Abstract
Viral therapy of cancer (viral oncolysis) is dependent on selective destruction of the tumor tissue compared with healthy tissues. Several factors, including receptor expression, extracellular components, and intracellular mechanisms, may influence viral oncolysis. In the present work, we studied the potential oncolytic activity of herpes simplex virus type 1 (HSV-1), using an organ culture system derived from colon carcinoma and healthy colon tissues of mouse and human origin. HSV-1 infected normal colons ex vivo at a very low efficiency, in contrast to high-efficiency infection of colon carcinoma tissue. In contrast, adenoviral and lentiviral vectors infected both tissues equally well. To investigate the mechanisms underlying the preferential affinity of HSV-1 for the carcinoma tissue, intracellular and extracellular factors were investigated. Two extracellular components, collagen and mucin molecules, were found to restrict HSV-1 infectivity in the healthy colon. The mucin layer of the healthy colon binds to HSV-1 and thereby blocks viral interaction with the epithelial cells of the tissue. In contrast, colon carcinomas express small amounts of collagen and mucin molecules and are thus permissive to HSV-1 infection. In agreement with the ex vivo system, HSV-1 injected into a mouse colon carcinoma in vivo significantly reduced the volume of the tumor. In conclusion, we describe a novel mechanism of viral selectivity for malignant tissues that is based on variance of the extracellular matrix between tumor and healthy tissues. These insights may facilitate new approaches to the application of HSV-1 as an oncolytic virus.
The application of oncolytic viruses in cancer therapy is an emerging experimental approach that still requires a better understanding of viral interactions with tumor tissue compared to those with healthy tissue (44). Adenovirus type 5 (adenovirus) and herpes simplex virus type 1 (HSV-1) have been studied extensively as potential oncolytic viruses, and various genetic manipulations were introduced to improve their selective oncolytic activities. First, certain viral genes were deleted in order to facilitate preferential replication of the virus in the tumor cell compared to the normal cell (3, 26, 39, 52). Second, these viruses were applied as vectors to express cytotoxic genes or cytokines under the control of a tumor-specific promoter (32, 52, 54). Third, genetic modifications were introduced in order to redirect receptor usage of the virus from the native receptor to tumor-specific cell surface proteins (15, 33, 51). Studies along these lines are usually carried out with transformed cells in culture, and if such studies are successful, mouse tumor models are applied to determine the selective oncolytic activity of the virus. However, determination of the selective killing activity of cultured tumor cells by the virus is not optimal, as the normal counterpart of the carcinoma cell is usually not available in culture. In addition, the tropism of these oncolytic viruses cannot be studied with cultured cells, as these viruses use receptors that are expressed ubiquitously on most cell types in culture. Infection by adenovirus is initially mediated by attachment through its capsid fiber protein to the cell surface receptor coxsackievirus-adenovirus receptor (CAR). Subsequently, interactions of the adenovirus penton base protein with αv-integrin trigger endocytosis of the virus (30). In contrast, several cell surface molecules serve as receptors for HSV-1 entry into cells. While heparan sulfate mediates the attachment of HSV-1 virions to cells, the repertoire of HSV-1 entry receptors includes herpesvirus entry mediator, nectin-1, nectin-2, and 3-O-sulfated heparan sulfate (45). Application of animal models to studies related to the tissue tropism of these human viruses is also complicated due to species specificity, immune response mechanisms, and circulating factors that interfere with the infection in vivo.
To elucidate factors that determine viral tropism in solid tissues, we developed an organ culture system from mouse and human tissues. Such a system may serve as a means to study viral tropism in the context of a three-dimensional tissue structure (8, 14). Organ cultures derived from normal liver tissue and from ovarian and breast carcinomas have also been applied to the study of gene transfer by viral vectors (21, 41, 46, 47). The organ culture method described here is unique because it allows direct comparison of viral infection of tumor tissue versus the corresponding normal tissue (22). We employed this system as a platform to study viral tropism for colon carcinomas. Colon carcinoma is a relatively common and lethal malignancy affecting the adult population (29), and colorectal carcinoma often requires extensive adjuvant therapy before surgical removal. In this context, a viral oncolytic gene therapy approach for colon carcinoma is especially attractive, as the primary tumor is directly accessible to local administration of the vector.
