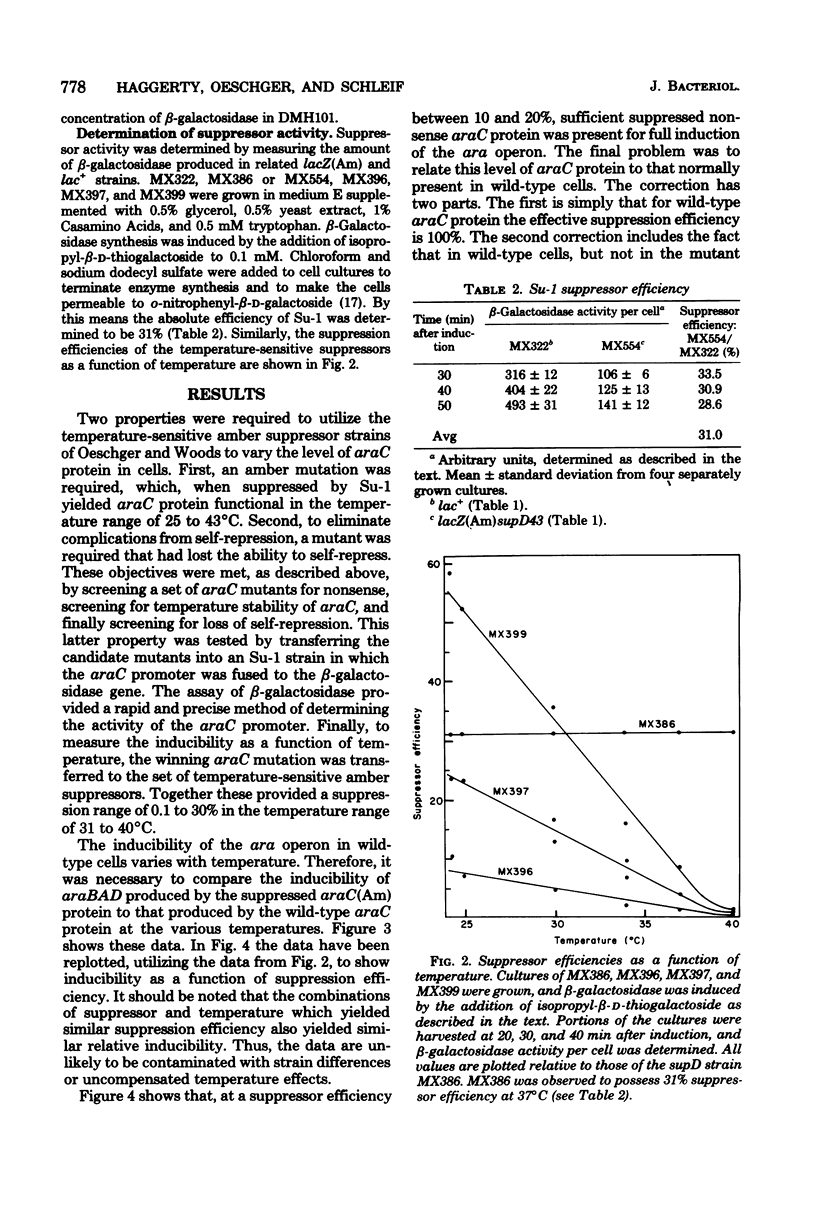
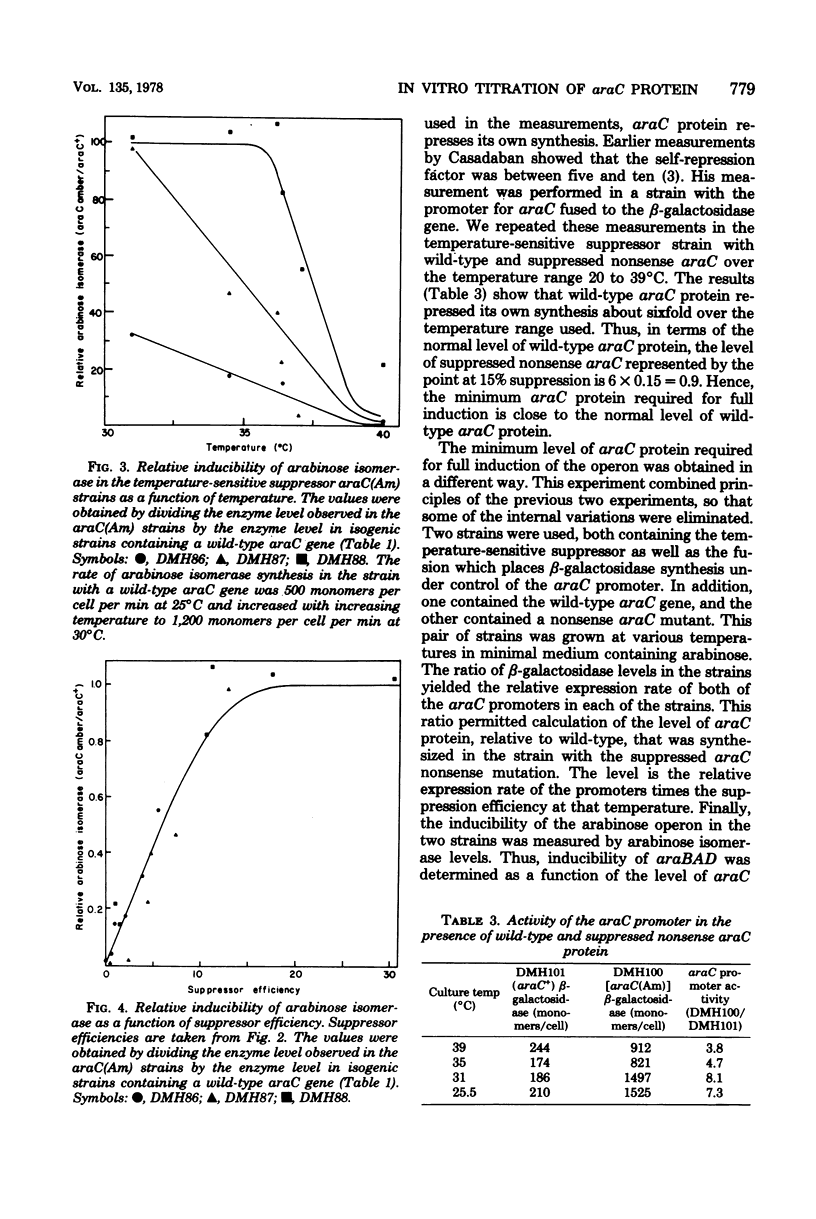
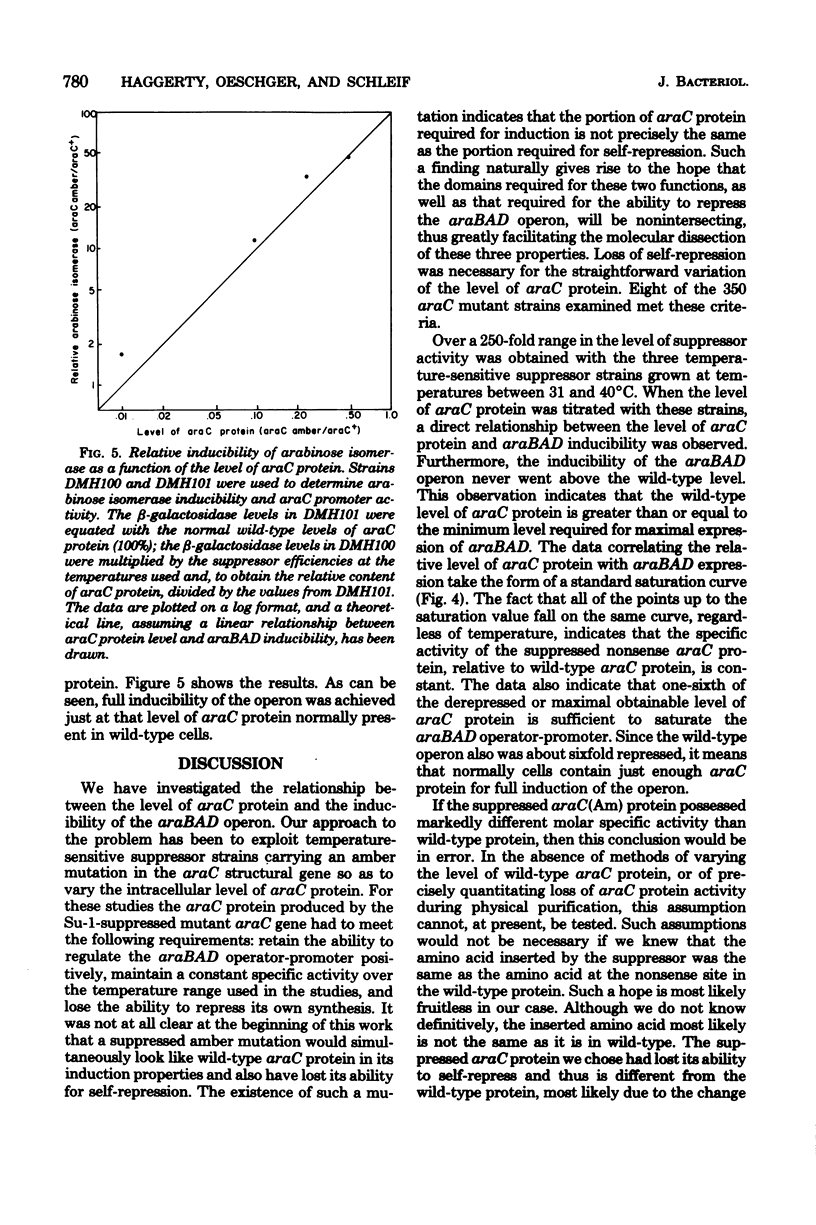
The requirement for araC protein in the induction of the araBAD operon was investigated. Strains of Escherichia coli carrying an araC(Am) mutation and temperature-sensitive amber suppressors were used to vary the intracellular level of araC protein. The levels of araC protein studied ranged from 0.007 to 1.8 times the normal amount. The results indicate that the normal level of araC protein is just sufficient to provide maximal expression of the araBAD operon.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J. Regulation of the regulatory gene for the arabinose pathway, araC. J Mol Biol. 1976 Jul 5;104(3):557–566. doi: 10.1016/0022-2836(76)90120-0. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Englesberg E., Squires C., Meronk F., Jr The L-arabinose operon in Escherichia coli B-r: a genetic demonstration of two functional states of the product of a regulator gene. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1100–1107. doi: 10.1073/pnas.62.4.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein S. I., Altman S. Coding properties of an ochre-suppressing derivative of Escherichia coli tRNAITyr. J Mol Biol. 1977 May 25;112(3):453–470. doi: 10.1016/s0022-2836(77)80192-7. [DOI] [PubMed] [Google Scholar]
- Feinstein S. I., Altman S. Context effects on nonsense codon suppression in Escherichia coli. Genetics. 1978 Feb;88(2):201–219. doi: 10.1093/genetics/88.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt J., Schleif R. Arabinose C protein: regulation of the arabinose operon in vitro. Nat New Biol. 1971 Oct 6;233(40):166–170. doi: 10.1038/newbio233166a0. [DOI] [PubMed] [Google Scholar]
