Abstract
Identification of anti-hepatitis C virus (anti-HCV) human antibody clones with broad neutralizing activity is important for a better understanding of the interplay between the virus and host and for the design of an effective passive immunotherapy and an effective vaccine. We report the identification of a human monoclonal Fab (e137) able to bind the HCV E2 glycoprotein of all HCV genotypes but genotype 5. The results of antibody competition assays and testing the reactivity to alanine mutant E2 proteins confirmed that the e137 epitope includes residues (T416, W420, W529, G530, and D535) highly conserved across all HCV genotypes. Fab e137 neutralized HCV pseudoparticles bearing genotype 1a, 1b, and 4 E1-E2 proteins and to a lesser extent, genotype 2b. Fab e137 was also able to inhibit cell culture-grown HCV (genotype 2a). These data indicate that broadly cross-reacting and cross-neutralizing antibodies are generated during HCV infection.
It is widely accepted that antibodies play a crucial role in the prevention and treatment of many viral infections of humans, including respiratory syncytial virus (16), rabies virus (34), and hepatitis B virus (35) infections. In contrast, a protective role of antibodies during infections by several persistent RNA viruses has not been widely accepted. In hepatitis C virus (HCV) infection, the frequent inability of the host to clear the virus and the possible reinfection after virus clearance (21) have been considered evidence against a protective role of specific antibodies. However, it has recently been shown that the anti-HCV antibody repertoire includes neutralizing and cross-reactive clones that are dispersed within a majority of antibody molecules that have minimal benefit for the host (8, 9, 25, 39, 36). Parallel analyses have recently suggested that antibodies play a crucial role in different phases of the natural history of HCV infection (3, 14, 15, 19, 30, 31).
In the present study, we characterized the anti-HCV E2 human monoclonal antibody (MAb) e137, which was cloned as a Fab fragment by phage display from the immunoglobulin G1 (IgG1) light-chain κ repertoire of an infected patient (7, 11). The E2-binding activity of Fab e137 is inhibited by sera of patients infected with different HCV genotypes (9, 25, 26), suggesting that this human MAb could recognize E2 proteins of a wide range of HCV genotypes and subtypes.
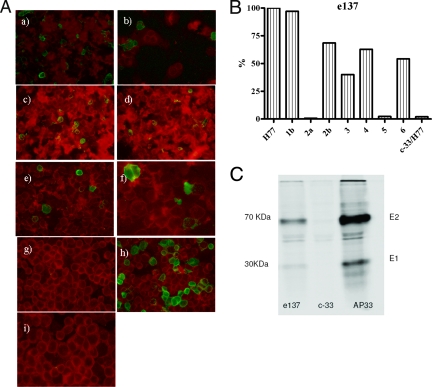
In order to better define the breadth of e137 cross-reactivity, we used human epithelial kidney (HEK) 293T cells expressing HCV E1-E2 of different genotypes (23). In detail, the HEK 293T cells were transfected with 3 μg of pcDNA3.1 vector (23), encoding E1-E2 glycoproteins from different HCV genotypes. The binding of e137 was assayed by immunofluorescence using a fluorescein isothiocyanate-conjugated anti-human Fab (Sigma) (18). Fab e137 was able to bind all HCV genotypes but genotype 5 (Fig. 1A). The data were confirmed using cells expressing HCV E1-E2 from other isolates (Fig. 1B). In only one case, e137 did not recognize HCV of genotype 2a (strain UKN2A2.4). The isolate UKN2A2.4 E2 sequence diverges by 17% from that derived from UKN2A1.2 (which was recognized by e137). These sequence differences likely cause a loss of contact residues or conformational changes that could make the epitope of e137 less accessible. The broad cross-reactivity of e137 was also confirmed by an immunoprecipitation assay performed on lysates of HEK 293 cells expressing E1-E2 glycoproteins from all genotypes (Fig. 1C). The immunoprecipitation assay was performed as previously described (28).
FIG. 1.
