Abstract
The human immunodeficiency virus type 1 (HIV-1) Vpu accessory protein is a transmembrane protein that down regulates CD4 expression and promotes the release of new virions. We screened a human leukocyte-specific yeast two-hybrid expression library to discover novel Vpu-interacting cellular proteins. The major histocompatibility complex class II (MHC II) invariant chain, also called Ii or CD74, was found to be one such protein. We show direct binding of Vpu and CD74 by using a yeast two-hybrid assay and coimmunoprecipitation from HIV-1-infected cells. The cytoplasmic region of Vpu was found to interact with the 30-amino-acid cytoplasmic tail of CD74. Human monocytic U937 cells infected with wild-type or Vpu-defective HIV-1 and transfected cells showed that Vpu down modulated the surface expression of mature MHC II molecules. The reduction in cell surface mature MHC II molecules correlated with decreased antigen presentation to T cells in culture. Thus, the Vpu protein also contributes to viral persistence by attenuating immune responses during HIV infection. This report further exemplifies the rich diversity and redundancy shown by HIV in immune evasion.
Human immunodeficiency virus type 1 (HIV-1) is a complex retrovirus and is the causative agent of AIDS (14). Besides coding for the typical retroviral Gag, Pol, and Env proteins, the HIV-1 genome also encodes the regulatory Tat and Rev proteins and the accessory Vif, Vpr, Vpu, and Nef proteins (14). The accessory proteins are required for establishment and persistence of infection in the host but are dispensable for HIV replication in vitro (14). The vpu gene is found exclusively in HIV-1 and some HIV-1-related simian immunodeficiency virus (SIV) isolates, such as SIVcpz, SIVgsn, and SIVmon, but not in HIV-2 or the majority of SIV isolates (10-12, 23). Two main functions have been assigned to the Vpu protein; they are enhancement of virus release from infected cells (26, 41, 47, 50) and degradation of the HIV receptor CD4 protein in the endoplasmic reticulum (ER) (53). Whether Vpu is a virulence factor remains to be established, but compared to HIV-1, closely related retroviruses, such as HIV-2 and SIV, that lack expression of a fully functional Vpu protein also cause less severe disease outcomes. Vpu-defective HIV-1 mutants replicate poorly in CD4+ T cells and macrophages, and recent studies with macaques have demonstrated that Vpu-defective simian-human immunodeficiency virus (SHIV) strains are attenuated in vivo.
The Vpu protein is an 81-amino-acid (81-aa) type I integral membrane protein with two major domains, namely, an N-terminal transmembrane (TM) domain that anchors it in the cellular membrane and appears to form a cation channel and a carboxyl-terminal cytoplasmic domain that contains two amphipathic alpha helices (10, 47). Between these helices are positioned two serine residues (S52 and S56) that are phosphorylated by cellular casein kinase II (42). The ability of Vpu to induce CD4 degradation depends on these phosphoserines through binding of beta-transducin repeat-containing protein (βTrCP) and the formation of an E3 ubiquitin ligase complex at the ER (2, 31). This association polyubiquitinates CD4 at its cytoplasmic tail and marks it for proteasomal degradation (16, 40). βTrCP is part of a complex that also regulates degradation of various cellular substrates, including β-catenin and inhibitor of kappa B (IκB); its stable association with Vpu affects the Wnt and nuclear factor kappa B (NF-κB) signaling pathways (1, 5). In HIV-infected cells, Vpu is synthesized from a bicistronic mRNA that also codes for the viral envelope (Env) protein gp160. While these two proteins are synthesized at similar rates, unlike the Env protein, which is packaged into virions and exported from the cell, Vpu remains largely cell associated and accumulates over time in infected cells (6).
Another function of Vpu is to enhance viral particle release from the cell (26), and this depends largely on the TM domain of Vpu, which also shows ion channel activity (13). Early studies showed that expression of Vpu could enhance the release of diverse retroviral particles from human cells, irrespective of whether those retroviruses normally carry a Vpu protein (17). More recent studies suggest that Vpu, as well as the Vpu-like activity of HIV-2 envelope proteins, acts by overcoming a novel species-specific host restriction to HIV release (51). The expression of Vpu in trans rescued Vpu-defective HIV-1 release to levels similar to those of the wild-type virus. The pericentriolar recycling endosome has been shown to be important for Vpu-mediated particle release and was proposed to be a potential site for interaction between Vpu and the host restriction factor (52). The Vpu protein prevents accumulation of HIV-1 and murine leukemia virus Gag in endosomal compartments and instead constrains Gag accumulation to the plasma membrane (36). This is likely to positively influence the assembly and release of newly synthesized virions.
Viral proteins are known to perform multiple functions in infected cells by interacting with a variety of cellular proteins. In this study, we used a yeast two-hybrid screen to identify novel host proteins that might bind Vpu. One such protein, the major histocompatibility complex class II (MHC II) invariant chain (Ii), also called CD74, was further characterized for its interaction with HIV-1 Vpu and for functional effects on MHC II following HIV infection. Our results support a role for the Vpu protein in attenuating immune responses and thus contributing to viral persistence.
MATERIALS AND METHODS
Cells and viruses.
The human monocytic cell line U937 was maintained in RPMI containing 10% fetal bovine serum in a 5% CO2 environment. HIV-1 stocks were generated by electroporation of HeLa cells with the HIV-1 infectious molecular clones pNL4-3 (wild type) and pNL4-3U35 (vpu deleted), obtained from the NIH AIDS Reagent and Reference Program. The culture supernatants were collected 72 h later and filter sterilized. The viral titers were quantitated either by a reverse transcriptase assay (32) or through p24 enzyme immunoassay (Beckman-Coulter). Prior to infection, U937 cells were serum starved for 90 min, and 2 × 106 cells were infected with about 100,000 to 200,000 reverse transcriptase counts. After 2 h of infection, complete medium was added. Cells were collected at various times postinfection and used for immunoprecipitation or flow cytometry.
Cloning and expression of vpu.
The vpu gene was PCR amplified with Pfu polymerase (Stratagene, La Jolla, CA), using a 3.5-kb fragment encompassing the vpr-to-env region, previously amplified and cloned from a primary isolate of HIV-1 subtype C, as a template. The PCR primers used were as follows (with restriction sites shown in italics): Vpu-R5-F, GGATCCATGTTAAATTTAGATTATAAATTAGGAGTAGG; and Vpu-R5-R, GAATTCATTACAAATCATTAACATCCAAAAGCC. The amplified fragment designated R5 vpu was cloned into plasmid pGEM-T Easy (Promega, Madison, WI) and sequenced in both directions. The gene fragment corresponding to the TM domain of Vpu was assembled from the synthetic oligonucleotides R5-TM-F (CATGGAGATGTTAAATTTAGATTATAAATTAGGAGTAGGAGCATTGATAGTAGCACTA ATCATAGCAATAGTCGTGTGGACCATAGTATATATAGAATAGG) and R5-TM-R (AATTCCTATTCTATATATACTATGGTCCACACGACTATTGCTATGATTAGTGCTACTATCAATGCTCCTACTCCTAATTTATAATCTAAAT0054TAACATCTC). The gene fragment corresponding to the cytoplasmic domain of Vpu was PCR amplified using primers R5-Cyto-F (CCATGGAGTATAGGAAATTGGTACAAC) and Vpu-R5-R.
