Abstract
Current vaccine efforts to elicit cross-reactive neutralizing antibodies (NAbs) against human immunodeficiency virus (HIV) focus on the engineering of soluble mimetics of the trimeric HIV Env glycoprotein (commonly termed gp140 immunogens). Such immunogens are thought to be more effective than previously tested monomeric gp120 immunogens at eliciting cross-reactive NAbs. Still, the breadth of neutralizing antibody responses elicited by gp140 immunogens is narrow. Understanding why antibodies elicited by gp140 immunogens fail to neutralize a wide range of heterologous primary HIV isolates is necessary for improving the design of such immunogens. We previously reported that antibodies elicited in macaques by SF162 Env-derived gp140 immunogens fail to neutralize several heterologous “neutralization-resistant” primary HIV type 1 isolates, such as JRFL, ADA, and YU2. Here we show that by replacing the V1 region of Env on these heterologous viruses with that of SF162, we render them highly susceptible to neutralization by the SF162gp140-elicited antibodies. We observed that viral neutralization was mediated not only by vaccine-elicited anti-V1 but also by anti-V3 antibodies and antibodies directed against as yet unidentified Env regions, depending on the heterologous Env background. Our study indicates that common neutralization epitopes are differentially exposed on diverse primary HIV isolates and that the V1 loop contributes to this differential exposure. Therefore, the antibody responses elicited by soluble gp140 immunogens will have to overcome several distinct obstacles in order to neutralize diverse primary HIV isolates.
The Env glycoprotein (Env) of human immunodeficiency virus (HIV) plays critical roles in several steps of the viral life cycle, including its transmissibility, cellular tropism, and replication kinetics, and it is the target of both cell-mediated and antibody-mediated antiviral immune responses. Env is produced as a single, heavily glycosylated polypeptide that during intracellular processing is cleaved by cellular enzymes into two noncovalently associated subunits: a transmembrane subunit (gp41) and an extracellular subunit (gp120) (14). During processing, the Env oligomerizes into trimers of gp120/gp41 heterodimers (9, 23, 48), and it is this trimeric Env form that allows the viral lipid envelope to fuse with target cell plasma membranes expressing appropriate receptor molecules during the initial steps of infection.
Antibodies to almost every Env region have been isolated from infected patients (28). However, not every Env region on the virion-associated trimers is optimally available for antibody binding (5, 27, 31, 35, 39). In general, neutralizing antibodies (NAbs) bind to epitopes that are exposed on the virion-associated Env trimer, although NAbs have also been described that preferentially bind to their epitopes once Env attaches itself to cell surface CD4 and undergoes specific conformational changes (29, 42, 46). Most patients infected with HIV develop NAbs, and many monoclonal antibodies (MAbs) with neutralizing activity have been isolated from HIV-infected patients. However only a few of these MAbs display cross-neutralizing reactivity, i.e., can neutralize diverse HIV isolates (4, 8). These are the types of antibodies one would want to elicit during immunization, but thus far this goal has not been achieved (1-3, 12, 13, 15-17, 20, 22, 38, 40, 45).
Since the target of NAbs is HIV Env, several variations of this viral antigen have been tested over the years as immunogens to elicit cross-reactive anti-HIV NAbs. Soluble monomeric gp120 immunogens are ineffective at eliciting such antibodies (18, 19, 24). Overall, soluble oligomeric forms of Env (comprising the gp120 subunit and the extracellular region of gp41, termed gp140s) are capable of eliciting cross-reactive NAbs but of limited breadth (1, 3, 12, 16, 17, 22, 45), although a recent study indicated that immunization with an Env protein (designated R2) derived from an HIV-infected subject who developed cross-reactive neutralizing antibody responses resulted in the elicitation of cross-neutralizing antibody responses against many heterologous viruses (47).
Structural and antigenic studies of HIV Env and of certain broadly reactive NAbs provide valuable information on the presentation of neutralization epitopes on Env and on their interaction with NAbs. The immunogenic properties of HIV Env immunogens are not, however, predictable by using structural or antigenic studies. For instance, even though the SF162 Env contains epitopes recognized by many broadly reactive MAbs (such as IgG1b12, 2G12, 2F5, or 4E10) and the SF162 virus is susceptible to neutralization by such antibodies (33, 34, 37), immunization with SF162 Env-derived immunogens does not result in the generation of IgG1b12-, 2G12-, 2F5-, or 4E10-like antibodies (1, 7, 12).
A large fraction of the homologous neutralizing antibodies elicited by SF162gp140 bind to the V1 loop of gp120 (12). Such antibodies are not expected to recognize the V1 loop on heterologous isolates since the V1 loop is highly variable. As a result, sera from animals immunized with SF162gp140-derived immunogens display a narrow breadth of neutralizing activities (12). An alternative possibility is that some of the anti-V1 antibodies elicited by the SF162gp140-derived immunogens are cross-reactive but their epitopes are differentially exposed on the V1 loops of heterologous isolates, i.e., they are less accessible to antibody binding.
By examining the specificities of antibodies elicited by gp140 immunogens and by identifying obstacles that interfere with the ability of antibodies to interact with their targets on heterologous isolates, a systematic and rational optimization of the gp140 constructs can be achieved.
Here we examine in detail how the positioning of the V1 loop on heterologous virions influences the neutralizing activity of SF162 gp140-elicited antibodies. Our results indicate that the V1 loop presents a major hurdle in our efforts to elicit cross-reactive NAbs by immunization, not only because it is highly variable and gp140-elicited anti-V1 antibodies do not bind to the V1 loops of heterologous isolates but also because it prevents the binding of cross-reactive NAbs to potentially conserved targets outside the V1 loop.
MATERIALS AND METHODS
Cell lines.
Human epithelial cell-like embryonic kidney 293T cells and TZM-bl cells were propagated as previously described (12).
Antibodies and sera.
MAbs P1H6, P3B2, P4D7, and P3C8 were isolated from mice immunized with SF162ΔV2 gp140, while MAb P3E1 was isolated from mice immunized with SF162 gp140 (11). IgG1b12 MAb was provided by Dennis Burton (Scripps Institute). IgGCD4 was purchased from Progenics (Tarrytown, NY). MAb 2G12 was purchased from Polymun Scientific (Vienna, Austria). MAb 447D was provided by Susan Zolla-Pazner and Mirek Gorny (New York University,). Macaque sera were collected from animals previously immunized with SF162gp140-derived immunogens (12).
Env-expressing plasmids.
The full-length SF162 env gene was cloned in the pEMC* vector (25, 32). The Env glycoproteins encoding genes of JR-FL, YU-2, and ADA were provided by J. Sodroski (Dana Faber Cancer Institute, Harvard, MA).
Replacement of the V1 loop of JRFL, ADA, YU2, HxB2, and 89.6 with that of SF162.
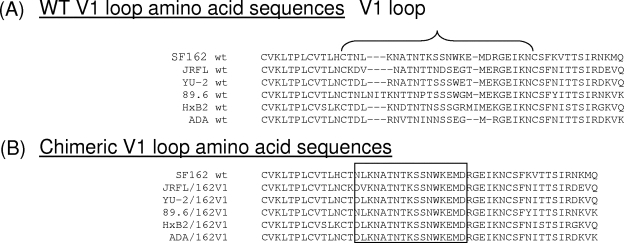
The V1 loop amino acid sequences of the six wild-type (WT) Envs examined here are shown in Fig. 1A.