(A) Analysis of binding of the Fab e137 by immunofluorescence staining of cells expressing E1-E2 proteins derived from different HCV genotypes. The cells were counterstained with Evans blue (red-stained cells). (a) Genotype 1a isolate UKN1A20.8; (b) genotype 1b isolate UKN1B5.23; (c) genotype 2a isolate UKN2A1.2; (d) genotype 2b isolate UKN2B1.1; (e) genotype 3 isolate UKN3A13.6; (f) genotype 4 isolate UKN4.21.16; (g) genotype 5 isolate UKN5.15.11; (h) genotype 6 isolate UKN6.5.8; (i) a human recombinant Fab (c33-3) specific for a nonstructural antigen of HCV (NS3) was included as a negative control (data generated on UKN1A20.8 are shown). Fab fragments were tested at a concentration of 10 μg/ml. (B) Binding activity of anti-HCV E2 Fab e137 on E1-E2 proteins derived from HCV isolates with different genotypes (genotypes 1a, 1b, 2a, 2b, 3, 4, 5, and 6): H77.20, UKN1B12.16, UKN2A.2.4, UKN2B2.8, UKN3A1.28c, UKN4.21.16, UKN5.15.11, and UKN6.5.8. Binding activity was expressed as a percentage of reactivity of the e137 Fab on E1-E2 proteins of genotype 1a (H77 strain). A human recombinant Fab (c33-3) specific for a nonstructural antigen of HCV (NS3) was included as a negative control (data generated on H77 are shown). The binding was assayed by fluorescence-activated cell sorting, using a fluorescein isothiocyanate-conjugated secondary anti-human Fab (Sigma) and measured by analysis of the percentage of cells with a higher fluorescence signal than cells without Fab. Fab e137 was also tested using untransfected cells, and this fluorescence was subtracted as background. The broadly cross-reactive AP33 was used in order to analyze the efficiency of transfection. The percentage of AP33-incubated cells with a higher fluorescence signal than untreated cells was at the same level among cells expressing E1-E2 proteins of different genotypes (data not shown). Fab e137 was tested at 10 μg/ml. (C) Radiolabeled proteins in the lysate of HEK 293T expressing E1-E2 glycoproteins of all genotypes were immunoprecipitated using e137. AP33 and c-33 were used as positive and negative controls, respectively. The immune complexes were analyzed by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis under reducing conditions. The protein sizes (in kilodaltons) are shown to the left of the gel. Data for E1-E2 of genotype 1a are shown.
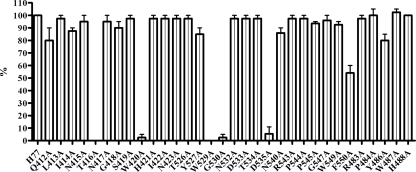
Considering these data, an important point is the definition of the HCV E2 regions having the potential of eliciting the cross-reactive antibody. Our previous attempts to identify the epitope recognized by e137 using multiple antigenic peptides of HCV envelope glycoprotein E2 were not successful (11). Furthermore, Fab e137 did not bind to recombinant maltose-binding protein-E2 fusion protein or to hypervariable region (HVR) multiple antigenic peptides using an enzyme-linked immunosorbent assay (ELISA) (data not shown). These data suggest that e137 is directed against a conformational epitope retained in the full-length HCV E2, as usually seen in broadly neutralizing antibodies (1, 5, 17, 18). Accordingly, as an alternative strategy for mapping the epitope recognized by e137, we used an ELISA competition assay with a panel of mouse and rat MAbs directed against known epitopes of genotype 1a HCV E2 (Table 1). Competition experiments were performed as described previously (5). Using this approach, binding of e137 to HCV E2 was shown to be inhibited by the mouse MAb AP33 and two rat MAbs (2/64a and 9/75) each recognizing linear epitopes spanning E2 regions from amino acid (aa) 421 to 423, 524 to 531, and 528 to 535, respectively. Interestingly, the regions from aa 412 to 423 and 524 to 535 have been reported to be crucial for CD81 binding and retroviral pseudoparticle (HCVpp) infectivity (29). To confirm these data, we used a panel of H77-derived E1-E2 (genotype 1a) proteins containing alanine replacement mutants, some of which have been previously shown to be important for CD81 binding (29). The analysis of e137 binding of the panel of E1-E2 mutants (Fig. 2) confirmed that the e137 epitope is centered in aa 412 to 423 and aa 528 to 535 of HCV E2 regions, since substitutions at conserved positions 416, 420, 529, 530, and 535 reduced binding by greater than 90%. These data confirm that the conformational epitope bound by e137 includes conserved residues that are crucial for CD81 binding and HCVpp infectivity. These data are interesting, considering that e137 has been described to be an antibody with neutralization of binding activity (11). Furthermore, the data highlight that the epitope of e137 includes two conserved residues (aa 416 and 420) that were described to be critical within the epitope recognized by MAb AP33 (36). Interestingly, among the genotype 2a-derived E2 sequences studied in this paper (UKN2A1.2, UKN2A2.4, and JFH-1), a mutation from threonine to serine at position 416 was present only in the isolate not bound in the binding assay analyzed by fluorescence-activated cell sorting (UKN2A2.4), thus confirming that this mutation plays a crucial role in the lack of e137 binding to this strain. Indeed, T416 is quite conserved among different E2 genotypes, being always present in genotypes 1a, 1b, 2b, 3, 5, and 6. However, the T416S replacement has been reported in 59% of E2 sequences derived from genotype 2a and in 40% of E2 sequences derived from genotype 4 (37). As far as the other unbound genotype is concerned, all available HCV E2 sequences of genotype 5 present in online databases and those belonging to all isolates tested in our study have been aligned, confirming the constant presence of unmutated T416. This suggests the possibility that the lack of binding to this single genotype is due to mutations outside the regions examined by our approach.
