Abstract
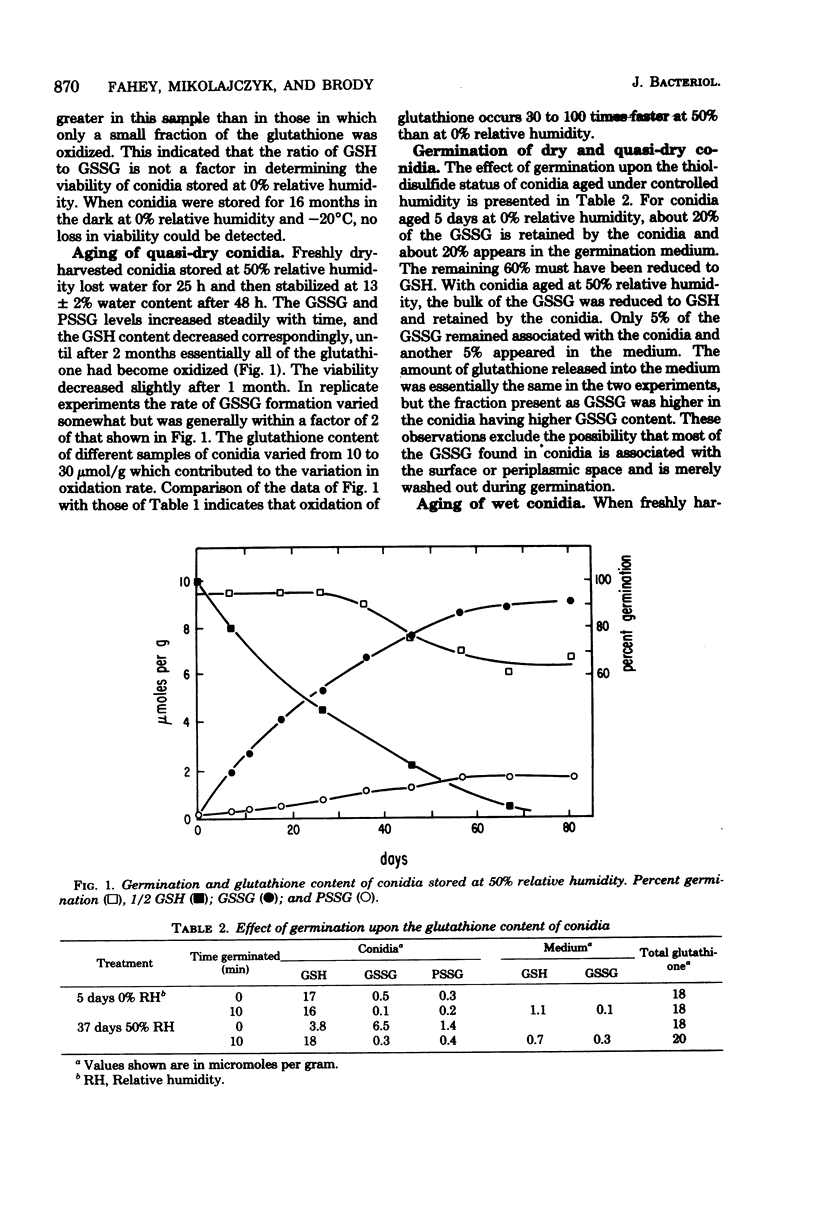
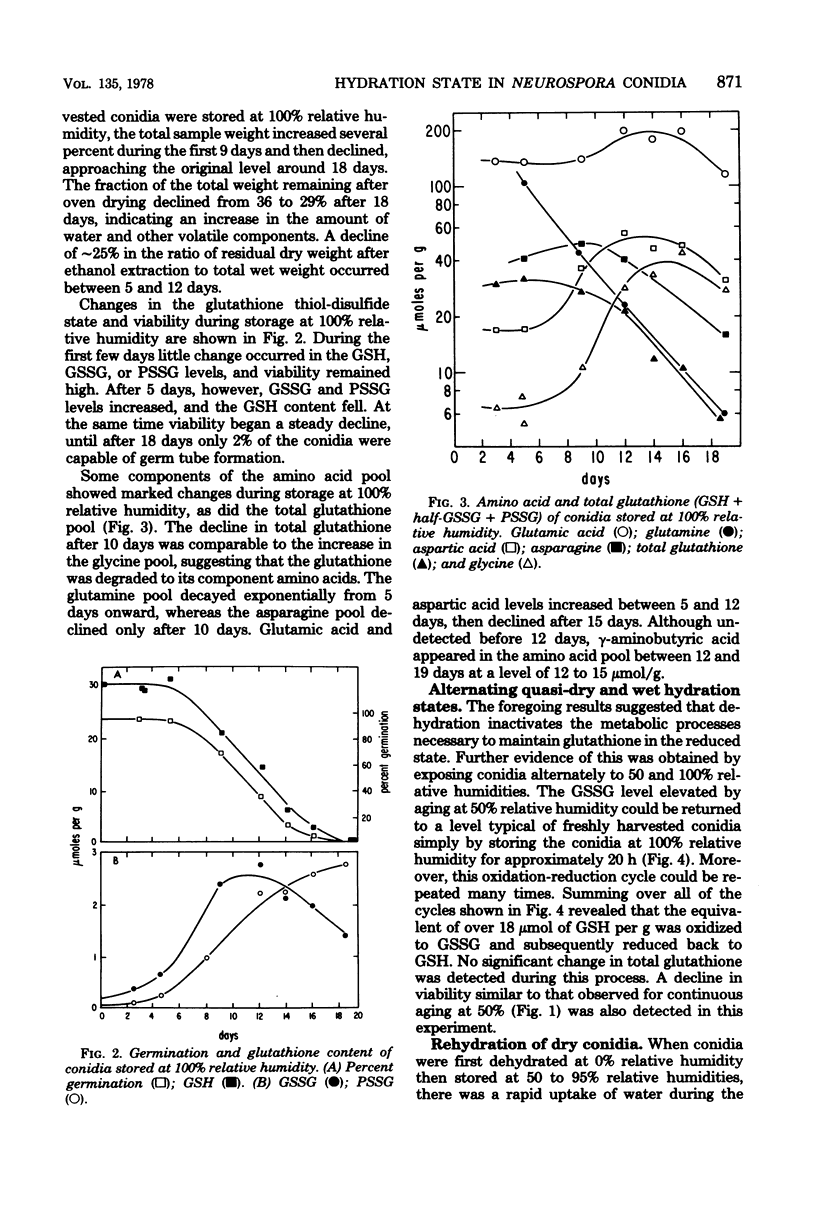
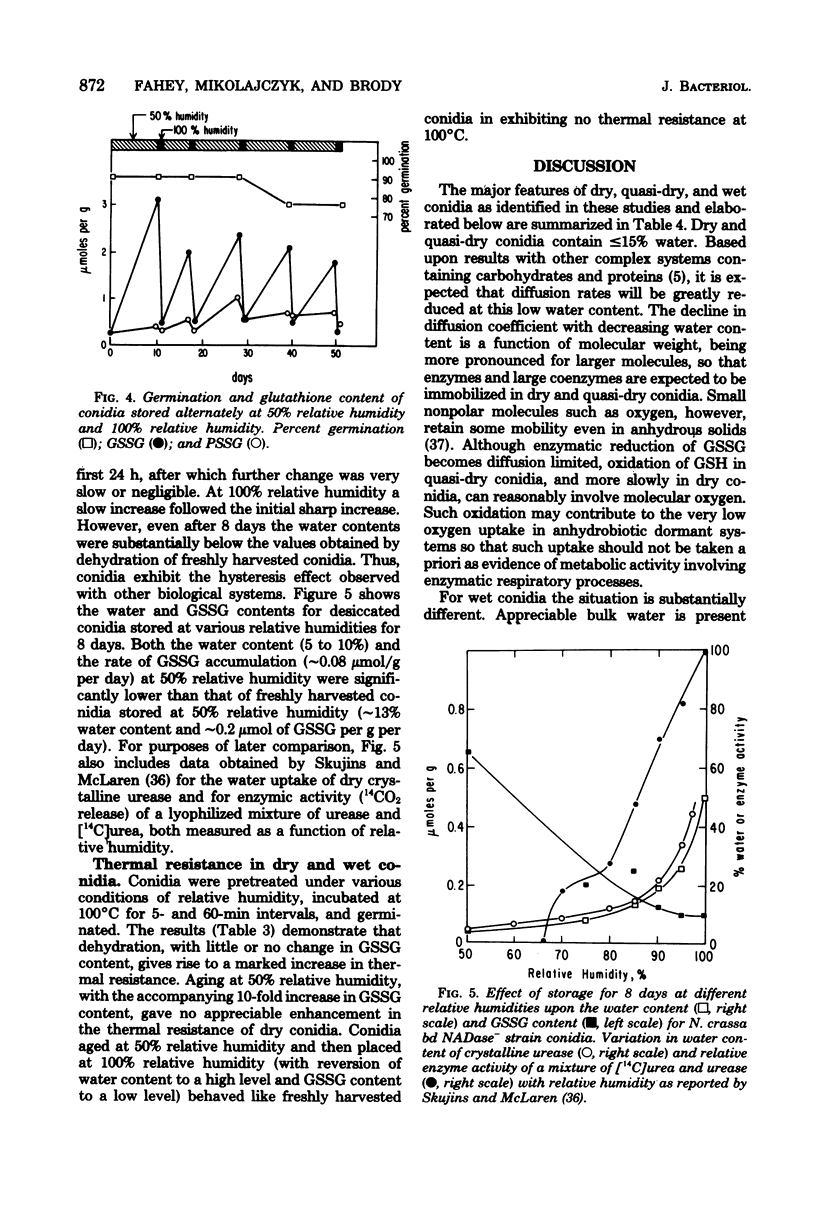
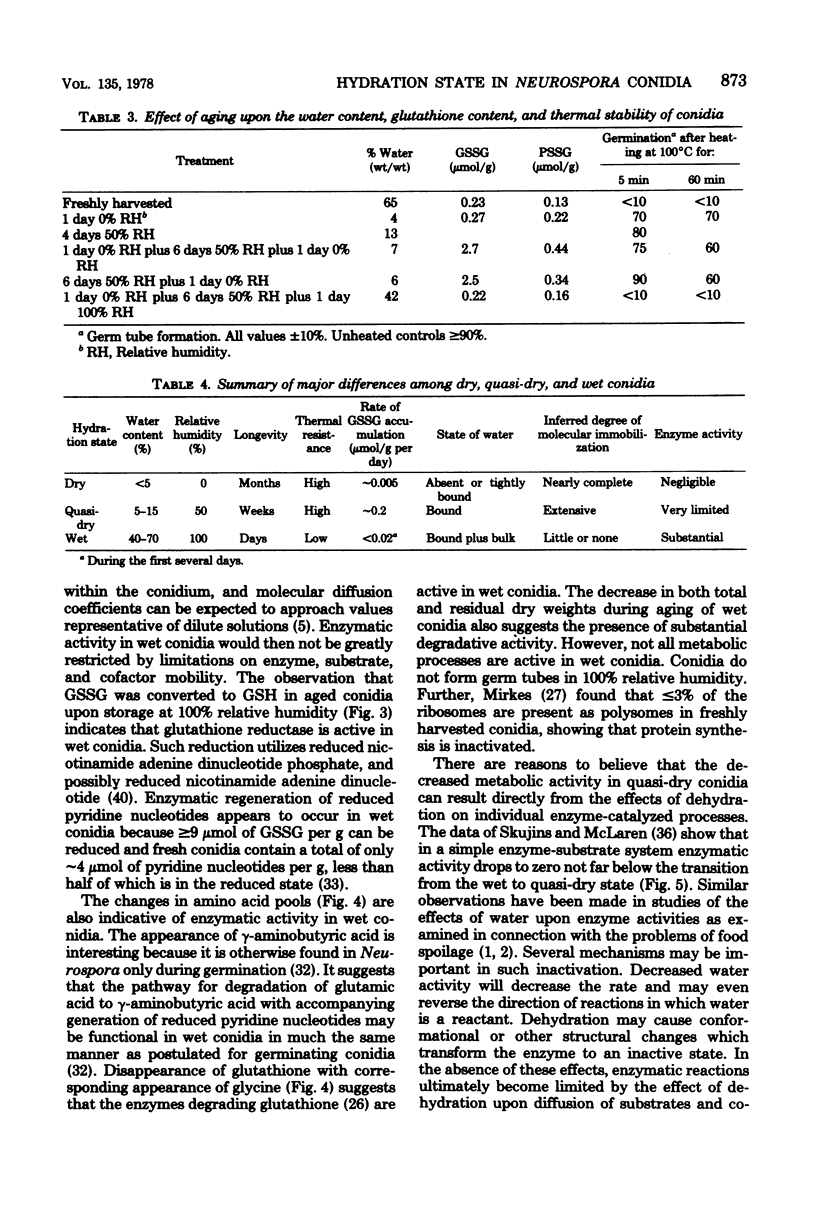
Ungerminated Neurospora crassa conidia were incubated at 0, 50, and 100% relative humidity, giving rise to conidia in dry, quasi-dry, and wet hydration states, respectively. Metabolic activity was detected by monitoring levels of reduced glutathione (GSH), oxidized glutathione (GSSG), and the soluble-amino acid pools as a function of incubation time. Wet conidia (approximately 65% water content) exhibited significant metabolic activity as evidenced by: (i) reduction of GSSG to GSH, (ii) degradation of GSH, and (iii) changes in the pool sizes of certain amino acids. GSSG accumulated slowly in dry conidia (less than 5% water content) and more rapidly in quasi-dry conidia (approximately 13% water content), indicating that enzymatic reduction of GSSG is inactive in these states. Longevity and thermal resistance were high for dry conidia and low for wet conidia, but were not influenced by variation in GSSG content. The water content of conidia exhibited a hysteresis effect in that at a given relative humidity previously dried conidia attained a lower water content than freshly harvested conidia.