Abstract
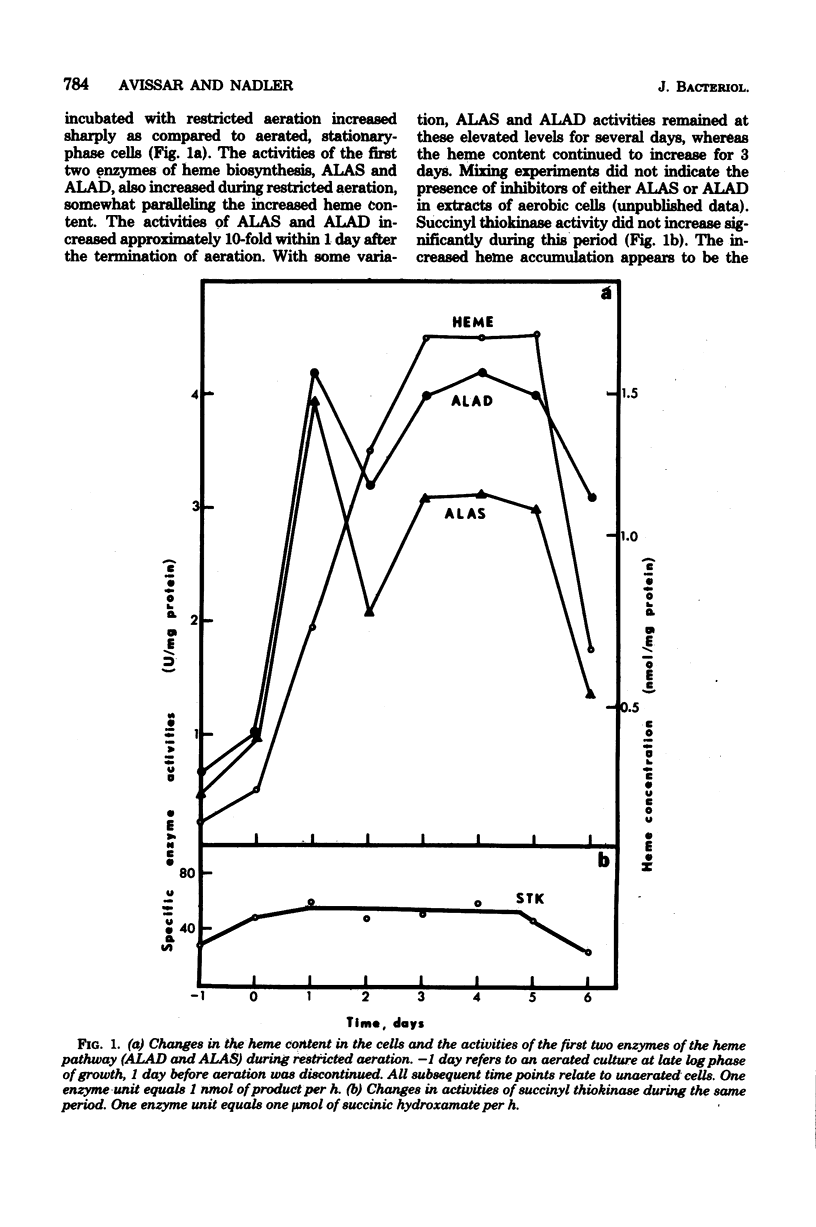
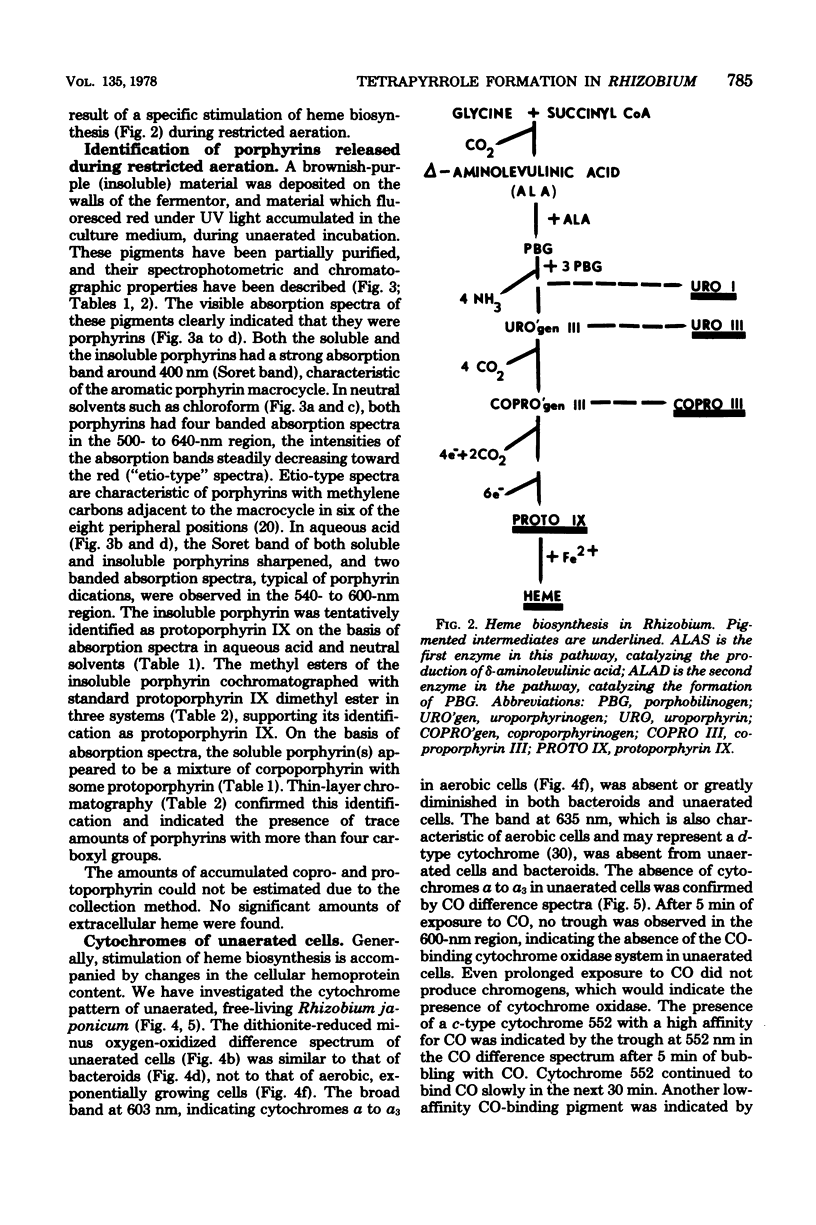
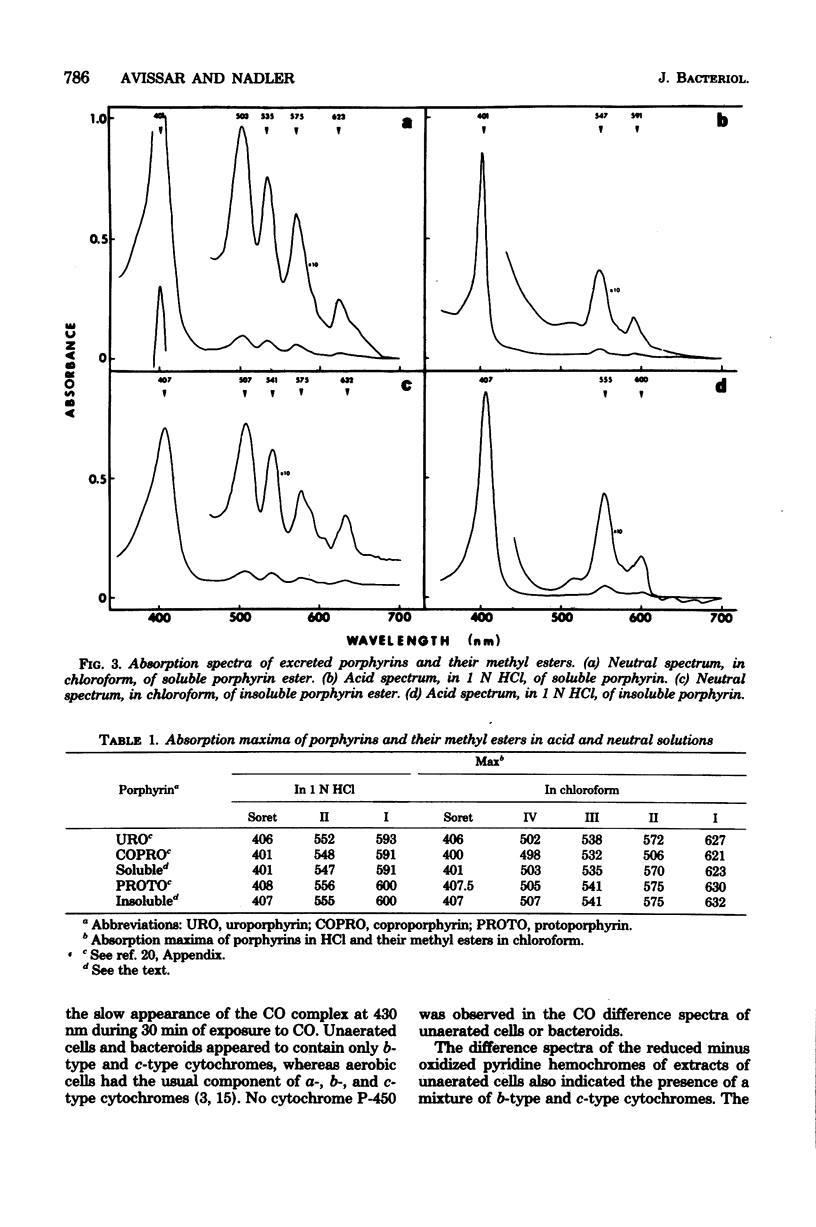
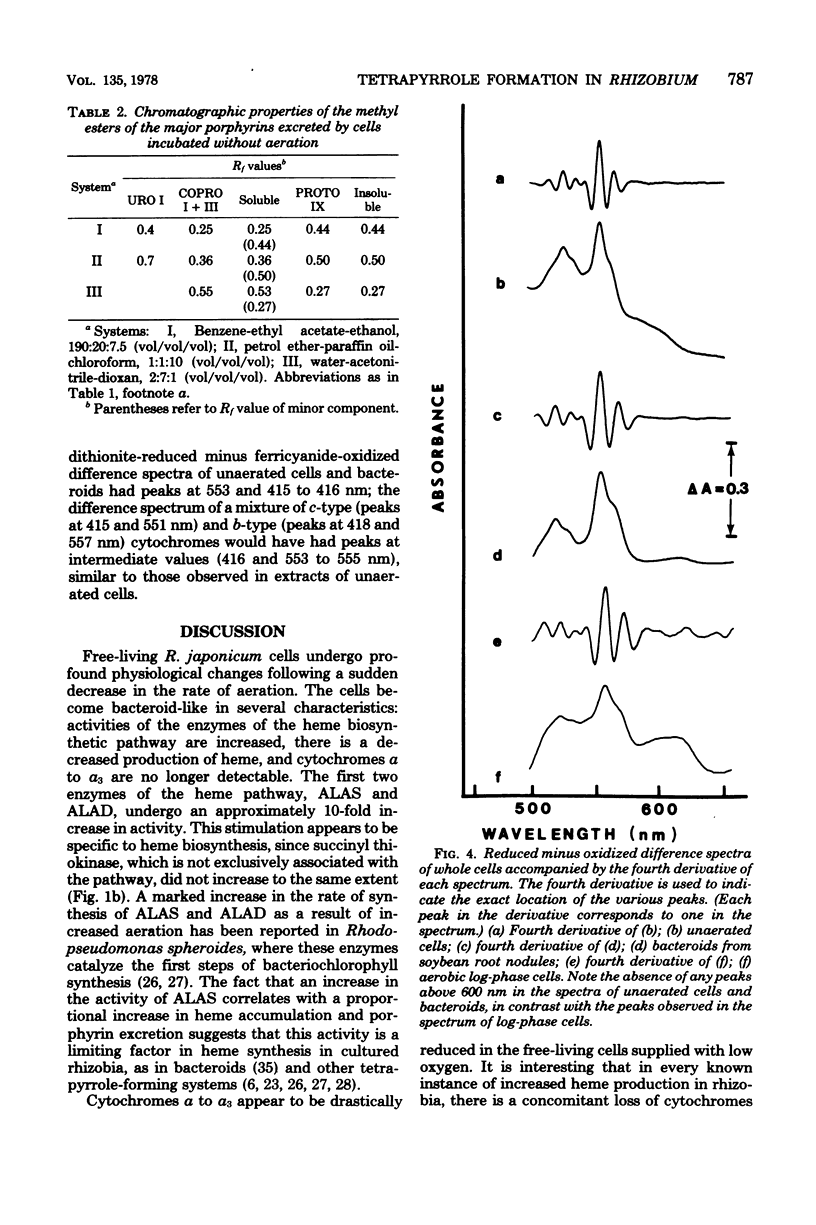
Cultures of Rhizobium japonicum were grown with vigorous aeration to stationary phase and were then incubated under restricted aeration for several days. Under these "microaerobic" conditions, cellular heme content increased 10-fold, and visible amounts of porphyrins were released into the culture medium. The two predominant porphyrins produced were identified, on the basis of their spectrophotometric and chromatographic properties, as protoporphyrin and coproporphyrin. The cytochrome complement of microaerobic cells partially resembled that of the symbiotic bacteria in that cytochromes alpha-alpha3 were absent and a CO-binding cytochrome 552 was present. During the period of restricted aeration, at the time that the heme content was increasing, there was a similar 10-fold increase in the activities of the first two enzymes of heme biosynthesis, delta-aminolevulinic acid synthase and delta-aminolevulinic acid dehydrase. However, during the same period, the activity of succinyl thiokinase (an enzyme that is required in large amounts whether or not heme is being produced) increased only twofold. These results suggest that reduced oxygen tension may play a role in inducing heme synthesis necessary for leghemoglobin formation and bacterial differentiation in soybean root nodules.