To express a maltose-binding protein-Vpu (MBP-Vpu) fusion protein, the vpu gene was cloned as a BamHI-EcoRI fragment into the pMal-c2 vector (New England Biolabs, Beverly, MA). Following induction of a freshly diluted overnight culture of transformed Escherichia coli BL21(DE3) cells with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) for 4 h at 37°C, the cells were harvested, resuspended in phosphate-buffered saline (PBS) containing 0.1% Triton X-100, 10 μg/ml lysozyme, and 1 mM phenylmethylsulfonyl fluoride, and subjected to five cycles each of freeze-thaw and sonication. The sample was centrifuged at 12,000 rpm at 4°C in a microcentrifuge (Biofuge 17RS; Heraeus, Germany). To the clarified lysate, amylose resin (New England Biolabs, Beverly, MA) was added, and the MBP-Vpu protein was allowed to bind for 2 h at 4°C. The resin was washed with 10 volumes of wash buffer (20 mM Tris-HCl, pH 7.4, 0.2 M NaCl, 10 mM β-mercaptoethanol, and 1 mM EDTA) to remove nonspecifically bound proteins. The bound proteins were eluted with wash buffer containing 50 mM maltose. Similarly purified MBP was used as a control.
The full-length Vpu protein and its cytoplasmic or transmembrane mutant proteins were also synthesized in vitro and labeled with [35S]methionine-cysteine. For this purpose, a coupled in vitro transcription-translation system (TNT; Promega, Madison, WI) was used according to the manufacturer's guidelines. The in vitro system was programmed with pGBKT7 plasmids carrying the required gene fragments, whose details are given in the following section.
Yeast two-hybrid library screening and assays.
The GAL4-based two-hybrid system contained the DNA binding domain (BD) vector pGBKT7 and the activation domain (AD) vector pGADT7. The R5 vpu gene was cloned into the pGBKT7 vector as an EcoRI-BamHI fragment. The nucleotide sequences corresponding to the TM and cytoplasmic domains of the Vpu proteins were similarly cloned into the two-hybrid vectors as NcoI-BamHI fragments. Expression of the relevant fusion proteins from each of the Vpu two-hybrid constructs was tested in a T7 polymerase-based in vitro coupled transcription-translation system (Promega, Madison, WI), followed by immunoprecipitation with anti-Vpu antibodies. A commercial human leukocyte-specific yeast two-hybrid cDNA library (Matchmaker; Clontech, Germany) derived from mRNAs isolated from healthy peripheral blood leukocytes pooled from 550 Caucasians was used for screening. The library, containing 2 × 106 independent clones, was amplified to 3× redundancy and titrated as suggested by the supplier. The plasmid DNA pool was isolated by CsCl density gradient centrifugation. For the screen, plasmids pGBKT7-vpu (BD-Vpu) and pAct-leukocyte cDNA (AD) were cotransformed into the Saccharomyces cerevisiae strain AH109 (MATa trp1-901 his3 leu2-3,112 ura3-52 ade2 gal4 gal80 URA3::GAL-lacZ LYS2::GAL-HIS3), containing the HIS3 and lacZ reporter genes under the control of GAL4-binding sites. The host strain, containing plasmids pAS2-SNF1 and pACT2-SNF4, served as the positive control (19). Various negative controls that included single or dual transformants were also run in the same assay. AH109 yeast cells were transformed using lithium acetate and plated on synthetic dextrose in the absence of leucine, tryptophan, and histidine (SD/LTH−) to grow cotransformants containing Vpu interactors. The clones that grew were replica plated on SD/LTH− plates containing 20 mM 3-amino-1,2,3-triazole (SD/LTH−3AT) to select strong interactors. β-Galactosidase filter lift and liquid assays were carried out as described earlier (24). From individual positive yeast clones on SD/LTH−3AT plates, the plasmid DNAs were isolated and used to transform E. coli DH5α, selected on LB-ampicillin plates to eliminate cells containing a plasmid(s) other than pACT2. The plasmid DNAs so obtained were purified, digested with EcoRI and XhoI, and analyzed in agarose gels. The inserts from these positive clones were sequenced, and the sequences obtained were analyzed by BLAST searches.
A direct yeast two-hybrid assay was carried out using pGBKT7-vpu and pACT-CD74 to confirm the interaction, essentially as described earlier (24). To identify the CD74-interacting domain of Vpu, the assay was carried out using pACT-CD74 and either pGBKT7-vpu(TM) or pGBKT7-vpu(cyto). AH109 yeast cells were transformed using the lithium acetate procedure and plated on SD/LTH− plates to test protein interactions. Various negative controls that included single or dual transformants were run in the same assay, with the host strain containing plasmids pAS2-SNF1 and pACT2-SNF4 used as a positive control. The interaction specificity was tested by replica plating on SD/LTH−3AT plates and through filter lift and liquid β-galactosidase assays.
Synthesis of CD74 peptide and binding assays.
The 30-aa cytoplasmic domain of CD74 was synthesized in vitro using solid-phase 9-fluorenylmethoxy carbonyl chemistry. The final synthesized peptide was cleaved from the resin with 94.5% trifluoroacetic acid-1.25% EDTA for 2 h at room temperature, with gentle shaking. The cleavage mixture with resin was filtered and the peptide precipitated with cold ether. The peptide was dissolved in 5% acetic acid and lyophilized for 16 h. The size and purity of the synthesized peptide were confirmed by sodium dodecyl sulfate (SDS)-15% polyacrylamide gel electrophoresis and by mass spectrometry. The peptide sequence was MHRRRSRSCREDQKPVMDDQRDLISNNEQL.
For the binding assay, 200 ng of the CD74 cytoplasmic peptide, a nonspecific adrenocorticotropin (ACTH) peptide, or the MBP-Vpu protein was used to coat the wells of an enzyme-linked immunosorbent assay (ELISA) plate for 12 h at 4°C, and the plate was then blocked for 2 h at room temperature with 5% nonfat milk in Tris-buffered saline containing 0.1% Tween 20 (TBST). The reticulocyte reaction mixtures for 35S-labeled full-length, cytoplasmic, and transmembrane Vpu proteins were added and incubated for 12 h at 4°C. A blank 35S reaction mixture was used as a negative control. After three washes with TBST, the retained 35S-labeled proteins were eluted in 100 μl of TBS containing 1% SDS and quantitated by scintillation counting. MBP-Vpu-coated wells were used as a positive control. For another binding assay, an ELISA plate similarly coated with CD74 and ACTH peptides was used to evaluate the binding of purified MBP-Vpu or MBP (as a control). The purified proteins were diluted in TBST containing 5% nonfat milk, and 400 ng of each protein was incubated in the well for 2 h at room temperature. After one wash, diluted (1:1,000) rabbit anti-MBP antibodies in TBST containing 5% bovine serum albumin (BSA) were added and incubated for 2 h at room temperature. This was followed by three washes with TBST and incubation for 90 min at room temperature with diluted (1:2,000) horseradish peroxidase-linked anti-rabbit immunoglobulin G in TBST containing 5% nonfat milk. Following three washes with TBST, the color was developed with diaminobenzidine, and the optical density at 490 nm was measured.
Immunoprecipitation and Western blotting.
