Abstract
Most of the simian virus 40 (SV40) genome is conserved among isolates, but the noncoding regulatory region and the genomic region encoding the large T-antigen C terminus (T-ag-C) may exhibit considerable variation. We demonstrate here that SV40 isolates differ in their oncogenic potentials in Syrian golden hamsters. Experimental animals were inoculated intraperitoneally with 107 PFU of parental or recombinant SV40 viruses and were observed for 12 months to identify genetic determinants of oncogenicity. The viral regulatory region was found to exert a statistically significant influence on tumor incidence, whereas the T-ag-C played a minor role. Viruses with a single enhancer (1E) were more oncogenic than those with a two-enhancer (2E) structure. Rearrangements in the 1E viral regulatory region were detected in 4 of 60 (6.7%) tumors. Viral loads in tumors varied, with a median of 5.4 SV40 genome copies per cell. Infectious SV40 was rescued from 15 of 37 (40%) cell lines established from tumors. Most hamsters with tumors and many without tumors produced antibodies to T antigen. All viruses displayed similar transforming frequencies in vitro, suggesting that differences in oncogenic potential in vivo were due to host responses to viral infection. This study shows that SV40 strains differ in their biological properties, suggests that SV40 replicates to some level in hamsters, and indicates that the outcome of an SV40 infection may depend on the viral strain present.
Simian virus 40 (SV40) is a member of the family Polyomaviridae and is known for its ability to induce malignancies in the Syrian golden hamster (Mesocricetus auratus) model (9-11, 16, 20, 32). SV40 was discovered as an inadvertent contaminant of early forms of poliovirus and adenovirus vaccines (9, 46) that were prepared in primary cultures of kidney cells from rhesus monkeys, which are often naturally infected with the virus (9, 43, 51). Since its discovery, SV40 has been an important model for studies of virus-induced cancers and of viral effects on eukaryotic cell processes (1, 5). SV40 has been found to cause human infections and to be associated with some human malignancies (9, 21, 52).
Phylogenetic analysis has recently established that strains of SV40 exist and can be grouped into clades or genogroups (18). Strains are identified by nucleotide differences at the C terminus of the large tumor antigen (T-ag) gene (T-ag-C) that result in amino acid changes in the protein. The SV40 large T-ag protein is essential for viral replication and is the major viral oncoprotein (1, 9, 32). Strains of SV40 can diverge in the structures of their noncoding regulatory regions (9, 23, 26, 45), generating what are termed variants. SV40 variants containing a partial or complete duplication of the 72-bp enhancer element or other sequence rearrangements are designated as having complex regulatory regions, and those with one enhancer are designated as having a simple or archetypal regulatory region structure (23, 26). It has been demonstrated that increased numbers of enhancer elements in the regulatory region of SV40 enhance the replication of the virus in cell cultures (26, 29). However, any potential contribution of the structure of the SV40 regulatory region to viral pathogenesis in vivo is unknown.
As multiple strains and variants of SV40 appear to be present in humans (7, 27, 30, 31, 36, 45), it is important to understand their potential range of biologic properties. The paradigm of viral strain effects on disease development is exemplified by human papillomaviruses, as only a limited number of strains, termed high-risk types, are able to cause human cancer (4, 56). This report describes the oncogenic properties of different SV40 strains and variants in the hamster model as well as a detailed characterization of tumors induced by those viruses. We discovered an influence of the viral regulatory region on SV40 pathogenesis in vivo that is not evident in transformation assays in vitro.
MATERIALS AND METHODS
Experimental animals.
Outbred weanling male and female hamsters that belonged to the HSD:HAN:AURA stocks of Syrian golden hamsters (M. auratus) were purchased from Harlan Sprague Dawley. Animals were housed in the biohazard facility at the Center for Comparative Medicine at Baylor College of Medicine. Animals were maintained according to approved protocols, and all care was in accordance with established national guidelines as outlined in DHEW publication no. 78-23, Guide for the Care and Use of Laboratory Animals (NIH) (20a).
Viruses.
Natural SV40 strains from different phylogenetic groups and sources were studied. These included (i) SVCPC (GenBank accession number AF156108), (ii) SVPML-1 (GenBank accession number AY271816), (iii) VA45-54 variant 2E (GenBank accession number AF156105), (iv) 777 (GenBank accession number AF332562), (v) Baylor variants 1E (GenBank accession number AF155359) and 2E (GenBank accession number AF155358), and (vi) 776 variants 1E and 2E (GenBank accession number J02400). Sequence differences in the viral regulatory region, the small tumor antigen (t-ag), and the large T-ag variable domain for the different parental viruses have been described previously (23, 24, 29, 44, 45). T-ag-C recombinant viruses were constructed using backbones from parental strains 776(2E) and SVCPC. The cloning strategy consisted of excising part of the early region from strains 776(2E) and SVCPC using restriction enzymes BstXI and BamHI and replacing that fragment with the corresponding fragment from SVCPC, SVPML-1, VA45-54, Baylor, and 776 strains to create the recombinant viruses. The small t-ag coding sequence in a recombinant was that of the viral regulatory region strain. Each construct was sequenced across the joints to confirm that recombination events had not induced mutations; the regulatory and T-ag-C regions were also sequenced. The infectivity of each recombinant construct was confirmed by transfecting plasmid DNA into TC-7 cells (an AGMK cell line) using the transfection reagent Effectene (Qiagen). Cells were harvested by freezing and thawing when cytopathic effects were widespread. Viral stocks of SV40 strains and recombinant viruses were prepared in TC-7 cells, and virus titers were quantitated by plaque assay in the same cells (8).
Induction of tumors in hamsters.
Following aseptic techniques (50), 21-day-old male and female animals were injected by the intraperitoneal (i.p.) route with 1.0 × 107 PFU of virus in 0.5 ml. Control animal groups of the same age and sex included one group inoculated with 0.5 ml of uninfected TC-7 cell lysates and another group that was not inoculated. Animals were observed three times weekly and were sacrificed when there was evidence of neoplasia or debility or at 12 months postinoculation (p.i.) at the termination of the experiment. Euthanasia was done via isoflurane overdose and exsanguination by cardiac puncture. Necropsy included gross examination and collection of organs and neoplasms. Tissues were fixed in 10% neutral buffered formalin or zinc formalin, trimmed and processed into paraffin blocks, sectioned at 5 μm, and stained with hematoxylin and eosin. Tumor morphology was characterized by microscopic evaluation without knowledge of the virus group.
Tumor cell lines.
Tumor samples were removed aseptically from hamsters at the time of necropsy; cells were dissociated using trypsin and were cultured using Dulbecco's modified Eagle's medium containing 10% fetal bovine serum. When cultures reached confluence, cells were subcultured using trypsin and the same medium.
Virus rescue.
Flasks containing tumor cell lines between passages 1 and 10 were incubated until cell detachment (10 to 21 days after seeding), at which time the cultures were harvested by freeze-thaw cycles, and the cell lysates were clarified by low-speed centrifugation. The lysates were analyzed directly by plaque assay or were inoculated into TC-7 cell cultures and incubated for up to 12 days before harvest and plaque assay.