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleby C. A. Electron transport systems of Rhizobium japonicum. II. Rhizobium haemoglobin, cytochromes and oxidases in free-living (cultured) cells. Biochim Biophys Acta. 1969 Jan 14;172(1):88–105. doi: 10.1016/0005-2728(69)90094-2. [DOI] [PubMed] [Google Scholar]
- BURNHAM B. F., PIERCE W. S., WILLIAMS K. R., BOYER M. H., KIRBY C. K. delta-aminolaevulate dehydratase from Rhodopseudomonas spheroides. Biochem J. 1963 Jun;87:462–472. doi: 10.1042/bj0870462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergersen F. J., Turner G. L., Appleby C. A. Studies of the physiological role of leghaemoglobin in soybean root nodules. Biochim Biophys Acta. 1973 Jan 18;292(1):271–282. doi: 10.1016/0005-2728(73)90271-5. [DOI] [PubMed] [Google Scholar]
- Butler W. L. Absorption spectroscopy of biological materials. Methods Enzymol. 1972;24:3–25. doi: 10.1016/0076-6879(72)24052-6. [DOI] [PubMed] [Google Scholar]
- Chu T. C., Chu E. J. Thin-layer chromatography of methyl esters of porphyrins, chlorins and related compounds with Eastman "chromagram". J Chromatogr. 1966 Jan;21(1):46–51. doi: 10.1016/s0021-9673(01)91259-2. [DOI] [PubMed] [Google Scholar]