FIG. 1.
Amino acid sequence of WT and chimeric Envs. (A) The amino acid composition of the V1 loops from the various WT Envs used is indicated. (B) The amino acid sequences of the chimeric Envs expressing the SF162 V1 loop (Env/162V1 constructs) is shown.
(i) Introduction by mutagenesis of FseI and NotI restriction sites.
In order to replace the V1 loops of the heterologous Envs with that of SF162, FseI and NotI restriction sites flanking the V1 region were inserted in the env genes of SF162, JRFL, ADA, YU2, HxB2, and 89.6. The FseI restriction site was created by inserting nine nucleotides in the 5′ end of the V1 region of each env gene by using the QuikChange II site-directed mutagenesis kit (Stratagene). The following primers were used: for SF162, SF162 FseI F (5′-CATTGCACTAATTTGGGCCGGCCTAAGAATGCTACTAATACC-3′) and SF162 FseI R (5′-GGTATTAGTAGCATTCTTAGGCGGGCCCAAATTAGTGCAATG-3′) (the restriction site is shown in bold; one extra nucleotide [underlined] was inserted directly after the restriction site in order to maintain the SF162 env open reading frame); for JRFL, JRFL FseI F (5′-AATTGCAAGGATGTGGGCCGGCCTAATGCTACTAATACC-3′) and JRFL FseI R (5′-GGTATTAGTAGCATTAGGCCGGCCCACATCCTTGCAATT-3′); for ADA, ADA FseI F (5′-AATTGCACTGATTTGGGCCGGCCAAGGAATGTTACTAAT-3′) and ADA FseI R (5′-ATTAGTAACATTCCTTGGCCGGCCCAA ATCAGTGCAATT-3′); for YU2, YU2 FseI F (5′-AATTGCACTGATTTAGGCCGGCCAAGGAATGCTACTAAT-3′) and YU2 FseI R (5′-ATTAGTAGCATTCCTTGGCCGGCCTAAATCAGTGCAATT-3′); for 89.6, 89.6 FseI F (5′-TGCACTAATTTGGGCCGGCCAAATATCACTAAGAATACT-3′) and 89.6 FseI R (5′-AGTATTCTTAGTGATATTTGGCCGGCCCAAATTAGTGCA-3′); and for HxB2, HxB2 FseI F (5′-AAGTGCACTGATTTGGGCCGGCCAAAGAATGATACTAATACC-3′) and HxB2 FseI R (5′-GGTATTAGTATCATTCTTTGGCCGGCCCAAATCAGTGCACTT-3′).
The NotI site was created in a similar fashion using the QuikChange II site-directed mutagenesis kit (Stratagene). The restriction site is shown again in bold. The following primers were used: for SF162, SF162 NotI F (5′-GAGATGGACAGAGGATGCGGCCGCGAAATAAAAAATTGC-3′) and SF162 NotI R (5′-GCAATTTTTTATTTCGCGGCCGCATCCTCTGTCCATCTC-3′); for JRFL, JRFL NotI F (5′-ACGATGGAGAGAGGATGCGGCCGCGAA ATAAAAAACTGC-3′) and JRFL NotI R (5′-GCAGTTTTTTATTTCGCGGCCGCATCCTCTCTCCATCGT-3′); for ADA, ADA NotI F (5′-GAGGGAATGAGAG GATGCGGCCGCGAAATAAAAAACTGC-3′) and ADA NotI R (5′-GCAGTTTTTTATTTCGCGGCCGCATCCT CTCATTCCCTC-3′); for YU2, YU2 NotI F (5′-ACGATGGAGAAAGGATGCGGCCGCGA AATAAAAAACTGC-3′) and YU2 NotI R (5′-GCAGTTTTTTATTTCGCGGCCGCATCCTTTCTCCATCGT-3′); for 89.6, 89.6 NotI F (5′-ATGATGGAGAAAGGATGCGGCCGCGAAATAAAAAATTGC-3′) and 89.6 NotI R (5′-GCAATTTTTTATTTCGCGGCCGCATCCTTTCTCCATCAT-3′); and for HxB2, HxB2 NotI F (5′-ATAATGGAGAAAGGATGCGGCCGCGAGATAAAAAAC-3′) and HxB2 NotI R (5′-GTTTTTTATCTCGCGGCCGCATCCTTTCTCCATTAT-3′).
Introduction of these two restriction sites was verified first by enzymatic digestion with the FseI and NotI enzymes and then by sequencing.
(ii) Amplification of SF162 V1.
Forward primer 216 (5′-AACCCACAAGAAATAGTATTG-3′) and reverse primer 218 (5′-ATCATTACACTTTAGAATCGC-3′) were used to amplify (“Hot start” Platinum Pfx DNA polymerase; Invitrogen) a region of approximately 450 bp from the SF162 FseI/NotI (F/N) construct. The PCR product was separated from the plasmid by agarose gel electrophoresis. The QIAquick gel extraction kit (Qiagen) was used to recover the 450bp amplified product from the agarose gel. The purified PCR product containing V1 of SF162 (51 bp) flanked by the FseI and NotI cleavage sites was simultaneously digested with the FseI and NotI restriction enzymes. This double digestion creates a fragment with a 3′ FseI protruding site, a fragment with a 5′ NotI protruding site, and a small middle fragment containing the V1 region of SF162 (SF162 V1). This last fragment was purified.
(iii) Creation of chimeric clade B env genes that contain V1 of SF162.
Following double digestion of the JRFL F/N, ADA F/N, YU-2 F/N, 89.6 F/N, and HxB2 F/N env sequences and the elimination of the V1 sequences, the SF162V1 F/N fragment was inserted into these constructs. The entire env genes were then sequenced. These chimeric constructs were designated as follows: the name of the heterologous Env, the introduced SF162 V1, and the existence of the FseI and NotI sites (for example: JRFL/162V1 F/N).
(iv) Elimination of the FseI and NotI restriction sites from the chimeric env genes.
The FseI and NotI sites were removed from the above-mentioned chimeric Envs (Fig. 1B).