Human monocytic U937 cells were infected with HIV-1 NL4-3 and NL4-3U35. Cells were collected at various times postinfection and washed with PBS, and ∼4 × 106 cells were lysed using 450 μl of radioimmunoprecipitation assay buffer lacking SDS. After incubation on ice for 45 min, the lysates were clarified at 12,000 rpm for 10 min and the supernatants incubated on ice for 1 h with 2.5 μg of either mouse anti-human CD74 (Serotech, Oxford, United Kingdom) or mouse anti-human epidermal growth factor receptor (EGFR) (Santa Cruz Biotechnology, Santa Cruz, CA). To this mixture, 50 μl of a 50% suspension of radioimmunoprecipitation assay buffer-washed protein A-Sepharose beads was added, and the mixture was incubated with constant shaking at 4°C for 90 min. The beads were washed five times with 0.5 ml of the lysis buffer, resuspended in 30 μl of SDS-polyacrylamide gel electrophoresis loading buffer, heated at 100°C for 5 min, and centrifuged, and the supernatants were loaded on an SDS-12% polyacrylamide gel. For Western blotting, proteins separated by SDS-polyacrylamide gel electrophoresis were transferred to a nitrocellulose membrane (Hybond ECL). After being blocked with TBS containing 10% BLOTTO (Bio-Rad) for 12 h at 4°C, the membrane was washed with TBST and incubated for 2 h at room temperature with a rabbit polyclonal anti-Vpu antibody diluted 1:1,000 in TBST containing 5% BSA. The blot was washed thrice for 10 min each with TBST and then incubated for 90 min at room temperature with horseradish peroxidase-linked anti-rabbit immunoglobulin G diluted 1:2,000 in TBST containing 5% BLOTTO. The blot was again washed as described above, and chemiluminescence detection of proteins was carried out using a Western blot detection system (Santa Cruz Biotechnology, Santa Cruz, CA) according to the supplier's guidelines.
Flow cytometry.
Infected U937 cells were collected at the indicated times postinfection, washed with PBS, and costained for either total or surface MHC II together with p24 to score HIV-infected cells. The L243 monoclonal antibody recognizes mature MHC II, while anti-CD74 measures levels of immature MHC II as well as free CD74. The U937 cells were washed twice with PBS and then fixed with 100 μl of fixative (Dako IntraStain kit) for 15 min at room temperature. To this mixture, 100 μl of L243 culture supernatant or 5 μg of anti-CD74 was added, and the mixture was incubated at room temperature for 15 min. The cells were washed in PBS and stained with 1 μg (in 100 μl) of Alexa 594-conjugated anti-mouse (Molecular Probes) for 15 min at room temperature. Following this step, the cells were permeabilized with 100 μl of permeabilization solution (Dako IntraStain kit) for 15 min and stained with 2 μl fluorescein isothiocyanate-conjugated anti-p24 (Beckman-Coulter). For total MHC II intracellular staining, the cells were fixed, permeabilized, and stained with L243 as described above. Following two washes in PBS containing 1% BSA, the same permeabilized cells were stained for p24. The cells were then washed twice in PBS containing 1% BSA and resuspended in 500 μl of the same for acquisition. For total CD74 and p24, fixed and permeabilized cells were simultaneously costained with 2 μl each of phycoerythrin-conjugated anti-CD74 (Santa Cruz) and fluorescein isothiocyanate-conjugated anti-p24. The stained cells were acquired using a Cyan-ADP flow cytometer (Dako, Denmark), and data were analyzed using Summit software, version 4.3. U937 cells transfected with expression vectors for a Vpu-enhanced green fluorescent protein (Vpu-EGFP) fusion protein or EGFP as a control were similarly stained for surface or total levels of MHC II or CD74.
Antigen presentation assay.
The Ova-myc plasmid has been described previously (8). Murine BMC-2 cells were transfected with the appropriate plasmids, and 12 h later, the dead cells were removed by flotation on Ficoll-Hypaque. The live cells obtained (3 × 106 cells/ml) were then used to stimulate OT-II T-cell-receptor-transgenic mouse splenic CD4+ T cells (3 × 106 cells/ml) for 24 h as described earlier (8). Activation-induced proliferation of CD4+ T cells was measured by [3H]thymidine incorporation. As controls for cell death during the assay, the transfected BMC-2 cells were stained with propidium iodide 12 h and 24 h into the stimulation assay, and apoptotic cells were quantitated by flow cytometry.
RESULTS
The Vpu protein interacts with CD74.
To identify novel cellular proteins that would interact with it, the HIV-1 Vpu protein was used in a yeast two-hybrid screen of a human leukocyte cDNA library. As described in Materials and Methods, the screen was made stringent by selecting only strongly interacting proteins by growing cotransformants on SD/LTH− plates containing 20 mM 3-AT and then assaying for reporter β-galactosidase activity. From an activation domain library of ∼2 × 106 clones, about 1,000 cotransformants with BD-Vpu grew on SD/LTH− plates, and this number was reduced to 350 on SD/LTH−3AT plates. Of these, 80 clones showed reporter β-galactosidase activity. Plasmid DNAs isolated from these yeast clones were amplified in E. coli, transformed back into S. cerevisiae AH109 cells, grown on yeast extract-peptone-dextrose plates, and replica plated on SD/LH− plates. Thirty clones did not grow on SD/LH− plates and represented the true interactors. Plasmid DNAs isolated from these were restriction digested, and those with unique inserts were sequenced.
Through this process, the genes for four unique proteins, hitherto not reported to bind Vpu, were identified. One of these was CD74, a type II integral membrane protein. It is also known as Ii, the invariant chain that binds to MHC II molecules before antigen loading in the endolysosomal compartment. The CD74 protein has an N-terminal cytoplasmic domain of 30 aa followed by a 26-aa TM domain and a 160-aa domain that either projects into the lumen or is extracellular, depending upon its subcellular location. The association of CD74 and MHC II occurs inside the ER, but the complex is also found on the cell surface, possibly en route to the endolysosomal compartment, where CD74 is degraded and MHC II is loaded with exogenously acquired peptides. Since Vpu is also localized to the ER-Golgi region, it is likely that the CD74-Vpu interaction takes place in this compartment.
Vpu and CD74 cytoplasmic domains are required for their interaction.
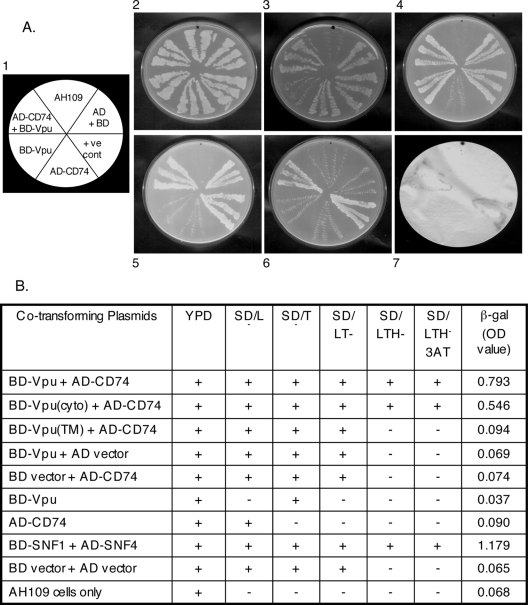
A direct yeast two-hybrid assay was carried out to confirm the CD74-Vpu interaction. As shown in Fig. 1A, a strong interaction was observed between the two proteins. Besides growing on SD/LTH− plates containing 20 mM 3-AT (Fig. 1A, panel 6), the AD-CD74/BD-Vpu cotransformants showed β-galactosidase reporter activity in a filter assay (Fig. 1A, panel 7) as well as in a semiquantitative liquid β-galactosidase chromogenic assay (Fig. 1B). To identify the CD74-interacting domain of Vpu, regions of the vpu gene corresponding to the TM and cytoplasmic domains were cloned into plasmid pGBKT7. As described in Materials and Methods, the yeast two-hybrid assay was repeated using pACT-CD74 and either pGBKT7-vpu(TM) or pGBKT7-vpu(cyto). The results summarized in Fig. 1B show that the Vpu cytoplasmic domain, but not its TM domain, was responsible for the interaction with CD74.
FIG. 1.
