Abstract
Epidemiological studies suggest that cigarette smoke carcinogens are cofactors which synergize with human papillomavirus (HPV) to increase the risk of cervical cancer progression. Benzo[a]pyrene (BaP), a major carcinogen in cigarette smoke, is detected in the cervical mucus and may interact with HPV. Exposure of cervical cells to high concentrations of BaP resulted in a 10-fold increase in HPV type 31 (HPV31) viral titers, whereas treatment with low concentrations of BaP resulted in an increased number of HPV genome copies but not an increase in virion morphogenesis. BaP exposure also increased HPV16 and HPV18 viral titers. Overall, BaP modulation of the HPV life cycle could potentially enhance viral persistence, host tissue carcinogenesis, and permissiveness for cancer progression.
Worldwide, cervical cancer is the third most prevalent type of female cancer and ranks second as a cause of cancer-related deaths in women (49). Human papillomavirus (HPV) is causatively linked to over 90% of all cervical cancer cases examined (5, 20, 42, 51, 55). Most cases of HPV-induced dysplasia spontaneously regress over time (18), and only a small percentage of women infected with oncogenic “high-risk” HPV types, such as HPV type 16 (HPV16), HPV18, and HPV31, progress to high-grade squamous intraepithelial lesions and cervical cancer (4, 10). Epidemiological studies suggest that environmental and host-related cofactors act in conjunction with HPV to promote malignant progression of cervical lesions (7). It has been proposed that cigarette smoking among HPV-positive women is one of the cofactors which likely influences the risk for cervical cancer progression (15, 21, 27, 38, 41, 50, 52, 54). Tobacco-specific polycyclic aromatic hydrocarbons such as benzo[a]pyrene (BaP) (31) and nitrosamines such as 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) (43), which are potent inducers of carcinogenesis (12, 16, 28), have been detected in the cervical mucus of women who smoke and exhibit cervical dysplasias (19, 29, 47). Quantitative levels of noncarcinogenic nicotine and its metabolite cotinine in cervical mucus were correlated with smoking intensity (29, 47, 48) and were shown to be concentrated more strongly in cervical mucus than in blood (17). Colocalization of HPV and cigarette smoke carcinogens in the cervix may present an opportunity for virus/carcinogen interaction (15).
Molecular mechanisms targeted by cigarette smoke carcinogens which potentially deregulate the HPV life cycle and which may promote cervical cancer progression have not been addressed. In this study, we demonstrate for the first time that BaP, a major carcinogenic constituent of cigarette smoke, stimulates high levels of virion synthesis in cell lines productively infected with HPV.
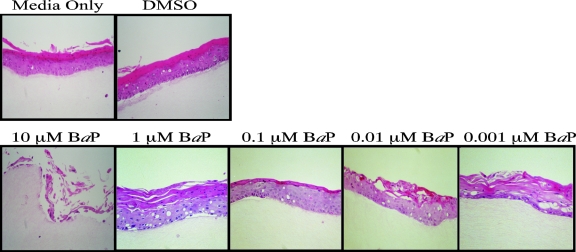
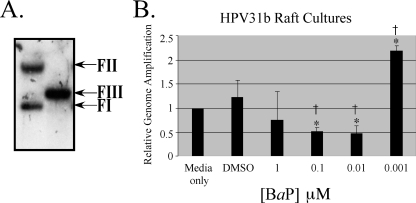
We have previously demonstrated that the complete HPV life cycle is strictly dependent on host tissue differentiation (2, 14, 32, 33, 35, 36, 40). Utilizing the in vitro organotypic raft culture system and the CIN-612 9E cervical intraepithelial neoplasia type I biopsy-derived cell line, our laboratory was the first to report the in vitro propagation of infectious HPV31b (36). Using CIN-612 9E raft cultures, we studied the effect of BaP exposure on the complete HPV31b life cycle. Since BaP has been detected in cervical mucus but not quantitated (31), its physiological concentrations are unknown but may be estimated by considering other cigarette carcinogens which have been quantitated in the cervical mucus. For example, NNK has been detected at concentrations as high as 0.56 μM (43), nicotine at 3 μM, and its metabolite cotinine at 0.36 μM (48). On the other hand, treatment with BaP concentration levels as low as 0.01 μM were shown to be effective in