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clegg J. S. Interrelationships between Water and cellular metabolism in Artemia cysts. VIII Sorption isotherms and derived thermodynamic quantities. J Cell Physiol. 1978 Feb;94(2):123–137. doi: 10.1002/jcp.1040940202. [DOI] [PubMed] [Google Scholar]
- Cooke R., Kuntz I. D. The properties of water in biological systems. Annu Rev Biophys Bioeng. 1974;3(0):95–126. doi: 10.1146/annurev.bb.03.060174.000523. [DOI] [PubMed] [Google Scholar]
- Fahey R. C. Biologically important thiol-disulfide reactions and the role of cyst(e)ine in proteins: an evolutionary perspective. Adv Exp Med Biol. 1977;86A:1–30. doi: 10.1007/978-1-4684-3282-4_1. [DOI] [PubMed] [Google Scholar]
- Fahey R. C., Brody S., Mikolajczyk S. D. Changes in the glutathione thiol-disulfide status of Neurospora crassa conidia during germination and aging. J Bacteriol. 1975 Jan;121(1):144–151. doi: 10.1128/jb.121.1.144-151.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey R. C., Brown W. C., Adams W. B., Worsham M. B. Occurrence of glutathione in bacteria. J Bacteriol. 1978 Mar;133(3):1126–1129. doi: 10.1128/jb.133.3.1126-1129.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould G. W., Measures J. C. Water relations in single cells. Philos Trans R Soc Lond B Biol Sci. 1977 Mar 29;278(959):151–166. doi: 10.1098/rstb.1977.0035. [DOI] [PubMed] [Google Scholar]