TABLE 1.
Inhibition of human anti-HCV E2 Fab e137 binding by competing anti-HCV E2 rat or mouse MAbs directed against known regions of E2
| Competing mouse or rat MAb | Location (aa) or type of HCV E2 epitopea | % of inhibition of Fab 137 bindingb |
|---|---|---|
| 7/59 | 384-391 | 25 |
| 3/11 | 412-423 | 21 |
| AP33c | 412-423 | 55 |
| 1/39 | 436-443 | 33 |
| 11/20 | 436-447 | 3 |
| 7/16b | 436-447 | 5 |
| H47 | 452-459 | 2 |
| 6/1a | 464-471 | 0 |
| 6/41a | 480-493 | 0 |
| 2/64a | 524-531 | 45 |
| 9/75 | 528-535 | 81 |
| 6/53 | 544-551 | 0 |
| H62 | 644-655 | 8 |
| H60 | Conf. | 4 |
| H53 | Conf. | 3 |
| H61 | Conf. | 4 |
| H33 | Conf. | 0 |
| H44 | Conf. | 0 |
| H50 | Conf. | 58 |
| None | 2 |
Conf., conformational epitope.
Inhibition data between 20% and 50% are shown in italic type, and Inhibition data more than 50% are shown in bold type.
The only MAb able to react with all HCV genotypes is AP33, a mouse MAb that is capable of potent neutralization of HCVpp representing a broad variety of HCV genotypes.
FIG. 2.
Reactivity of anti-HCV E2 Fab e137 on a panel of mutated E1-E2 glycoproteins. Binding activity expressed as a percentage of the reactivity on wild-type E1-E2 (H77 strain) is shown on the y axis, and the wild type E1-E2 protein and the mutations are shown on the x axis. The means plus standard errors of the means (error bars) for three replicate assays are reported. Fab e137 was tested at 10 μg/ml.
We have previously shown that e137 is able to strongly neutralize the infection of pseudoparticles derived from vesicular stomatitis virus expressing E2 of HCV genotype 1a (10). However, concerns about the reliability of the vesicular stomatitis virus model system (6), together with the broad E2 reactivity reported here, prompted us to define neutralizing activity of e137 using alternative strategies. First, Fab e137 activity was tested against pseudoparticles derived from murine leukemia virus displaying unmodified and functional full-length E1-E2 proteins of all HCV genotypes. The pseudoparticle neutralization analysis was performed as previously described (4).
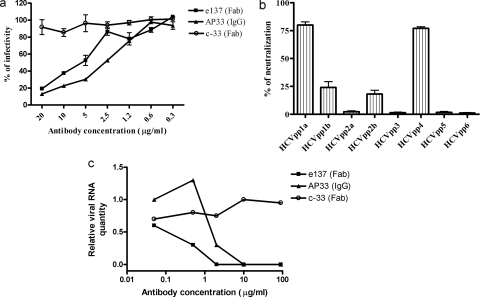
This approach confirmed that e137 is a strong neutralizer of HCV genotype 1a, with a 50% neutralization activity (50% inhibitory concentration [IC50]) at concentrations of 5 μg/ml (Fig. 3a). Furthermore, when tested at a single concentration of 15 μg/ml, e137 was able to neutralize the infectivity of HCVpp bearing E1-E2 of genotypes 1b, 2b, and 4 by 25%, 20%, and 75%, respectively (Fig. 3b). Although e137 was cloned from a HCV genotype 1b-infected patient, the neutralizing activity of HCVpp displaying E1-E2 of genotype 1b was lower than that of pseudoparticles displaying E1-E2 of genotype 1a. It is possible that differences in terms of density of e137 epitope on the pseudoparticle surface affect the neutralizing activity of e137. Another possible explanation is that E1-E2-derived UKN1B5.23 may show some mutation within or near the e137 epitope that can affect the neutralizing activity of e137.