Two rounds of mutagenesis PCR were performed on the chimeric env genes to eliminate the FseI and NotI sites from the above-described constructs. We first removed the FseI site and then the NotI site. The primers used for removing the FseI site were as follows: JRFL-RF F (5′ AATTGCAAGGATGTGAAGAATGCTACTAATACC 3′) and R (5′ GGTATTAGTAGCATTCTTCACATCCTTGCAATT 3′); ADA-RF F (5′ AATTGCACTGATTTGAAGAATGCTACTAATACC 3′) and R (5′ GGTATTAGTAGCATTCTTCAAATCAGTGCAATT 3′); YU-2-RF F (5′ AATTGCACTGATTTAAAGAATGCTACTAA TACC 3′) and R (5′ GGTATTAGTAGCATTCTTTAAATCAGTGCAATT 3′); 89.6-RF F (5′ AATT GCACTAATTTGAAGAATGCTACTAATACC 3′) and R (5′ GGTATTAGTAGCATTCTTCAAATTAGTGCAATT 3′); and HxB2-RF F (5′ AAGTGCACTGATTTGAAGAATGCTACTAATACC 3′) and R (5′ GGTATTAGTAGCATTCTTCAAATCAGTGCACTT 3′). Each mutagenesis reaction was performed using the QuikChange II site-directed mutagenesis kit (Stratagene) and 60 ng of plasmid as a template. Bovine serum albumin (0.4%) was also added to each master mix. The reaction conditions consisted of 1 cycle at 95°C for 30 s (initial denaturation), followed by 18 cycles of successive denaturation, annealing, and elongation steps performed at 95°C for 30 s, 55°C for 1 min, and 68°C for 7.5 min, respectively, and 1 final cycle at 70°C for 7 min (final elongation). All reactions were digested with DpnI overnight, and the mixture was used to transform competent cells (One Shot MAX Efficiency DH5α-T1R; Invitrogen). Prior to transformation, 1 μl of β-mercaptoethanol (1:10 dilution in H2O; Sigma) was added directly to the competent cells, followed by 10 min of incubation on ice.
The primers used to remove the NotI sites were as follows: JRFL-RN F (5′ GAGATGGACAGAGGAGAAATAAAAAACTGC 3′) and R (5′ GCAGTTTTTTATTTCTCCTCTGTCCATCTC 3′), used to remove the NotI restriction site from JRFL/162V1 without FseI, ADA/162V1 without FseI, and YU-2/162 without FseI env genes. Primers 89.6-RN F (5′ GAGATGGACAGAGGAGAA A TAAAAAATTGC 3′) and R (5′ GCAATTTTTTATTTCTCCTCTGTCCATCTC 3′) were used to remove the NotI restriction site from 89.6/162V1 without the FseI env gene, and primers HxB2-RN F (5′ GAGATGGACAGAGGAG AGATAAAAAACTGC 3′) and R (5′ GCAGTTTTTTATCTCTCCTCTGTCCATCTC 3′) were used to remove the NotI restriction site from HxB2/162V1 without FseI. Sixty nanograms of plasmid DNA was used as a template for each reaction. The thermocycler conditions were the same as the ones described above for the first mutagenesis. Following mutagenesis, the entire env genes were sequenced.
For clarification purposes, the chimeric Envs containing SF162 V1 are designated as JRFL/162V1, HxB2/162V1, ADA/162V1, YU-2/162V1, and 89.6/162V1 (Fig. 1B). The nucleotide sequences of the entire Env genes of all of these constructs were verified by direct sequencing.
Generation of single-round competent virions expressing SF162-, JRFL-, ADA-, YU-2-, 89.6-, and HxB2-derived envelopes. Luciferase reporter pseudoviruses, capable of a single round of replication and expressing various envelope gp160 glycoproteins, were generated as previously described (12, 25, 32) using the pNL4-3 luciferase-positive, Vpr-negative, Env-negative construct as a backbone. The ratio of gp160-expressing:pNL4-3-expressing vectors used during transfection varied depending on the type of vector that carried the env gene. For env genes cloned in the pEMC* vector (SF162-derived env genes), that ratio was 1:20. For env genes cloned in the pSV7d vector (ADA-, HxB2-, YU-2-, and JRFL-derived env genes), the ratio was 1:1. For 89.6-derived env genes (cloned in vector E7), the ratio was 1:50. The p24 antigen concentration of each pseudovirus preparation was determined with the HIV type 1 p24 Antigen Capture Assay kit (AIDS Vaccine Program; NCI-Frederick Cancer Research and Development Center).
Entry assays.
The ability of each Env construct to mediate virus-cell fusion was evaluated using TZM-bl cells as targets as previously described (12). Briefly, twofold serial dilutions of the various single-round competent virions (starting at 30 ng p24) were added to polybrene-treated cells (3 × 103 cells per well of a 96-well U-bottomed tissue culture plate; Falcon). Following a 72-h incubation, the cell-associated luciferase was determined as previously described using a Fluoroskan Ascent FL luminometer (Thermo Labsystems) and the Ascent software for Fluoroskan Ascent FL (12, 25, 32). The results were processed, analyzed, and plotted using GraphPad Prism 4.03 software.
Virus neutralization.
The neutralization susceptibilities of virions expressing the various Envs to MAbs and sera from macaques immunized with SF162gp140 was determined using TZM-bl cells as targets as previously described (12, 25, 32). Briefly, 3 × 103 TZM-bl cells were plated per well in a 96-well flat-bottomed cell culture cluster (Corning Incorporated) and incubated at 37°C with 5% CO2 for approximately 24 h. Viruses were preincubated with an equal volume of serially diluted MAb or sera (in complete Dulbecco's modified Eagle's medium) for 90 min at 37°C in 96-well U-bottomed tissue culture plate (Falcon). For each MAb or serum dilution, duplicate wells were used. The cells were first treated with Polybrene (2 μg/ml) for 30 min at 37°C and incubated either alone or with virus in the absence of MAbs (negative and positive controls for entry, respectively). In neutralization assays with sera from SHIVSF162P4-infected rhesus macaques (6, 12), each virus was also incubated with sera collected from the same animals prior to immunization (prebleed, which serves as an internal control for potential nonspecific inhibition of HIV entry). The virus/antibody mixture was incubated with cells for 72 h at 37°C. At that point, the cell supernatant was removed and cell-associated luciferase was determined as discussed above. After subtracting the average number of relative light units (RLU) in the negative control wells from all of the values, the percent neutralization was calculated as follows: [(average number of RLU in the positive control wells − number of RLU in the presence of MAb or serum)/average number of RLU in the positive control wells] × 100.
The contribution of anti-V1- and anti-V3-directed antibodies present in immune sera was determined as previously described (12). Briefly, serial dilutions of sera were incubated with peptide (10 μg/ml) for 1 h at 37°C and then with single-round competent virus for 1 h at 37°C. This mixture was then incubated with polybrene-treated TZM-bl cells for 72 h at 37°C, and cell-associated luciferase was determined. The percent reduction in neutralization in the presence of peptide was determined at the serum dilution that resulted in 80% inhibition of infection in the absence of peptide.
RESULTS
Neutralization susceptibility of viruses expressing the chimeric Envs.
Pinter et al. reported that replacement of the entire V1V2 region of JRFL with that of SF162 renders the virus susceptible not only to V1 and V2 antibodies but also to anti-V3 antibodies (30). This substitution had no effect on neutralization by antibodies such as b12 or 2G12. We wanted to determine whether substitution of the V1 loop alone would have similar effects on the neutralization susceptibilities of all the additional isolates studied here, including JRFL.
(i) Susceptibility to neutralization by MAbs.