- Kosower E. M., Correa W., Kinon B. J., Kosower N. S. Glutathione. VII. Differentiation among substrates by the thiol-oxidizing agent, diamide. Biochim Biophys Acta. 1972 Mar 30;264(1):39–44. doi: 10.1016/0304-4165(72)90114-6. [DOI] [PubMed] [Google Scholar]
- Kuntz I. D., Jr, Kauzmann W. Hydration of proteins and polypeptides. Adv Protein Chem. 1974;28:239–345. doi: 10.1016/s0065-3233(08)60232-6. [DOI] [PubMed] [Google Scholar]
- Mirkes P. E. Polysomes, ribonucleic acid, and protein synthesis during germination of Neurospora crassa conidia. J Bacteriol. 1974 Jan;117(1):196–202. doi: 10.1128/jb.117.1.196-202.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell W. G., Scott W. J. The heat resistance of bacterial spores at various water activities. J Gen Microbiol. 1966 Jun;43(3):411–425. doi: 10.1099/00221287-43-3-411. [DOI] [PubMed] [Google Scholar]
- Nelson R. E., Selitrennikoff C. P., Siegel R. W. Mutants of Neurospora deficient in nicotinamide adenine dinucleotide (phosphate) glycohydrolase. J Bacteriol. 1975 May;122(2):695–709. doi: 10.1128/jb.122.2.695-709.