Abstract
Dengue viruses are distributed widely in the tropical and subtropical areas of the world and cause dengue fever and its severer form, dengue hemorrhagic fever. While neutralizing antibodies are considered to play a major role in protection from these diseases, antibody-dependent enhancement (ADE) of infection is an important mechanism involved in disease severity, in addition to the involvement of T lymphocytes. Here, we analyzed relationships between neutralizing and enhancing activities at a clonal level using models of dengue type 2 virus (DENV2) and dengue type 4 virus (DENV4). Totals of 33 monoclonal antibodies (MAbs) against DENV2 and 43 against DENV4 were generated, all directed to the envelope protein. In these MAbs, enhancing activities were shown at subneutralizing doses under normal ADE assay conditions where test samples were heat inactivated. However, the inclusion of commercial rabbit complement or fresh sera from healthy humans in the ADE assay system abolished the enhancing activities of all these MAbs. The reductive effect of fresh sera on enhancing activities was significantly reduced by their heat inactivation or the use of C1q- or C3-depleted sera. In some fresh sera, enhancing activities were shown within a range of 20 to 80% of normal complement levels in a dose-dependent fashion. These results indicate that a single antibody species possesses two distinct activities (neutralizing/enhancing), which are controlled by the level of complement, suggesting the involvement of complement in dengue disease severity. Fresh human sera also tended to reduce enhancing activities more effectively in homologous than heterologous combinations of viruses (DENV2/DENV4) and MAbs (against DENV2/DENV4).
Dengue fever (DF) and dengue hemorrhagic fever (DHF) are mosquito-borne diseases caused by infection with any one of the four dengue viruses, dengue virus types 1 to 4 (DENV1 to DENV4) (2, 12, 15). These viruses are distributed throughout the tropical and subtropical areas of the world, with an estimated 50 to 100 million DF cases and 250 to 500 thousand DHF cases reported every year (31, 47). Most infections with dengue viruses result in asymptomatic infections. Clinical cases usually take a benign form (DF) and occasionally a severe form (DHF). DF patients develop high fever, headache, and muscle and joint pain from which almost all recover, whereas DHF patients, though the occurrence and progress of disease are similar to those of DF patients, develop mainly plasma leakage and hemorrhagic manifestations, which may lead to shock (11). The case-fatality rate of DHF is roughly 5% (31), and a large proportion (90%) of DHF patients are children under 15 years of age (53).
Four dengue viruses belong to the genus Flavivirus of the family Flaviviridae. The flavivirus virion consists of a nucleocapsid structure surrounded by a lipid bilayer containing an envelope (E) and a membrane (M) protein (34). The E protein is the major surface protein, containing many neutralizing epitopes. Most members of the genus Flavivirus are grouped into eight antigenic complexes, and four dengue viruses belong to the dengue virus serocomplex (2). Four dengue viruses are antigenically cross-reactive with each other, while they also possess type-specific epitopes as recognized by neutralization tests that provide the highest specificity among existing serological tests (29). Thus, antibodies induced by dengue virus infections can be roughly divided into type-specific neutralizing antibodies, cross-reactive nonneutralizing antibodies, and cross-neutralizing antibodies among the four dengue viruses (31, 47).
Epidemiological evidence indicates that people once infected with one type of dengue virus are usually protected from subsequent infection with the same type of dengue virus (hereafter called homotypic infection) (13, 15, 44). Cross-protection against infections with different types (hereafter called heterotypic infection) is only shown in the short term following infection. It has been considered that neutralizing antibody is important for protection against dengue virus infection, since passive transfer of neutralizing monoclonal antibodies (MAbs) can confer protection from lethal challenge in a murine model (22, 23). On the other hand, secondary infection with a different type of dengue virus may cause DHF, as demonstrated by several epidemiological studies (reviewed in reference 15): secondary infections cause 40 times more DHF cases than primary infections. Therefore, cross-reactive nonneutralizing antibodies induced by the primary infection have been considered to serve as “enhancing” antibodies causing increased disease severity upon secondary infection with a different type of dengue virus.
The initial targets of dengue virus infections following infective mosquito bites have been reported to be immature monocyte-derived dendritic cells (39, 54). A virus replicated and released from these cells may enter the bloodstream and circulate throughout the host body, where monocytes/macrophages (4, 14) and the liver (9) are considered to be principal targets. Several different hypotheses have been proposed for the mechanisms of DHF development (11, 31), including virus virulence (40, 42), cross-reactive T lymphocytes (8, 36), etc. However, most agree that the level of viremia correlates to disease severity (33, 47, 52). One of the host factors relating to increased viremia levels is antibody-dependent enhancement (ADE) of infection (16, 24), which is mediated by Fc gamma receptors on the monocytes/macrophages in the presence of “enhancing” antibodies (30).
It is widely believed that neutralizing antibodies can reduce viremia levels, whereas cross-reactive nonneutralizing (enhancing) antibodies may increase them. Therefore, the balance of these antibody species has been considered important for determining the outcome of the disease; that is, protection or pathogenesis. However, a recent paper reported that some individuals who had neutralizing antibodies against DENV2 develop symptoms upon infection with DENV2, suggesting that neutralizing antibodies do not always work for protection from the subsequent homologous infection (7). Another study reported that enhancing antibodies do not correlate to the viremia level (32). Thus, detailed analyses of the relationships between neutralizing and enhancing activities are needed to elucidate the mechanisms increasing disease severity.
Most previous studies dealing with neutralizing and/or enhancing activities have used serum specimens. However, approaches at a polyclonal level seem to limit detailed analyses. In the present study, MAbs against DENV2 (D2MAbs) or DENV4 (D4MAbs) were generated from immune mice and characterized for enhancing and neutralizing activities against homologous and heterologous types. The results indicated that all MAbs showing enhancing activities showed neutralizing activities. Furthermore, the addition of commercial rabbit complement or fresh human sera into the ADE assay systems abolished the enhancing activities of all MAbs.
MATERIALS AND METHODS
Cells.
Vero cells (26) were cultivated in Eagle's minimum essential medium (MEM) supplemented with 10% fetal bovine serum (FBS) and 60 μg/ml kanamycin. C6/36 cells (26) were cultivated in MEM supplemented with 10% FBS, nonessential amino acids, and 60 μg/ml kanamycin. The U937 human monocytic cell line (48) and the K562 erythroleukemia cell line (35), both provided by Ichiro Kurane of the National Institute of Infectious Diseases (NIID), Japan, were cultivated in RPMI 1640 medium supplemented with 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin (RPMI-10% FBS). The P3-X63-Ag8-U1 (P3U1) mouse myeloma cell line and hybridoma cells were cultivated in RPMI 1640 medium supplemented with 10 to 20% FBS, 100 units/ml penicillin, 100 μg/ml streptomycin, and 5 × 10−5 M 2-mercaptoethanol. All cells were cultivated in a humidified atmosphere of 5% CO2 95% air at 37°C, except for the C6/36 cells, which were cultivated at 28°C.
Viruses.
The New Guinea C strain of DENV2 and the H241 strain of DENV4 (27) were used. Viruses harvested from culture fluids of infected Vero cells were used as antigens for the competition assay, hemagglutination inhibition (HAI) test, and enzyme-linked immunosorbent assay (ELISA) to quantify antibody levels. Viruses harvested from culture fluids of infected C6/36 cells were used for immunoprecipitation, the neutralization test, and the ADE assay. Viruses in the form of an infected mouse brain homogenate at a 10% emulsion in 7.5% bovine serum albumin in phosphate-buffered saline were used for booster immunization of mice to generate hybridomas.
Rabbit hyperimmune sera.
Sera from rabbits hyperimmune to the New Guinea C strain of DENV2 (26) or the H241 strain of DENV4 (27) have been described previously.
Human sera.
Serum samples were collected from 14 healthy humans aged 21 to 52 years, average 26.1 years, with no history of travel in countries where dengue virus is endemic. Heat inactivation of sera was performed at 56°C for 30 min. Complement C1q-depleted human serum and C3-depleted human serum were purchased from Merck, Darmstadt, Germany. All human sera used in the present study were negative for neutralizing antibodies against DENV2 and DENV4 as determined by 50% focus reduction assay (data not shown). The use of human sera was approved by the ethical committee of Kobe University School of Medicine.