FIG. 3.
(a) Neutralizing activity of Fab e137 using virus pseudoparticles displaying E1-E2 genotype 1a (UKN1A20.8). Data obtained from HCVpp infection in the presence of c33-3 (negative control [neg]), AP33, and in the presence of different concentrations (in micrograms per milliliter) of immunoaffinity-purified (>90%) Fab e137 are presented as a percentage of the infection detected in the absence of antibody. Neutralization activity was expressed by percent reduction of luciferase activity relative to the value for the control without competing antibodies. The experiment was performed three times, and the means ± standard errors of the means (error bars) from the three replicate assays are reported. (b) Neutralizing activity of Fab e137 at 15 μg/ml using virus pseudoparticles displaying E1-E2 proteins of different genotypes (HCV genotypes 1a to 6) (UKN1A20.8, UKN1B5.23, UKN2A1.2, UKN2B1.1, UKN3A13.6, UKN4.21.16, UKN5.15.11, and UKN6.5.8). The means plus standard errors of the means (error bars) of two replicate assays are reported. (c) Neutralization activity of e137 using the HCVcc system (genotype 2a). The infectivity of JFH-1 in the presence of e137, negative-control Fab (c33-3), and AP33 is presented as the viral RNA quantity normalized against glyceraldehyde-3-phosphate dehydrogenase RNA, as determined by quantitative reverse transcription-PCR. The virus infectivity was evaluated by measuring the levels of positive-stranded HCV RNA (24).
The cross-neutralizing activity of e137 was also analyzed using the authentic cell culture infectious HCV (HCVcc) system based on HCV genotype 2a strain JFH-1 (12). The transfection of Huh-7 cells with in vitro-transcribed strain JFH-1 genomic RNA was performed as previously described (20). The Fab e137 showed a strong neutralizing activity, since at a concentration as low as 1 μg/ml, it was able to completely abrogate the infectivity of HCV genotype 2a (Fig. 3c). These data showed that e137 was capable of potent neutralization of the genotype 2 JFH-1 isolate. This is intriguing, as e137 was unable to neutralize the genotype 2a sample UKN2A1.2 in the HCVpp assay. Likely explanations for this are differences in the E2 sequences within or close to the e137 epitope. Alternatively, it may reflect differences between the two assays, for example, E1-E2 glycosylation pattern and packaging of viral pseudoparticles, which may affect the arrangement of the envelope glycoproteins and consequently modulate the accessibility of the E2 epitopes. Differences in terms of the neutralization profiles between HCVpp and HCVcc assays have been previously described (20). Which system is more predictive of neutralization in vivo is not known, but HCVcc should be more similar to plasma-derived virions than HCVpp.
The data shown here document that MAb e137 is a broadly cross-reactive and cross-neutralizing human antibody clone generated during the natural course of HCV infection. This antibody is directed against a conformational epitope centered on the conserved HCV E2 regions from aa 412 to 420 and aa 528 to 535 and therefore outside hypervariable region 1 (HVR1). Importantly, the regions recognized by e137 show a lower variability rate than HVR1 does, and some E2 amino acid residues crucial for HCV infection are also critical for e137 binding. In particular, mutations of these residues generate variants able to escape from the e137 binding, but in parallel abrogate the infectivity of HCVpp (29). These data suggest that viral mutants able to escape e137 could have a reduced replication capacity. To date, the only MAb able to react with all HCV genotypes is AP33, a mouse MAb that is capable of potent neutralization of HCVpp representing a broad variety of HCV genotypes (28).Two intriguing points are that the epitope recognized by e137 partially overlaps with that of AP33 and that it is a broadly cross-neutralizing antibody in the pseudovirus-based neutralization assay. Indeed, e137 is able to neutralize HCVpp bearing E1-E2 of genotypes 1a, 1b, and 4 and to a lesser extent, genotype 2b. Moreover, e137 is able to neutralize HCVcc at a lower concentration than AP33 is. Notably, AP33 is a full-length immunoglobulin, while e137 is a Fab fragment, and the activity of a Fab molecule may increase in the whole immunoglobulin format (22, 38). Should the HCVcc neutralizing activity be a projection of the in vivo neutralizing potential and the IgG1 format increase the Fab neutralization activity by only 10-fold, a passive administration of e137-derived IgG MAb could easily reach serum levels potentially beneficial for the patient (2). Moreover, using e137 in combination with other neutralizing antibodies might result in an enhancement of the neutralizing activity and in a broadening of the panel of HCV genotypes neutralized.