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. L., Woodward D. O. Genetic determinants of circadian rhythmicity in Neurospora. J Bacteriol. 1969 Feb;97(2):861–866. doi: 10.1128/jb.97.2.861-866.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmit J. C., Brody S. Neurospora crassa conidial germination: role of endogenous amino acid pools. J Bacteriol. 1975 Oct;124(1):232–242. doi: 10.1128/jb.124.1.232-242.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B., Setlow P. Levels of acetyl coenzyme A, reduced and oxidized coenzyme A, and coenzyme A in disulfide linkage to protein in dormant and germinated spores and growing and sporulating cells of Bacillus megaterium. J Bacteriol. 1977 Nov;132(2):444–452. doi: 10.1128/jb.132.2.444-452.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B., Setlow P. Most of the coenzyme A in dormant spores of Bacillus megaterium is in disulfide linkage to protein. Biochem Biophys Res Commun. 1977 Mar 7;75(1):45–50. doi: 10.1016/0006-291x(77)91286-4. [DOI] [PubMed] [Google Scholar]
- Skujins J. J., McLaren A. D. Enzyme reaction rates at limited water activities. Science. 1967 Dec 22;158(3808):1569–1570. doi: 10.1126/science.158.3808.1569. [DOI] [PubMed] [Google Scholar]