DNA extraction and PCR analysis of SV40 DNA.
Small pieces of tumor samples were minced and processed by overnight incubation at 55°C with proteinase K (Roche Diagnostics) and nucleus lysate solution (Promega). Protein was removed, and DNA was precipitated by isopropanol, resuspended in Tris-EDTA buffer (pH 8.0), and stored at −20°C. Real-time quantitative PCR assays were used to determine the viral genome content in tumor cells (33) using the hamster vimentin gene to normalize viral gene copy numbers to cell numbers (N. Patel et al., unpublished data). Primer sets RA1/RA2, RA3/RA4 (for regulatory region), and TA1/TA2 (for T-ag-C region) were used in conventional PCR to amplify SV40 DNA from tumors and virus stocks (25). Direct sequence analysis of PCR products and alignment of sequences with that of the original inoculated virus revealed any mutations and/or genome rearrangements.
Viral transformation.
Confluent primary mouse embryo fibroblasts were infected with SV40 at a multiplicity of infection of 10 PFU/cell. Virus was allowed to adsorb for 2 h at 37°C, the inoculum was then washed off, medium containing 10% fetal bovine serum was added, and incubation continued at 37°C. Twenty to 24 h p.i., the cells were trypsinized, and 5 × 104 cells were seeded into 60-mm2 plates; four to seven replicate plates per sample were prepared. Cell culture medium was changed every 3 to 4 days. At 3 and 6 weeks p.i., replicate cultures were washed, fixed with 10% formalin, and stained with hematoxylin. The numbers of transformed foci were counted and normalized to foci per 106 cells.
SV40 antibody responses.
Experimental animals were tested for the development of antibodies against virus-specific proteins. Antibodies against SV40 T-ag were detected by indirect immunofluorescence using T-ag-expressing transformed cells; the antibody titer was the highest serum dilution that gave a detectable T-ag reaction (50). SV40-neutralizing antibodies were detected using a plaque reduction test (8). Serum samples were screened at a final serum dilution of 1:10, and titers were determined for sera that reduced the number of plaques by >50% in repeat experiments.
Statistics.
Continuous data are presented as the median and range, and categorical data are presented as proportions. The standard two-sample t test was used to test differences between medians, while differences in proportions were tested using the Z test of proportions. Significance was defined as a P value of <0.05.
RESULTS
Tumor induction in hamsters by SV40.
Groups of 21-day-old Syrian golden hamsters were inoculated i.p. with 1.0 × 107 PFU of SV40 parental isolates or recombinant viruses. Parental isolates included the following: (i) 776, the historical reference strain of SV40, isolated from an adenovirus type 1 vaccine seed stock (48) {two variants of this strain were tested, one with a complex [776(2E)] and the other with a simple [776(1E)] regulatory region}; (ii) Baylor, recovered from a 1956 type 2 Sabin oral polio vaccine (18, 34) (variants with simple and complex regulatory regions were tested); (iii) 777, with a simple regulatory region, isolated from an inactivated polio vaccine (18); (iv) SVCPC, a strain with a simple regulatory region isolated and detected in several human malignant specimens (22, 27, 53) and in an oral polio vaccine seed stock from the former Soviet Union (14); (v) VA45-54, with a complex regulatory region [VA45-54(2E)], originally isolated from monkey kidney cells (20) and detected in a human malignant specimen (28) and in the polio vaccine seed stock from the former Soviet Union; and (vi) SVPML, with a complex regulatory region, recovered from a human patient with progressive multifocal leukoencephalopathy (54). Recombinant viruses were constructed to examine the effects of regulatory region structures and of unique T-ag-C regions from different SV40 isolates on tumor induction in the absence of other differences among the viral genomes. The backbone viruses for these constructs were strains 776 with the complex regulatory region [776(2E)] and SVCPC with a simple regulatory region structure. The T-ag C-terminal coding regions of natural isolates SVCPC, 776, Baylor, VA45-54, and SVPML were moved onto the two viral backgrounds. The constructs were viable and replicated well in monkey kidney cells.
A total of 307 hamsters were inoculated with virus, and 187 animals served as controls (Table 1). Tumors developed only among animals exposed to SV40 and not in the control groups (84/307 [27%] versus 0/187 [0%]; P = 0.0001). Malignancies developed in groups exposed to SV40, with frequencies ranging from 0% to 83% (Table 1). The median latency period (time to tumor appearance) for animals inoculated with different virus isolates varied from 23 to 37 weeks, with individual tumors appearing from 15 to 52 weeks p.i.
TABLE 1.
Tumor development in Syrian golden hamsters following i.p. inoculation with SV40 parental strains and recombinant virusesa
| Viral isolate | No. of tumors/no. of animals | % Tumors | Median time to tumors (wk) (range) |
|---|---|---|---|
| Parental viruses | |||
| 776(1E) | 5/16 | 31 | 33 (29-46) |
| 776(2E) | 3/30 | 10 | 27 (24-41) |
| Baylor(1E) | 10/12 | 83 | 27 (21-30) |
| Baylor(2E) | 1/17 | 6 | 23 |
| 777 | 5/19 | 26 | 35 (21-39) |
| SVCPCb | 18/33 | 54 | 32 (15-52) |
| VA45-54(2E)b | 3/13 | 23 | 26 (23-30) |
| SVPML | 0/16 | 0 | |
| Subtotal | 45/156 | 29 | |
| Recombinant viruses | |||
| 776-CPC(2E) | 1/15 | 7 | 27 |
| 776-Baylor(2E) | 5/15 | 33 | 28 (17-52) |
| 776-VA(2E) | 4/31 | 13 | 32 (26-41) |
| 776-PML(2E) | 1/18 | 6 | 36 |
| CPC-776(1E) | 6/17 | 35 | 30 (15-46) |
| CPC-Baylor(1E) | 7/19 | 37 | 30 (17-40) |
| CPC-VA(1E) | 4/17 | 24 | 36 (22-48) |
| CPC-PML(1E) | 11/19 | 58 | 37 (25-51) |
| Subtotal | 39/151 | 26 | |
| Controls | |||
| TC7 cell lysate | 0/109 | 0 | |
| Uninoculated | 0/78 | 0 |
A total of 1 × 107 PFU of each virus was inoculated i.p. into 21-day-old weanling hamsters, and animals were observed for 1 year. Only autopsy-proven tumors are reported; if an animal died and no autopsy was performed, that animal was removed from the group count.
Some animals of the SVCPC and VA45-54 groups were described previously (50).
Influence of the SV40 regulatory region and T-ag variable domain on tumor induction.