To evaluate the effect of V1 loop substitution on the overall neutralization susceptibilities of different Envs, we employed four anti-V1-directed MAbs (P1H6, P3C8, P4D7, and P3B2), two anti-V3-directed MAbs (P3E1 and 447D), one anti-CD4-binding-site MAb (IgG1b12), the chimeric IgGCD4 protein, and the anti-glycan-directed MAb 2G12. A summary of the neutralization results with all the heterologous and chimeric Envs is presented in Table 1. The anti-V1 MAbs tested here neutralized SF162 but none of the other WT viruses. This is expected, since these MAbs were isolated from mice immunized with SF162gp140-derived constructs and are specific for the SF162 V1 region (11). As anticipated, the replacement of the V1 loop on these Envs with that of SF162 rendered all viruses expressing the chimeric Envs susceptible to anti-V1 MAb neutralization. In certain cases, viruses expressing the chimeric Envs were even more susceptible to anti-V1 MAb neutralization than the SF162 virus. For example, the 50% inhibitory concentration (IC50) for MAb P1H6 against SF162 was 0.4 μg/ml, while the IC50 for the HxB2/162V1 virus was 0.04 μg/ml (Table 1). These results, although expected, indicate that the introduced SF162V1 loop did not become masked by other regions or glycosylation sites present on the different heterologous Env backgrounds.
TABLE 1.
Susceptibilities of WT and chimeric viruses to neutralization by MAbs
| MAb | Epitope | IC50 (μg/ml) of MAb for virusa
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SF162 | YU2
|
89.6
|
JRFL
|
HxB2
|
ADA
|
|||||||
| WT | 162V1 | WT | 162V1 | WT | 162V1 | WT | 162V1 | WT | 162V1 | |||
| P1H6 | V1 | 0.4 | — | 0.1 | — | 0.03 | — | 0.7 | — | 0.04 | — | 20 |
| P3C8 | V1 | 0.3 | — | 3 | — | 0.02 | — | 2.5 | — | 0.2 | — | — |
| P4D7 | V1 | 0.9 | — | 7 | — | 0.1 | — | 4 | — | 1 | — | — |
| P3B2 | V1 | 1 | — | 8 | — | 0.3 | — | 1.5 | — | 1 | — | — |
| P3E1 | V3 | 0.2 | — | — | 7 | 0.2 | — | 10 | — | — | — | — |
| 447-52D | V3 | 0.04 | — | 2 | 0.01 | <0.01 | 20 | 0.5 | 0.2 | 0.01 | — | 20 |
| IgGCD4 | CD4bs | 0.04 | 0.6 | 0.04 | 0.01 | 0.01 | 0.03 | 0.01 | 0.02 | <0.01 | 0.8 | 0.6 |
| B12 | CD4bs | 0.01 | 10 | 1 | 0.001 | 0.001 | 0.005 | 0.003 | <0.01 | <0.01 | 1 | 0.5 |
| 2G12 | Glycan | 0.3 | — | — | 0.03 | 0.06 | 0.1 | 0.05 | 0.03 | 0.03 | 0.3 | 0.03 |
Bold numbers indicate changes in neutralization susceptibility of at least fourfold. —, 50% inhibition of infection was not observed with the highest MAb concentration tested (20 μg/ml); <0.01, 50% inhibition of neutralization was not reached even at the lowest MAb concentration tested (0.01 μg/ml).
Replacement of the heterologous V1 loops by that of SF162 also altered the neutralizing susceptibility to antibodies that recognize epitopes outside the V1 loop. For example, viruses expressing these chimeric Envs became susceptible to neutralization by certain anti-V3 MAbs, such as the human anti-V3 MAb 447D and the mouse anti-V3 MAb P3E1. The epitope of P3E1 overlaps that of 447D, and both MAbs recognize V3 sequences that contain the GPGR motif (11), which is present on all Envs examined here. For example, incorporating the SF162 V1 into all the heterologous Envs examined here rendered the viruses highly susceptible to 447D-mediated neutralization. Most likely the 447D epitope is present but not exposed within these WT Env trimers because of the particular positioning of the homologous V1 loops. P3E1 neutralized only the 89.6- and JRFL-derived chimeric Envs expressing the SF162 V1 loop. This result suggests therefore that the P3E1 epitope (and potentially other V3 loop epitopes) is oriented differentially among the heterologous Envs examined here.
All viruses expressing the WT Envs were susceptible to neutralization by IgGCD4 and b12. Replacement of the V1 loops by that of SF162 did not alter the neutralization susceptibilities of the viruses, with the exception of YU2, which became more (by approximately 1 log10) susceptible to IgGCD4 and b12 neutralization.
MAb 2G12, which binds to a complex epitope formed by glycans in the C3 and V4 regions of gp120, neutralized (with the exception of YU2) the viruses expressing the WT and the corresponding JRFL, 89.6, and HxB2 chimeric Envs similarly.
The chimeric ADA virus behaved somewhat differently from JRFL, YU2, 89.6, and HxB2. The WT ADA virus was resistant to neutralization with all four anti-V1 MAbs tested. Replacement of its V1 loop with that of SF162 rendered the virus highly susceptible to only one anti-V1 MAb (P1H6). Also, in contrast to the other viruses, replacement of the V1 loop of ADA with that of SF162 rendered the virus more susceptible (by approximately 1 log10) to MAb 2G12. As recorded with the other viruses, replacement of the V1 loop of ADA with that of SF162 rendered the virus susceptible to 447D-mediated neutralization.
(ii) Susceptibility to neutralization by sera from SF162gp140-immunized macaques.
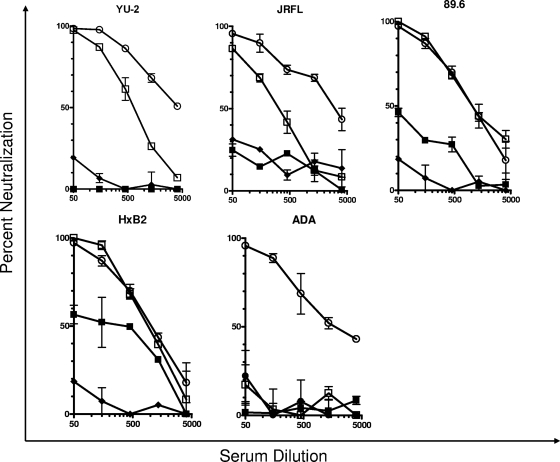
JRFL, ADA, and YU2 are resistant to neutralization by antibodies elicited by SF162gp140 immunogens (12), but weak anti-HXB2 and anti-89.6 neutralizing antibody responses are recorded for some macaques immunized with SF162gp140. Here we examined whether these heterologous isolates would become susceptible to neutralization by such antibodies once their V1 loop was replaced with that of SF162. Indeed, all chimeric heterologous viruses became highly susceptible to neutralization by antibodies elicited by the SF162gp140 immunogen (Fig. 2). In particular, 89.6 and HxB2 became as susceptible to neutralization as the SF162 virus. In the case of YU2 and JRFL, the viruses expressing the chimeric Env protein were also highly susceptible to neutralization by the polyclonal sera but to a lesser extent than SF162.
FIG. 2.