Generation of mouse MAbs.
Four-week-old female BALB/c mice were immunized twice at intervals of 3 weeks by inoculation with 100 μg of a DNA vaccine using a needle-free jet injector (ShimaJET; Shimadzu, Kyoto, Japan). The DNA vaccines were pcDNA3-based plasmids expressing the premembrane and E proteins of DENV2 (26) or DENV4 (27). One to 2 months after the second immunization, the mice were boosted with dengue virus antigens of the corresponding type (infected mouse brain homogenate; 1 × 107 PFU/mouse), and spleen cells were collected 3 to 4 days after the booster immunization. Hybridoma cells were generated essentially as described by Kohler and Milstein (25) with some modifications (28). Briefly, spleen cells were fused to P3U1 cells using polyethylene glycol-4000 (Merck, Darmstadt, Germany). Hybridoma cells were screened by ELISA for the production of specific antibodies (see below) and cloned by limiting dilution. For MAb production, hybridoma clones were grown as ascites tumors by intraperitoneal inoculation of 6- to 8-week-old pristane-primed male BALB/c mice with 107 cells from culture. One to three weeks later, ascitic fluids were collected, clarified, and stored at −30°C until use as D2MAbs or D4MAbs.
Affinity purification of IgG from ascitic fluids and biotinylation.
Immunoglobulin G (IgG) was purified from ascitic fluids by using protein G Sepharose 4 Fast Flow (GE Healthcare Bio-Sciences, Piscataway, NJ) and dialyzed against phosphate-buffered saline. Purified IgG was biotinylated with EZ-Link Sulfo-NHS-LC-Biotin (Pierce, Rockford, IL) following the instructions provided by the manufacturer.
Competition assay.
Microplates were sensitized by incubation at 4°C overnight with a 1:1,000 dilution of rabbit hyperimmune serum against DENV2 or DENV4. The sensitized plates were then incubated with culture fluids of Vero cells infected with the corresponding virus (DENV2 or DENV4), a 1:1,000 dilution of the first antibody (ascitic fluid), a 1:100 dilution of the biotinylated second antibody (IgG fraction), a 1:1,000 dilution of avidin-alkaline phosphatase conjugate, and then p-nitrophenyl phosphate. When the absorbance was less than 70% of the control absorbance obtained without incubation with the first antibody, the reaction was determined to be competitive.
ELISA for measuring antibodies to DENV2 or DENV4.
Antibody levels in hybridoma culture fluids or mouse ascitic fluids were measured by a conventional ELISA as described previously (27). Briefly, microplates sensitized with rabbit hyperimmune sera against DENV2 or DENV4 were incubated serially with the corresponding (DENV2 or DENV4) antigen, ascitic fluid samples at a 1:1,000 dilution or culture fluid samples at the original dilution, alkaline phosphatase-conjugated antimouse IgG, and then p-nitrophenyl phosphate. Samples were determined to be positive when the absorbance was higher than the average plus two times the standard deviation (SD) of absorbances obtained with six negative control samples.
ELISA for measuring IgG concentrations.
A sandwich ELISA was performed as described previously (28). Briefly, microplates sensitized with goat anti-mouse IgG were incubated with serial 10-fold dilutions of test samples, alkaline phosphatase-conjugated goat anti-mouse IgG, and then p-nitrophenyl phosphate. The IgG concentrations were calculated by comparing the absorbances with those obtained for the standard mouse sera with known IgG concentrations and expressed as ng/ml.
Determination of isotypes.
The isotype of each MAb was determined by using an ImmunoPure monoclonal antibody isotyping kit II (Pierce, Rockford, IL) according to the manufacturer's instructions.
Immunoprecipitation.
The immunoprecipitation of viral proteins with MAbs was performed by using protein A agarose (Invitrogen, Carlsbad, CA) as described previously (26). Briefly, protein A-coated agarose beads were incubated with each MAb sample, rinsed, and then incubated with culture fluids of C6/36 cells infected with DENV2 or DENV4. Precipitated proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis under nonreducing conditions and detected by silver staining. E and M proteins were confirmed as bands with predicted molecular masses of approximately 63 and 10 kDa, respectively, which were calculated from the amino acid compositions of the E and M proteins based on the nucleotide sequences of the dengue virus genomes registered in GenBank: accession number M19197 for DENV2 NGC and AF326825 for DENV4 H241.
HAI test.
HAI tests were performed by using a microplate modification of the method of Clarke and Casals (5).
Titration of viral infectivity and neutralization test.
Infective titers were determined on Vero cells by counting infectious foci after immunostaining (see below) and expressed as focus-forming units (FFU). Neutralizing antibody titers were determined by using focus reduction assays performed with DENV2 or DENV4 essentially as described previously (27). Briefly, the virus-antibody mixture was incubated with rabbit complement at a final concentration of 5%. In some experiments, the neutralization test was performed without complement. The neutralizing activities were expressed as percentages of focus reduction or neutralizing antibody titers. The percentage of focus reduction was calculated relative to the results for virus controls without test samples. The neutralizing antibody titer was expressed as the maximum sample dilution yielding a 50% reduction in focus number, unless otherwise specified.
Immunostaining.
Immunochemical staining was performed essentially as described previously (26). Briefly, cells infected with DENV2 or DENV4 were fixed with acetone-methanol (1:1) for use as antigens in the immunostaining. These cells were incubated serially with MAbs to DENV2 or DENV4, biotinylated anti-mouse IgG, ABC (avidin-biotinylated peroxidase complex) reagents, and VIP substrate (Vector Laboratories, Burlingame, CA). This method was used for the screening of MAbs, titration of viral infectivity, and ADE assays. The MAbs used for the virus titration and ADE assays were D2-4G2 (E specific, flavivirus group cross-reactive [19]; provided by Tomohiko Takasaki of NIID, Japan) and D2-II-11H4 (E specific, reactive with both DENV2 and DENV4), which was generated from a DENV2-immune mouse in the present study (refer to Table 1).
TABLE 1.
Characterization of D2MAbs and D4MAbsa
| Hybridoma clone group | Clone code | Isotype | Neutralizing Ab titerb | HAI titerc | ELISA reactivityd
|
No. of clonese | |||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||||||
| D2-1 | D2-II-1B3 | IgG2a | 1:11,449 | 1:2,560 | + | + | − | + | 2 |
| D2-2 | D2-II-6D6 | IgG2a | 1:1,810 | <1:10 | + | + | − | + | 1 |
| D2-3 | D2-II-15A4 | IgG2a | 1:1,280 | 1:320 | − | + | − | + | 1 |
| D2-4 | D2-III-1B6 | IgG2a | 1:90,510 | 1:10 | − | + | − | + | 1 |
| D2-5 | D2-III-13F2 | IgG2a | 1:10,119 | 1:1,280 | + | + | − | + | 2 |
| D2-6 | D2-II-13A3 | IgG2b | 1:160 | 1:20 | − | + | + | + | 1 |
| D2-7 | D2-V-10F1 | IgG2a | 1:3,620 | 1:640 | + | + | − | + | 1 |
| D2-8 | D2-IX-5B10 | IgG2a | 1:7,241 | 1:10 | − | + | − | − | 1 |
| D2-9 | D2-II-9A7 | IgG2b | 1:28 | <1:10 | − | + | − | − | 1 |
| D2-10 | D2-II-11H4 | IgG2a | 1:40 | 1:20 | − | + | + | + | 4 |
| D2-11 | D2-II-11D1 | IgG1 | 1:40 | <1:10 | − | + | + | + | 3 |
| D2-12 | D2-II-11E1 | IgG1 | <1:10 | <1:10 | − | + | + | + | 6 |
| D2-13 | D2-VI-4A1 | IgG1 | <1:10 | <1:10 | − | + | − | − | 9 |
| D4-1 | D4-I-1D6 | IgG2a | 1:40 | 1:160 | − | + | − | + | 1 |
| D4-2 | D4-II-12B2 | IgG2a | 1:2,024 | 1:10 | − | − | − | + | 3 |
| D4-3 | D4-IV-10E10 | IgG2a | 1:160 | 1:80 | − | − | − | + | 3 |
| D4-4 | D4-IV-10E5 | IgG2a | 1:28 | 1:20 | − | + | + | + | 3 |
| D4-5 | D4-III-10E10 | IgG2b | 1:113 | 1:10 | − | − | − | + | 1 |
| D4-6 | D4-I-11D11 | IgG2a | 1:5,634 | <1:10 | − | − | − | + | 3 |
| D4-7 | D4-III-1A12 | IgG1 | 1:113 | <1:10 | + | + | + | + | 2 |
| D4-8 | D4-IV-3G12 | IgG2a | 1:505 | <1:10 | + | + | − | + | 1 |
| D4-9 | D4-I-14F1 | IgG2a | <1:10 | 1:160 | − | − | − | + | 3 |
| D4-10 | D4-III-2F1 | IgG2a | <1:10 | 1:10 | + | + | + | + | 4 |
| D4-11 | D4-IV-9D4 | IgG1 | <1:10 | <1:10 | − | − | − | + | 7 |
| D4-12 | D4-I-9B4 | IgG2a | <1:10 | <1:10 | − | − | − | + | 5 |
| D4-13 | D4-III-5C8 | IgG1 | <1:10 | <1:10 | − | + | − | + | 7 |
All MAbs were used in ascitic form.