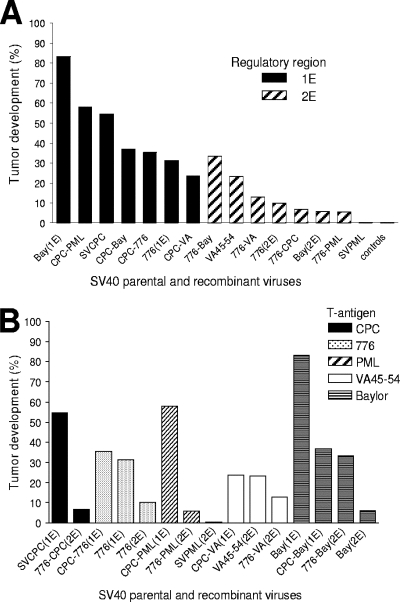
The SV40 regulatory region controls viral transcription and replication (26). Molecular analyses have established that natural isolates of SV40 may possess different regulatory region structures (26). In vitro studies showed that an SV40 isolate with a complex regulatory region replicated better than a derivative with a single enhancer (29). In addition, we showed previously that two SV40 strains with dissimilar regulatory regions and T-ag variable domains differed in their oncogenic potentials in vivo (50). These findings provided the rationale for asking whether SV40 viruses with differences in the structures of the regulatory regions diverged in their tumor-inducing abilities in vivo (Table 1 and Fig. 1A). The results showed that a significantly larger proportion of hamsters developed tumors p.i. with parental viruses containing simple regulatory regions (1E) than after exposure to parental viruses with complex or rearranged regulatory regions (2E) (38/80 [48%] versus 7/76 [9%]; P = 0.0001). There was no difference in the median time to appearance of tumors induced by 1E and 2E viruses (32.5 versus 27 weeks, respectively; P = 0.50). The recombinant viruses confirmed this difference. The SVCPC-based recombinants with a single enhancer were more oncogenic than the 776 recombinants that have a complex enhancer (28/72 [39%] versus 11/79 [14%]; P = 0.0005).
FIG. 1.
Tumor induction in hamsters by parental and recombinant SV40 viruses. (A) Influence of the viral regulatory region. Black bars indicate viruses with simple regulatory regions (1E); shaded bars indicate viruses with complex regulatory regions (2E). The difference between the two groups (1E versus 2E) was statistically significant (66/152 [43%] versus 18/155 [12%]; P = 0.0001). (B) Influence of the T-ag variable domain. Sequences unique to a given strain are indicated by markings shown in the figure.
The variable domain at the extreme C terminus of the T-ag gene contains the highest proportion of variable sites in the SV40 genome, and phylogenetic analyses established that this region can be used for the identification of SV40 strains (18). Embedded within the variable domain of T-ag is a functional domain, defined as the host range/adenovirus helper function (hr/hf) domain (12, 37). Those observations plus our previous tumorigenicity studies prompted an examination of whether different T-ag-C sequences may influence viral oncogenicity. All the T-ag-C recombinant viruses tested were oncogenic, but no patterns emerged when tumor frequencies were compared (Fig. 1B). A direct comparison of parental and recombinant viruses was complicated by the strong influence of viral regulatory region structures on tumor induction. Therefore, a limited comparison between the recombinant constructs with the SVCPC(1E) and the 776(2E) regulatory regions was performed (Table 2). This analysis showed that the T-ag-C domain exhibited only a modest influence on SV40 tumorigenicity in hamsters.
TABLE 2.
Effect of the T-ag variable domain on SV40 tumor development in hamsters
| T-ag | Cladea | Constructb | No. of tumors/no. of animals | % Tumors |
|---|---|---|---|---|
| CPC | B | CPC-CPC | 18/33 | 54 |
| 776-CPC | 1/15 | 7 | ||
| Total | 19/48 | 40c | ||
| Baylor | B | CPC-Baylor | 7/19 | 37 |
| 776-Baylor | 5/15 | 33 | ||
| Total | 12/34 | 35 | ||
| PML | C | CPC-PML | 11/19 | 58 |
| 776-PML | 1/18 | 6 | ||
| Total | 12/37 | 32 | ||
| 776 | A | CPC-776 | 6/17 | 35 |
| 776-776 | 3/30 | 10 | ||
| Total | 9/47 | 19c | ||
| VA | Ungrouped | CPC-VA | 4/17 | 24 |
| 776-VA | 4/31 | 13 | ||
| Total | 8/48 | 17c |
Transformation in vitro.
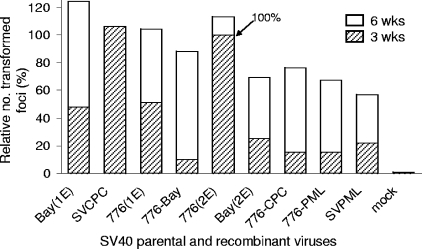
Transformation experiments using cultured mouse embryo fibroblasts were carried out with selected viruses to determine if the viral regulatory region effects exhibited in vivo were detectable under in vitro conditions. Parental viruses tested included those with high [Baylor(1E) and SVCPC] or intermediate [776(1E)] oncogenic potential and simple regulatory regions, those with low oncogenic potential and complex regulatory regions [Baylor(2E) and 776(2E)], and the nononcogenic isolate (SVPML). Several recombinant viruses with low oncogenic potential were also included (Fig. 2). All the parental and recombinant viruses were able to transform mouse embryo fibroblasts in culture with similar transforming frequencies. By 6 weeks p.i., there was a twofold or less difference in the relative number of transformed foci produced by the isolates tested. All showed transforming frequencies of about 1 per 1,000 cells.
FIG. 2.
Transformation in vitro by SV40 parental and recombinant viruses. Primary mouse embryo fibroblasts were infected at 10 PFU per cell. Replicate plates were stained, and transformed foci were counted at 3 and 6 weeks p.i. The relative numbers of transformed foci are plotted and normalized to the value for strain 776(2E) at 3 weeks. Bay(1E), Baylor(1E).
Viral analysis of hamster tumors.
Based on the frequency of tumor induction observed in the hamsters, parental viruses and recombinant constructs were divided into tumor risk groups (high, intermediate, and low), and tumors (n = 78) were molecularly analyzed to determine if virus-related properties of the tumors correlated with the category of inciting virus (Table 3). Viral loads were determined by a real-time quantitative PCR assay using the hamster vimentin gene to calculate cell equivalents. A broad range was observed in SV40 genome copies/cell (0.8 to 326) among individual tumors; median viral loads were higher in tumors from the high-risk (4.6 viral copies/cell) and intermediate-risk (6.8 viral copies/cell) groups than in tumors induced by low-risk viruses (1.9 viral copies/cell), although fewer tumors were available for analysis in the latter category. The state of the viral genomes (episomal or integrated) in these tumors was not determined.
TABLE 3.