Neutralization of WT and chimeric viruses by SF162gp140-elicited antibodies. The effect of replacing the V1 loop of the YU2, JRFL, 89.6, HxB2, and ADA viruses with that of SF162 on the susceptibilities of these viruses to neutralization by antibodies elicited in rhesus macaques immunized with SF162 gp140 (12) is shown. ○, SF162WT; ▪, YU2 WT, JRFL WT, 89.6 WT, HxB2 WT, or ADA WT; □, YU2, JRFL, 89.6, HxB2, or ADA expressing the V1 loop; ⧫, virions pseudotyped with murine leukemia virus Env.
Once again the chimeric ADA virus behaved differently from the other chimeric viruses, in that it was as resistant to neutralization as the WT ADA virus. Therefore, presentation of the V1 loop on the ADA Env protein differs significantly from that of the other four Envs tested here.
Epitope specificity of NAbs on heterologous Envs.
As discussed above, replacement of the V1 loop of JRFL, ADA, YU2, 89.6, and HxB2 with that of SF162 rendered the viruses susceptible not only to anti-V1 NAbs but also to anti-V3 NAbs (Table 1), suggesting a potential role of the V1 loop in regulating access to anti-V3 neutralizing epitopes. Also, in the case of YU2, substitution of the V1 loop increased the neutralization susceptibility to certain anti-CD4-binding-site (anti-CD4-BS) antibodies (Table 1). Immunization with SF162gp140 elicits the generation of anti-V1 NAbs but also anti-V3 NAbs and anti-CD4 BS antibodies (12). Therefore, the high susceptibility of the viruses expressing the chimeric Envs to neutralization by SF162gp140-elicited serum antibodies could be due (entirely or in part) to the presence of anti-V3- or anti-CD4-BS-directed NAbs.
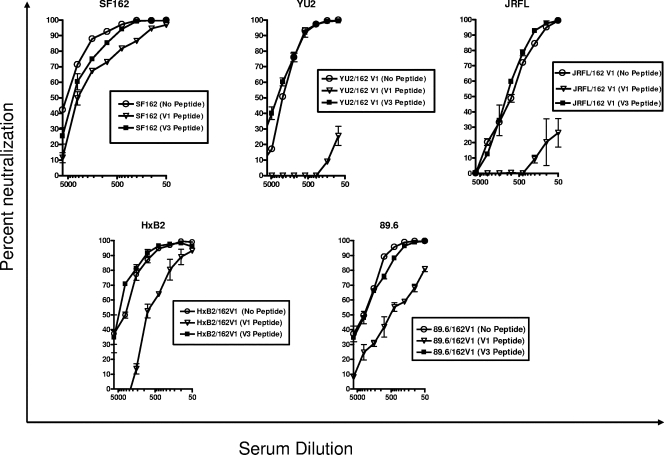
To define the relative contributions of the various antibodies present in the macaque immune sera to neutralization of the chimeric viruses, we performed adsorption experiments during which sera were preincubated with V1- or V3-derived peptides before they were incubated with virus (Fig. 3). SF162 neutralization was reduced by approximately 40% when the sera were preincubated with the anti-V1 peptide and by approximately 27% when the sera were preincubated with the anti-V3 peptide. The WT JRFL, ADA, and YU2 viruses were resistant to neutralization by the SF162gp140-elicited sera, and as expected, serum preincubation with the V1 or V3 peptide did not have any effect on the neutralization phenotype of these viruses (data not shown). The neutralizing potency of these sera against the chimeric YU2 virus was completely eliminated following preincubation with the V1 peptide. In contrast, preincubation with the V3 peptide had no effect on the neutralizing potency of these sera. A similar result was obtained in the case of the chimeric JRFL virus. In the case of ADA, the chimeric virus expressing the SF162 V1 loop was resistant to neutralization by the SF162gp140 sera (Fig. 2), and incubation of sera with either the V3 peptide or the V1 peptide had no effect on the neutralization of the chimeric ADA virus which expresses the SF162 V1 loop (data not shown). As recorded with the JRFL and YU2 chimeric viruses, preincubation with the V3 peptide did not reduce the neutralizing potency of sera against the chimeric HxB2 and 89.6 viruses. In contrast, however, to what we recorded with the chimeric JRFL and YU2 viruses, preincubation with the V1 peptide reduced the neutralizing potencies of sera against the HxB2 and 89.6 chimeric viruses by only 75% and 54%, respectively.
FIG. 3.
Neutralization competition with V1 and V3 derived peptides. V1 and V3 peptides derived from SF162 Env were used to block the activities of neutralizing antibodies in sera from macaques immunized with SF162gp140 (12). The percent reduction in serum neutralizing activity in the presence of the V1 or V3 peptide was determined at the concentration resulting in 80% inhibition of infection, as discussed in Materials and Methods.
Therefore, antibodies with epitope specificities other than the V1 and V3 loops are present in the SF162gp140-elicited sera, and these antibodies can bind their targets on the 89.6 and HxB2 viruses but not on the YU2 and JRFL viruses. These antibodies could be targeting the CD4-BS of Env. Overall, our results indicate that different isolates can be neutralized by different antibodies present in a given immune serum.
DISCUSSION
In this study we aimed to better understand the way HIV resists neutralization by antibodies elicited by Env gp140 immunogens. Specifically, we examined how the V1 loop of Env influences the neutralization susceptibilities of heterologous viruses to antibodies elicited during immunization of macaques with the SF162gp140 immunogen (36). This immunogen elicits a polyclonal anti-Env antibody response that includes antibodies to the V1 and V3 loops, the CD4-BS, CD4-induced (CD4i) epitopes, the gp41 external region, and additional but as of yet unidentified Env regions (12, 43, 44).
Overall, our study indicates that the V1 loop as expressed on the surface of virion-associated Env trimers plays a major role in the resistance of heterologous viruses to neutralization by gp140-elicited NAbs. With the exception of ADA, when the V1 loop of the heterologous isolates tested here was replaced by the V1 loop present on the SF162gp140 immunogen, these isolates became highly susceptible to neutralization by the SF162gp140-elicited serum antibodies. The protective role of the V1 loop is through direct and indirect mechanisms. Due to the high amino acid variability within the V1 loop of HIV Env, anti-V1 antibodies elicited by SF162gp140 do not bind to heterologous V1 loops. Most likely these antibodies recognize linear epitopes and not common conformational epitopes that could exist among the isolates studied here. In addition, the V1 loop, due to its orientation within the trimeric HIV Env protein and due to the extent of its glycosylation, limits the accessibility of other Env regions to NAbs elicited by SF162gp140. As a result, anti-V3 and anti-CD4-BS NAbs elicited by SF162gp140 do not access their epitopes on heterologous Envs.
Our results support observations made by others using viruses expressing chimeric Envs between SF162 and JRFL. Pinter et al. (30) showed that the V1V2 Env region of the neutralization-resistant isolate JRFL protects this virus from NAbs that recognize certain epitopes within the V2 and V3 loops. Replacement of the entire V1V2 region of JRFL with that of SF162 (which is a neutralization-susceptible primary isolate) renders the former virus susceptible to neutralization by anti-V2 and anti-V3 MAbs. Our results obtained with MAb 447D indicate that its epitope is not well expressed on numerous isolates and that this is due in part to the orientation and/or glycosylation pattern of the V1 loop. So even though immunogens such as SF162gp140 may elicit cross-binding anti-V3 antibodies, such antibodies will have limited neutralizing potency unless strategies are developed to circumvent obstructions due to the V1 loop.