Yeast two-hybrid analysis of Vpu-CD74 interaction. (A) Representative plates showing interactions of Vpu and CD74. Panel 1 shows the template for panels 2 to 7, which in turn show transformants streaked on each section of the following plates: 2, yeast extract-peptone-dextrose; 3, SDLeu−; 4, SDTrp−; 5, SDLeu−Trp−; and 6, SDLeu−Trp−His−. Panel 7 shows a β-galactosidase filter assay. Growth is seen as light streaks on a dark background (panels 2 to 6). The β-galactosidase signal (panel 7) is seen as dark streaks on a white background. (B) Complete results for the entire screen, using CD74 and full-length, TM domain, and cytoplasmic domain (cyto) Vpu fusions to the Gal4 protein AD or BD. Growth (+) or no growth (−) of transformants on various media is shown. LTH−3AT represents growth on SDLeu−Trp−His− plates containing 20 mM 3-AT. The liquid β-galactosidase assay values represent averages of two independent measurements. Various negative and positive controls are also shown.
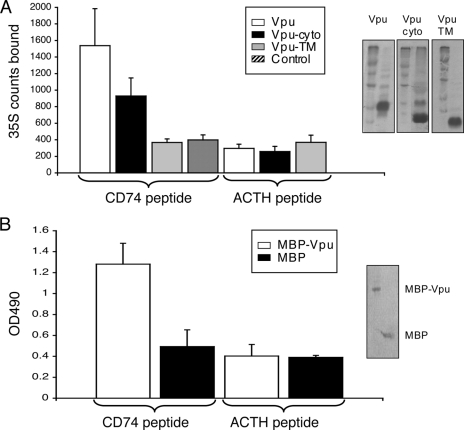
Due to the topology of the two proteins, we reasoned that the cytoplasmic domain of Vpu is likely to bind the N-terminal cytoplasmic domain of CD74. To test this, a 30-aa peptide corresponding to the entire cytoplasmic domain of CD74 (aa 1 to 30) was chemically synthesized. This peptide was used as the coating antigen on an ELISA plate to test Vpu binding. A nonspecific peptide was used as the coating control. In the first assay, [35S]methionine-cysteine-labeled Vpu proteins were synthesized in vitro in a coupled transcription-translation system and used to bind to peptide-coated wells. As shown in Fig. 2A, significantly higher 35S counts were retained in CD74 peptide-coated wells that received either full-length Vpu or its cytoplasmic portion, but not its TM domain. On the other hand, only background counts were retained in wells coated with the nonspecific ACTH peptide. Similarly, the MBP-Vpu fusion protein, but not control MBP, was preferentially retained in CD74 peptide-coated wells but not in wells coated with the ACTH peptide (Fig. 2B). These in vitro assays support the yeast two-hybrid results of Vpu-CD74 interaction and show that the cytoplasmic regions of the two proteins are required for their interaction.
FIG. 2.
Vpu binding to CD74 cytoplasmic domain peptide. (A) In vitro-synthesized 35S-labeled Vpu proteins retained in wells coated with either a CD74 cytoplasmic domain peptide or a nonspecific peptide (ACTH). The results shown are averages of triplicate measurements. Autoradiograms on the right show crude in vitro translation mixtures run together with molecular size markers. (B) Purified MBP-Vpu or MBP control retained in wells coated with either a CD74 cytoplasmic domain peptide or the nonspecific ACTH peptide. The results shown are averages of triplicate measurements. The Coomassie blue-stained SDS gel on the right shows purified proteins.
Vpu binds to CD74 in HIV-1-infected cells.
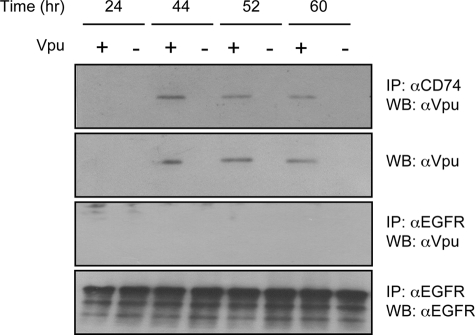
To detect the interaction of Vpu and CD74 in HIV-1-infected cells, the human monocytic cell line U937 was used. U937 cells constitutively express endogenous CD74 as well as the MHC II α and β chains. The U937 cells were infected with HIV-1 NL4-3 or the Vpu-defective mutant NL4-3U35. Cells were collected at various times postinfection, and the lysates were immunoprecipitated with anti-CD74 antibodies and then Western blotted with anti-Vpu antibodies. As shown in Fig. 3, anti-CD74 antibodies efficiently precipitated Vpu from NL4-3-infected cells, while no precipitation was seen from NL4-3U35-infected cell lysates. It is known that Vpu is expressed late in viral infection, starting at about 40 h postinfection. While at early times postinfection (24 h) no Vpu could be immunoprecipitated from NL4-3-infected cells, it could be precipitated efficiently later in infection. Direct Western blotting of infected cell lysates showed a similar expression pattern for Vpu (Fig. 3). To confirm that Vpu does not bind nonspecifically to cellular proteins, we used an isotype-matched anti-EGFR antibody for immunoprecipitation, followed by Western blotting for Vpu. No Vpu was immunoprecipitated under conditions that brought down large amounts of EGFR (Fig. 3). Together, these results confirm a direct interaction between the HIV-1 Vpu protein and host cell CD74 in virus-infected cells and support the in vitro results shown above.
FIG. 3.
Coimmunoprecipitation of Vpu and CD74 from HIV-infected cells. U937 cells were infected with HIV-1 NL4-3 (Vpu+) or NL4-3U35 (Vpu−), and cell lysates were prepared at the indicated times postinfection. The lysates were then immunoprecipitated (IP) with anti-CD74 followed by Western blotting (WB) with anti-Vpu. The immunoprecipitated Vpu proteins are shown. Alternatively, infected cell lysates were directly Western blotted with anti-Vpu antibodies. As nonspecific controls, the same lysates were also immunoprecipitated with an isotype-matched anti-EGFR rabbit polyclonal antibody followed by Western blotting with either anti-Vpu or anti-EGFR antibody.
Vpu modulates MHC II levels in HIV-infected and transfected cells.
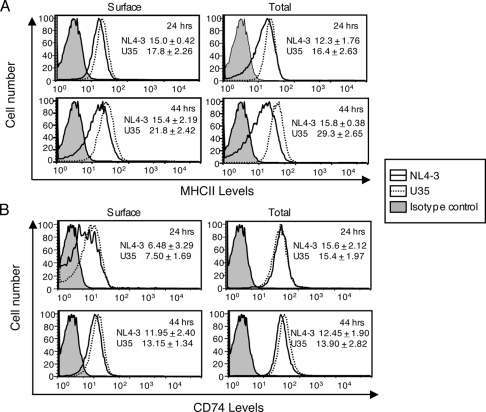
Since CD74 is critical for MHC II presentation, we tested the effects of Vpu on MHC II levels in infected and transfected cells. Human monocytic U937 cells were activated with 10 ng/ml phorbol myristic acid for 24 h to induce MHC II expression and then infected with either HIV-1 NL4-3 or the Vpu-defective NL4-3U35 virus. The infected cells were collected at 24 and 44 h postinfection, stained for surface and total MHC II, using either the L243 antibody, which recognizes mature MHC II, or the MCA2364 antibody, which recognizes Ii (CD74) present on immature MHC II, and analyzed by flow cytometry by gating on the p24-positive population. The anti-CD74 antibody is directed to the luminal region of the CD74 protein and would also stain CD74 protein present on the cell surface or in subcellular compartments. The results are shown in Fig. 4. At 24 h postinfection, a slight decrease in mature MHC II was observed on the surfaces of cells infected with NL4-3 compared to that on cells infected with NL4-3U35; this was also the case with total MHC II (Fig. 4A). In earlier experiments wherein cells were not costained for p24, the surface MHC II decrease at this early time point was found to be independent of Vpu (not shown). At 44 h postinfection, a larger decrease in mature MHC II staining was observed on the surfaces of NL4-3-infected cells than on those of cells infected with the Vpu-defective NL4-3U35 virus (Fig. 4A). A similar pattern was observed when mature MHC II levels were estimated for permeabilized U937 cells stained with the L243 antibody. No significant decrease in surface or total levels of immature MHC II (or CD74) was observed in infected cells at 24 or 44 h postinfection (Fig. 4B). Together, these results suggest that Vpu down regulates the surface as well as total levels of mature MHC II in HIV-infected cells. This effect was observed late but not early in infection, again suggesting the involvement of Vpu, which is expressed late during the viral life cycle.