- Cutting J. A., Schulman H. M. The biogenesis of leghemoglobin. The determinant in the Rhizobium-legume symbiosis for leghemoglobin specificity. Biochim Biophys Acta. 1971 Jan 19;229(1):58–62. [PubMed] [Google Scholar]
- Cutting J. A., Schulman H. M. The control of heme synthesis in soybean root nodules. Biochim Biophys Acta. 1971 Feb 28;261(2):321–327. doi: 10.1016/0304-4165(72)90054-2. [DOI] [PubMed] [Google Scholar]
- Cutting J. A., Schulman H. M. The site of heme synthesis in soybean root nodules. Biochim Biophys Acta. 1969 Dec 30;192(3):486–493. doi: 10.1016/0304-4165(69)90398-5. [DOI] [PubMed] [Google Scholar]
- DRESEL E. I., FALK J. E. Studies on the biosynthesis of blood pigments. 2. Haem and porphyrin formation in intact chicken erythrocytes. Biochem J. 1956 May;63(1):72–79. doi: 10.1042/bj0630072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel R. M., Appleby C. A. Anaerobic-nitrate, symbiotic and aerobic growth of Rhizobium japonicum: effects on cytochrome P 450 , other haemoproteins, nitrate and nitrite reductases. Biochim Biophys Acta. 1972 Sep 20;275(3):347–354. doi: 10.1016/0005-2728(72)90215-0. [DOI] [PubMed] [Google Scholar]
- Dilworth M. J. The plant as the genetic determinant of leghaemoglobin production in the legume root nodule. Biochim Biophys Acta. 1969 Jul 30;184(2):432–441. doi: 10.1016/0304-4165(69)90047-6. [DOI] [PubMed] [Google Scholar]