The fact that the 447D epitope is not as accessible to MAb 447D binding on the WT YU2, JRFL, and ADA trimeric Envs as it is on the corresponding chimeric Envs expressing the SF162V1 loop suggests that the orientation of the V1 loop of SF162 on the heterologous backbone may not be the same as that of the WT V1 loops. This difference does not appear to be linked to the “size” of the V1 loops studied here. With the exception of 89.6 (which has 29 amino acids in the V1 loop), all other V1 loops have between 24 and 26 amino acids. Similarly, this difference does not appear to correlate with the number of PNLGS within the V1 loops of the Envs studied here.
Previously we reported that immunization with SF162gp140-derived immunogens results in the generation of high titers of antibodies to CD4i epitopes, including epitopes within the V3 loop (12). These antibodies do not neutralize neutralization-resistant viruses such as JRFL unless the viruses are preincubated with suboptimal sCD4 concentrations, which results in the exposure of the CD4i epitopes (12). Our current results indicate that this resistance is due in part to the orientation and glycosylation patterns of the V1 loop and suggest that Env-based immunization approaches aimed at eliciting antibodies to CD4i epitopes will be ineffective at eliciting broad cross-reactive NAbs.
Regarding the effect of V1 loop substitution on the susceptibility to neutralization by anti-CD4-BS antibodies, we observed that on the background of the JRFL, ADA, 89.6, and HxB2 Envs, this substitution did not appear to alter the overall exposure of the b12 epitope. However, in the case of YU2, that same substitution rendered the virus more susceptible to both IgGCD4 and b12-mediated neutralization. Since the V1V2 region appears to be positioned in close proximity to the CD4-BS (21), replacement of the YU2 V1 loop with that of SF162 could have enhanced neutralization by IgGCD4 and MAb b12 by either a direct effect of the V1 loop or indirectly through a repositioning of the V2 loop over the CD4-BS of this isolate. It was reported, for example, that escape from b12-mediated neutralization can be achieved though mutations in the V1V2 region (26). However, regardless of how this enhancement in CD4-BS exposure occurred, the difference observed between YU2 and the other isolates tested here suggests that subtle differences exist in the exposure of the CD4-BS within the trimeric Env among diverse HIV isolates. It is possible that these differences in Env organization among primary isolates, however subtle they appear to be, may result in the neutralization of diverse isolates by the same immune sera through the binding of antibodies to distinct epitopes. Also, differences in structure and organization of the trimeric Env protein may have profound implications for the design of Env-based immunogens that are hoped to elicit cross-neutralizing antibody responses.
The ADA Env protein behaved differently from JRFL, YU2, 89.6, and HxB2 Envs. The chimeric ADA Env expressing the SF162 V1 loop was as resistant to neutralization by the SF162-gp140-elicited antibodies as the WT ADA virus. Also, the chimeric ADA virus was resistant to almost all anti-V1 MAbs we tested, while the other four chimeric viruses were susceptible to all anti-V1 MAbs. Also, replacement of the V1 loop of ADA with that of SF162 rendered the viruses highly susceptible to neutralization by MAb 2G12, something that we did not observe with the other Envs. This is another indication that the organization of the ADA Env trimer may differ from that of the other Envs examined here.
In addition to SF162-based immunogens, other clade B immunogens (22, 41) appear to elicit high titers of V1-directed NAbs. Our current results are highly relevant to improving the design of Env-based immunogens. The future design of such immunogens needs to overcome not only the high immunogenicity of the V1 loop on these constructs but also several structural barriers that exist on the target Envs that are due to the overall positioning and glycosylation of the V1 loops on these targets. We believe that the main target of the antibodies elicited by Env-based immunogens should be the CD4-BS due to its conserved overall structure. However, our results indicate that the V1 loops of diverse isolates are variably positioned over the CD4-BS so that conserved epitopes within that site are differentially exposed among diverse isolates. This differential exposure renders the development of a single Env immunogen, capable of eliciting antibodies that would overcome the diverse positioning of the V1 loops on heterologous isolates, extremely difficult.
Acknowledgments
This study was funded by NIH grant R01 AI47708 (L.S.). We acknowledge the financial support of the M. J. Murdock Charitable Trust and the J. B. Pendleton Charitable Trust.
We express our gratitude to those who contributed reagents for this study.
Footnotes
Published ahead of print on 14 November 2007.
REFERENCES
- 1.Barnett, S. W., S. Lu, I. Srivastava, S. Cherpelis, A. Gettie, J. Blanchard, S. Wang, I. Mboudjeka, L. Leung, Y. Lian, A. Fong, C. Buckner, A. Ly, S. Hilt, J. Ulmer, C. T. Wild, J. R. Mascola, and L. Stamatatos. 2001. The ability of an oligomeric human immunodeficiency virus type 1 (HIV-1) envelope antigen to elicit neutralizing antibodies against primary HIV-1 isolates is improved following partial deletion of the second hypervariable region. J. Virol. 755526-5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beddows, S., M. Franti, A. K. Dey, M. Kirschner, S. P. Iyer, D. C. Fisch, T. Ketas, E. Yuste, R. C. Desrosiers, P. J. Klasse, P. J. Maddon, W. C. Olson, and J. P. Moore. 2007. A comparative immunogenicity study in rabbits of disulfide-stabilized, proteolytically cleaved, soluble trimeric human immunodeficiency virus type 1 gp140, trimeric cleavage-defective gp140 and monomeric gp120. Virology 360329-340. [DOI] [PubMed] [Google Scholar]
- 3.Beddows, S., N. Schulke, M. Kirschner, K. Barnes, M. Franti, E. Michael, T. Ketas, R. W. Sanders, P. J. Maddon, W. C. Olson, and J. P. Moore. 2005. Evaluating the immunogenicity of a disulfide-stabilized, cleaved, trimeric form of the envelope glycoprotein complex of human immunodeficiency virus type 1. J. Virol. 798812-8827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binley, J. M., T. Wrin, B. Korber, M. B. Zwick, M. Wang, C. Chappey, G. Stiegler, R. Kunert, S. Zolla-Pazner, H. Katinger, C. J. Petropoulos, and D. R. Burton. 2004. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J. Virol. 7813232-13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bou-Habib, D. C., G. Roderiquez, T. Oravesz, P. W. Berman, P. Lusso, and M. A. Norcross. 1994. Cryptic nature of envelope V3 region epitopes protects primary monocytotropic human immunodeficiency virus type 1 from antibody neutralization. J. Virol. 686006-6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckner, C., L. G. Gines, C. J. Saunders, L. Vojtech, I. Srivastava, A. Gettie, R. Bohm, J. Blanchard, S. W. Barnett, J. T. Safrit, and L. Stamatatos. 2004. Priming B cell-mediated anti-HIV envelope responses by vaccination allows for the long-term control of infection in macaques exposed to a R5-tropic SHIV. Virology 320167-180. [DOI] [PubMed] [Google Scholar]