Different mechanisms, such as physical barriers (tight junctions and mucin secretion) and the immune system, protect the normal colon from infection by most microorganisms, including viruses (42).
We hypothesized that unlike the normal colon, the carcinoma tissue may be susceptible to preferential infection by several viruses. The rationale for a possible differential susceptibility of malignant tissues to viral infection may include an altered cell cycle, extracellular matrix modifications, such as diminished mucin-2 secretion, and changes in cell receptor expression and polarity (16, 19, 27).
In the present study, we describe the molecular interactions of HSV-1 with the normal colon and respective malignant tissues of mouse and human origin. In contrast to nonselective infection of the malignant and normal tissues by adenovirus and lentivirus, we found that HSV-1 specifically infects carcinoma tissues. We further show that this cancer-specific tropism is due mainly to a decrease of extracellular matrix components, including collagen and mucin molecules, compared with those in the normal colon. Furthermore, direct injection of HSV-1 into colon carcinoma tissue combined with ganciclovir treatment in a mouse model resulted in a specific inhibition of tumor growth. Taken together, these findings define, for the first time, the molecular interactions of HSV-1 with the normal colon and corresponding carcinoma tissue and set the background for further development of this oncolytic virus as a colon carcinoma-specific agent.
MATERIALS AND METHODS
Cells.
Cell lines were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum (FCS), 1% minimal essential medium vitamin solution (Biological Industries, Beit Haemek, Israel), penicillin (100 U/ml), and streptomycin sulfate (100 μg/ml) at 37°C in 5% CO2. The cell lines used in this work were the BALB/c mouse colon carcinoma line CT26, obtained from Y. Keisari, Tel Aviv University, human kidney 293T cells, and monkey kidney Vero cells.
Vectors.
HSV 17+/pR20.5/5 contains the β-galactosidase (β-Gal) gene under the control of the Rous sarcoma virus (RSV) promoter and the green fluorescent protein (GFP) gene under the control of the cytomegalovirus (CMV) promoter, and it is referred to as HSV-(RSVβ-gal). Both genes were inserted at the Us5 gene locus and are expressed constitutively. It was previously shown that inactivation of Us5 does not otherwise affect virus growth in vitro or virulence or the kinetics of latency in vivo (50).
HSV-1/VP26-GFP is a recombinant HSV-1 strain 17 that expresses the capsid protein VP26 as a fusion with GFP (18). The virus was provided by D. Knebel-Mörsdorf (Max-Planck-Institute for Neurological Research, University of Cologne, Cologne, Germany).
HSV-1 was propagated in Vero cells, harvested from the cell pellet, purified on a 10% sucrose cushion, and titrated on Vero cells (1).
Adenovirus Ad5CMVlacZ, containing the β-Gal gene under the control of the immediate-early CMV promoter and deleted of the E1A gene, is a replication-defective virus propagated in 293 cells that express E1A (36). The virus was propagated, purified, and concentrated as described before and then titrated on Vero cells (1).
Lentiviral pseudotype vectors were produced by cotransfection of 293T cells essentially as described previously (34), using a packaging plasmid (pCMVΔR8.9), a plasmid encoding the envelope glycoprotein of vesicular stomatitis virus (pMDG), and the transfer plasmid pHR-NLS-LacZ, encoding β-Gal under the control of the phosphoglycerate kinase promoter. Infectious lentiviruses were harvested at 48 and 72 h posttransfection, filtered through 0.45-μm-pore-size filters, propagated, purified, and concentrated as described before, and titrated on 293T cells (1).
All viruses were purified by sedimentation through a 10% sucrose cushion for 2 h at 4°C. The virus pellet was suspended in phosphate-buffered saline (PBS) and kept at −70°C.
Tissues.
Human tissues were obtained within 2 h of surgery (under approval 20-01/08/03 of the Hadassah Hospital IRB committee). For preparation of organ cultures from mouse orthotopic colon tumors, BALB/c mice were anesthetized with ketamine-xylazine and injected with colon carcinoma CT26 cells through the rectum into the colon mucosa and submucosa (2 × 106 cells/50 μl PBS) or subcutaneously (s.c.) into the low lateral side of the back (1 × 105 cells/100 μl PBS). Orthotopic colon tumors usually developed after 3 weeks, and s.c. tumors developed in 10 days. Mice were sacrificed, and the normal colon and adjacent tumor tissues were harvested and prepared for ex vivo organ culture.