mediating cellular effects in lymphocytes (24). CIN-612 9E raft cultures were generated as described previously (36), using rat tail collagen type 1 (4 mg/ml; Becton Dickinson), except that 2 × 105 epithelial cells were seeded on top of collagen matrices. Raft cultures were fed every other day with E medium as described previously (36), supplemented with BaP (Aldrich; catalog no. 410632), using a range of final concentrations, as follows: 10 μM, 1 μM, 0.1 μM, 0.01 μM, and 0.001 μM. A growth medium-only control was used, in addition to dimethyl sulfoxide (DMSO; Sigma; catalog no. D5879) as a vehicle control. A final concentration of 0.0125% DMSO was determined to have no effect on HPV or cellular functions, and we used this concentration for our vehicle. We also added 1,2-dioctanoyl-sn-glycerol (C8:0), a synthetic diacylglycerol (DAG) which induces activity of the protein kinase C (PKC) pathway (36), which results in a more complete differentiation of raft tissue derived from low-grade or invasive carcinomas, characterized by the increased expression of physiologic markers of keratinocyte differentiation (40), with the concomitant induction of virion synthesis (36). On day 12, the raft tissues were harvested by removing the epithelial layer. Histochemical analysis of raft tissues was performed as previously described (36). We observed that 10 μM BaP was cytotoxic to the HPV31b raft culture tissue (Fig. 1). We isolated total DNA from raft cultures treated with BaP concentrations lower than 10 μM and performed Southern blotting analysis to detect HPV31b genome amplification, using protocols described previously (39). We performed densitometric analysis of the form III (HindIII-digested) linear bands, with the growth medium-only control bands set at 1 (Fig. 2). Treatment with decreasing concentrations of BaP resulted in a nonlinear dose response with respect to changes in HPV genome copies. Raft cultures treated with 1 μM BaP resulted in genome amplification which was about 25% lower than that of the medium-only controls (Fig. 2). Treatment with 0.1 μM and 0.01 μM BaP resulted in genome amplification which was 50% lower than that of medium-only controls (Fig. 2). Surprisingly, treatment with 0.001 μM BaP resulted in a greater than twofold increase in genome amplification (Fig. 2). The data presented are representative of three independent experiments, with standard error bars above the mean.
FIG. 1.
Histochemical analysis. Shown are hematoxylin and eosin-stained CIN-612 9E raft culture tissue sections treated with a range of BaP concentrations as indicated.
FIG. 2.
Southern blotting and densitometric analysis were performed to determine the effect of BaP treatment on HPV31b genome copies. (A) A representative Southern blot showing the F1 (episomal), FII (nicked), and FIII (HindIII linearized) forms of the HPV31b genome. Five micrograms of total cellular DNA was digested with HindIII, which linearizes the HPV31b genome at nucleotide 2455. Blots were detected with a 32P-labeled total HPV31-specific DNA probe generated by random primer extension, followed by autoradiography. (B) Comparative densitometric analysis of the FIII bands from Southern blots of BaP treatments compared with those of DMSO treatments and medium-only controls. The medium-only control was set to 1. Results indicate the averages of three independent experiments ± standard error of the means (*, P < 0.005 relative to that of medium-only control samples; †, P < 0.005 relative to that of DMSO-treated control samples). The paired Student t test was used to calculate P values.
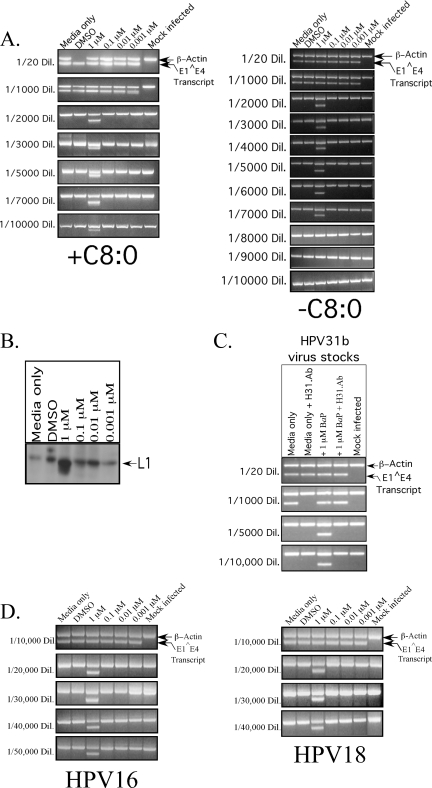