FIG. 4.
Vpu alters MHC II expression in virus-infected APCs. Activated U937 cells were infected with either HIV-1 NL4-3 or NL4-3U35. (A) At 24 h or 44 h postinfection, cells were fixed and labeled with anti-p24 and the L243 antibody (for mature MHC II), either without permeabilization for surface levels or after permeabilization for total levels. (B) The cells were similarly stained with anti-p24 and anti-CD74 (for immature MHC II) antibodies. Labeled cells were quantitated by flow cytometry. Single-parameter plots are shown for cells gated on p24 expression. The inset data show mean fluorescence intensities (± standard deviations) calculated over two separate experiments, each with duplicate samples.
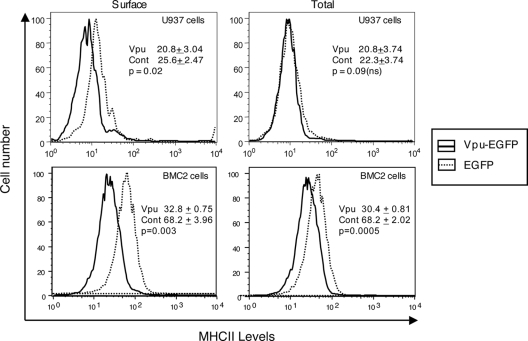
The effects of ectopic expression of Vpu on MHC II were also analyzed. Activated human U937 macrophages or murine BMC-2 macrophages were transfected to express either a Vpu-EGFP fusion protein or EGFP as a control. Cells were stained for surface and total levels of mature MHC II with L243 antibody and quantitated by flow cytometry. Among cells gated for EGFP expression, Vpu reduced mature MHC II on the surfaces of transfected cells (P = 0.02), but the apparent differences in immature surface MHC II (or CD74) were not statistically significant (P = 0.09) (Fig. 5). In BMC-2 cells, the effects were more pronounced, with Vpu showing larger down modulation of both surface and total MHC II levels. The levels of immature MHC II (or CD74) were not statistically different between Vpu-expressing and control cells (not shown).
FIG. 5.
Effect of transfected Vpu on MHC II expression. Activated U937 or BMC-2 cells were transfected to express either Vpu-EGFP or EGFP, and at 24 h posttransfection, cells were stained for surface or total levels of mature MHC II as in Fig. 4. Single-parameter plots are shown for cells gated on EGFP expression. The inset data show mean fluorescence intensities (± standard deviations) calculated over three separate experiments, each with duplicate samples. The P values were calculated by Student's t test, and values of ≥0.05 were considered not significant (ns).
Vpu attenuates T-cell activation.
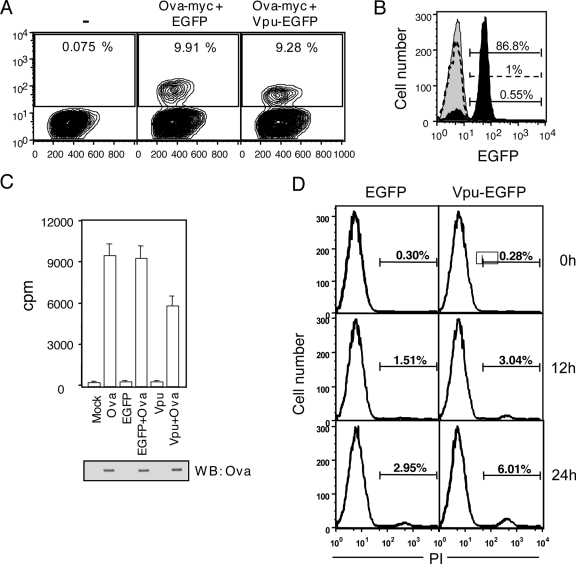
Down regulation of mature MHC II is likely to result in reduced antigen presentation to T cells. This was tested using a mouse cell system. The mouse macrophage cell line BMC-2 was cotransfected with plasmids that express a Vpu-EGFP fusion protein and myc-tagged ovalbumin (Ova-myc). As controls, BMC-2 cells were also transfected with individual expression plasmids and a cotransfection control comprising of Ova-myc and EGFP expression plasmids. The expression of Ova-myc and Vpu-EGFP in BMC-2 cells is shown in Fig. 6A and B. The cotransfected BMC-2 cells were then purified to remove dead cells, and the live cells were used as stimulator antigen-presenting cells (APCs) for Ova-specific MHC II-restricted CD4+ T cells from the OT-II transgenic mouse. Stimulator APC-directed T-cell activation was measured by [3H]thymidine incorporation. As shown in Fig. 6C, BMC-2 cells coexpressing Ova-myc and Vpu-EGFP attenuated activation of the CD4+ T cells compared to BMC-2 cells that coexpressed Ova-myc and EGFP or expressed only the Ova-myc protein. Since Vpu is known to be proapoptotic, it was important to also demonstrate that attenuated T-cell activation by Vpu-expressing cells was not due to apoptotic death of these APCs. We therefore stained cells coexpressing Ova-myc plus EGFP and Ova-myc plus Vpu-EGFP with propidium iodide and quantitated the apoptotic cells at different times during the activation assay. Although Vpu-expressing cells showed about twice as much apoptosis as control cells, these were still a very small fraction of the total cells (Fig. 6D) and would not account for the differences in T-cell stimulation observed in Fig. 6C. Thus, the reduced surface expression of mature MHC II in Vpu-expressing cells is also functionally relevant in down modulating T-cell activation.
FIG. 6.
Vpu attenuates T-cell activation by APCs. (A) Two-parameter plots showing frequencies of Ova-myc expression in untransfected BMC-2 cells (−) or BMC-2 cells transfected to express Ova-myc plus EGFP or Ova-myc plus Vpu-EGFP, as indicated. The gates for OVA-myc-P+ cells are also shown. (B) Histograms show staining levels for Vpu-EGFP in Ova-myc-P+ cells from Ova-myc- plus EGFP-transfected (dotted curve) or Ova-myc- plus Vpu-EGFP-transfected (black shaded curve) cultures. The gray shaded curve is the isotype control curve. (C) Ova-specific MHC II-restricted responses of primary T cells from OT-II transgenic mice to variously transfected BMC-2 cells, as indicated. All data are means ± standard errors for triplicate cultures. T-cell activation was measured as [3H]thymidine incorporation into cellular DNA. The Western blot (WB) shows the expression of Ova in transfected cells stained with anti-myc antibodies. (D) Histograms showing propidium iodide staining of BMC-2 cells transfected with either Ova-myc plus EGFP or Ova-myc plus Vpu-EGFP at different times into the stimulation assay. Cells at 0 h correspond to those immediately after Ficoll-Hypaque purification or at ∼12 h posttransfection.
DISCUSSION
Primate lentiviruses carry a number of accessory proteins not commonly found in other retroviruses. While these proteins are not required for viral replication in all cells in vitro, they modulate replication, virus spread, and survival of the host cell in the background of some cell types. However, in vivo studies have clearly demonstrated a role for these proteins in viral pathogenesis, with their inactivation leading to dramatic attenuation of disease progression and severity (18). These proteins appear to work as multifunctional adaptors that recruit cellular proteins as a means to modulate host cellular processes. Such modulation translates into optimization of viral replication through wide-ranging effects on infectivity, gene expression, and production of new virions.