Neutralizing Ab titer obtained by a 50% focus reduction assay against the homologous virus type in the absence of complement. Geometric means of the results obtained in two separate experiments are shown.
HAI antibody titer against the homologous virus type.
ELISA reactivities to dengue virus antigens. Numbers 1 to 4 correspond to DENV1 to DENV4.
The number of clones generated in each group.
ADE assay.
Three assay methods, based on the infection rate, yield, and number of infectious centers, were used to evaluate the enhancing activities of MAbs.
(i) Infection rate assay.
Based on the method described by Wu et al. (54), enhancing activity was assessed by the percentage of infected cells 4 to 6 days after cells were infected with virus in the presence of antibody. U937 or K562 cells, 1 × 105 in number, were suspended in 50 μl of MAb diluted in RPMI-10% FBS and immediately mixed with 150 μl of DENV2 or DENV4 containing 1 × 105 FFU. The IgG concentrations of MAbs included in the virus-antibody-cell mixture ranged from 100 to 106 ng/ml, and concentrations that showed the highest enhancing activities were mainly used. Following incubation at 37°C for 2 h, cells were washed three times and cultivated at 37°C for 4 to 6 days in RPMI-10% FBS including the MAb identical to that used in the virus-antibody-cell mixture at the same concentration. Then, cells were washed, fixed on a slide glass, and immunostained. Stained and unstained cells contained in three random microscopic fields were counted to calculate percentages of infected cells: the mean number of the total cell counts per field was approximately 600. As a negative control, ascitic fluids from mice inoculated with P3U1 cells were used. The borderline differentiating enhancing from nonenhancing activities was the average plus two times the SD of the percentages of infected cells obtained with six negative controls. In experiments where rabbit complement, fresh human serum, or C1q/C3-depleted human serum was added to the virus-antibody-cell mixture, complement or serum was added to the cell-antibody mixture before being mixed with the virus. To make up the final volume of the virus-antibody-cell mixture to 200 μl, the volume of the virus was adjusted. In experiments to prepare the cell-antibody mixture under dense serum conditions, the volumes of both the MAb and virus were adjusted to achieve the final concentration of the serum at a maximum of 80%. Rabbit complement or fresh human serum was not included in RPMI-10% FBS during cultivation for 4 to 6 days because of the rapid inactivation of the complement at 37°C and the limited amount of human sera available for this experiment.
(ii) Yield assay.
U937 cells mixed with MAb and virus were incubated, washed, and cultivated as described above. Following the method described by Halstead and O'Rourke (17), the enhancing activity was evaluated by the infective titer contained in the culture fluid.
(iii) Infectious center assay.
U937 cells were used for this assay. Based on the method described by Halstead and O'Rourke (18), the virus-antibody-cell mixture prepared as described above for the infection rate assay was serially diluted twofold and incubated at 37°C for 2 h. The mixture was then mixed with 2.5 × 105 Vero cells in wells of a 24-well microplate and further incubated at 37°C for 3 h, allowing cells to attach onto the bottoms of wells. An overlay medium (1% methyl cellulose in MEM) replaced the culture fluid, and the cells were cultivated at 37°C for 3 to 4 days. The cells were then fixed and immunostained to count the foci. The number of infectious centers was expressed as the number of foci included in 1 × 105 cells used for the virus-antibody-cell mixture.
Complement, complement component, and measurement of complement levels.
Low-Tox-M rabbit complement (designated “rabbit complement”) (Cedarlane, Hornby, Canada) was used as a source of complement. Complement C1q and C3 components were purchased from Merck, Darmstadt, Germany. Complement hemolytic activities were measured using sheep red blood cells treated with antibodies to sheep red blood cells in a CH50 “SEIKEN” kit (Denka Seiken, Niigata, Japan) according to the manufacturer's instructions. Absorbances measured following hemolysis were expressed as a 50% hemolytic unit of complement (CH50). Levels of C1q or C3 in C1q- or C3-depleted sera were measured by a sandwich ELISA essentially as described for measuring IgG concentrations (see above). Microplates sensitized with goat anticomplement C1q or C3 were incubated with serial 10-fold dilutions of test samples, peroxidase-conjugated anticomplement C1q or C3 (sheep anti-human C1q:horseradish peroxidase [AbD Serotec, Oxford, United Kingdom] or peroxidase-conjugated goat IgG fraction to human complement C3 [Cappel, West Chester, PA]), and then o-phenylenediamine dihydrochloride. The C1q and C3 concentrations were calculated by comparing absorbances with those obtained from the standard human complement component: C1q and C3 (Merck, Darmstadt, Germany) with known concentrations.
Statistical analysis.
The significances of differences in percentages of infected cells were evaluated by the Student's t test. Probability values (P) of less than 0.05 were considered significant.
RESULTS
Generation and basic characterization of D2MAbs and D4MAbs.
BALB/c mice immunized twice with 100 μg of DENV2 or DENV4 DNA vaccine developed low neutralizing antibody titers of 1:40 to 1:80 in a 70% focus reduction assay (<1:10 to 1:10 in a 90% focus reduction assay). Booster immunization with homologous types of dengue viruses at a dose of 1 × 107 PFU elicited significantly higher neutralizing antibody titers (1:320 or more in a 90% focus reduction assay), which were considered enough for generating a relatively large number of hybridoma clones.
Following screening by ELISA using homologous antigens, we obtained 33 hybridoma clones from nine DENV2-immune mice and 43 clones from four DENV4-immune mice. These clones were grouped into 13 distinct groups each for D2MAbs and D4MAbs, based on neutralizing and HAI activities against DENV2 or DENV4 antigens and ELISA reactivities to four dengue virus antigens, as well as competition assays. Table 1 lists the results of these basic characterizations of representative MAbs in each group. Neutralizing activities were shown in MAbs from 11 (D2MAbs) or 8 (D4MAbs) of the 13 groups, and the MAbs showing neutralizing activities were mostly of the IgG2a subclass in both D2MAbs and D4MAbs. The total numbers of MAbs showing neutralizing activities were 18 of the 33 (55%) for D2MAbs and 17 of the 43 (40%) for D4MAbs. Although the data are not described here, all MAbs recognized the E protein as determined by immunoprecipitation.
Comparison of three ADE assay methods.
Three representative methods based on the infection rate (54), yield (17), and number of infectious centers (18) have been reported for measuring enhancing activities. In comparison to the infection rate assay, the yield assay represents the actual production of progeny viruses. The infectious center assay represents the number of cells with primary infection releasing progeny viruses, different from the infection rate or yield assay that represents the outcome of secondary infections or later ones. To select indicator cells (K562 or U937) and one ADE assay method to be used for the subsequent characterization of our MAbs, we compared the usefulness of these two indicator cells and three ADE assays. The MAbs used for this comparison were D2-II-1B3, D4-I-1D6, and D4-IV-10E5, all of which showed relatively high enhancing activities in a pilot experiment.