Since increased genome amplification is thought to be correlated directly with virion synthesis (13, 55), we wanted to determine whether the observed changes with viral genome copies were indicative of changes in infectious viral titers. We prepared viral stocks from BaP-treated and control raft cultures, using protocols described previously (11), with the following modifications. A total of three raft tissues were added to a ground homogenizer containing 500 μl 1 M NaCl-0.05 M Na-phosphate buffer (pH 8). Tissue was uniformly homogenized, followed by douncing 10 times. The tissue suspension was transferred to a 1.5-ml Eppendorf tube. An additional 200 μl of buffer was added to the homogenizer to collect residual material and added to the Eppendorf tube. The tissue suspension was centrifuged at 10,500 rpm for 10 min at 4°C. The supernatant was removed and stored at −20°C. Infectious viral titers were determined by infecting HaCat cells with serially diluted viral stocks, followed by performing infectivity assays to amplify HPV31 E1̂E4 spliced transcript cDNA as described previously (30), using HPV31-specific primers (Table 1). An additional set of primers specific for β-actin was included in the PCR mixture as a control for mRNA detection and were used as described previously (30) (Table 1). All PCR products were visualized by electrophoresis in a 2% agarose-ethidium bromide gel. We determined that treatment with 1 μM BaP resulted in a 10-fold increase in HPV31b viral titers compared with those of the control cultures (Fig. 3A, left panel), even though at this concentration, the magnitude of viral genome amplification was slightly lower than that of the growth medium-only controls (Fig. 2). Conversely, treatment with 0.001 μM BaP resulted in the highest magnitude of HPV31b genome amplification (Fig. 2), while viral titers remained the same as that of the control cultures (Fig. 3A, left panel). Raft cultures treated with 0.1 μM and 0.01 μM BaP also displayed viral titers which were the same as that of the medium-only and the DMSO-treated cultures (Fig. 3A, left panel), although in these samples, genome amplification was reduced by 50% compared with that of the medium-only controls (Fig. 2).
TABLE 1.
PCR primers and cycling profiles used for performing infectivity assays to detect E1̂E4 splice transcript cDNA in HPV31b, HPV16, and HPV18 raft cultures
| PCR cycle | HPV31 cycling profile
|
HPV16 and HPV18 cycling profile
|
Strain F and R primersa | Sequence (nt position)b | ||||
|---|---|---|---|---|---|---|---|---|
| Cycle | Temp (°C) | Time | Cycle | Temp (°C) | Time | |||
| PCR 1 | 1 | 95 | 2 min | 1 | 95 | 5 min | HPV31 F | 5′GTGTGTACAGCACACAAGT3′ (760-780) |
| 2 | 95 | 30 s | 2 | 95 | 30 s | HPV31 R | 5′TTGGTTTGTGCATGCAGCTGC3′ (3521-3541) | |
| 3 | 60 | 30 s | 3 | 60 | 30 s | HPV16 F | 5′TGGAAGACCTGTTAATGGGCACAC3′ (797-820) | |
| 4 | 72 | 30 s | 4 | 72 | 1 min | HPV16 R | 5′GTTACTATTACAGTTAATCCGTCC3′ (3584-3607) | |
| Repeat cycles 2 to 4 (39 times) | Repeat cycles 2 to 4 (39 times) | HPV18 F | 5′GTTGTGTATGTGTTGTAAGTGTGA3′ (772-795) | |||||
| 5 | 72 | 10 min | 5 | 72 | 10 min | HPV18 R | 5′GTCCACAATGCTGCTTCTCCG3′ (3580-3600) | |
| 6 | 4 | 6 | 4 | β-actin F | 5′GATGACCCAGACATGTTTG3′ (778-798) | |||
| β-actin R | 5′GGAGCAATGATCTTGATCTTC3′ (3665-3684) | |||||||
| PCR 2 | 1 | 95 | 2 min | 1 | 95 | 5 min | HPV31 F | 5′CGCATATTGCAAGAGCTGTTA3′ (788-808) |
| 2 | 95 | 30 s | 2 | 95 | 30 s | HPV31 R | 5′AGTTGACACTGTCCACGGAGT3′ (3486-3506) | |
| 3 | 60 | 30 s | 3 | 60 | 30 s | HPV16 F | 5′GGAATTGTGTGCCCCATCTGTTC3′ (823-845) | |
| 4 | 72 | 30 s | 4 | 72 | 1 min | HPV16 R | 5′GCAACAACTTAGTGGTGTGGC3′ (3507-3527) | |
| Repeat cycles 2 to 4 (39 times) | Repeat cycles 2 to 4 (40 times) | HPV18 F | 5′GAATTGGCTAGTAGTAGAAAGCT3′ (801-824) | |||||
| 5 | 72 | 10 min | 5 | 72 | 10 min | HPV18 R | 5′TCCCACGTGTCCAGGTCGTGT3′ (3555-3575) | |
| 6 | 4 | 6 | 4 | β-actin F | 5′AACACCCCAGCCATGTACGTTG3′ (808-828) | |||
| β-actin R | 5′ACTCCATGCCCAGGAAGGAAGG3′ (3559-3578) | |||||||
F, forward; R, reverse.
nt, nucleotide.