The primary role of Vpu is to increase the synthesis and release of new virions (4). This is based on two independent activities of Vpu. The first is its ability to mediate interaction of CD4 with the βTrCP-Skp1-Cul1 complex at the ER, leading to ubiquitination, retrotranslocation, and proteasomal degradation of the CD4 protein (53). This prevents formation of a CD4-gp160 complex in the ER, allowing the HIV envelope glycoprotein to transit to the plasma membrane. Vpu is also a cation-selective ion channel (13), and its interaction with the TASK-1 K+ channel was shown to suppress the latter activity and promote the release of new virions (22). The strongest evidence for a role of Vpu in HIV-1 pathogenesis comes from studies of monkeys infected with SHIV. Deletion of the vpu gene in this background led to decreased viral loads in animals infected with SHIV (30). Furthermore, an intact vpu gene was found to be important for the CD4+ T-cell loss during infection with pathogenic SHIV (46). Current models of Vpu action suggest that by increasing viral loads, it contributes to virus spread, which in turn results in increased rates of mutation in the env and nef genes (33, 45). Accumulating mutations in env would contribute positively to the development of neutralization escape variants that drive disease progression and virus transmission to a naïve host (35).
While searching for novel Vpu-interacting cellular proteins in a yeast two-hybrid screen, we found the MHC II Ii or CD74 protein to bind Vpu. This protein is a type II transmembrane protein that binds the MHC II α and β chains in the ER (3). This complex moves through the Golgi complex to the MHC II compartment, where peptide loading occurs by displacement of the class II-associated Ii peptide (CLIP) domain of CD74 before the mature MHC II-peptide complex is presented on the cell surface (39). The displaced CLIP domain is further cleaved by I-CLIP proteases to release a 42-aa cytoplasmic peptide that translocates to the nucleus to modulate transcription of critical survival genes (27). The CD74 protein is also expressed as a trimer on the cell surface and is responsible for mediating signaling initiated by the macrophage migration inhibitory factor (28). Thus, CD74 or Ii is involved in multiple cellular functions that involve MHC II presentation as well as signal transduction. Here we explored the effects of HIV-1 Vpu on MHC II presentation.
In an earlier report, using the HIV-2 Vpx protein as a bait, Pancio et al. (38) similarly pulled out CD74 from a yeast two-hybrid screen. Their results showed that a C-terminal 83-aa region of CD74 interacted with a 20-aa amphipathic helix in the Vpx protein. We observed that the extreme C-terminal 30 aa that comprise the cytoplasmic domain of CD74 interacted with the cytoplasmic domain of Vpu (Fig. 1 and 2). This region of the Vpu protein is made up of two helices, of which helix 1 is amphipathic (6) and, by analogy, is the likely interaction site for CD74. Taken together, our results and those of Pancio et al. (38) suggest that binding CD74 is likely to be important for the HIV/SIV group of lentiviruses. While Vpu carries out this function for HIV-1, the Vpx protein fulfils this role for HIV-2 and SIV.
What might be the functional significance of binding CD74? Since it is the class II invariant chain, this interaction is likely to affect MHC II presentation during HIV infection. In human monocytic U937 cells infected with wild-type and Vpu-defective HIV-1, we observed a slight decrease in surface MHC II presentation early in infection. This effect was Vpu independent and may have been due to Nef, an accessory protein of HIV that is expressed early in infection. Besides promoting the endocytosis of immunologically important cell surface molecules, such as MHC I molecules (43) and the B7 family proteins (8, 9), Nef also down regulates surface MHC II molecules (49), albeit with slow kinetics and a half-life of about 24 h (Chaudhry et al., unpublished data). At later times following infection, Vpu-dependent down modulation of surface as well as total MHC II was observed in infected U937 cells. This also correlated with the timing of Vpu expression and its interaction with CD74 following infection. Ectopic expression of Vpu in transiently transfected U937 or BMC-2 cells also showed surface down modulation of mature MHC II, in agreement with the infection results.
Viruses have evolved various strategies to evade the host immune response. One critical element in the adaptive immune response is the ability of CD4+ T cells to provide help for differentiation of B cells and CD8+ T cells. This, in turn, depends upon the ability of CD4+ T cells to recognize exogenously acquired antigens presented efficiently on the surfaces of APCs in association with MHC II α and β chains. Our results show that HIV-1 Vpu reduces the cell surface levels of mature MHC II molecules in infected APCs and that this interferes with the ability of Vpu-transfected APCs to efficiently present Ova peptides to Ova-specific MHC II-restricted T cells. However, during HIV infection, only a small number of APCs are infected; the remaining, uninfected APCs would continue to express normal levels of cell surface MHC II and to provide T-cell help. While Vpu can down modulate the antiviral immune response and contribute to pathogenesis and viral persistence, this is likely to be a minor pathway. Nevertheless, these results show the rich diversity and functional redundancy of HIV in its immune evasion strategies.
There are several examples of viral down modulation of the MHC presentation pathway (39). For example, the adenovirus E3-19K protein binds and retains the MHC I heavy chain in the ER (7), while herpes simplex virus ICP47 inhibits the ER peptide transporters TAP1 and -2, thus blocking peptide access to the ER lumen (15, 21). Cytomegaloviruses (CMVs) carry multiple proteins that interfere with the MHC I pathway. HIV also uses its Nef and Vpu proteins to interfere with the MHC I pathway. While Nef, an early protein, promotes endocytosis of MHC I from the surfaces of APCs (43), the late Vpu protein interferes with biosynthesis of the MHC I heavy chain (25). In studies on viral down regulation of the MHC II pathway, CMV was shown to interfere with expression of the MHC II heavy chain through repression of class II transactivator expression (20, 29, 34). Direct effects of CMV on global protein secretion were also shown to inhibit MHC II trafficking to the cell surface. Herpes simplex virus infection strongly reduces invariant chain (Ii; CD74) expression (37), and the viral glycoprotein B competes with Ii for binding to HLA-DR (37, 44). The Nef protein of HIV also impairs MHC II presentation by reducing the surface levels of mature MHC II while increasing the levels of functionally incompetent immature MHC II (49). We observed similar effects of the HIV-1 Vpu protein.
We report here a role for the HIV-1 Vpu protein in down modulating MHC II presentation on APCs. We propose that by binding the class II invariant chain (CD74), Vpu prevents maturation of MHC II, something monitored in our experiments with the L243 monoclonal antibody. This would be a novel strategy for interfering with MHC II presentation, unlike the effects of Nef, which are likely due to the modification of intracellular MHC II trafficking (49). An instance of direct binding of CD74 was demonstrated for the HIV-2 Vpx protein (38), but the functional relevance of that binding for MHC II presentation was not studied. The results presented here add to the growing list of strategies used by HIV to evade the host immune response towards the goal of establishing a successful persistent infection.
Acknowledgments
We thank Dinkar Sahal for help with peptide synthesis and Satyajit Rath for T-cell assays and useful discussions. We also thank Malcolm Martin for pNL4-3, Klaus Strebel for pNL-U35, and Frank Maldarelli and Klaus Strebel for the HIV-1 NL4-3 Vpu antiserum, obtained through the NIH AIDS Research and Reference Reagent Program.
This work was supported by a grant from the Department of Biotechnology, Government of India. A.H. received a research fellowship from the University Grants Commission, Government of India. ICGEB and NII receive core funding from the Department of Biotechnology, Government of India.
Footnotes
Published ahead of print on 24 October 2007.