Although several human MAbs against HCV have been described, the evidence of a broad cross-reactivity is still limited. Only a few anti-HCV E2 human MAbs have been shown to have cross-neutralizing activity. In particular, Fab 4, showed an IC50 from 0.3 to 10 μg/ml on HCVpp bearing E1-E2 of HCV genotypes 1a, 1b, and 2a, while data on HCVcc are not available (33). A group of anti-HCV IgG1 exhibited an IC50 ranging from 1.3 to 16 μg/ml and from 0.05 to 0.2 μg/ml, using HCVpp (bearing genotype 1b E1-E2) and the HCVcc system (genotype 2a), respectively (20); however, the antibodies were unable to neutralize HCV genotype 1a (27). Additionally, a recent clinical trial evaluated the use of a human MAb directed against HCV E2 as support in preventing the reinfection of patients with liver transplant for the end stage of the HCV liver disease (32). The trial showed an efficacy limited to the patients receiving very high doses; this could be due to the fact that the molecule used in this trial neutralizes HCVpp bearing E1-E2 of genotype 1a at 20 μg/ml (13), a dose difficult to reach in passive immunotherapy, leaving room for the expectation that a powerful antibody could possibly exert a beneficial effect in a similar clinical setting.
Overall, the availability of cross-reactive MAbs with strong neutralizing activity (i) allows a better understanding of the virus-host interplay, (ii) provides new opportunities to develop antigens potentially able to elicit a broadly neutralizing immune response, and (iii) may assist in the development of an effective passive immunotherapy for HCV infection.
Footnotes
Published ahead of print on 7 November 2007.
REFERENCES
- 1.Allander, T., K. Drakenberg, A. Beyene, D. Rosa, S. Abrignani, M. Houghton, A. Widell, L. Grillner, and M. A. A. Persson. 2000. Recombinant human monoclonal antibodies against different conformational epitopes of the E2 envelope glycoprotein of hepatitis C virus that inhibit its interaction with CD81. J. Gen. Virol. 812451-2459. [DOI] [PubMed] [Google Scholar]
- 2.Barbas, C. F., III, E. Bjorling, F. Chiodi, N. Dunlop, D. Cababa, T. M. Jones, S. L. Zebedee, M. A. Persson, P. L. Nara, E. Norrby, et al. 1992. Recombinant human Fab fragments neutralize human type 1 immunodeficiency virus in vitro. Proc. Natl. Acad. Sci. USA 899339-9343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartosch, B., J. Bukh, J. C. Meunier, C. Granier, R. E. Engle, W. C. Blackwelder, S. U. Emerson, F. L. Cosset, and R. H. Purcell. 2003. In vitro assay for neutralizing antibody to hepatitis C virus: evidence for broadly conserved neutralization epitopes. Proc. Natl. Acad. Sci. USA 10014199-14204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartosch, B., J. Dubuisson, and F. L. Cosset. 2003. Infectious hepatitis C virus pseudo-particles containing functional E1-E2 envelope protein complexes. J. Exp. Med. 197633-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bugli, F., N. Mancini, C. Y. Kang, C. Di Campli, A. Grieco, A. Manzin, A. Gabrielli, A. Gasbarrini, G. Fadda, P. E. Varaldo, M. Clementi, and R. Burioni. 2001. Mapping B-cell epitopes of hepatitis C virus E2 glycoprotein using human monoclonal antibodies from phage display libraries. J. Virol. 759986-9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buonocore, L., K. J. Blight, C. M. Rice, and J. K. Rose. 2002. Characterization of vesicular stomatitis virus recombinants that express and incorporate high levels of hepatitis C virus glycoproteins. J. Virol. 766865-6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burioni, R., F. Bugli, N. Mancini, D. Rosa, C. Di Campli, G. Moroncini, A. Manzin, S. Abrignani, P. E. Varaldo, M. Clementi, and G. Fadda. 2001. Nonneutralizing human antibody fragments against hepatitis C virus E2 glycoprotein modulate neutralization of binding activity of human recombinant Fabs. Virology 28829-35. [DOI] [PubMed] [Google Scholar]