The comparison of two indicator cells for an ADE assay using DENV2 indicated a higher sensitivity of U937 than of K562 cells in the infection rate assay. Specifically, the maximum percentages of infected cells obtained with D4-I-1D6 at optimal concentrations (1:103 to 1:104 dilutions of ascitic fluids and IgG concentrations of 2,800 to 280 ng/ml) were 30 to 40% in K562 cells but approximately 90% in U937 cells, whereas the average percentages of infected cells obtained without enhancing antibodies were 1 to 5% in both cells (data not shown). The comparison of U937 with K562 cells using DENV4 provided results consistent with those obtained with DENV2 (data not shown). We therefore used U937 cells for most of the subsequent experiments.
Three ADE assay methods were compared at various dilutions of MAbs (IgG concentrations ranging from 100 to 106 ng/ml). After the virus-antibody-cell mixture was incubated at 37°C for 2 h, half of the cells were used for infection rate and yield assays and the other half for the infectious center assay. For infection rate and yield assays, cells were cultivated at 37°C for 4 days for DENV2 and 6 days for DENV4. Half of the cells were used for the infection rate assay, while the other half were kept in cultivation for one more day and the culture fluids used for the yield assay. For the infectious center assay, cells were cultivated for 3 days for DENV2 and 4 days for DENV4. A pilot experiment using DENV2 and two MAbs in homologous (D2-II-1B3) and heterologous (D4-I-1D6) combinations indicated that the highest enhancing activities were shown at 32 or 2,800 ng/ml in homologous and heterologous combinations, respectively, in all assay methods (data not shown). Then, we examined each of the MAbs against DENV2 (D2-II-1B3) and DENV4 (D4-I-1D6 and D4-IV-10E5) for enhancing activities against DENV2 or DENV4; thus, ADE assays were performed in two homologous and two heterologous combinations.
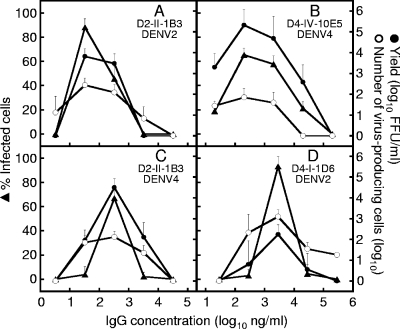
As shown in Fig. 1, three dose-dependent curves were roughly in parallel in any combination. Particularly, the highest enhancing activities were shown at the same IgG concentration in all three ADE assays in both homologous and heterologous combinations. The consistent results for the three methods indicate the reliability of each assay system. Although the data are not shown, examinations using affinity-purified IgG fractions of these MAbs showed dose-response curves similar to those obtained with the ascitic fluids that are shown in Fig. 1.
FIG. 1.
Comparison of three assay methods for evaluating enhancing activities of MAbs against DENV2 or DENV4 using U937 cells. One D2MAb (D2-II-1B3) and two D4MAbs (D4-I-1D6 and D4-IV-10E5) were used in homologous (panels A and B) and heterologous (panels C and D) combinations with DENV2 and DENV4. The left ordinate indicates percentages of infected cells obtained by the infection rate assay (closed triangles). The right ordinate indicates infective titers (FFU/ml) contained in the culture fluid obtained by the yield assay (closed circles) and the number of virus-producing cells included in 105 cells obtained by the infectious center assay (open circles). The abscissa indicates the final concentration of IgG included in the virus-antibody-cell mixture. The assays were done in triplicate (infection rate assay) or duplicate (other assays). Each datum represents an average obtained in two separate assays, with SDs indicated by error bars.
Since the infection rate assay took less time than the yield assay and allowed more samples to be tested at one time than the infectious center assay, we selected the infection rate assay to test enhancing activities. In this assay, we included MAbs in the medium during cultivation for 4 to 6 days at the same concentration as used in the virus-antibody-cell mixture, since the addition of MAbs in the culture provided clearer differentiation between enhancing and nonenhancing activities.
Relationship between enhancing and neutralizing activities.
All representative D2MAbs and D4MAbs were tested for enhancing activities with the infection rate assay using DENV2 and DENV4 in homologous and heterologous combinations (see closed triangles in Fig. 2). Of 13 D2MAbs, 10 showed enhancing activities against DENV2, including 4 also showing activities against DENV4. On the other hand, only 4 of the 13 D4MAbs showed enhancing activities against the homologous type, none of which showed activities against the heterologous type. One MAb (D4-I-1D6) showed enhancing activities only against the heterologous, but not the homologous, type. In the subsequent ADE assays, IgG concentrations showing the highest ADE activities, as shown in Fig. 2, were used for each MAb.
FIG. 2.
Relationship between enhancing and neutralizing activities in 13 representative D2MAbs and 13 representative D4MAbs. Enhancing activities were determined by an infection rate assay using U937 cells mixed with virus and serial 10-fold dilutions of each MAb and expressed as percentages of infected cells (open and closed triangles; left ordinate). Neutralizing activities were determined by a focus reduction assay using Vero cells infected with mixtures of virus and serial twofold dilutions of each MAb and expressed as percentages of focus reduction (open and closed circles; right ordinate). Only percentages of focus reduction between 40 and 100% were plotted. IgG fractions purified from ascitic fluids were used in neutralization tests when neutralizing antibody titers in ascitic fluids were 1:40 or lower as determined by a 90% focus reduction assay, since control ascitic fluids obtained from P3U1-inoculated mice frequently showed positive results in a 50% focus reduction assay. Closed triangles and circles indicate results obtained without inclusion of rabbit complement in the assay system, whereas open triangles and circles indicate results obtained in the presence of rabbit complement at a final concentration of 5%. Both assays were done in duplicate. Each datum represents an average obtained in three (for the infection rate assay) or two (for the focus reduction assay) separate experiments, with SDs indicated by error bars. The mean borderlines for enhancing activities obtained from three experiments using negative controls without MAbs were 18.0% for DENV2 and 14.3% for DENV4 in three separate assays.
Comparison with neutralizing activities (see open circles in Fig. 2) showed that all MAbs showing enhancing activities against homologous types showed neutralizing activities against homologous types. As well, all MAbs showing enhancing activities against heterologous types showed neutralizing activities against heterologous types. Most MAbs showed enhancing activities at subneutralizing doses; however, some exceptions (e.g., D2-II-9A7 and D4-IV-10E5) showed the highest enhancing activities at IgG concentrations approximately 100-fold different from those showing neutralizing activities at 50% focus reduction. In addition, some MAbs (e.g., D2-II-11D1 and D4-IV-3G12) showed neutralizing activities in a certain range of IgG concentrations but did not show enhancing activities at any IgG concentrations examined in the present study.
Effect of rabbit complement on enhancing and neutralizing activities.
Since rabbit complement was included in the virus-antibody mixture to increase sensitivity in our neutralization test, we attempted to include rabbit complement in the virus-antibody-cell mixture in the ADE assay at a final concentration of 5%. As shown in Fig. 2, enhancing activities shown in the absence of complement were abolished when complement was added to the assay system in all MAbs that showed enhancing activities. This abolishment was shown in both homologous and heterologous combinations.
We also attempted not including the rabbit complement in the virus-antibody mixture in neutralization tests. In the absence of the complement, neutralizing activities were decreased approximately 2- to 50-fold in all MAbs (Fig. 2).
Effect of fresh human serum on enhancing activities.
Based on the results obtained by the inclusion of rabbit complement, we next included fresh human serum in the ADE assay system. Heterologous combinations of MAbs and dengue virus types were used: D2-II-1B3 with DENV4 and D4-I-1D6 with DENV2 (Fig. 3). The enhancing activity was reduced depending on the final concentration of fresh serum in the virus-antibody-cell mixture, within a range of 0.1 to 10%. As a reference, rabbit complement was included in the virus-antibody-cell mixture at various concentrations and showed a pattern of dose-dependent effects similar to those obtained by the inclusion of fresh human serum (Fig. 3).