REFERENCES
- 1.Akari, H., S. Bour, S. Kao, A. Adachi, and K. Strebel. 2001. The human immunodeficiency virus type 1 accessory protein Vpu induces apoptosis by suppressing the nuclear factor kappa B-dependent expression of antiapoptotic factors. J. Exp. Med. 1941299-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai, C., P. Sen, K. Hofmann, L. Ma, M. Goebl, J. W. Harper, and S. J. Elledge. 1996. SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell 86263-274. [DOI] [PubMed] [Google Scholar]
- 3.Becker-Herman, S., G. Arie, H. Medvedovsky, A. Kerem, and I. Shachar. 2005. CD74 is a member of the regulated intramembrane proteolysis-processed protein family. Mol. Biol. Cell 165061-5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binette, J., and E. A. Cohen. 2004. Recent advances in the understanding of HIV-1 Vpu accessory protein functions. Curr. Drug Targets 4297-307. [DOI] [PubMed] [Google Scholar]
- 5.Bour, S., C. Perrin, H. Akari, and K. Strebel. 2001. The human immunodeficiency virus type 1 Vpu protein inhibits NF-kappa B activation by interfering with beta TrCP-mediated degradation of Ikappa B. J. Biol. Chem. 27615920-15928. [DOI] [PubMed] [Google Scholar]
- 6.Bour, S., and K. Strebel. 2003. The HIV-1 Vpu protein: a multifunctional enhancer of viral particle release. Microbes Infect. 51029-1039. [DOI] [PubMed] [Google Scholar]
- 7.Burgert, H. G., and S. Kvist. 1985. An adenovirus type 2 glycoprotein blocks cell surface expression of human histocompatibility class I antigens. Cell 41987-997. [DOI] [PubMed] [Google Scholar]
- 8.Chaudhry, A., S. R. Das, A. Hussain, S. Mayor, A. George, V. Bal, S. Jameel, and S. Rath. 2005. The Nef protein of HIV-1 induces loss of cell surface costimulatory molecules CD80 and CD86 in APCs. J. Immunol. 1754566-4574. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhry, A., S. R. Das, S. Jameel, A. George, V. Bal, S. Mayor, and S. Rath. 2007. A two-pronged mechanism for HIV-1 Nef-mediated endocytosis of immune costimulatory molecules CD80 and CD86. Cell Host Microbe 137-49. [DOI] [PubMed] [Google Scholar]
- 10.Cohen, E. A., E. F. Terwilliger, J. G. Sodroski, and W. A. Haseltine. 1988. Identification of a protein encoded by the vpu gene of HIV-1. Nature 334532-534. [DOI] [PubMed] [Google Scholar]
- 11.Courgnaud, V., B. Abela, X. Pourrut, E. Mpoudi-Ngole, S. Loul, E. Delaporte, and M. Peeters. 2003. Identification of a new simian immunodeficiency virus lineage with a vpu gene present among different Cercopithecus monkeys (C. mona, C. cephus, and C. nictitans) from Cameroon. J. Virol. 7712523-12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Courgnaud, V., M. Salemi, X. Pourrut, E. Mpoudi-Ngole, B. Abela, P. Auzel, F. Bibollet-Ruche, B. Hahn, A. M. Vandamme, E. Delaporte, and M. Peeters. 2002. Characterization of a novel simian immunodeficiency virus with a vpu gene from greater spot-nosed monkeys (Cercopithecus nictitans) provides new insights into simian/human immunodeficiency virus phylogeny. J. Virol. 768298-8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ewart, G. D., T. Sutherland, P. W. Gage, and G. B. Cox. 1996. The Vpu protein of human immunodeficiency virus type 1 forms cation-selective ion channels. J. Virol. 707108-7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freed, E. O., and M. A. Martin. 2001. Human immunodeficiency viruses and their replication, p. 1971-2041. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 15.Fruh, K., K. Ahn, H. Djaballah, P. Sempe, P. M. vanEndert, R. Tampe, P. A. Peterson, and Y. Yang. 1995. A viral inhibitor of peptide transporters for antigen presentation. Nature 375415-418. [DOI] [PubMed] [Google Scholar]
- 16.Fujita, K., S. Omura, and J. Silver. 1997. Rapid degradation of CD4 in cells expressing human immunodeficiency virus type 1 Env and Vpu is blocked by proteasome inhibitors. J. Gen. Virol. 78619-625. [DOI] [PubMed] [Google Scholar]
- 17.Gottlinger, H. G., T. Dorfman, E. A. Cohen, and W. A. Haseltine. 1993. Vpu protein of human immunodeficiency virus type 1 enhances the release of capsids produced by gag gene constructs of widely divergent retroviruses. Proc. Natl. Acad. Sci. USA 907381-7385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greene, W. C., and B. M. Peterlin. 2002. Charting HIV's remarkable voyage through the cell: basic science as a passport to future therapy. Nat. Med. 8673-680. [DOI] [PubMed] [Google Scholar]
- 19.Harper, J. W., G. R. Adami, N. Wei, K. Keyomarsi, and S. J. Elledge. 1993. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 75805-816. [DOI] [PubMed] [Google Scholar]
- 20.Heise, M. T., M. Connick, and H. W. Virgin. 1998. Murine cytomegalovirus inhibits interferon gamma-induced antigen presentation to CD4 T cells by macrophages via regulation of expression of major histocompatibility complex class II-associated genes. J. Exp. Med. 1871-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill, A., P. Jugovic, I. York, G. Russ, J. Bennink, J. Yewdell, H. Ploegh, and D. Johnson. 1995. Herpes simplex virus turns off TAP to evade host immunity. Nature 375411-415. [DOI] [PubMed] [Google Scholar]
- 22.Hsu, K., J. Seharaseyon, P. Dong, S. Bour, and E. Marban. 2004. Mutual functional destruction of HIV-1 Vpu and host TASK-1 channel. Mol. Cell 14259-267. [DOI] [PubMed] [Google Scholar]
- 23.Huet, T., R. Cheynier, A. Meyerhans, G. Roelants, and S. Wain-Hobson. 1990. Genetic organization of a chimpanzee lentivirus related to HIV-1. Nature 345356-359. [DOI] [PubMed] [Google Scholar]
- 24.Kar-Roy, A., H. Korkaya, R. Oberoi, S. K. Lal, and S. Jameel. 2004. The hepatitis E virus open reading frame 3 protein activates ERK through binding and inhibition of the MAPK phosphatase. J. Biol. Chem. 27928345-28357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kerkau, T., I. Bacik, J. R. Bennink, J. W. Yewdell, T. Hunig, A. Schimpl, and U. Schubert. 1997. The human immunodeficiency virus type 1 (HIV-1) Vpu protein interferes with an early step in the biosynthesis of major histocompatibility complex (MHC) class I molecules. J. Exp. Med. 1851295-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klimkait, T., K. Strebel, M. D. Hoggan, M. A. Martin, and J. M. Orenstein. 1990. The human immunodeficiency virus type 1-specific protein Vpu is required for efficient virus maturation and release. J. Virol. 64621-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leng, L., and R. Bucala. 2006. Insight into the biology of macrophage migration inhibitory factor (MIF) revealed by the cloning of its cell surface receptor. Cell Res. 16162-168. [DOI] [PubMed] [Google Scholar]