FIG. 3.
HPV infectivity assay and L1 expression. (A) Infectious titer of the HPV31b control raft tissues and tissues treated with BaP in the presence of C8:0 (left panel) and in the absence of C8:0 (right panel). Shown is a 2% agarose gel of nested reverse transcription (RT)-PCR-amplified HPV31 E1̂E4 spliced transcript and β-actin in reactions with increasing dilutions of the virus stocks shown on the left. (B) The effect of BaP on L1 capsid protein expression as determined from Western blots. (C) Neutralization of HPV31b virus particles from BaP-treated and control raft cultures, using monoclonal H31.Ab, followed by detection of the E1̂E4 spliced transcript in the infectivity assay is shown. The H31.Ab was used at a dilution of 1:20 in HaCat medium. Virus stocks were used at the dilutions shown on the left. (D) Infectious titers of HPV16 and HPV18 control raft tissues and tissues treated with BaP. HPV16- and HPV18-positive cell lines HPV16(114/b)wt:3 and HPV18wt:4, respectively, were used to generate raft cultures for these assays. Shown are 2% agarose gels of nested RT-PCR-amplified HPV16 and HPV18 E1̂E4 spliced transcript and β-actin bands. Reactions were performed with increasing dilutions of the virus stocks shown on the left of each panel.
We then determined L1 capsid protein expression in total viral extracts. A 100-μl aliquot of the virus prep was transferred into a 1.5-ml microcentrifuge tube, followed by the addition of 100 μl buffer A as described previously (1), followed by the addition of 200 μl of buffer B, as described previously (1), and boiled in a water bath for 8 min. To each sample, 100 μl of a 5× sample loading buffer was added as described previously (1). Total protein concentrations were measured by using the Lowry method as described previously (34). To determine capsid protein expression, 60 μg of the whole-virus extracts were loaded onto 7.5% sodium dodecyl sulfate-polyacrylamide gels. The H31.Ab monoclonal antibody (a kind gift from N. Christensen) was used for detecting HPV31 L1 in Western blots and was used at a dilution of 1:500. We observed that L1 protein expression was increased in 1 μM BaP-treated raft tissue (Fig. 3B), which correlated with increased virion synthesis (Fig. 3A, left panel).
To demonstrate the authenticity of virions detected in the infectivity assay, we performed neutralization assays using a conformation-dependent monoclonal antibody against an HPV31 L1 epitope. Neutralization assays were performed as previously described (30), except that the H.31Ab monoclonal antibody was used. Virus stocks derived from both BaP-treated and control raft cultures were diluted as shown in Fig. 3C and preincubated for 1 h with the H.31Ab monoclonal antibody diluted 1:20 in HaCat medium as described previously (30). Following preincubation, HaCat cells were infected with the virus-antibody mixture and incubated for 48 h as described previously (30). Total RNA was then extracted, and the viral E1̂E4-spliced transcript was detected as described above. Antibody treatment effectively neutralized virions produced in both BaP-treated and untreated tissues, as determined by their interference with the infection of HaCat cells (Fig. 3C). Notably, because of the higher number of infectious particles present, higher dilutions of the 1 μM BaP-treated viral stocks were required for the complete neutralization of virions than that required for control viral stocks. Cumulatively, our data suggest that exposure to cigarette smoke carcinogens may culminate in an increased viral burden that results from increased virion morphogenesis as well as from the amplification of viral genome copies.
In order to demonstrate that the effect of BaP enhancement of virion synthesis was not specific to HPV31, BaP treatments were repeated with HPV16- and HPV18-positive cell lines. Both cell lines were derived by electroporating human foreskin keratinocytes with HPV16 and HPV18 genomic DNA, respectively, using protocols described elsewhere (30). Infectious viral titers for both virus types were determined by using techniques described previously (30), using HPV16- and HPV18-specific primers (Table 1). We observed that treatment with 1 μM BaP resulted in a four- to fivefold increase in viral titers for both of these viral types (Fig. 3D).