FIG. 3.
Effect of fresh human serum on enhancing activities of MAbs. Enhancing activities were evaluated in the infection rate assay using U937 cells and expressed as percentages of infected cells. Heterologous combinations of MAbs and the viruses were used: D2-II-1B3 and DENV4 (open circles) and D4-I-1D6 and DENV2 (closed circles). The final concentrations of IgG included in the virus-antibody-cell mixture for D2-II-1B3 and D4-I-1D6 were 32 and 2,800 ng/ml, respectively, which showed the highest enhancing activities in the experiments whose results are shown in Fig. 2. Rabbit complement was also examined for its effect on enhancing activities in a heterologous combination of D4-I-1D6 and DENV2 (closed triangles). The abscissa indicates the final concentration of human serum or rabbit complement in the virus-antibody-cell mixture. The assays were done in duplicate, and each datum represents an average obtained in three separate assays, with SDs indicated by error bars.
Heat-inactivated or complement component-depleted sera reduced the effect on enhancing activities.
To confirm that the complement contained in fresh human serum could be a factor involved in the effect on enhancing activities, heat-inactivated human serum was included in the ADE assay system using D4-I-1D6 and DENV2. As a reference, heat-inactivated rabbit complement was tested in parallel. As shown in Fig. 4A, there was no significant reduction of enhancing activities by the addition of rabbit complement when the complement was heat inactivated (P > 0.05). Although heat-inactivated fresh human serum showed reduction of enhancing activities with low statistical significance (P < 0.05), this reduction was considerably smaller than the reduction shown with noninactivated fresh human serum.
FIG. 4.
Effect of heat inactivation or depletion of C1q or C3 on fresh human serum's capacity to reduce enhancing activities. Infection rate assay was performed using U937 cells with a combination of D4-I-1D6 and DENV2. The D4-I-1D6 was included in the virus-antibody-cell mixture at a final IgG concentration of 2,800 ng/ml. (A) Effect of heat inactivation. Rabbit complement was used as a reference. Fresh human serum and rabbit complement were inactivated at 56°C for 30 min. The assay was performed in the absence (open bars) or presence of fresh (hatched bars) or inactivated (cross-hatched bars) human serum or rabbit complement at a final concentration of 5%. (B) Effect of depletion of C1q or C3. The assay was performed in the absence (open bars) or presence of fresh (hatched bars) or C1q- or C3-depleted (cross-hatched bars) human serum at a final concentration of 1%. (C) Effect of complementation by addition of purified C1q or C3 to C1q- or C3-depleted human serum. Enhancing activities were obtained with the virus-antibody-cell mixture including C1q- or C3-depleted serum at a final concentration of 1% and various concentrations of C1q or C3 (open triangles). The abscissa indicates C1q or C3 concentrations adjusted to those contained in depleted and complemented human sera. The depleted and complemented human sera were also examined for CH50 values (closed diamonds). As controls, enhancing activities obtained with the virus-antibody-cell mixture including only C1q or C3 at a final concentration of 100 or 1,000 μg/ml, respectively, are shown (closed circles). The C1q and C3 in C1q- and C3-depleted sera, respectively, were not detectable in a sandwich ELISA (<0.02 μg/ml for both). All assays were done in duplicate. Each datum in panel A represents an average obtained in three separate assays, and each datum shown in panels B and C represents an average obtained in two separate assays, with SDs indicated by error bars. For panels A and B, asterisks indicate significant differences from percentages of infected cells obtained in the absence of serum in each experimental group: *, P > 0.05; **, P < 0.05; ***, P < 0.001. For panel C, asterisks indicate significant differences from percentages of infected cells obtained without addition of C1q or C3 in each experimental group: *, P > 0.05; **, P < 0.01; ***, P < 0.001.
To confirm that the heat-labile factor associated with reduction in enhancing activity is the complement, commercial C1q- or C3-depleted human sera were used for an infection rate assay comparing them with nondepleted human sera (fresh human sera used in the experiments whose results are shown in Fig. 3 and 4A). These commercial sera did not contain detectable antibodies to DENV2 or DENV4 as determined by a 50% focus reduction neutralization test (data not shown). As shown in Fig. 4B, the significant reduction in enhancing activities shown with the use of nondepleted serum was not shown with the use of C1q- or C3-depleted serum.
Furthermore, the addition of purified C1q or C3 to the C1q- or C3-depleted serum, respectively, reduced enhancing activities in a dose-dependent manner (Fig. 4C). In this experiment, C1q levels of 7.5 to 240 μg/ml and C3 levels of 75 to 2,400 μg/ml were used, based on the standard range for normal individuals (mostly 70 to 150 μg/ml for C1q and 850 to 1,500 μg/ml for C3) since the original C1q or C3 levels in the commercial C1q- or C3-depleted sera were unknown. Although complementation by C1q was more effective than that by C3, the decrease in enhancing activities was closely related to the increase in CH50 values (Fig. 4C). Specifically, enhancing activities were significantly reduced when CH50 values were within the normal range of 25 to 45. These results indicated that the complement was responsible for the effect of fresh human serum on enhancing activities.
Effect of fresh human serum on enhancing activities under dense serum conditions.
To create an assay condition closer to the in vivo environment, the serum concentration in the virus-antibody-cell mixture was increased to 80%. To achieve different complement levels at a constant serum concentration, heat-inactivated serum was mixed with fresh serum at various ratios. The complement level in each inactivated/fresh serum mixture was confirmed by measuring CH50 values. Four fresh human sera were examined under a combination of D4-I-1D6 and DENV2 (Fig. 5A). Under the dense serum condition, the percentages of infected cells shown in the absence of complement activity (40 to 70%) were lower than those shown under the normal assay condition without the inclusion of serum in the virus-antibody-cell mixture (over 90%) (Fig. 3). Accordingly, the borderline provided under the dense serum condition (8.0%) (Fig. 5) was lower than that obtained under the normal condition (18.0%) (Fig. 2).
FIG. 5.
Dose-dependent reduction of enhancing activities by inclusion of fresh human serum in the virus-antibody-cell mixture under dense serum conditions. Enhancing activities were evaluated in an infection rate assay using U937 or K562 cells mixed with D4-I-1D6 and DENV2 and expressed as percentages of infected cells (left ordinate). The D4-I-1D6 was included in the virus-antibody-cell mixture at a final IgG concentration of 2,800 ng/ml. The dense serum condition was achieved by including the fresh/inactivated serum mixture in the virus-antibody-cell mixture at a final concentration of 80%. Fresh human serum was included in the fresh/inactivated serum mixture at various concentrations as indicated by the abscissa. The complement activity in the fresh/inactivated serum mixture was measured by a standard CH50 hemolytic assay and expressed as CH50 values (right ordinate). (A) Evaluation with sera from four healthy humans using U937 cells. Enhancing activities obtained with these sera are indicated by open circles and triangles and closed triangles and squares. The dotted line indicates the mean borderline (8.0%) for enhancing activities, obtained from three separate assays using negative controls without MAbs. The CH50 values were similar in four serum samples; one result is shown (closed diamonds). (B) Evaluation using K562 cells. One serum used in the above experiment (indicated by closed triangles in panel A) was used for this evaluation. Enhancing activities obtained using K562 cells are indicated by closed circles, while those obtained using U937 cells (the same data as shown in panel A) are indicated by closed triangles for reference. The assays were done in duplicate, and each datum represents an average obtained in three (for data shown in closed triangles) or two (for other data) separate assays, with SDs indicated by error bars. The broken line indicates the mean borderline (7.4%) for enhancing activities obtained from two separate assays using K562 cells. Asterisks indicate significant differences from percentages of infected cells obtained at a fresh serum concentration of 100% in each serum sample, except for data obtained at 0%: *, P < 0.05; **, P < 0.01.