- 8.Burioni, R., N. Mancini, F. Canducci, S. Carletti, A. Grieco, M. Perotti, G. Serafini, E. Berardinelli, S. Bighi, P. E. Varaldo, and M. Clementi. 2003. Humoral immune response against hepatitis C virus. J. Biol. Regul. Homeost. Agents 17125-127. [PubMed] [Google Scholar]
- 9.Burioni, R., N. Mancini, S. Carletti, M. Perotti, A. Grieco, F. Canducci, P. E. Varaldo, and M. Clementi. 2004. Cross-reactive pseudovirus-neutralizing anti-envelope antibodies coexist with antibodies devoid of such activity in persistent hepatitis C virus infection. Virology 327242-248. [DOI] [PubMed] [Google Scholar]
- 10.Burioni, R., Y. Matsuura, N. Mancini, H. Tani, T. Miyamura, P. E. Varaldo, and M. Clementi. 2002. Diverging effects of human recombinant anti-hepatitis C virus (HCV) antibody fragments derived from a single patient on the infectivity of a vesicular stomatitis virus/HCV pseudotype. J. Virol. 7611775-11779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burioni, R., P. Plaisant, A. Manzin, D. Rosa, V. Delli Carri, F. Bugli, L. Solforosi, S. Abrignani, P. E. Varaldo, G. Fadda, and M. Clementi. 1998. Dissection of human humoral immune response against hepatitis C virus E2 glycoprotein by repertoire cloning and generation of recombinant Fab fragments. Hepatology 28810-814. [DOI] [PubMed] [Google Scholar]
- 12.Cai, Z., C. Zhang, K. S. Chang, J. Jiang, B. C. Ahn, T. Wakita, T. J. Liang, and G. Luo. 2005. Robust production of infectious hepatitis C virus (HCV) from stably HCV cDNA-transfected human hepatoma cells. J. Virol. 7913963-13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eren, R., D. Landstein, D. Terkieltaub, O. Nussbaum, A. Zauberman, J. Ben-Porath, J. Gopher, R. Buchnick, R. Kovjazin, Z. Rosenthal-Galili, S. Aviel, E. Ilan, Y. Hoshany, L. Neville, T. Waisman, O. Ben-Moshe, A. Kischitsky, S. K. Foung, Z. Y. Keck, O. Pappo, A. Eid, O. Jurim, G. Zamir, E. Galun, and S. Dagan. 2006. Preclinical evaluation of two neutralizing human monoclonal antibodies against hepatitis C virus (HCV): a potential treatment to prevent HCV reinfection in liver transplant patients. J. Virol. 802654-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farci, P., H. J. Alter, D. C. Wong, R. H. Miller, S. Govindarajan, R. Engle, M. Shapiro, and R. H. Purcell. 1994. Prevention of hepatitis C virus infection in chimpanzees after antibody-mediated in vitro neutralization. Proc. Natl. Acad. Sci. USA 917792-7796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farci, P., A. Shimoda, D. Wong, T. Cabezon, D. De Gioannis, A. Strazzera, Y. Shimizu, M. Shapiro, H. J. Alter, and R. H. Purcell. 1996. Prevention of hepatitis C virus infection in chimpanzees by hyperimmune serum against the hypervariable region 1 of the envelope 2 protein. Proc. Natl. Acad. Sci. USA 9315394-15399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groothuis, J. R., E. Simoes, M. J. Levin, C. B. Hall, C. E. Long, W. J. Rodriguez, J. Arrobio, H. C. Meissner, D. R. Fulton, R. C. Welliver, D. A. Tristram, G. R. Siber, G. A. Prince, M. Van Raden, and V. G. Hemming for The Respiratory Syncytial Virus Immune Globulin Study Group. 1993. Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants and young children. N. Engl. J. Med. 3291524-1530. [DOI] [PubMed] [Google Scholar]