- Doss M., Ulshöfer B. Porphyrin stability as a function of the number of carboxylic acid side chains. Biochim Biophys Acta. 1971 May 18;237(2):356–360. doi: 10.1016/0304-4165(71)90330-8. [DOI] [PubMed] [Google Scholar]
- Hendry G. S., Jordan D. C. Coproporphyrin excretion by Rhizobium meliloti. Can J Microbiol. 1969 Feb;15(2):242–244. doi: 10.1139/m69-043. [DOI] [PubMed] [Google Scholar]
- LASCELLES J. The synthesis of enzymes concerned in bacteriochlorophyll formation in growing cultures of Rhodopseudomonas spheroides. J Gen Microbiol. 1960 Dec;23:487–498. doi: 10.1099/00221287-23-3-487. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lascelles J., Altschuler T. Mutant strains of Rhodopseudomonas spheroides lacking delta-aminolevulinate synthase: growth, heme, and bacteriochlorophyll synthesis. J Bacteriol. 1969 May;98(2):721–727. doi: 10.1128/jb.98.2.721-727.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lascelles J., Wertlieb D. Mutant strains of Rhodopseudomonas spheroides which form photosynthetic pigments aerobically in the dark. Growth characteristics and enzymic activities. Biochim Biophys Acta. 1971 Mar 2;226(2):328–340. doi: 10.1016/0005-2728(71)90100-9. [DOI] [PubMed] [Google Scholar]