Two of four fresh human sera showed enhancing activities within complement levels of 20 to 80%, whereas two other sera did not show enhancing activities in any complement levels, except at 0% (Fig. 5A). One of the two sera that showed enhancing activities showed higher activities at 50 and 75% than at 10 and 30% for the fresh serum concentration, whereas another serum showed higher activities at 20 and 40% than at 60 and 80%. These results indicated that the dose-dependent curves under dense serum conditions differed according to the individual sera and that a decrease in the complement level to 50 to 75% can induce enhancing activities in some sera.
To seek the reason for enhancing activities with relatively high complement levels under dense serum conditions, one of the sera that showed enhancing activities when using U937 cells (closed triangles, Fig. 5A) was examined for enhancing activities when using K562 cells. As shown in Fig. 5B, enhancing activities were not detectable within complement levels of 30 to 100% when K562 cells were used. Since K562 cells do not have complement receptor 3 (CR3), which is possessed by U937 cells, this result suggested that enhancing activities shown with relatively high complement levels under dense serum conditions were associated with a mechanism of CR3-mediated enhancement (3).
Comparison between effects of fresh human sera on enhancing activities of homologous and heterologous MAb-virus combinations.
The experiment detailed above was performed in a heterologous MAb-virus combination. To compare the effect of fresh sera on enhancing activities between homologous and heterologous combinations, MAbs D2-II-1B3, D4-I-1D6, and D4-IV-10E5 were used in various combinations with DENV2 and DENV4, using 14 fresh human sera. In this experiment, each fresh serum was included in the virus-antibody-cell mixture at a final concentration of 50%. Although there were individual variations, the heterologous combinations (Fig. 6B) showed significantly higher enhancing activities than the homologous combinations (Fig. 6A) in 8 of 28 (29%) MAb-virus combinations (P < 0.05 or P < 0.01). These 14 sera had similar complement levels, as determined by the CH50 assay (data not shown).
FIG. 6.
Effect of fresh human sera on enhancing activities under dense serum conditions in homologous and heterologous MAb-virus combinations. Infection rate assay was performed in duplicate using U937 cells. (A and B) Effect of sera from 14 healthy humans. One D2MAb (D2-II-1B3) and two D4MAbs (D4-I-1D6 and D4-IV-10E5) were used in homologous (A) and heterologous (B) combinations with DENV2 and DENV4. The final concentrations of IgG in the virus-antibody-cell mixture were 32 (for D2-II-1B3), 2,800 (for D4-I-1D6), and 200 (for D4-IV-10E5) ng/ml. Fresh human sera were included in the virus-antibody-cell mixture at a final concentration of 50%. Closed and hatched bars indicate percentages of infected cells obtained with DENV2 and DENV4, respectively. Each datum represents an average obtained in two separate assays, with SDs indicated by error bars. Asterisks indicate significant differences between percentages of infected cells shown in homologous (A) and heterologous (B) combinations: *, P < 0.05; **, P < 0.01. For controls, mean percentages of infected cells obtained from two separate experiments without inclusion of human sera were 98.6% for DENV2 and 72.2% for DENV4 in homologous combinations and 97.3% for DENV2 and 71.6% for DENV4 in heterologous combinations, while those obtained with negative controls without MAbs were 4.6% for DENV2 and 1.4% for DENV4. (C) Comparison of dose-dependent enhancing activities under various fresh serum concentrations. Serum number 4 used in the experiment shown in panels A and B was used in this experiment. The abscissa indicates concentrations of fresh serum in the fresh/inactivated serum mixture. The left ordinate indicates percentages of infected cells obtained with D2-II-1B3 (closed triangles), D2-IX-5B10 (open squares), and D4-I-1D6 (closed circles). The final concentrations of IgG in the virus-antibody-cell mixture for D2-II-1B3 and D4-I-1D6 were the same as described above, and that for D2-IX-5B10 was 300 ng/ml. The right ordinate indicates complement activities in the fresh/inactivated serum mixture as determined by the CH50 assay (closed diamonds). Each datum represents an average obtained in four separate experiments, with SDs indicated by error bars. Asterisks indicate significant differences between percentages of infected cells shown in homologous (closed triangles) and heterologous (closed circles) combinations: *, P < 0.05; **, P < 0.001. The mean baseline enhancement in the presence of MAb and the absence of fresh serum was 60.7% for D2-II-1B3, 50.5% for D2-IX-5B10, and 71.0% for D4-I-1D6. The dotted line indicates the mean borderline (7.9%) for enhancing activities obtained from four separate assays using negative controls without MAbs.
Among the 14 sera, serum number 4 was selected for further investigation of the effect of fresh sera on enhancing activities under dense serum conditions in homologous and heterologous combinations with DENV2. The fresh serum was mixed with heat-inactivated serum at various ratios as in the experiment whose results are shown in Fig. 5. D2-II-1B3 and D2-IX-5B10 were used for homologous combinations and D4-I-1D6 for a heterologous combination. As shown in Fig. 6C, the heterologous combination showed higher percentages of infected cells than did the homologous combinations under all experimental conditions for this serum sample (within the fresh serum concentrations of 20 to 100%). The percentages of infected cells were higher than the borderline within a range of 20 to 80% in the heterologous combination: even at 100%, the percentage of infected cells was close to the borderline. These results indicate that the heterologous combination provided higher enhancing activities than the homologous combination in some serum samples under dense serum conditions, so long as DENV2 was used for the assay.
DISCUSSION
Infection enhancement is a critical factor involved in the pathogenesis of many viral infections (49). An enhancing phenomenon was first reported with flaviviruses and, thereafter, with human immunodeficiency virus (HIV), Ebola virus, etc. Flaviviruses usually have a mechanism of Fc receptor-dependent enhancement (30, 41, 45), whereas HIV has a mechanism of Fc receptor-independent, complement receptor-mediated enhancement, in addition to an Fc receptor-dependent one (10). In Ebola virus, antibody-dependent, C1q receptor-mediated mechanisms are involved in the enhancement (50). In some HIV and most Ebola virus studies, enhancing assays have been performed using fresh sera. In the present study, we used fresh sera in ADE assays, although, to date, heat-inactivated sera have been almost exclusively used for dengue virus ADE studies. Use of fresh sera in an in vitro assay is considered to provide an assay condition closer to the in vivo environment.
Cross-reactive nonneutralizing antibodies have been generally considered a major factor involved in enhancing activities in dengue virus infections (15, 31, 47). In the present study, however, all MAbs showing enhancing activities had neutralizing activities irrespective of the homologous or heterologous combination of the MAb and virus used in the ADE assay. That is, nonneutralizing antibody species showing enhancing activities could not be found for the MAbs generated against DENV2 or DENV4. A similar result has been reported in an influenza A virus system in which all MAbs showing enhancing activities showed high or low levels of neutralizing activities (51). As well, the enhancing activities at subneutralizing doses were abolished or dramatically decreased when the assay was performed in the presence of fresh sera, although enhancing activities exhibited at subneutralizing doses were consistent with the results of previous reports where test samples were heat inactivated before the ADE assay (15). A similar result has been reported in a measles virus system in which ADE was almost completely blocked by the addition of guinea pig or rabbit complement in the virus-antibody mixture (21). Further, MAbs that showed neutralizing, but not enhancing, activities irrespective of the presence or absence of complement were generated in the present study. Although most of these MAbs had relatively low neutralizing activities, these might be new antibody species that have a protective role, since MAbs showing neutralizing activities have been generally considered to show enhancing activities (37).
Several experimental results in the present study demonstrated that the reductive effect of fresh sera on enhancing activities is attributed to the complement. Specifically, the reductive effects of fresh sera were reduced by their heat inactivation or by depletion of the complement component, C1q or C3. The most probable mechanism underlying this effect is virolysis, since MAbs showing enhancing activity had neutralizing activity that was increased in the presence of complement in all cases. Immune complexes consisting of MAb and the E protein on the surface of virus particles may activate complement pathways, followed by formation of the C5b-C9 membrane attack complex that may have lysed the viral envelope (20). On the other hand, the possibility that cell lysis may occur through complement activation on the cell surface to which the virus is attached along with the immune complex can be considered negligible, since over 99% of cells survived after incubation with the virus, antibody, and rabbit complement as determined by trypan blue inclusion (data not shown). Under both the normal and dense serum conditions, fresh serum reduced enhancing activities in a dose-dependent manner (Fig. 3, 5), indicating that the function of antibodies (neutralizing or enhancing activities) is regulated by the level of complement in the assay system. Specifically, neutralizing/enhancing antibody shows neutralizing activities under normal complement levels, in contrast to the enhancing activities under reduced complement levels. Since some sera showed enhancing activities with relatively high complement levels under dense serum conditions (Fig. 5), a decrease in complement levels to a certain extent within the physiological range may enhance infection by a CR3-mediated mechanism in some individuals, through the binding of iC3b, a major cleavage product of activated C3 (3).