- 17.Habersetzer, F., A. Fournillier, J. Dubuisson, D. Rosa, S. Abrignani, C. Wychowski, I. Nakano, C. Trepo, C. Desgranges, and G. Inchauspe. 1998. Characterization of human monoclonal antibodies specific to the hepatitis C virus glycoprotein E2 with in vitro binding neutralization properties. Virology 24932-41. [DOI] [PubMed] [Google Scholar]
- 18.Hadlock, K. G., R. E. Lanford, S. Perkins, J. Rowe, Q. Yang, S. Levy, P. Pileri, S. Abrignani, and S. K. H. Foung. 2000. Human monoclonal antibodies that inhibit binding of hepatitis C virus E2 protein to CD81 and recognize conserved conformational epitopes. J. Virol. 7410407-10416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishii, K., D. Rosa, Y. Watanabe, T. Katayama, H. Harada, C. Wyatt, K. Kiyosawa, H. Aizaki, Y. Matsuura, M. Houghton, S. Abrignani, and T. Miyamura. 1998. High titers of antibodies inhibiting the binding of envelope to human cells correlate with natural resolution of chronic hepatitis C. Hepatology 281117-1120. [DOI] [PubMed] [Google Scholar]
- 20.Keck, Z. Y., J. Xia, Z. Cai, T. K. Li, A. M. Owsianka, A. H. Patel, G. Luo, and S. K. Foung. 2007. Immunogenic and functional organization of hepatitis C virus (HCV) glycoprotein E2 on infectious HCV virions. J. Virol. 811043-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai, M. E., A. P. Mazzoleni, F. Argiolu, S. De Virgilis, A. Balestrieri, R. H. Purcell, A. Cao, and P. Farci. 1994. Hepatitis C virus in multiple episodes of acute hepatitis in polytransfused thalassaemic children. Lancet 343388-390. [DOI] [PubMed] [Google Scholar]
- 22.Lamarre, A., and P. J. Talbot. 1995. Protection from lethal coronavirus infection by immunoglobulin fragments. J. Immunol. 1543975-3984. [PubMed] [Google Scholar]
- 23.Lavillette, D., A. W. Tarr, C. Voisset, P. Donot, B. Bartosch, C. Bain, A. H. Patel, J. Dubuisson, J. K. Ball, and F. L. Cosset. 2005. Characterization of host-range and cell entry properties of the major genotypes and subtypes of hepatitis C virus. Hepatology 41265-274. [DOI] [PubMed] [Google Scholar]
- 24.Lindenbach, B. D., M. J. Evans, A. J. Syder, B. Wolk, T. L. Tellinghuisen, C. C. Liu, T. Maruyama, R. O. Hynes, D. R. Burton, J. A. McKeating, and C. M. Rice. 2005. Complete replication of hepatitis C virus in cell culture. Science 309623-626. [DOI] [PubMed] [Google Scholar]
- 25.Mancini, N., F. Canducci, S. Carletti, E. Berardinelli, G. Serafini, A. Grieco, M. Perotti, G. Malcangi, M. G. Danieli, P. E. Varaldo, M. Clementi, and R. Burioni. 2003. Heterogeneity of the humoral anti-HCV/E2 response in persistently infected patients as demonstrated by divergent patterns of inhibition of the binding of anti-HCV/E2 human monoclonal antibodies. J. Biol. Regul. Homeost. Agents 17183-187. [PubMed] [Google Scholar]
- 26.Mancini, N., S. Carletti, M. Perotti, L. Romanò, R. Di Stefano Craxì, A. Craxì, A. R. Zanetti, M. Clementi, and R. Burioni. 2006. Modulation of epitope-specific anti-hepatitis C virus E2 (anti-HCV/E2) antibodies by anti-viral treatment. J. Med. Virol. 781304-1311. [DOI] [PubMed] [Google Scholar]