- Maier R. J., Brill W. J. Ineffective and non-nodulating mutant strains of Rhizobium japonicum. J Bacteriol. 1976 Aug;127(2):763–769. doi: 10.1128/jb.127.2.763-769.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs B., Gest H. Regulation of bacteriochlorophyll synthesis by oxygen in respiratory mutants of Rhodopseudomonas capsulata. J Bacteriol. 1973 Jun;114(3):1052–1057. doi: 10.1128/jb.114.3.1052-1057.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler K. D., Avissar Y. J. Heme Synthesis in Soybean Root Nodules: I. On the Role of Bacteroid delta-Aminolevulinic Acid Synthase and delta-Aminolevulinic Acid Dehydrase in the Synthesis of the Heme of Leghemoglobin. Plant Physiol. 1977 Sep;60(3):433–436. doi: 10.1104/pp.60.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjepkema J., Evans H. J. Nitrogen fixation by free-living Rhizobium in a defined liquid medium. Biochem Biophys Res Commun. 1975 Jul 22;65(2):625–628. doi: 10.1016/s0006-291x(75)80192-6. [DOI] [PubMed] [Google Scholar]
- Verma D. P., Bal A. K. Intracellular site of synthesis and localization of leghemoglobin in root nodules. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3843–3847. doi: 10.1073/pnas.73.11.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg J. B. Facilitated oxygen diffusion. The role of leghemoglobin in nitrogen fixation by bacteroids isolated from soybean root nodules. J Biol Chem. 1974 Jul 10;249(13):4057–4066. [PubMed] [Google Scholar]