Identification of tumor risk groups and viral analysis of tumors for SV40 viruses in hamsters
| Risk levela | Virus | No. of tumors (%)b | Virus load
|
Regulatory regionc
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of tumors tested | Median no. of SV40 genome copies/tumor cell (range) | No. of tumors tested | PCR band
|
Sequence analysis
|
||||||
| WT | R | No. tested | WT | R | ||||||
| High | Baylor(1E) | 10 (83) | 10 | 4.3 (0.3-524.6) | 10 | 10 | 1 | 2 | 2 | 1 |
| SVCPC | 18 (54) | 16 | 4.2 (0.0-23.1) | 15 | 15 | 4 | 4 | |||
| CPC-PML | 11 (58) | 11 | 5.4 (0.7-24.5) | 11 | 11 | 1 | 1 | 1 | 1 | |
| Intermediate | CPC-Baylor | 7 (37) | 7 | 6.9 (1.2-18.0) | 7 | 7 | 1 | 1 | 1 | 1 |
| CPC-776 | 6 (35) | 6 | 4.2 (0.0-17.5) | 4 | 4 | 1 | 1 | |||
| CPC-VA | 4 (24) | 4 | 5.8 (0.0-48.6) | 3 | 3 | 0 | ||||
| 777 | 5 (26) | 5 | 6.3 (0.1-11.9) | 5 | 5 | 1 | 1 | |||
| 776(1E) | 5 (31) | 5 | 6.3 (0.4-10.7) | 5 | 4 | 1 | 2 | 1 | 1 | |
| 776-Baylor | 5 (33) | 4 | 16.0 (9.8-19.7) | 4 | 4 | 0 | ||||
| VA45-54(2E) | 3 (23) | 3 | 14.7 (2.7-25.3) | 3 | 3 | 1 | 1 | |||
| Low | Baylor(2E) | 1 (6) | 1 | 4.0 | 1 | 1 | 1 | 1 | ||
| 776(2E) | 3 (10) | 3 | 1.8 (1.0-4.6) | 3 | 3 | 1 | 1 | |||
| 776-CPC | 1 (7) | 1 | 0.8 | 1 | 1 | 1 | 1 | |||
| 776-VA | 4 (13) | 2 | 2.5 (1.9-3.0) | 1 | 1 | 0 | ||||
| 776-PML | 1 (6) | 0 | ||||||||
| SVPML | 0 (0) | 0 | ||||||||
| Total | 84 | 78 | 5.4 | 73 | 72 | 4 | 16 | 15 | 4 | |
Risk levels were defined as follows: high, >50% tumors following i.p. inoculation of weanling Syrian golden hamsters with 1 × 107 PFU of virus; intermediate, 20 to 40% tumors; low, <20% tumors.
Data from Table 1.
Abbreviations: WT, wild type (same as virus inoculated); R, rearranged regulatory region (based on gel migration of PCR product and sequence analysis).
Regulatory region sequence rearrangements.
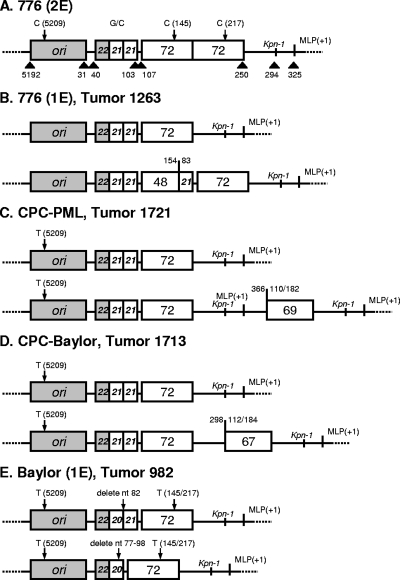
Data suggest that SV40 variants with a complex regulatory region can arise de novo during infection of immunocompromised monkeys (15, 23). The SV40 DNAs in hamster tumor specimens were examined to determine if spontaneous genetic changes occurred in the regulatory region in vivo during the course of the experiments. Seventy-three tumors, including 60 induced by viruses with a simple regulatory region, were tested by PCR; this was followed by DNA sequence analysis on 16 of the tumors (Table 3). Four tumor samples yielded evidence of rearranged viral sequences; the others contained SV40 regulatory region sequences identical to those of the virus variant inoculated into the hamsters. Sequence analysis revealed the structure of each of the rearranged regulatory regions to be unique (Fig. 3). In three of four tumors, the input viral genetic structure was detected, as was the rearranged sequence. In one tumor induced by 776(1E), the original viral sequence was not detected. The detection of rearrangements in four independent neoplasms out of 60 tumors induced by viruses with simple regulatory regions suggests a frequency of rearrangement of 6.7% under the experimental conditions described here.
FIG. 3.
SV40 regulatory region rearrangements detected in hamster tumors. (A) Schematic representation of the SV40 reference strain [776(2E)] showing segments within the regulatory region. ori, origin of DNA replication; G/C, G/C-rich region; 72, 72-bp enhancer element; MLP(+1), major late promoter start site. Numbers above or below the figure identify specific nucleotide positions in 776(2E). The relative borders of specific regulatory region segments are indicated by arrows above or below the figure. Tandemly repeated sequences are depicted by white boxes inscribed with the numbers 21 or 72. (B to E) Regulatory region structures of viruses inoculated (top row) and sequences recovered (bottom row) from hamster tumors. In the tumor induced by 776(1E) in hamster 1263, only the rearranged regulatory region was detected; the original structure was not detected. In tumors from hamsters 1721, 1713, and 982, both original and rearranged regulatory region sequences were detected.
One or two tumors from each virus group were analyzed for the viral T-ag C-terminal sequence, and the expected sequence was recovered in each case. This survey confirmed the identity of the virus strain inoculated into each group of animals. No sequence changes were detected, an observation that is compatible with previous indications that a T-ag variable domain sequence is genetically stable (15, 18, 23, 24).
Recovery of infectious SV40 from hamster tumor cell lines.
Cell lines were established in tissue culture from primary hamster tumors during the course of this study. Attempts to recover infectious SV40 from the cell lines were made. Cell cultures were disrupted, and the cell lysates were either plaqued directly or passaged once in TC-7 cells before plaque assay. Infectious SV40 was recovered from 15 of 37 (40%) tumor cell lines (Table 4). The viral load (SV40 genome copies per tumor cell) was similar for rescue-positive (median, 4.9) and rescue-negative (median, 6.2) cell lines. The median latency periods (time to tumor appearance) for the tumors from which cell lines were established were comparable for rescue-positive (median, 25 weeks) and rescue-negative (median, 30 weeks) cell lines. The frequencies of virus recovery were similar for cell lines from tumors induced by viruses with complex regulatory regions and with simple regulatory regions (5/9 [56%] versus 10/28 [36%]; P = 0.44).
TABLE 4.
Recovery of infectious SV40 from cell lines established from hamster tumors
| Virus | No. of tumor cell lines tested | No. of rescue-positive tumor cell lines | No. of SV40 genome copies/cella
|
Time to tumors (wk) from which cell lines were established
|
||
|---|---|---|---|---|---|---|
| Rescue-positive tumor cell lines | Rescue-negative tumor cell lines | Rescue-positive tumors | Rescue-negative tumors | |||
| SVCPC | 12 | 1 | ND | ND | 16 | 14, 26, 27, 30, 32, 33, 35, 35, 37, 39, 39 |
| Baylor(1E) | 2 | 0 | 5.9, 326.3 | 21, 21 | ||
| 777 | 2 | 2 | 7.6, 16.8 | 21, 33 | ||
| CPC-776 | 2 | 2 | 2.3, 23.4 | 20, 24 | ||
| CPC-VA | 2 | 1 | 17.3 | 3.4 | 22 | 30 |
| CPC-PML | 4 | 4 | 0.8, 0.9, 1.1, 4.9 | 25, 28, 30, 30 | ||
| 776(1E) | 4 | 0 | 3.1, 6.4, 7.5, 10.8 | 29, 31, 33, 51 | ||
| 776(2E) | 2 | 1 | 1.3 | 2.5 | 24 | 27 |
| 776-CPC | 1 | 0 | 3.7 | 27 | ||
| 776-Baylor | 2 | 1 | 2.8 | 13.7 | 21 | 17 |
| 776-VA | 2 | 2 | 13.0, 33.4 | 26, 41 | ||
| VA45-54 | 2 | 1 | ND | ND | 33 | 34 |
| Total | 37 | 15 | ||||
| Median | 40b | 4.9 | 6.2 | 25 | 30 | |
ND, not done.