- Haggerty D. M., Schleif R. F. Kinetics of the onset of catabolite repression in Escherichia coli as determined by lac messenger ribonucleic acid initiations and intracellular cyclic adenosine 3',5'-monophosphate levels. J Bacteriol. 1975 Sep;123(3):946–953. doi: 10.1128/jb.123.3.946-953.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh J., Schleif R. In vivo experiments on the mechanism of action of L-arabinose C gene activator and lactose repressor. J Mol Biol. 1973 Nov 5;80(3):433–444. doi: 10.1016/0022-2836(73)90414-2. [DOI] [PubMed] [Google Scholar]
- Hofnung M., Schwartz M., Hatfield D. Complementation studies in the maltose-A region of the Escherichia coli K12 genetic map. J Mol Biol. 1971 Nov 14;61(3):681–694. doi: 10.1016/0022-2836(71)90072-6. [DOI] [PubMed] [Google Scholar]
- Kessler D. P., Englesberg E. Arabinose-leucine deletion mutants of Escherichia coli B-r. J Bacteriol. 1969 Jun;98(3):1159–1169. doi: 10.1128/jb.98.3.1159-1169.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N., Wilcox G., Gielow W., Arnold J., Cleary P., Englesberg E. In vitro activation of the transcription of araBAD operon by araC activator. Proc Natl Acad Sci U S A. 1974 Mar;71(3):634–638. doi: 10.1073/pnas.71.3.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson N. M., Schleif R. Paucity of sites mutable to constitutivity in the araC activator gene of the L-arabinose operon of Escherichia coli. J Mol Biol. 1975 Jul 25;96(1):185–199. doi: 10.1016/0022-2836(75)90190-4. [DOI] [PubMed] [Google Scholar]