- 7.Burke, B., N. R. Derby, Z. Kraft, C. J. Saunders, C. Dai, N. Llewellyn, I. Zharkikh, L. Vojtech, T. Zhu, I. K. Srivastava, S. W. Barnett, and L. Stamatatos. 2006. Viral evolution in macaques coinfected with CCR5- and CXCR4-tropic SHIVs in the presence or absence of vaccine-elicited anti-CCR5 SHIV neutralizing antibodies. Virology 355138-151. [DOI] [PubMed] [Google Scholar]
- 8.Burton, D. R., R. L. Stanfield, and I. A. Wilson. 2005. Antibody vs. HIV in a clash of evolutionary titans. Proc. Natl. Acad. Sci. USA 10214943-14948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Center, R. J., R. D. Leapman, J. Lebowitz, L. O. Arthur, P. L. Earl, and B. Moss. 2002. Oligomeric structure of the human immunodeficiency virus type 1 envelope protein on the virion surface. J. Virol. 767863-7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denisova, G., B. Stern, D. Raviv, J. Zwickel, N. I. Smorodinsky, and J. M. Gershoni. 1996. Humoral immune response to immunocomplexed HIV envelope glycoprotein 120. AIDS Res. Hum. Retrovir. 12901-909. [DOI] [PubMed] [Google Scholar]
- 11.Derby, N. R., S. Gray, E. Wayner, D. Campogan, G. Vlahogiannis, Z. Kraft, S. W. Barnett, I. K. Srivastava, and L. Stamatatos. 2007. Isolation and characterization of monoclonal antibodies elicited by trimeric HIV-1 Env gp140 protein immunogens. Virology 366433-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derby, N. R., Z. Kraft, E. Kan, E. T. Crooks, S. W. Barnett, I. K. Srivastava, J. M. Binley, and L. Stamatatos. 2006. Antibody responses elicited in macaques immunized with human immunodeficiency virus type 1 (HIV-1) SF162-derived gp140 envelope immunogens: comparison with those elicited during homologous simian/human immunodeficiency virus SHIVSF162P4 and heterologous HIV-1 infection. J. Virol. 808745-8762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doria-Rose, N. A., G. H. Learn, A. G. Rodrigo, D. C. Nickle, F. Li, M. Mahalanabis, M. T. Hensel, S. McLaughlin, P. F. Edmonson, D. Montefiori, S. W. Barnett, N. L. Haigwood, and J. I. Mullins. 2005. Human immunodeficiency virus type 1 subtype B ancestral envelope protein is functional and elicits neutralizing antibodies in rabbits similar to those elicited by a circulating subtype B envelope. J. Virol. 7911214-11224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Earl, P., L, B. Moss, and R. W. Doms. 1991. Folding, interaction with GRP78-BiP, assembly, and transport of the human immunodeficiency virus type 1 envelope protein. J. Virol. 652047-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Earl, P. L., W. Sugiura, D. C. Montefiori, C. C. Broder, S. A. Lee, C. Wild, J. Lifson, and B. Moss. 2001. Immunogenicity and protective efficacy of oligomeric human immunodeficiency virus type 1 gp140. J. Virol. 75645-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao, F., E. A. Weaver, Z. Lu, Y. Li, H. X. Liao, B. Ma, S. M. Alam, R. M. Scearce, L. L. Sutherland, J. S. Yu, J. M. Decker, G. M. Shaw, D. C. Montefiori, B. T. Korber, B. H. Hahn, and B. F. Haynes. 2005. Antigenicity and immunogenicity of a synthetic human immunodeficiency virus type 1 group m consensus envelope glycoprotein. J. Virol. 791154-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grundner, C., Y. Li, M. Louder, J. Mascola, X. Yang, J. Sodroski, and R. Wyatt. 2005. Analysis of the neutralizing antibody response elicited in rabbits by repeated inoculation with trimeric HIV-1 envelope glycoproteins. Virology 33133-46. [DOI] [PubMed] [Google Scholar]
- 18.Haigwood, N. L., P. L. Nara, E. Brooks, G. A. V. Nest, G. Ott, K. W. Higgins, N. Dunlop, C. J. Scandella, J. W. Eichberg, and K. S. Steimer. 1992. Native but not denatured recombinant human immunodeficiency virus type 1 gp120 generates broad-spectrum neutralizing antibodies in baboons. J. Virol. 66172-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanson, C. V. 1994. Measuring vaccine-induced HIV neutralization: report of a workshop. AIDS Res. Hum. Retrovir. 10645-648. [DOI] [PubMed] [Google Scholar]
- 20.Kim, M., Z. S. Qiao, D. C. Montefiori, B. F. Haynes, E. L. Reinherz, and H. X. Liao. 2005. Comparison of HIV Type 1 ADA gp120 monomers versus gp140 trimers as immunogens for the induction of neutralizing antibodies. AIDS Res. Hum. Retrovir. 2158-67. [DOI] [PubMed] [Google Scholar]
- 21.Kwong, P. D., R. Wyatt, J. Robinson, R. W. Sweet, J. Sodroski, and W. A. Hendrickson. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature (London) 393648-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, Y., K. Svehla, N. L. Mathy, G. Voss, J. R. Mascola, and R. Wyatt. 2006. Characterization of antibody responses elicited by human immunodeficiency virus type 1 primary isolate trimeric and monomeric envelope glycoproteins in selected adjuvants. J. Virol. 801414-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu, M., S. C. Blacklow, and P. S. Kim. 1995. A trimeric structural domain of the HIV-1 transmembrane glycoprotein. Nat. Struct. Biol. 21075-1082. [DOI] [PubMed] [Google Scholar]
- 24.Mascola, J. R., S. W. Snyder, O. S. Weislow, S. M. Belay, R. B. Belshe, D. H. Schwartz, M. L. Clements, R. Dolin, B. S. Graham, G. J. Gorse, M. C. Keefer, M. J. McElrath, M. C. Walker, K. F. Wagner, J. G. McNeil, F. E. McCutchan, D. S. Burke, and the NIAID AIDS Vaccine Evaluation Group. 1996. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. J. Infect. Dis. 173340-348. [DOI] [PubMed] [Google Scholar]