Shown as a percentage.
Viral serologic responses in hamsters.
Serologic responses to SV40 proteins were determined for many of the hamsters (n = 304). The majority of tumor-bearing hamsters produced antibodies to T-ag, irrespective of the responsible virus strain, as measured by an indirect immunofluorescence assay (Table 5). The percentage of T-antibody positivity approached 100% for tumor-bearing animals inoculated with viruses that had either simple or complex regulatory regions. Many of the virus-inoculated, non-tumor-bearing hamsters also developed T antibodies. The percentage of positive responders among tumor-free hamsters was higher for those inoculated with viruses with complex regulatory regions than for those exposed to viruses with simple enhancers (99/136 [73%] versus 35/86 [41%]; P = 0.0001). SV40-neutralizing antibody responses were determined using a plaque reduction assay. With few exceptions, the tumor-bearing animals contained viral neutralizing antibody. In contrast, only about 60% of the non-tumor-bearing animals produced a detectable SV40-neutralizing antibody, regardless of the virus inoculum. Median titers for both T antibody and neutralizing antibody were generally higher in the tumor-bearing animals than in those without tumors. The uninoculated control animals tested (n = 7) possessed no virus-specific antibodies.
TABLE 5.
Antibody responses in Syrian golden hamsters inoculated i.p. as weanlings with SV40 parental and recombinant virusesa
| Type of regulatory region and virus | Presence of tumors | No. of hamsters tested | Anti-T-agb
|
Neutralizing antibodyb
|
||
|---|---|---|---|---|---|---|
| No. of positive hamsters (%) | Median titerc (range) | No. of positive hamsters (%) | Median titerc (range) | |||
| Simple | ||||||
| Baylor(1E) | Yes | 10 | 10 (100) | 100 (10-5,000) | 10 (100) | 8,000 (1,000-10,000) |
| No | 2 | 1 (50) | 10 (10) | 2 (100) | 3,200 (200-6,000) | |
| SVCPC | Yes | 18 | 16 (89) | 100 (5-5,000) | 18 (100) | 9,000 (100-10,000) |
| No | 15 | 7 (47) | 100 (10-1,000) | 8 (53) | 1,000 (1,000) | |
| 777 | Yes | 5 | 5 (100) | 100 (10-1,000) | 5 (100) | 2,000 (100-5,000) |
| No | 14 | 3 (21) | 10 (5-100) | 5 (36) | 100 (100-1,000) | |
| 776(1E) | Yes | 4 | 4 (100) | 550 (100-5,000) | 3 (75) | 2,000 (1,000-10,000) |
| No | 11 | 5 (45) | 100 (100) | 7 (64) | 1,000 (100-2,000) | |
| CPC-Baylor | Yes | 7 | 7 (100) | 1,000 (5-1,000) | 7 (100) | 5,000 (1,000-65,000) |
| No | 12 | 9 (75) | 100 (5-100) | 9 (75) | 1,000 (100-2,000) | |
| CPC-PML | Yes | 11 | 10 (91) | 1,000 (10-5,000) | 10 (91) | 2,000 (100-6,000) |
| No | 8 | 4 (50) | 100 (10-100) | 6 (75) | 1,500 (100-6,000) | |
| CPC-776 | Yes | 6 | 3 (50) | 100 (10-5,000) | 5 (83) | 25,000 (1,000-200,000) |
| No | 11 | 4 (36) | 100 (5-1,000) | 4 (36) | 1,000 (100-2,000) | |
| CPC-VA | Yes | 3 | 2 (67) | 2,550 (10-5,000) | 1 (33) | 10,000 (10,000) |
| No | 13 | 2 (15) | 55 (10-100) | 5 (38) | 100 (100-1,000) | |
| Complex | ||||||
| Baylor(2E) | Yes | 1 | 1 (100) | 100 (100) | 1 (100) | 2,000 (2,000) |
| No | 16 | 11 (69) | 5 (2-100) | 8 (50) | 1,000 (100-2,000) | |
| 776(2E) | Yes | 3 | 3 (100) | 1,000 (1,000) | 3 (100) | 1,000 (1,000-70,000) |
| No | 27 | 22 (81) | 100 (5-5,000) | 16 (59) | 1,000 (100-70,000) | |
| VA45-54 | Yes | 3 | 3 (100) | 2,000 (2,000-5,000) | 3 (100) | 6,000 (6,000-10,000) |
| No | 10 | 7 (70) | 10 (5-1,000) | 2 (20) | 550 (10-1,000) | |
| SVPML | Yes | 0 | ||||
| No | 16 | 8 (50) | 100 (5-1,000) | 3 (100)d | 1,000 (100-2,000) | |
| 776-Baylor | Yes | 5 | 5 (100) | 1,000 (10-5,000) | 5 (100) | 2,000 (1,000-6,000) |
| No | 9 | 9 (100) | 100 (10-1,000) | 9 (100) | 1,000 (200-6,000) | |
| 776-CPC | Yes | 1 | 1 (100) | 1,000 (1,000) | 1 (100) | 6,000 (6,000) |
| No | 14 | 12 (86) | 10 (5-100) | 12 (86) | 1,000 (100-2,000) | |
| 776-VA | Yes | 4 | 4 (100) | 100 (100-5,000) | 4 (100) | 1,000 (100-1,000) |
| No | 27 | 15 (56) | 100 (5-100) | 6 (32)d | 1,000 (1,000) | |
| 776-PML | Yes | 1 | 1 (100) | 100 (100) | 1 (100) | 1,000 (1,000) |
| No | 17 | 15 (88) | 10 (5-1,000) | 16 (94) | 550 (100-6,000) | |
| None | ||||||
| Controls | No | 7 | 0 (0) | 0 (0) | ||
Sera were collected when tumor-bearing animals were sacrificed or when the experiments were terminated at 12 months after inoculation.
Indirect immunofluorescence was performed to detect antibodies against SV40 T-ag, and a specific plaque reduction assay was used to measure neutralizing antibodies against SV40.
Titers are expressed as the reciprocal of the highest serum dilution that tested positive. Median titers given are for positive sera only.
Neutralizing antibodies were measured in only 3 and 19 tumor-free animals from the SVPML and 776-VA groups, respectively.
Pathology.