Although there have been only a few reports describing complement levels in dengue virus-infected patients, marked reductions in levels of the complement component C3 have been shown in DHF patients (1, 38). Additionally, increasing levels of immune complexes have been reported in dengue virus-infected patients (43): antibodies induced by infection may form immune complexes with viral antigens that may consume the complement (20). Finally, complement hemolytic activities (CH50 values) have been shown to be low in the acute phase of DHF (38), in contrast to most DF patients, in whom the complement activities were not altered (46). As well, mass complement components are produced mainly in the liver and monocytes/macrophages (6), both known as principal targets of dengue virus infection (4, 9, 14). The infection may decrease the level of complement production, possibly facilitating further decreases in the complement levels in circulation.
Most of the MAbs generated in the present study had higher neutralizing antibody titers in homologous than heterologous combinations with the viruses (Fig. 2). As well, using 14 healthy human sera, cross-reactive antibodies provided higher enhancing activities than did specific antibodies under dense serum conditions in 29% of MAb-virus combinations (Fig. 6A and B). Further, in one particular serum, cross-reactive antibodies provided significantly higher enhancing activities than did specific antibodies within the complement levels of 20 to 80% of the original fresh serum in the assay system using DENV2 (Fig. 6C). These results suggested that, although there are wide individual variations, some sera give an environment that provides higher enhancing activities against heterologous than homologous antibodies, consistent with epidemiological evidence showing that disease severity is usually greater upon heterologous secondary infection (15).
In conclusion, the present study demonstrated in DENV2 and DENV4 models that antibody species that have enhancing activities also have neutralizing activities and that these two distinct activities are controlled in vitro by levels of complement within the physiological range. Therefore, complement levels are considered an additional factor involved in the in vivo ADE phenomenon, suggesting that the disease outcome may be determined by the complement level in the early stage of the disease: protection at a normal level, but increased severity at a reduced level. However, the existence of other mechanisms or factors, in particular, nonneutralizing cross-reactive antibody species against DENV1 and DENV3, is not ruled out. Reports concerning the inclusion of complement in an ADE assay system, though limited in early studies, have described its boosting (14) or not detectable (17) effects on enhancing activities in a polyclonal system. We are currently investigating this complement-dependent ADE phenomenon using a mouse model and also planning to monitor the serum complement level in DF and DHF patients to confirm the relationship of the complement level to the level of viremia.
Acknowledgments
We thank Ichiro Kurane and Tomohiko Takasaki of NIID, Japan, for providing cell lines and antibodies.
This research project received financial support from WHO Vaccines and Biologicals (V&B).
Footnotes
Published ahead of print on 14 November 2007.
REFERENCES
- 1.Bokisch, V. A., F. H. Top, Jr., P. K. Russell, F. J. Dixon, and H. J. Muller-Eberhard. 1973. The potential pathogenic role of complement in dengue hemorrhagic shock syndrome. N. Engl. J. Med. 289996-1000. [DOI] [PubMed] [Google Scholar]
- 2.Burke, D. S., and T. P. Monath. 2001. Flaviviruses, p. 1043-1125. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott-Raven, Philadelphia, PA.
- 3.Cardosa, M. J., J. S. Porterfield, and S. Gordon. 1983. Complement receptor mediates enhanced flavivirus replication in macrophages. J. Exp. Med. 158258-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaturvedi, U. C., R. Nagar, and R. Shrivastava. 2006. Macrophage and dengue virus: friend or foe? Ind. J. Med. Res. 12423-40. [PubMed] [Google Scholar]
- 5.Clarke, D. H., and J. Casals. 1958. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am. J. Trop. Med. Hyg. 7561-573. [DOI] [PubMed] [Google Scholar]
- 6.Colten, H. R. 1976. Biosynthesis of complement. Adv. Immunol. 2267-118. [DOI] [PubMed] [Google Scholar]
- 7.Endy, T. P., A. Nisalak, S. Chunsuttitwat, D. E. Vaughn, S. Green, F. A. Ennis, A. L. Rothman, and D. H. Libraty. 2004. Relationship of preexisting dengue virus (DV) neutralizing antibody levels to viremia and severity of disease in prospective cohort study of DV infection in Thailand. J. Infect. Dis. 189990-1000. [DOI] [PubMed] [Google Scholar]
- 8.Fink, J., F. Gu, and S. G. Vasudevan. 2006. Role of T cells, cytokines and antibody in dengue fever and dengue haemorrhagic fever. Rev. Med. Virol. 16263-275. [DOI] [PubMed] [Google Scholar]
- 9.George, R., and L. C. S. Lum. 1997. Clinical spectrum of dengue infection, p. 89-113. In D. J. Gubler and G. Kuno (ed.), Dengue and dengue hemorrhagic fever. CAB International, New York, NY.
- 10.Gras, G. S., and D. Dormont. 1991. Antibody-dependent and antibody-independent complement-mediated enhancement of human immunodeficiency virus type 1 infection in a human, Epstein-Barr virus-transformed B-lymphocytic cell line. J. Virol. 65541-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green, S., and A. Rothman. 2006. Immunopathological mechanisms in dengue and dengue hemorrhagic fever. Curr. Opin. Infect. Dis. 19429-436. [DOI] [PubMed] [Google Scholar]
- 12.Gubler, D. J. 1998. Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 113480-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guzman, M. G., G. P. Kouri, J. Bravo, M. Soler, S. Vazquez, and L. Morier. 1990. Dengue hemorrhagic fever in Cuba, 1981: a retrospective seroepidemiologic study. Am. J. Trop. Med. Hyg. 42179-184. [DOI] [PubMed] [Google Scholar]
- 14.Halstead, S. B. 1982. Immune enhancement of viral infection. Prog. Allergy 31301-364. [PubMed] [Google Scholar]
- 15.Halstead, S. B. 2003. Neutralization and antibody-dependent enhancement of dengue viruses. Adv. Virus Res. 60421-467. [DOI] [PubMed] [Google Scholar]
- 16.Halstead, S. B., and E. J. O'Rourke. 1977. Antibody-enhanced dengue virus infection in primate leukocytes. Nature 265739-741. [DOI] [PubMed] [Google Scholar]
- 17.Halstead, S. B., and E. J. O'Rourke. 1977. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J. Exp. Med. 146201-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halstead, S. B., and E. J. O'Rourke. 1977. Dengue viruses and mononuclear phagocytes. II. Identity of blood and tissue leukocytes supporting in vitro infection. J. Exp. Med. 146218-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henchal, E. A., J. M. McCown, D. S. Burke, M. C. Seguin, and W. E. Brandt. 1985. Epitopic analysis of antigenic determinants on the surface of dengue-2 virions using monoclonal antibodies. Am. J. Trop. Med. Hyg. 34162-169. [DOI] [PubMed] [Google Scholar]