Since the late 1970s, a close correlation has been observed between cigarette smoking and the incidence of cervical cancer (52). These studies suggested that the carcinogens present in cigarette smoke may modify important cellular proteins and thus act as cofactors in cervical cancer progression. Our current study provides molecular evidence that links the dosage-dependent effects of a known tobacco carcinogen, implicated by epidemiology to play a causative role in cervical cancer progression, to measurable milestones in the HPV life cycle events such as changes in genome amplification and virion synthesis. Epidemiologists define HPV viral load in qualitative terms with respect to genome copies, both episomal and integrated, in exfoliated cervical cells and tissue biopsies (53). Our results are significant because they support epidemiological studies which first correlated the relationship between cigarette smoke as a cofactor for the development of cervical cancer (38).
A paradoxical observation is that high concentrations of BaP are favorable for enhancing virion synthesis and not genome amplification, but low concentrations of BaP favor increasing genome amplification and not virion synthesis. These results could be explained if we consider two reported studies. First, we have previously demonstrated that under differentiating conditions, tyrosine kinase inhibitors negatively regulate HPV genome amplification (3), suggesting that regulation via growth factor- activated pathways is requisite for genome maintenance. Second, BaP has been shown to induce activation of the epidermal growth factor receptor/RAS/extracellular signal-regulated kinase (EGFR/RAS/ERK)-regulated pathways of proliferation in multiple cell types (6, 9, 22, 23, 26, 45, 46), including possible growth factor-regulated activation of phospholipase C-PKC pathways (44). Based on our data presented, the BaP stimulation of increased virion synthesis is an intriguing observation and could reflect the inherent property of BaP to release intracellular Ca2+ stores, as reported in studies elsewhere (8), which has the additional potential to activate Ca2+-sensitive PKC isoenzymes and associated pathways (37). We routinely add C8:0 to HPV-positive raft cultures to induce DAG-sensitive PKC isoenzymes, in addition to those PKC pathways that are endogenously regulated within the host cells, which are necessary for virion synthesis (36). Thus, BaP may enhance the activation of Ca2+-sensitive PKC pathways, thus boosting PKC activity levels regulated by DAG, resulting in HPV virion synthesis synergistic enhancement of compared with that of controls. We have explored this idea further by performing BaP treatment of raft cultures in the absence of C8:0 and have observed that in the presence of 1 μM BaP, HPV virion synthesis was approximately seven- to eightfold higher than that of controls (Fig. 3A, right panel), whereas for identical experiments performed in the presence of C8:0, virion synthesis was 10-fold higher in BaP-treated raft cultures than in controls (Fig. 3A, left panel).
BaP-mediated regulation of the HPV life cycle may implement the carcinogenic potential of the host tissue. Upon BaP exposure, increased virus production may increase the chances of infecting secondary sites, thus increasing viral persistence, which is thought to be necessary for progression (53). On the other hand, low BaP concentrations correlated with genome amplification, which could potentially result in increased templates from which the E6 and E7 oncogene transcripts are produced. Increased oncogene expression is also directly correlated with increased carcinogenic potential of the tissue (25). Our findings suggest the possibility that BaP-mediated manipulation of multiple HPV life cycle functions, such as the induction of genome copies, the possible stimulation and/or stabilization of late gene transcripts/capsid proteins, and the concomitant virion assembly, may cumulatively determine the risk for cervical cancer progression.
Acknowledgments
We thank N. Christensen for the H31.Ab L1 monoclonal antibody. We also thank Lynn Budgeon for excellent technical assistance.
Research described in this article was supported by Philip Morris USA, Inc., and Philip Morris International.
Footnotes
Published ahead of print on 7 November 2007.