Macroscopic and microscopic examinations revealed morphologically solid tumors as well as neoplasms that covered thickened peritoneal surfaces, with visceral adhesions and with invasion of viscera, retroperitoneal and perirenal regions, and the abdominal wall for all virus groups. No morphological differences were observed in tumors arising from the different parental and recombinant viruses.
DISCUSSION
This report describes an extensive analysis of the pathogenesis of SV40-induced tumors in the Syrian golden hamster model. Natural viral strains from monkey kidneys, contaminated virus vaccines, and human malignant and nonmalignant specimens were examined. The results show that distinct SV40 strains differ in their oncogenic potentials in vivo. An influence of the viral regulatory region on tumorigenicity was demonstrated, with parental and recombinant viruses possessing a simple regulatory region being significantly more oncogenic than those with complex regulatory region structures. It appears, therefore, that the SV40 regulatory region may represent a biological determinant of pathogenesis of viral infection and/or disease.
In the murine polyomavirus (MuPyV) system, the viral enhancer region has been shown to influence the level of virus replication and organ specificity during acute infections in newborn mice (40). The MuPyV B enhancer affected the level of persistent infection in mouse kidneys (41) but was not required for replication in several adult tissues (2). These effects were interpreted as reflecting differentially expressed host transcription factors. In addition, a duplication in the viral regulatory region outside the enhancer was sufficient to drive the development of thymic tumors in mice (19). However, mice are highly susceptible hosts for infection by MuPyV, so it is not clear whether observations from that system would be predictive for SV40 infections in hamsters.
It has not been established if SV40 undergoes limited replication in hamsters, although there is indirect evidence that SV40 may be able to replicate in the animals. Infectious SV40 was rescued from 40% of the tumor cell lines tested in this study. The tumors from which the cell lines were established had arisen 5 to 8 months p.i., so it is unlikely that residual input virus was resident in the tumors. These data confirmed decades-old reports of recovery of infectious SV40 from virus-induced hamster tumors (50). Many of the virus-inoculated, non-tumor-bearing animals developed antibodies to T-ag that persisted until the termination of the experiment 1 year later. We speculate that the induction of detectable T antibodies in the absence of tumor formation is a reflection of virus replication in those animals. This study showed that not all animals responded to a given virus exposure in the same way, which is not surprising, as the animals were from an outbred population. A similar diversity of responses might be predicted following human infections due to differences in host responses.
Rearrangements in the simple regulatory region of inoculated SV40 strains were detected in four tumors in this study. The mechanisms responsible for rearrangements in the polyomavirus regulatory region are unknown. Whether viral DNA replication is a prerequisite for the occurrence of such rearrangements is unclear, but it has been proposed that recombination occurs between two newly synthesized daughter strands during replication (13, 55). Studies of monkeys (15, 23) found variability in viral regulatory regions in peripheral blood mononuclear cells, so lymphoid cells may promote such rearrangements. As both parental and rearranged versions were found in three tumors reported here, perhaps DNA repair systems within tumor cells were involved. Viral rearrangements have been detected in SV40-infected monkeys immunocompromised due to infection with simian immunodeficiency virus (15, 23), but the significance for natural infection processes is unknown. Particles of the JC and BK (BKV) polyomaviruses shed in the urine tend to contain archetypal regulatory region structures, whereas JCV brain isolates from patients with progressive multifocal leukoencephalopathy usually have complex enhancer structures (55). Many rearrangements have been found in BKV isolates but have not been linked to any BKV-associated disease to date (13, 35).
The differing oncogenic potentials of 1E and 2E variants of SV40 in hamsters were not reflected in differences in transforming activities in vitro. We conclude that the in vivo results must reflect some aspect of the host response to viral infection. One possible explanation is that differences in oncogenicity reflect dissimilar rates of viral replication. Studies of cell cultures have shown that SV40 and BK viruses with complex regulatory regions replicate faster and to higher titers than those with simple regulatory regions (29, 47). One can speculate that a faster-replicating virus would be recognized and cleared more efficiently by the host's antiviral immune response than a slower-replicating virus. This explanation is supported by studies with MuPyV, which showed that faster-growing viral variants were associated with high viral replication in mouse kidneys and a strong immune response, whereas slower-growing variants persisted with low-level viral replication and no notable host response (42). Furthermore, evidence from the lymphocytic choriomeningitis virus model indicated that the speed of viral replication influenced virus persistence; a slower-replicating virus was more apt to evade immune surveillance and persist (3). We suggest that poorer clearance and more frequent residual persistent infection by SV40 variants with a simple regulatory region account for their higher frequency of subsequent tumor development.
Analysis of tumors produced by SV40 strains in different tumor risk groups did not reveal any characteristic differences. We speculate that if a virus of any given genetic type initiates the transformation process and the altered cells escape host clearance, then the ensuing tumor development is similar. However, only one route of animal inoculation (i.p.) was used in this study. It is possible that with other routes of inoculation, different cell types would be exposed to virus, and some tissue specificity for tumor induction might be detected among viral strains. It was reported previously for MuPyV that different organs became persistently infected when mice were inoculated by different routes (intranasal versus i.p.) (17).
SV40 large T-ag is required for viral replication as well as for virus-induced cellular transformation. The experiments described here involving recombinant viruses containing T-ag-C regions from different SV40 isolates revealed only a modest effect of this region of the viral genome on viral oncogenicity in vivo. This finding is corroborative of previous studies that suggested that although the T-ag-C is essential for the viral life cycle (39), it is not required for transformation in vitro (49).
Observations dating back several decades found SV40 to be associated with some human malignancies, but results among studies have been inconsistent, and the association remains under debate (9, 21, 38, 46). However, for malignant mesotheliomas, combined data from in vitro and in vivo studies strongly support a causal role for SV40, probably as a cofactor with asbestos (6). Discordances among some studies may reflect variations in technical aspects of the assays used for analyses (14), but a more important consideration is the probable variation in the prevalences of SV40 infections in different geographic regions (51). The recognition of SV40 genogroups (18) and differences in their biological properties (shown here) suggest that viral strain differences could also contribute to variable findings of SV40 associations with human cancers in different locales. Interestingly, an analysis of healthy Italian organ donors demonstrated SV40 sequence differences between individuals born before 1947 and those born after 1958 (36).
In conclusion, we have shown that differences in biological properties among SV40 strains exist. We found a strong influence of the structure of the SV40 regulatory region on tumor induction in hamsters. These findings raise the possibility there may be other, yet-unrecognized biological differences among SV40 strains that affect the pathogenesis of infection and disease. An important implication of these findings is that the risk and outcome of SV40 infections in susceptible hosts, including humans, may depend on the viral strain causing an infection.
Acknowledgments
This study was supported in part by grants R21 CA96951 and R01 CA104818 from the National Cancer Institute.
Footnotes
Published ahead of print on 31 October 2007.