- 20.Hirsch, R. L. 1982. The complement system: its importance in the host response to viral infection. Microbiol. Rev. 4671-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iankov, I. D., M. Pandey, M. Harvey, G. E. Griesmann, M. J. Federspiel, and S. J. Russell. 2006. Immunoglobulin G antibody-mediated enhancement of measles virus infection can bypass the protective antiviral immune response. J. Virol. 808530-8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaufman, B. M., P. L. Summers, D. R. Dubois, W. H. Cohen, M. K. Gentry, R. L. Timchak, D. S. Burke, and K. H. Eckels. 1989. Monoclonal antibodies for dengue virus prM glycoprotein protect mice against lethal dengue infection. Am. J. Trop. Med. Hyg. 41576-580. [DOI] [PubMed] [Google Scholar]
- 23.Kaufman, B. M., P. L. Summers, D. R. Dubois, and K. H. Eckels. 1987. Monoclonal antibodies against dengue 2 virus E-glycoprotein protect mice against lethal dengue infection. Am. J. Trop. Med. Hyg. 36427-434. [DOI] [PubMed] [Google Scholar]
- 24.Kliks, S. C., A. Nisalak, W. E. Brandt, L. Wahl, and D. S. Burke. 1989. Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. Am. J. Trop. Med. Hyg. 40444-451. [DOI] [PubMed] [Google Scholar]
- 25.Kohler, G., and C. Milstein. 1975. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256495-497. [DOI] [PubMed] [Google Scholar]
- 26.Konishi, E., and A. Fujii. 2002. Dengue type 2 virus subviral extracellular particles produced by a stably transfected mammalian cell line and their evaluation for a subunit vaccine. Vaccine 201058-1067. [DOI] [PubMed] [Google Scholar]
- 27.Konishi, E., S. Kosugi, and J. Imoto. 2006. Dengue tetravalent DNA vaccine inducing neutralizing antibody and anamnestic responses to four serotypes in mice. Vaccine 242200-2207. [DOI] [PubMed] [Google Scholar]
- 28.Konishi, E., and K. Uehara. 1990. Enzyme-linked immunosorbent assay for quantifying antigens of Dermatophagoides farinae and D. pteronyssinus (Acari: Pyroglyphidae) in house dust samples. J. Med. Entomol. 27993-998. [DOI] [PubMed] [Google Scholar]
- 29.Kuno, G. 2003. Serodiagnosis of flaviviral infections and vaccinations in humans. Adv. Virus Res. 613-65. [DOI] [PubMed] [Google Scholar]
- 30.Kurane, I., B. J. Mady, and F. A. Ennis. 1991. Antibody-dependent enhancement of dengue virus infection. Rev. Med. Virol. 1211-221. [Google Scholar]
- 31.Kurane, I., and T. Takasaki. 2001. Dengue fever and dengue haemorrhagic fever: challenges of controlling an enemy still at large. Rev. Med. Virol. 11301-311. [DOI] [PubMed] [Google Scholar]
- 32.Laoprasopwattana, K., D. H. Libraty, T. P. Endy, A. Nisalak, S. Chunsuttiwat, D. W. Vaughn, G. Reed, F. A. Ennis, A. L. Rothman, and S. Green. 2005. Dengue virus (DV) enhancing antibody activity in preillness plasma does not predict subsequent disease severity or viremia in secondary DV infection. J. Infect. Dis. 192510-519. [DOI] [PubMed] [Google Scholar]
- 33.Libraty, D. H., T. P. Endy, H. S. Houng, S. Green, S. Kalayanarooj, S. Suntayakorn, W. Chansiriwongs, D. W. Vaughn, A. Nisalak, F. A. Ennis, and A. L. Rothman. 2002. Differing influences of virus burden and immune activation on disease severity in secondary dengue-3 virus infections. J. Infect. Dis. 1851213-1221. [DOI] [PubMed] [Google Scholar]
- 34.Lindenbach, B. D., and C. M. Rice. 2001. Flaviviridae: the viruses and their replication, p. 991-1041. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott-Raven, Philadelphia, PA.
- 35.Lozzio, C. B., and B. B. Lozzio. 1975. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood 45321-334. [PubMed] [Google Scholar]
- 36.Mathew, A., I. Kurane, S. Green, H. A. Stephens, D. W. Vaughn, S. Kalayanarooj, S. Suntayakorn, D. Chandanayingyong, F. A. Ennis, and A. L. Rothman. 1998. Predominance of HLA-restricted cytotoxic T-lymphocyte responses to serotype-cross-reactive epitopes on nonstructural proteins following natural secondary dengue virus infection. J. Virol. 723999-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morens, D. M., S. B. Halstead, and N. J. Marchette. 1987. Profiles of antibody-dependent enhancement of dengue virus type 2 infection. Microb. Pathog. 3231-237. [DOI] [PubMed] [Google Scholar]
- 38.Nishioka, K. 1974-1975. Serum complement level in dengue hemorrhagic fever. Allerg. Immunol. 20-21:385-392. [PubMed]
- 39.Palucka, A. K. 2000. Dengue virus and dendritic cells. Nat. Med. 6748-749. [DOI] [PubMed] [Google Scholar]
- 40.Pandey, B. D., and A. Igarashi. 2000. Severity-related molecular differences among nineteen strains of dengue type 2 viruses. Microbiol. Immunol. 44179-188. [DOI] [PubMed] [Google Scholar]
- 41.Peiris, J. S., S. Gordon, J. C. Unkeless, and J. S. Porterfield. 1981. Monoclonal anti-Fc receptor IgG blocks antibody enhancement of viral replication in macrophages. Nature 289189-191. [DOI] [PubMed] [Google Scholar]
- 42.Rico-Hesse, R. 2003. Microevolution and virulence of dengue viruses. Adv. Virus Res. 59315-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruangjirachuporn, W., S. Boonpucknavig, and S. Nimmanitya. 1979. Circulating immune complexes in serum from patients with dengue haemorrhagic fever. Clin. Exp. Immunol. 3646-53. [PMC free article] [PubMed] [Google Scholar]
- 44.Sabin, A. B. 1952. Research on dengue during World War II. Am. J. Trop. Med. Hyg. 130-50. [DOI] [PubMed] [Google Scholar]
- 45.Schlesinger, J. J., and M. W. Brandriss. 1983. 17D yellow fever virus infection of P388D1 cells mediated by monoclonal antibodies: properties of the macrophage Fc receptor. J. Gen. Virol. 641255-1262. [DOI] [PubMed] [Google Scholar]
- 46.Shaio, M. F., F. Y. Chang, and S. C. Hou. 1992. Complement pathway activity in serum from patients with classical dengue fever. Trans. R. Soc. Trop. Med. Hyg. 86672-675. [DOI] [PubMed] [Google Scholar]
- 47.Stephenson, J. R. 2005. Understanding dengue pathogenesis: implications for vaccine design. Bull. W. H. O. 83308-314. [PMC free article] [PubMed] [Google Scholar]
- 48.Sundstrom, C., and K. Nilsson. 1976. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int. J. Cancer 17565-577. [DOI] [PubMed] [Google Scholar]
- 49.Takada, A., and Y. Kawaoka. 2003. Antibody-dependent enhancement of viral infection: molecular mechanisms and in vivo implications. Rev. Med. Virol. 13387-398. [DOI] [PubMed] [Google Scholar]
- 50.Takada, A., S. Watanabe, K. Okazaki, H. Kida, and Y. Kawaoka. 2001. Infectivity-enhancing antibodies to Ebola virus glycoprotein. J. Virol. 752324-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tamura, M., R. G. Webster, and F. A. Ennis. 1993. Neutralization and infection-enhancement epitopes of influenza A virus hemagglutinin. J. Immunol. 1511731-1738. [PubMed] [Google Scholar]
- 52.Vaughn, D. W., S. Green, S. Kalayanarooj, B. L. Innis, S. Nimmannitya, S. Suntayakorn, T. P. Endy, and A. Nisalak. 2000. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J. Infect. Dis. 1812-9. [DOI] [PubMed] [Google Scholar]
- 53.World Health Organization. April 2002. Fact sheet no.117. Dengue and dengue haemorrhagic fever. http://www.who.int/mediacentre/factsheets/fs117/en/.
- 54.Wu, S. J., G. Grouard-Vogel, W. Sun, J. R. Mascola, E. Brachtel, R. Putvatana, M. K. Louder, L. Filgueira, M. A. Marovich, H. K. Wong, A. Blauvelt, G. S. Murphy, M. L. Robb, B. L. Innes, D. L. Birx, C. G. Hayes, and S. S. Frankel. 2000. Human skin Langerhans cells are targets of dengue virus infection. Nat. Med. 6816-820. [DOI] [PubMed] [Google Scholar]