- Oeschger M. P., Woods S. L. A temperature-sensitive suppressor enabling the manipulation of the level of individual proteins in intact cells. Cell. 1976 Feb;7(2):205–212. doi: 10.1016/0092-8674(76)90019-2. [DOI] [PubMed] [Google Scholar]
- Ptashne M., Backman K., Humayun M. Z., Jeffrey A., Maurer R., Meyer B., Sauer R. T. Autoregulation and function of a repressor in bacteriophage lambda. Science. 1976 Oct 8;194(4261):156–161. doi: 10.1126/science.959843. [DOI] [PubMed] [Google Scholar]
- Schleif R. An L-arabinose binding protein and arabinose permeation in Escherichia coli. J Mol Biol. 1969 Nov 28;46(1):185–196. doi: 10.1016/0022-2836(69)90065-5. [DOI] [PubMed] [Google Scholar]
- Schleif R. Fine-structure deletion map of the Escherichia coli L-arabinose operon. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3479–3484. doi: 10.1073/pnas.69.11.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleif R., Lis J. T. The regulatory region of the L-arabinose operon: a physical, genetic and physiological study. J Mol Biol. 1975 Jul 5;95(3):417–431. doi: 10.1016/0022-2836(75)90200-4. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Wilcox G., Meuris P., Bass R., Englesberg E. Regulation of the L-arabinose operon BAD in vitro. J Biol Chem. 1974 May 10;249(9):2946–2952. [PubMed] [Google Scholar]
- Yahata H., Ocada Y., Tsugita A. Adjacent effect on suppression efficiency. II. Study on ochre and amber mutants of T4 phage lysozyme. Mol Gen Genet. 1970;106(3):208–212. doi: 10.1007/BF00340380. [DOI] [PubMed] [Google Scholar]