REFERENCES
- 1.Alam, S., E. Sen, H. Brashear, and C. Meyers. 2006. Adeno-associated virus type 2 increases proteosome-dependent degradation of p21WAF1 in a human papillomavirus type 31b-positive cervical carcinoma line. J. Virol. 804927-4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asselineau, D., and M. Prunieras. 1984. Reconstruction of ‘simplified’ skin: control of fabrication. Br. J. Dermatol. 111(Suppl. 27)219-222. [DOI] [PubMed] [Google Scholar]
- 3.Bodily, J. M., S. Alam, and C. Meyers. 2006. Regulation of human papillomavirus type 31 late promoter activation and genome amplification by protein kinase C. Virology 348328-340. [DOI] [PubMed] [Google Scholar]
- 4.Brinton, L. A., and J. F. Fraumeni, Jr. 1986. Epidemiology of uterine cervical cancer. J. Chronic Dis. 391051-1065. [DOI] [PubMed] [Google Scholar]
- 5.Broker, T., and M. Botchan. 1986. Papillomaviruses: retrospectives and prospectives. Cancer Cells 417-36. [Google Scholar]
- 6.Burdick, A. D., J. W. Davis II, K. J. Liu, L. G. Hudson, H. Shi, M. L. Monske, and S. W. Burchiel. 2003. Benzo(a)pyrene quinones increase cell proliferation, generate reactive oxygen species, and transactivate the epidermal growth factor receptor in breast epithelial cells. Cancer Res. 637825-7833. [PubMed] [Google Scholar]
- 7.Castellsague, X., F. X. Bosch, and N. Munoz. 2002. Environmental co-factors in HPV carcinogenesis. Virus Res. 89191-199. [DOI] [PubMed] [Google Scholar]
- 8.Davila, D. R., D. P. Davis, K. Campbell, J. C. Cambier, L. A. Zigmond, and S. W. Burchiel. 1995. Role of alterations in Ca(2+)-associated signaling pathways in the immunotoxicity of polycyclic aromatic hydrocarbons. J. Toxicol. Environ. Health 45101-126. [DOI] [PubMed] [Google Scholar]
- 9.De Buck, S. S., P. Augustijns, and C. P. Muller. 2005. Specific antibody modulates absorptive transport and metabolic activation of benzo[a]pyrene across Caco-2 monolayers. J. Pharmacol. Exp. Ther. 313640-646. [DOI] [PubMed] [Google Scholar]
- 10.Durst, M., L. Gissmann, H. Ikenberg, and H. zur Hausen. 1983. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc. Natl. Acad. Sci. USA 803812-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang, L., C. Meyers, L. R. Budgeon, and M. K. Howett. 2006. Induction of productive human papillomavirus type 11 life cycle in epithelial cells grown in organotypic raft cultures. Virology 34728-35. [DOI] [PubMed] [Google Scholar]
- 12.Fischer, S., B. Spiegelhalder, J. Eisenbarth, and R. Preussmann. 1990. Investigations on the origin of tobacco-specific nitrosamines in mainstream smoke of cigarettes. Carcinogenesis 11723-730. [DOI] [PubMed] [Google Scholar]
- 13.Frattini, M. G., H. B. Lim, and L. A. Laimins. 1996. In vitro synthesis of oncogenic human papillomaviruses requires episomal genomes for differentiation-dependent late expression. Proc. Natl. Acad. Sci. USA 933062-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuchs, E. 1990. Epidermal differentiation: the bare essentials. J. Cell Biol. 1112807-2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garrett, L. R., N. Perez-Reyes, P. P. Smith, and J. K. McDougall. 1993. Interaction of HPV-18 and nitrosomethylurea in the induction of squamous cell carcinoma. Carcinogenesis 14329-332. [DOI] [PubMed] [Google Scholar]
- 16.Hecht, S. S., A. Abbaspour, and D. Hoffman. 1988. A study of tobacco carcinogenesis. XLII. Bioassay in A/J. mice of some structural analogues of tobacco-specific nitrosamines. Cancer Lett. 42141-145. [DOI] [PubMed] [Google Scholar]
- 17.Hellberg, D., S. Nilsson, N. J. Haley, D. Hoffman, and E. Wynder. 1988. Smoking and cervical intraepithelial neoplasia: nicotine and cotinine in serum and cervical mucus in smokers and nonsmokers. Am. J. Obstet. Gynecol. 158910-913. [DOI] [PubMed] [Google Scholar]
- 18.Herrington, C. S. 1995. Human papillomaviruses and cervical neoplasia. II. Interaction of HPV with other factors. J. Clin. Pathol. 481-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holly, E. A., R. D. Cress, D. K. Ahn, D. A. Aston, J. J. Kristiansen, R. Wu, and J. S. Felton. 1993. Detection of mutagens in cervical mucus in smokers and nonsmokers. Cancer Epidemiol. Biomarkers Prev. 2223-228. [PubMed] [Google Scholar]
- 20.Howley, P. M. 1990. Papillomavirinae and their replication, p. 1625-1650. In B. N. Fields, D. M. Knipe, and R. M. e. a. Chanock (ed.), Fields virology. Raven Press, New York, NY.