- 28.Leng, L., C. N. Metz, Y. Fang, J. Xu, S. Donnelly, J. Baugh, T. Delohery, Y. Chen, R. A. Mitchell, and R. Bucala. 2003. MIF signal transduction initiated by binding to CD74. J. Exp. Med. 1971467-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LeRoy, E., A. Muhlethaler-Mottet, C. Davrinche, B. Mach, and J.-L. Davignon. 1999. Escape of human cytomegalovirus from HLA-DR-restricted CD41 T-cell response is mediated by repression of gamma interferon-induced class II transactivator expression. J. Virol. 736582-6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li, J. T., M. Halloran, C. I. Lord, A. Watson, J. Ranchalis, M. Fung, N. L. Letvin, and J. G. Sodroski. 1995. Persistent infection of macaques with simian-human immunodeficiency viruses. J. Virol. 697061-7067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Margottin, F., S. P. Bour, H. Durand, L. Selig, S. Benichou, V. Richard, D. Thomas, and K. Strebel. 1998. A novel human WD protein, h-beta TrCp, that interacts with HIV-1 Vpu connects CD4 to the ER degradation pathway through an F-box motif. Mol. Cell 1565-574. [DOI] [PubMed] [Google Scholar]
- 32.Matala, E., T. Hahn, V. R. Yedavalli, and N. Ahmad. 2001. Biological characterization of HIV type 1 envelope V3 regions from mothers and infants associated with perinatal transmission. AIDS Res. Hum. Retrovir. 171725-1735. [DOI] [PubMed] [Google Scholar]
- 33.McCormick-Davis, C., S. B. Dalton, D. R. Hout, D. K. Singh, N. E. Berman, C. Yong, D. M. Pinson, L. Foresman, and E. B. Stephens. 2000. A molecular clone of simian-human immunodeficiency virus (DeltavpuSHIV(KU-1bMC33)) with a truncated, non-membrane-bound vpu results in rapid CD4(+) T cell loss and neuro-AIDS in pig-tailed macaques. Virology 272112-126. [DOI] [PubMed] [Google Scholar]
- 34.Miller, D. M., B. M. Rahill, J. M. Boss, M. D. Lairmore, J. E. Durbin, W. J. Waldman, and D. D. Sedmak. 1998. Human cytomegalovirus inhibits major histocompatibility complex class II expression by disruption of the Jak/Stat pathway. J. Exp. Med. 187675-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Narayan, S. V., S. Mukherjee, F. Jia, Z. Li, C. Wang, L. Foresman, C. McCormick-Davis, E. B. Stephens, S. V. Joag, and O. Narayan. 1999. Characterization of a neutralization-escape variant of SHIVKU-1, a virus that causes acquired immune deficiency syndrome in pig-tailed macaques. Virology 25654-63. [DOI] [PubMed] [Google Scholar]
- 36.Neil, S. J., S. W. Eastman, N. Jouvenet, and P. D. Bieniasz. 2006. HIV-1 Vpu promotes release and prevents endocytosis of nascent retrovirus particles from the plasma membrane. PLoS Pathog. 2e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neumann, J., A. M. Eis-Hubinger, and N. Koch. 2003. Herpes simplex virus type 1 targets the MHC class II processing pathway for immune evasion. J. Immunol. 1713075-3083. [DOI] [PubMed] [Google Scholar]
- 38.Pancio, H. A., N. Vander Heyden, K. Kosuri, P. Cresswell, and L. Ratner. 2000. Interaction of human immunodeficiency virus type 2 Vpx and invariant chain. J. Virol. 746168-6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ploegh, H. L. 1998. Viral strategies of immune evasion. Science 280248-253. [DOI] [PubMed] [Google Scholar]
- 40.Schubert, U., L. C. Anton, I. Bacik, J. H. Cox, S. Bour, J. R. Bennink, M. Orlowski, K. Strebel, and J. W. Yewdell. 1998. CD4 glycoprotein degradation induced by human immunodeficiency virus type 1 Vpu protein requires the function of proteasomes and the ubiquitin-conjugating pathway. J. Virol. 722280-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schubert, U., S. Bour, A. V. Ferrer-Montiel, M. Montal, F. Maldarell, and K. Strebel. 1996. The two biological activities of human immunodeficiency virus type 1 Vpu protein involve two separable structural domains. J. Virol. 70809-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schubert, U., P. Henklein, B. Boldyreff, E. Wingender, K. Strebel, and T. Porstmann. 1994. The human immunodeficiency virus type 1 encoded Vpu protein is phosphorylated by casein kinase-2 (CK-2) at positions Ser52 and Ser56 within a predicted alpha-helix-turn-alpha-helix motif. J. Mol. Biol. 23616-25. [DOI] [PubMed] [Google Scholar]
- 43.Schwartz, O., V. Marechal, S. Le Gall, F. Lemonnier, and J. M. Heard. 1996. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat. Med. 2338-342. [DOI] [PubMed] [Google Scholar]
- 44.Sievers, E., J. Neumann, M. Raftery, G. Schnrich, A. M. Eis-Hubinger, and N. Koch. 2002. Glycoprotein B from strain 17 of herpes simplex virus type I contains an invariant chain homologous sequence that binds to MHC class II molecules. Immunology 107129-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh, D. K., C. McCormick, E. Pacyniak, K. Lawrence, S. B. Dalton, D. M. Pinson, F. Sun, N. E. Berman, M. Calvert, R. S. Gunderson, S. W. Wong, and E. B. Stephens. 2001. A simian human immunodeficiency virus with a nonfunctional Vpu (deltavpuSHIV(KU-1bMC33)) isolated from a macaque with neuroAIDS has selected for mutations in env and nef that contributed to its pathogenic phenotype. Virology 282123-140. [DOI] [PubMed] [Google Scholar]
- 46.Stephens, E. B., C. McCormick, E. Pacyniak, D. Griffin, D. M. Pinson, F. Sun, W. Nothnick, S. W. Wong, R. Gunderson, N. E. Berman, and D. K. Singh. 2002. Deletion of the vpu sequences prior to the env in a simian-human immunodeficiency virus results in enhanced Env precursor synthesis but is less pathogenic for pig-tailed macaques. Virology 293252-261. [DOI] [PubMed] [Google Scholar]
- 47.Strebel, K., T. Klimkait, and M. A. Martin. 1988. A novel gene of HIV-1, vpu, and its 16-kilodalton product. Science 2411221-1223. [DOI] [PubMed] [Google Scholar]
- 48.Stumptner-Cuvelette, P., and P. Benaroch. 2002. Multiple roles of the invariant chain in MHC class II function. Biochim. Biophys. Acta 15421-13. [DOI] [PubMed] [Google Scholar]
- 49.Stumptner-Cuvelette, P., S. Morchoisne, M. Dugast, S. Le Gall, G. Raposo, O. Schwartz, and P. Benaroch. 2001. HIV-1 Nef impairs MHC class II antigen presentation and surface expression. Proc. Natl. Acad. Sci. USA 9812144-12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Terwilliger, E. F., E. A. Cohen, Y. C. Lu, J. G. Sodroski, and W. A. Haseltine. 1989. Functional role of human immunodeficiency virus type 1 vpu. Proc. Natl. Acad. Sci. USA 865163-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Varthakavi, V., R. M. Smith, S. P. Bour, K. Strebel, and P. Spearman. 2003. Viral protein U counteracts a human host cell restriction that inhibits HIV-1 particle production. Proc. Natl. Acad. Sci. USA 10015154-15159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Varthakavi, V., R. M. Smith, K. L. Martin, A. Derdowski, L. A. Lapierre, J. R. Goldenring, and P. Spearman. 2006. The pericentriolar recycling endosome plays a key role in Vpu-mediated enhancement of HIV-1 particle release. Traffic 7298-307. [DOI] [PubMed] [Google Scholar]
- 53.Willey, R. L., F. Maldarelli, M. A. Martin, and K. Strebel. 1992. Human immunodeficiency virus type 1 Vpu protein induces rapid degradation of CD4. J. Virol. 667193-7200. [DOI] [PMC free article] [PubMed] [Google Scholar]