REFERENCES
- 1.Ahuja, D., M. T. Sáenz-Robles, and J. M. Pipas. 2005. SV40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene 247729-7745. [DOI] [PubMed] [Google Scholar]
- 2.Amalfitano, A., L. G. Martin, and M. M. Fluck. 1992. Different roles for two enhancer domains in the organ- and age-specific pattern of polyomavirus replication in the mouse. Mol. Cell. Biol. 123628-3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bocharov, G., B. Ludewig, A. Bertoletti, P. Klenerman, T. Junt, P. Krebs, T. Luzyanina, C. Fraser, and R. M. Anderson. 2004. Underwhelming the immune response: effect of slow virus growth on CD8+-T-lymphocyte responses. J. Virol. 782247-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burd, E. M. 2003. Human papillomavirus and cervical cancer. Clin. Microbiol. Rev. 161-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butel, J. S. 2000. Viral carcinogenesis: revelation of molecular mechanisms and etiology of human disease. Carcinogenesis 21405-426. [DOI] [PubMed] [Google Scholar]
- 6.Butel, J. S. SV40, human infections, and cancer: emerging concepts and causality considerations. In K. Khalili and K. T. Jeang (ed.), Viral oncology: basic science and clinical applications, in press. Blackwell Publishing Ltd., Oxford, United Kingdom.
- 7.Butel, J. S., A. S. Arrington, C. Wong, J. A. Lednicky, and M. J. Finegold. 1999. Molecular evidence of simian virus 40 infections in children. J. Infect. Dis. 180884-887. [DOI] [PubMed] [Google Scholar]
- 8.Butel, J. S., S. Jafar, C. Wong, A. S. Arrington, A. R. Opekun, M. J. Finegold, and E. Adam. 1999. Evidence of SV40 infections in hospitalized children. Hum. Pathol. 301496-1502. [DOI] [PubMed] [Google Scholar]
- 9.Butel, J. S., and J. A. Lednicky. 1999. Cell and molecular biology of simian virus 40: implications for human infections and disease. J. Natl. Cancer Inst. 91119-134. [DOI] [PubMed] [Google Scholar]
- 10.Butel, J. S., S. S. Tevethia, and J. L. Melnick. 1972. Oncogenicity and cell transformation by papovavirus SV40: the role of the viral genome. Adv. Cancer Res. 151-55. [DOI] [PubMed] [Google Scholar]
- 11.Cicala, C., F. Pompetti, and M. Carbone. 1993. SV40 induces mesotheliomas in hamsters. Am. J. Pathol. 1421524-1533. [PMC free article] [PubMed] [Google Scholar]
- 12.Cole, C. N., L. V. Crawford, and P. Berg. 1979. Simian virus 40 mutants with deletions at the 3′ end of the early region are defective in adenovirus helper function. J. Virol. 30683-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cubitt, C. L. 2006. Molecular genetics of the BK virus, p. 85-95. In N. Ahsan (ed.), Polyomaviruses and human diseases. Springer Science, Georgetown, TX.
- 14.Cutrone, R., J. Lednicky, G. Dunn, P. Rizzo, M. Bocchetta, K. Chumakov, P. Minor, and M. Carbone. 2005. Some oral poliovirus vaccines were contaminated with infectious SV40 after 1961. Cancer Res. 6510273-10279. [DOI] [PubMed] [Google Scholar]
- 15.Dang, X., M. K. Axthelm, N. L. Letvin, and I. J. Koralnik. 2005. Rearrangement of simian virus 40 regulatory region is not required for induction of progressive multifocal leukoencephalopathy in immunosuppressed rhesus monkeys. J. Virol. 791361-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diamandopoulos, G. T. 1973. Induction of lymphocytic leukemia, lymphosarcoma, reticulum cell sarcoma, and osteogenic sarcoma in the Syrian golden hamster by oncogenic DNA simian virus 40. J. Natl. Cancer Inst. 501347-1365. [DOI] [PubMed] [Google Scholar]
- 17.Dubensky, T. W., and L. P. Villarreal. 1984. The primary site of replication alters the eventual site of persistent infection by polyomavirus in mice. J. Virol. 50541-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forsman, Z. H., J. A. Lednicky, G. E. Fox, R. C. Willson, Z. S. White, S. J. Halvorson, C. Wong, A. M. Lewis, Jr., and J. S. Butel. 2004. Phylogenetic analysis of polyomavirus simian virus 40 from monkeys and humans reveals genetic variation. J. Virol. 789306-9316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freund, R., C. J. Dawe, and T. L. Benjamin. 1988. Duplication of noncoding sequences in polyomavirus specifically augments the development of thymic tumors in mice. J. Virol. 623896-3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Girardi, A. J., B. H. Sweet, V. B. Slotnick, and M. R. Hilleman. 1962. Development of tumors in hamsters inoculated in the neonatal period with vacuolating virus, SV40. Proc. Soc. Exp. Biol. Med. 109649-660. [DOI] [PubMed] [Google Scholar]
- 20a.Institute of Laboratory Animal Resources Committee on Care and Use of Laboratory Animals. 1978. Guide for the care and use of laboratory animals. Department of Health and Human Services publication no. (NIH) 78-23, Bethesda, MD.
- 21.Jasani, B., A. Cristaudo, S. A. Emri, A. F. Gazdar, A. Gibbs, B. Krynska, C. Miller, L. Mutti, C. Radu, M. Tognon, and A. Procopio. 2001. Association of SV40 with human tumours. Semin. Cancer Biol. 1149-61. [DOI] [PubMed] [Google Scholar]
- 22.Krieg, P., and G. Scherer. 1984. Cloning of SV40 genomes from human brain tumors. Virology 138336-340. [DOI] [PubMed] [Google Scholar]
- 23.Lednicky, J. A., A. S. Arrington, A. R. Stewart, X. M. Dai, C. Wong, S. Jafar, M. Murphey-Corb, and J. S. Butel. 1998. Natural isolates of simian virus 40 from immunocompromised monkeys display extensive genetic heterogeneity: new implications for polyomavirus disease. J. Virol. 723980-3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lednicky, J. A., and J. S. Butel. 1997. Tissue culture adaptation of natural isolates of simian virus 40: changes occur in viral regulatory region but not in carboxy-terminal domain of large T-antigen. J. Gen. Virol. 781697-1705. [DOI] [PubMed] [Google Scholar]
- 25.Lednicky, J. A., and J. S. Butel. 1998. Consideration of PCR methods for the detection of SV40 in tissue and DNA specimens. Dev. Biol. Stand. 94155-164. [PubMed] [Google Scholar]
- 26.Lednicky, J. A., and J. S. Butel. 2001. Simian virus 40 regulatory region structural diversity and the association of viral archetypal regulatory regions with human brain tumors. Semin. Cancer Biol. 1139-47. [DOI] [PubMed] [Google Scholar]
- 27.Lednicky, J. A., R. L. Garcea, D. J. Bergsagel, and J. S. Butel. 1995. Natural simian virus 40 strains are present in human choroid plexus and ependymoma tumors. Virology 212710-717. [DOI] [PubMed] [Google Scholar]
- 28.Lednicky, J. A., A. R. Stewart, J. J. Jenkins III, M. J. Finegold, and J. S. Butel. 1997. SV40 DNA in human osteosarcomas shows sequence variation among T-antigen genes. Int. J. Cancer 72791-800. [DOI] [PubMed] [Google Scholar]
- 29.Lednicky, J. A., C. Wong, and J. S. Butel. 1995. Artificial modification of the viral regulatory region improves tissue culture growth of SV40 strain 776. Virus Res. 35143-153. [DOI] [PubMed] [Google Scholar]
- 30.Li, R. M., M. H. Branton, S. Tanawattanacharoen, R. A. Falk, J. C. Jennette, and J. B. Kopp. 2002. Molecular identification of SV40 infection in human subjects and possible association with kidney disease. J. Am. Soc. Nephrol. 132320-2330. [DOI] [PubMed] [Google Scholar]
- 31.Li, R. M., R. B. Mannon, D. Kleiner, M. Tsokos, M. Bynum, A. D. Kirk, and J. B. Kopp. 2002. BK virus and SV40 co-infection in polyomavirus nephropathy. Transplantation 741497-1504. [DOI] [PubMed] [Google Scholar]
- 32.McNees, A. L., and J. S. Butel. Simian virus 40 (Polyomaviridae). In B. Mahy and M. Van Regenmortel (ed.), Encyclopedia of virology, in press. Elsevier, New York, NY.