- 21.Hurlin, P. J., P. Kaur, P. P. Smith, N. Perez-Reyes, R. A. Blanton, and J. K. McDougall. 1991. Progression of human papillomavirus type 18-immortalized human keratinocytes to a malignant phenotype. Proc. Natl. Acad. Sci. USA 88570-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jyonouchi, H., S. Sun, K. Iijima, M. Wang, and S. S. Hecht. 1999. Effects of anti-7,8-dihydroxy-9,10-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene on human small airway epithelial cells and the protective effects of myo-inositol. Carcinogenesis 20139-145. [DOI] [PubMed] [Google Scholar]
- 23.Ko, C. B., S. J. Kim, C. Park, B. R. Kim, C. H. Shin, S. Choi, S. Y. Chung, J. H. Noh, J. H. Jeun, N. S. Kim, and R. Park. 2004. Benzo(a) pyrene-induced apoptotic death of mouse hepatoma Hepa1c1c7 cells via activation of intrinsic caspase cascade and mitochondrial dysfunction. Toxicology 19935-46. [DOI] [PubMed] [Google Scholar]
- 24.Lambert, B., I. Berndtsson, J. Lindsten, M. Nordenskjold, S. Soderhall, B. Holmstedt, L. Palmer, B. Jernstrom, and L. Marsk. 1982. Smoking and sister chromatid exchange. Prog. Clin. Biol Res. 109401-414. [PubMed] [Google Scholar]
- 25.Lambert, P. F. 1991. Papillomavirus DNA replication. J. Virol. 653417-3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li, J., H. Chen, Q. Ke, Z. Feng, M. S. Tang, B. Liu, S. Amin, M. Costa, and C. Huang. 2004. Differential effects of polycyclic aromatic hydrocarbons on transactivation of AP-1 and NF-kappaB in mouse epidermal cl41 cells. Mol. Carcinog. 40104-115. [DOI] [PubMed] [Google Scholar]
- 27.Li, S. L., M. S. Kim, H. M. Cherrick, J. Doniger, and N. H. Park. 1992. Sequential combined tumorigenic effect of HPV-16 and chemical carcinogens. Carcinogenesis 131981-1987. [DOI] [PubMed] [Google Scholar]
- 28.Magee, P. N. 1989. The experimental basis for the role of nitroso compounds in human cancer. Cancer Surv. 8207-239. [PubMed] [Google Scholar]
- 29.McCann, M. F., D. E. Irwin, L. A. Walton, B. S. Hulka, J. L. Morton, and C. M. Axelrad. 1992. Nicotine and cotinine in the cervical mucus of smokers, passive smokers, and nonsmokers. Cancer Epidemiol. Biomarkers. Prev. 1125-129. [PubMed] [Google Scholar]
- 30.McLaughlin-Drubin, M. E., N. D. Christensen, and C. Meyers. 2004. Propagation, infection, and neutralization of authentic HPV16 virus. Virology 322213-219. [DOI] [PubMed] [Google Scholar]
- 31.Melikian, A. A., P. Sun, B. Prokopczyk, K. El-Bayoumy, D. Hoffmann, X. Wang, and S. Waggoner. 1999. Identification of benzo[a]pyrene metabolites in cervical mucus and DNA adducts in cervical tissues in humans by gas chromatography-mass spectrometry. Cancer Lett. 146127-134. [DOI] [PubMed] [Google Scholar]
- 32.Meyers, C. 2002. Epithelial cell culture: three-dimensional cervical system, p. 263-271. In A. Atala and R. P. Lanza (ed.), Methods of tissue engineering. Academic Press, San Diego, CA.
- 33.Meyers, C. 1996. Organotypic (raft) epithelial tissue culture system for the differentiation-dependent replication of papillomavirus. Methods Cell Sci. 181-10. [Google Scholar]
- 34.Meyers, C., S. Alam, M. Mane, and P. L. Hermonat. 2001. Altered biology of adeno-associated virus type 2 and human papillomavirus during dual infection of natural host tissue. Virology 28730-39. [DOI] [PubMed] [Google Scholar]
- 35.Meyers, C., J. L. Bromberg-White, J. Zhang, M. E. Kaupas, J. T. Bryan, R. S. Lowe, and K. U. Jansen. 2002. Infectious virions produced from a human papillomavirus type 18/16 genomic DNA chimera. J. Virol. 764723-4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyers, C., M. G. Frattini, J. B. Hudson, and L. A. Laimins. 1992. Biosynthesis of human papillomavirus from a continuous cell line upon epithelial differentiation. Science 257971-973. [DOI] [PubMed] [Google Scholar]
- 37.Michie, A. M., and R. Nakagawa. 2005. The link between PKCalpha regulation and cellular transformation. Immunol. Lett. 96155-162. [DOI] [PubMed] [Google Scholar]