- 25.McCaffrey, R. A., C. Saunders, M. Hensel, and L. Stamatatos. 2004. N-linked glycosylation of the V3 loop and the immunologically silent face of gp120 protects human immunodeficiency virus type 1 SF162 from neutralization by anti-gp120 and anti-gp41 antibodies. J. Virol. 783279-3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mo, H., L. Stamatatos, J. E. Ip, C. F. Barbas, P. W. H. I. Parren, D. R. Burton, J. P. Moore, and D. D. Ho. 1997. Human immunodeficiency virus type 1 mutants that escape neutralization by human monoclonal antibody IgG1b12. J. Virol. 716869-6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore, J. P., J. A. McKeating, Y. Huang, A. Askenazi, and D. D. Ho. 1992. Virions of primary human immunodeficiency virus type 1 isolates resistant to soluble CD4 (sCD4) neutralization differ in sCD4 binding and glycoprotein gp120 retention from sCD4-sensitive isolates. J. Virol. 66235-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore, J. P., and J. Sodroski. 1996. Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J. Virol. 701863-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moulard, M., S. K. Phogat, Y. Shu, A. F. Labrijn, X. Xiao, J. M. Binley, M. Y. Zhang, I. A. Sidorov, C. C. Broder, J. Robinson, P. W. Parren, D. R. Burton, and D. S. Dimitrov. 2002. Broadly cross-reactive HIV-1-neutralizing human monoclonal Fab selected for binding to gp120-CD4-CCR5 complexes. Proc. Natl. Acad. Sci. USA 996913-6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinter, A., W. J. Honnen, Y. He, M. K. Gorny, S. Zolla-Pazner, and S. C. Kayman. 2004. The V1/V2 domain of gp120 is a global regulator of the sensitivity of primary human immunodeficiency virus type 1 isolates to neutralization by antibodies commonly induced upon infection. J. Virol. 785205-5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sattentau, Q. J., and J. P. Moore. 1995. Human immunodeficiency virus type 1 neutralization is determined by epitope exposure on the gp120 oligomers. J. Exp. Med. 182185-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saunders, C. J., R. A. McCaffrey, I. Zharkikh, Z. Kraft, S. E. Malenbaum, B. Burke, C. Cheng-Mayer, and L. Stamatatos. 2005. The V1, V2, and V3 regions of the human immunodeficiency virus type 1 envelope differentially affect the viral phenotype in an isolate-dependent manner. J. Virol. 799069-9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stamatatos, L., and C. Cheng-Mayer. 1998. An envelope modification that renders a primary, neutralization resistant, clade B HIV-1 isolate highly susceptible to neutralization by sera from other clades. J. Virol. 727840-7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stamatatos, L., and C. Cheng-Mayer. 1993. Evidence that the structural conformation of envelope gp120 affects human immunodeficiency virus type 1 infectivity, host range, and syncytium-forming ability. J. Virol. 675635-5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stamatatos, L., and C. Cheng-Mayer. 1995. Structural modulations of the envelope gp120 glycoprotein of human immunodeficiency virus type 1 upon oligomerization and differential V3 loop epitope exposure of isolates displaying distinct tropism upon virion-soluble receptor binding. J. Virol. 696191-6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stamatatos, L., M. Lim, and C. Cheng-Mayer. 2000. Generation and structural analysis of soluble oligomeric envelope proteins derived from neutralization-resistant and neutralization-susceptible primary HIV-1 isolates. AIDS Res. Hum. Retrovir. 16981-994. [DOI] [PubMed] [Google Scholar]
- 37.Stamatatos, L., S. Zolla-Pazner, M. Gorny, and C. Cheng-Mayer. 1997. Binding of antibodies to virion-associated gp120 molecules of primary-like human immunodeficiency virus type 1 (HIV-1) isolates: effect on HIV-1 infection of macrophages and peripheral blood mononuclear cells. Virology 229360-369. [DOI] [PubMed] [Google Scholar]
- 38.Sugiura, W., C. C. Broder, B. Moss, and P. L. Earl. 1999. Characterization of conformation-dependent anti-gp120 murine monoclonal antibodies produced by immunization with monomeric and oligomeric human immunodeficiency virus type 1 envelope proteins. Virology 254257-267. [DOI] [PubMed] [Google Scholar]
- 39.Sullivan, N., Y. Sun, J. Li, W. Hofmann, and J. Sodroski. 1995. Replicative function and neutralization sensitivity of envelope glycoproteins from primary and T-cell line-passaged human immunodeficiency virus type 1 isolates. J. Virol. 694413-4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.VanCott, T. C., J. R. Mascola, R. W. Kaminski, V. Kalyanaraman, P. L. Hallberg, P. R. Burnett, J. T. Ulrich, D. J. Rechtman, and D. L. Birx. 1997. Antibodies with specificity to native gp120 and neutralization activity against primary human immunodeficiency virus type 1 isolates elicited by immunization with oligomeric gp160. J. Virol. 714319-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu, L., Z. Y. Yang, L. Xu, B. Welcher, S. Winfrey, Y. Shao, J. R. Mascola, and G. J. Nabel. 2006. Cross-clade recognition and neutralization by the V3 region from clade C human immunodeficiency virus-1 envelope. Vaccine 244995-5002. [DOI] [PubMed] [Google Scholar]
- 42.Xiang, S. H., N. Doka, R. K. Choudhary, J. Sodroski, and J. E. Robinson. 2002. Characterization of CD4-induced epitopes on the HIV type 1 gp120 envelope glycoprotein recognized by neutralizing human monoclonal antibodies. AIDS Res. Hum. Retrovir. 181207-1217. [DOI] [PubMed] [Google Scholar]
- 43.Xu, R., I. K. Srivastava, C. E. Greer, I. Zarkikh, Z. Kraft, L. Kuller, J. M. Polo, S. W. Barnett, and L. Stamatatos. 2006. Characterization of immune responses elicited in macaques immunized sequentially with chimeric VEE/SIN alphavirus replicon particles expressing SIVGag and/or HIVEnv and with recombinant HIVgp140Env protein. AIDS Res. Hum. Retrovir. 221022-1030. [DOI] [PubMed] [Google Scholar]
- 44.Xu, R., I. K. Srivastava, L. Kuller, I. Zarkikh, Z. Kraft, Z. Fagrouch, N. L. Letvin, J. L. Heeney, S. W. Barnett, and L. Stamatatos. 2006. Immunization with HIV-1 SF162-derived envelope gp140 proteins does not protect macaques from heterologous simian-human immunodeficiency virus SHIV89.6P infection. Virology 349276-289. [DOI] [PubMed] [Google Scholar]
- 45.Yang, X., R. Wyatt, and J. Sodroski. 2001. Improved elicitation of neutralizing antibodies against primary human immunodeficiency viruses by soluble stabilized envelope glycoprotein trimers. J. Virol. 751165-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang, M. Y., Y. Shu, I. Sidorov, and D. S. Dimitrov. 2004. Identification of a novel CD4i human monoclonal antibody Fab that neutralizes HIV-1 primary isolates from different clades. Antivir. Res. 61161-164. [DOI] [PubMed] [Google Scholar]
- 47.Zhang, P. F., F. Cham, M. Dong, A. Choudhary, P. Bouma, Z. Zhang, Y. Shao, Y. R. Feng, L. Wang, N. Mathy, G. Voss, C. C. Broder, and G. V. Quinnan, Jr. 2007. Extensively cross-reactive anti-HIV-1 neutralizing antibodies induced by gp140 immunization. Proc. Natl. Acad. Sci. USA 10410193-10198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu, P., E. Chertova, J. Bess, Jr., J. D. Lifson, L. O. Arthur, J. Liu, K. A. Taylor, and K. H. Roux. 2003. Electron tomography analysis of envelope glycoprotein trimers on HIV and simian immunodeficiency virus virions. Proc. Natl. Acad. Sci. USA 10015812-15817. [DOI] [PMC free article] [PubMed] [Google Scholar]