- 33.McNees, A. L., Z. S. White, P. Zanwar, R. A. Vilchez, and J. S. Butel. 2005. Specific and quantitative detection of human polyomaviruses BKV, JCV, and SV40 by real time PCR. J. Clin. Virol. 3452-62. [DOI] [PubMed] [Google Scholar]
- 34.Melnick, J. L., and S. Stinebaugh. 1962. Excretion of vacuolating SV-40 virus (papova virus group) after ingestion as a contaminant of oral poliovaccine. Proc. Soc. Exp. Biol. Med. 109965-968. [DOI] [PubMed] [Google Scholar]
- 35.Moens, U., and M. Van Ghelue. 2005. Polymorphisms in the genome of non-passaged human polyomavirus BK: implications for cell tropism and the pathological role of the virus. Virology 331209-231. [DOI] [PubMed] [Google Scholar]
- 36.Paracchini, V., S. Garte, P. Pedotti, F. Poli, S. Frison, and E. Taioli. 2005. Molecular identification of simian virus 40 infection in healthy Italian subjects by birth cohort. Mol. Med. 1148-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pipas, J. M. 1985. Mutations near the carboxyl terminus of the simian virus 40 large tumor antigen alter viral host range. J. Virol. 54569-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poulin, D. L., and J. A. DeCaprio. 2006. Is there a role for SV40 in human cancer? J. Clin. Oncol. 244356-4365. [DOI] [PubMed] [Google Scholar]
- 39.Poulin, D. L., and J. A. DeCaprio. 2006. The carboxyl-terminal domain of large T antigen rescues SV40 host range activity in trans independent of acetylation. Virology 349212-221. [DOI] [PubMed] [Google Scholar]
- 40.Rochford, R., B. A. Campbell, and L. P. Villarreal. 1990. Genetic analysis of the enhancer requirements for polyomavirus DNA replication in mice. J. Virol. 64476-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rochford, R., J. P. Moreno, M. L. Peake, and L. P. Villarreal. 1992. Enhancer dependence of polyomavirus persistence in mouse kidneys. J. Virol. 663287-3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rochford, R., and L. P. Villarreal. 1991. Polyomavirus DNA replication in the pancreas and in a transformed pancreas cell line has distinct enhancer requirements. J. Virol. 652108-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shah, K., and N. Nathanson. 1976. Human exposure to SV40: review and comment. Am. J. Epidemiol. 1031-12. [DOI] [PubMed] [Google Scholar]
- 44.Stewart, A. R., J. A. Lednicky, U. S. Benzick, M. J. Tevethia, and J. S. Butel. 1996. Identification of a variable region at the carboxy terminus of SV40 large T-antigen. Virology 221355-361. [DOI] [PubMed] [Google Scholar]
- 45.Stewart, A. R., J. A. Lednicky, and J. S. Butel. 1998. Sequence analyses of human tumor-associated SV40 DNAs and SV40 viral isolates from monkeys and humans. J. Neurovirol. 4182-193. [DOI] [PubMed] [Google Scholar]
- 46.Stratton, K., D. A. Alamario, and M. C. McCormick. 2003. Immunization safety review: SV40 contamination of polio vaccine and cancer. National Academies Press, Washington, DC. [PubMed]
- 47.Sugimoto, C., K. Hara, F. Taguchi, and Y. Yogo. 1989. Growth efficiency of naturally occurring BK virus variants in vivo and in vitro. J. Virol. 633195-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sweet, B. H., and M. R. Hilleman. 1960. The vacuolating virus, S.V.40. Proc. Soc. Exp. Biol. Med. 105420-427. [DOI] [PubMed] [Google Scholar]
- 49.Tevethia, M. J., J. M. Pipas, T. Kierstead, and C. Cole. 1988. Requirements for immortalization of primary mouse embryo fibroblasts probed with mutants bearing deletions in the 3′ end of SV40 gene A. Virology 16276-89. [DOI] [PubMed] [Google Scholar]
- 50.Vilchez, R. A., C. F. Brayton, C. Wong, P. Zanwar, D. E. Killen, J. L. Jorgensen, and J. S. Butel. 2004. Differential ability of two simian virus 40 strains to induce malignancies in weanling hamsters. Virology 330168-177. [DOI] [PubMed] [Google Scholar]
- 51.Vilchez, R. A., and J. S. Butel. 2004. Emergent human pathogen simian virus 40 and its role in cancer. Clin. Microbiol. Rev. 17495-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vilchez, R. A., C. A. Kozinetz, A. S. Arrington, C. R. Madden, and J. S. Butel. 2003. Simian virus 40 in human cancers. Am. J. Med. 114675-684. [DOI] [PubMed] [Google Scholar]
- 53.Vilchez, R. A., J. A. Lednicky, S. J. Halvorson, Z. S. White, C. A. Kozinetz, and J. S. Butel. 2002. Detection of polyomavirus simian virus 40 tumor antigen DNA in AIDS-related systemic non-Hodgkin lymphoma. J. Acquir. Immune Defic. Syndr. 29109-116. [DOI] [PubMed] [Google Scholar]
- 54.Weiner, L. P., R. M. Herndon, O. Narayan, R. T. Johnson, K. Shah, L. J. Rubinstein, T. J. Preziosi, and F. K. Conley. 1972. Isolation of virus related to SV40 from patients with progressive multifocal leukoencephalopathy. N. Engl. J. Med. 286385-390. [DOI] [PubMed] [Google Scholar]
- 55.Yogo, Y., and C. Sugimoto. 2001. The archetype concept and regulatory region rearrangement, p. 127-148. In K. Khalili and G. L. Stoner (ed.), Human polyomaviruses: molecular and clinical perspectives. Wiley-Liss, Inc., New York, NY.
- 56.zur Hausen, H. 2002. Papillomaviruses and cancer: from basic studies to clinical application. Nat. Rev. Cancer 2342-350. [DOI] [PubMed] [Google Scholar]