- 27.Op De Beeck, A., C. Voisset, B. Bartosch, Y. Ciczora, L. Cocquerel, Z. Keck, S. Foung, F. L. Cosset, and J. Dubuisson. 2004. Characterization of functional hepatitis C virus envelope glycoproteins. J. Virol. 782994-3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Owsianka, A., A. W. Tarr, V. S. Juttla, D. Lavillette, B. Bartosch, F. L. Cosset, J. K. Ball, and A. H. Patel. 2005. Monoclonal antibody AP33 defines a broadly neutralizing epitope on the hepatitis C virus E2 envelope glycoprotein. J. Virol. 7911095-11104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Owsianka, A. M., J. M. Timms, A. W. Tarr, R. J. Brown, T. P. Hickling, A. Szwejk, K. Bienkowska-Szewczyk, B. J. Thomson, A. H. Patel, and J. K. Ball. 2006. Identification of conserved residues in the E2 envelope glycoprotein of the hepatitis C virus that are critical for CD81 binding. J. Virol. 808695-8704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pestka, J. M., M. B. Zeisel, E. Blaser, P. Schurmann, B. Bartosch, F. L. Cosset, A. H. Patel, H. Meisel, J. Baumert, S. Viazov, K. Rispeter, H. E. Blum, M. Roggendorf, and T. F. Baumert. 2007. Rapid induction of virus-neutralizing antibodies and viral clearance in a single-source outbreak of hepatitis C. Proc. Natl. Acad. Sci. USA 1046025-6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piazza, M., L. Sagliocca, G. Tosone, V. Guadagnino, M. A. Stazi, R. Orlando, G. Borgia, D. Rosa, S. Abrignani, F. Palumbo, A. Manzin, and M. Clementi. 1997. Sexual transmission of the hepatitis C virus and efficacy of prophylaxis with intramuscular immune serum globulin. A randomized controlled trial. Arch. Intern. Med. 1571537-1544. [PubMed] [Google Scholar]
- 32.Schiano, T. D., M. Charlton, Z. Younossi, E. Galun, T. Pruett, R. Tur-Kaspa, R. Eren, S. Dagan, N. Graham, P. V. Williams, and J. Andrews. 2006. Monoclonal antibody HCV-AbXTL68 in patients undergoing liver transplantation for HCV: results of a phase 2 randomized study. Liver Transplant. 121381-1389. [DOI] [PubMed] [Google Scholar]
- 33.Schofield, D. J., B. Bartosch, Y. K. Shimizu, T. Allander, H. J. Alter, S. U. Emerson, F. L. Cosset, and R. H. Purcell. 2005. Human monoclonal antibodies that react with the E2 glycoprotein of hepatitis C virus and possess neutralizing activity. Hepatology 421055-1062. [DOI] [PubMed] [Google Scholar]
- 34.Servat, A., C. Lutsch, V. Delore, J. Lang, K. Veitch, and F. Cliquet. 2003. Efficacy of rabies immunoglobulins in an experimental post-exposure prophylaxis rodent model. Vaccine 22244-249. [DOI] [PubMed] [Google Scholar]
- 35.Shouval, D., and D. Samuel. 2000. Hepatitis B immune globulin to prevent hepatitis B virus graft reinfection following liver transplantation: a concise review. Hepatology 321189-1195. [DOI] [PubMed] [Google Scholar]
- 36.Tarr, A. W., A. M. Owsianka, J. M. Timms, C. P. McClure, R. J. Brown, T. P. Hickling, T. Pietschmann, R. Bartenschlager, A. H. Patel, and J. K. Ball. 2006. Characterization of the hepatitis C virus E2 epitope defined by the broadly neutralizing monoclonal antibody AP33. Hepatology 43592-601. [DOI] [PubMed] [Google Scholar]
- 37.Tarr, A. W., A. M. Owsianka, D. Jayaraj, R. J. Brown, T. P. Hickling, W. L. Irving, A. H. Patel, and J. K. Ball. 2007. Determination of the human antibody response to the epitope defined by the hepatitis C virus-neutralizing monoclonal antibody AP33. J. Gen. Virol. 882991-3001. [DOI] [PubMed] [Google Scholar]
- 38.Zhang, M. Y., X. Xiao, I. A. Sidorov, V. Choudhry, F. Cham, P. F. Zhang, P. Bouma, M. Zwick, A. Choudhary, D. C. Montefiori, C. C. Broder, D. R. Burton, G. V. Quinnan, Jr., and D. S. Dimitrov. 2004. Identification and characterization of a new cross-reactive human immunodeficiency virus type 1-neutralizing human monoclonal antibody. J. Virol. 789233-9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang, P., C. G. Wu, K. Mihalik, M. L. Virata-Theimer, M. Y. Yu, H. J. Alter, and S. M. Feinstone. 2007. Hepatitis C virus epitope-specific neutralizing antibodies in Igs prepared from human plasma. Proc. Natl. Acad. Sci. USA 1048449-8454. [DOI] [PMC free article] [PubMed] [Google Scholar]