- 38.Nischan, P., K. Ebeling, and C. Schindler. 1988. Smoking and invasive cervical cancer risk. Results from a case-control study. Am. J. Epidemiol. 12874-77. [DOI] [PubMed] [Google Scholar]
- 39.Ozbun, M. A., and C. Meyers. 1998. Human papillomavirus type 31b E1 and E2 transcript expression correlates with vegetative viral genome amplification. Virology 248218-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ozbun, M. A., and C. Meyers. 1996. Transforming growth factor β1 induces differentiation in human papillomavirus-positive keratinocytes. J. Virol. 705437-5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pecoraro, G., M. Lee, D. Morgan, and V. Defendi. 1991. Evolution of in vitro transformation and tumorigenesis of HPV16 and HPV18 immortalized primary cervical epithelial cells. Am. J. Pathol. 1381-8. [PMC free article] [PubMed] [Google Scholar]
- 42.Pfister, H. 1987. Human papillomaviruses and genital cancer. Adv. Cancer Res. 48113-147. [DOI] [PubMed] [Google Scholar]
- 43.Prokopczyk, B., J. E. Cox, D. Hoffmann, and S. E. Waggoner. 1997. Identification of tobacco-specific carcinogen in the cervical mucus of smokers and nonsmokers. J. Natl. Cancer Inst. 89868-873. [DOI] [PubMed] [Google Scholar]
- 44.Puga, A., A. Hoffer, S. Zhou, J. M. Bohm, G. D. Leikauf, and H. G. Shertzer. 1997. Sustained increase in intracellular free calcium and activation of cyclooxygenase-2 expression in mouse hepatoma cells treated with dioxin. Biochem. Pharmacol. 541287-1296. [DOI] [PubMed] [Google Scholar]
- 45.Rummel, A. M., J. E. Trosko, M. R. Wilson, and B. L. Upham. 1999. Polycyclic aromatic hydrocarbons with bay-like regions inhibited gap junctional intercellular communication and stimulated MAPK activity. Toxicol. Sci. 49232-240. [DOI] [PubMed] [Google Scholar]
- 46.Sadhu, D. N., M. Merchant, S. H. Safe, and K. S. Ramos. 1993. Modulation of protooncogene expression in rat aortic smooth muscle cells by benzo[a]pyrene. Arch. Biochem. Biophys. 300124-131. [DOI] [PubMed] [Google Scholar]
- 47.Sasson, I. M., N. J. Haley, D. Hoffmann, E. L. Wynder, D. Hellberg, and S. Nilsson. 1985. Cigarette smoking and neoplasia of the uterine cervix: smoke constituents in cervical mucus. N. Engl. J. Med. 312315-316. [DOI] [PubMed] [Google Scholar]
- 48.Schiffman, M. H., N. J. Haley, J. S. Felton, A. W. Andrews, R. A. Kaslow, W. D. Lancaster, R. J. Kurman, L. A. Brinton, L. B. Lannom, and D. Hoffmann. 1987. Biochemical epidemiology of cervical neoplasia: measuring cigarette smoke constituents in the cervix. Cancer Res. 473886-3888. [PubMed] [Google Scholar]
- 49.Schoell, W. M., M. F. Janicek, and R. Mirhashemi. 1999. Epidemiology and biology of cervical cancer. Semin. Surg. Oncol. 16203-211. [DOI] [PubMed] [Google Scholar]
- 50.Stockwell, H. G., and G. H. Lyman. 1987. Cigarette smoking and the risk of female reproductive cancer. Am. J. Obstet. Gynecol. 15735-40. [DOI] [PubMed] [Google Scholar]
- 51.Taichman, L. P., and R. F. LaPorta. 1986. The expression of papillomaviruses in epithelial cells, p. 109-139. In N. Salzman and P. M. Howley (ed.), The papillomaviruses, vol. 2. Plenum Press, New York, NY. [Google Scholar]
- 52.Winkelstein, W., Jr. 1977. Smoking and cancer of the uterine cervix: hypothesis. Am. J. Epidemiol. 106257-259. [DOI] [PubMed] [Google Scholar]
- 53.Woodman, C. B., S. I. Collins, and L. S. Young. 2007. The natural history of cervical HPV infection: unresolved issues. Nat. Rev. Cancer 711-22. [DOI] [PubMed] [Google Scholar]
- 54.zur Hausen, H. 1982. Human genital cancer: synergism between two virus infections or synergism between a virus infection and initiating events? Lancet 21370-1372. [DOI] [PubMed] [Google Scholar]
- 55.zur Hausen, H., and A. Schneider. 1987. The role of papillomaviruses in human anogenital cancer, p. 245-263. In N. Salzman and P. M. Howley (ed.), The papillomaviruses, vol. 2. Plenum Press, New York, NY. [Google Scholar]