Organ cultures.
Normal and tumor tissues of human and mouse (BALB/c male mice [6 to 7 weeks old]) origin were washed five times in supplemented hormonal epithelial medium (SHEM) (the normal mouse colon lumen was first opened with scissors, and tissues were cut in a microtome [TC-2 tissue sectioner; Sorvall Corp.] into 500-μm-thick slices). Tissues were incubated at 37°C in 5% CO2 (5 slices/well in a 48-well plate) (14, 22) in SHEM. The organ culture medium was SHEM (5), consisting of a 1:1 mixture of Dulbecco's modified Eagle's medium and Ham's F12 medium with 5% FCS, 10 mM HEPES, 0.5% dimethyl sulfoxide, 0.5 μg/ml hydrocortisone, 1% minimal essential medium vitamin solution (Biological Industries, Beit Haemek, Israel), 5 μg/ml insulin, 5 μg/ml transferrin, 5 ng/ml selenium, 2 ng/ml epidermal growth factor, 100 U/ml penicillin, 100 μg/ml streptomycin, 15 μg/ml gentamicin, and 15 μg/ml ciprofloxacin (Ciproxin).
Infection of organ cultures.
Organ culture tissues were infected with viruses in 300 μl SHEM/well in a 48-well plate. At 2 hours postinfection, 5% FCS was added to the medium, and tissues were incubated for a further 22 h at 37°C in 5% CO2 (22).
Tumor volume.
Mice were inoculated s.c. with CT26 cells (105 cells/100 μl PBS) in the low lateral side of the back. Local tumor growth was determined by measuring three mutually orthogonal tumor diameters with a caliper. The volume (V) of the tumors was calculated using the formula V = D1 × D2 × D3 × π/6, where D1, D2, and D3 represent the three mutually orthogonal tumor diameters (35).
MTT cell viability assay.
The MTT cell viability assay is based upon the ability of mitochondrial dehydrogenase to convert MTT substrate (3-[4,5-dimethylthiazol-2-yl-2,5-diphenyltetrazolium bromide]) (Sigma Corp.) into a blue formazan product. Tissue slices were incubated with the MTT substrate for 60 min at 37°C, followed by the addition of 500 μl ethanol to dissolve the product colored crystals. Samples were read using an enzyme-linked immunosorbent assay plate reader (Organon Teknika, The Netherlands) at a wavelength of 540 nm in reference to 650 nm (55). For protein determination, the same organ slices (ethanol extracted) were dissolved with lysis buffer (200 μl PBS, 0.1% Triton X-100), freeze-thawed twice, and sonicated, and the extract was clarified by centrifugation (3,000 × g for 10 min). The protein content of extracts was determined by the Bradford assay (6). Cell viability was determined after correction for the protein content of extracts. The amount of color produced is proportional to the number of viable cells.
X-Gal staining of tissues for in situ β-Gal detection.
Colon organ cultures were fixed for 10 min in 0.2% glutaraldehyde, 2% formaldehyde, and 2 mM MgCl2 in PBS (pH 7.4), washed in PBS, and incubated for 1 h at 37°C in 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 2 mM MgCl2, and 20 mg X-Gal substrate (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside), followed by fixation with 4% formaldehyde (7).
Beta-Glo assay for β-Gal quantitative analysis.
Infected organ culture tissues were extracted with lysis buffer (100 μl PBS, 0.1% Triton X-100), freeze-thawed three times, sonicated, and homogenized. The extract was clarified by centrifugation (3,000 × g for 10 min). Supernatant extracts were added to Beta-Glo substrate (20 μl) according to the manufacturer's instructions (Promega Corp.). Beta-Glo serves as a substrate for β-Gal, and the product serves as a substrate for the luciferase enzyme present in the assay mixture. Luciferase activity was examined with a luminometer (Mithras-LB940; Berthold Corp.). β-Gal enzyme specific activity was determined by correction for the protein content of the organ cultures by the Bradford assay (6).
Histological analysis.
Colon tissues were fixed in buffered formaldehyde (4%) and embedded in paraffin. Five-micrometer sections were stained with nuclear fast red stain, hematoxylin and eosin, or Masson Trichrome (Bio-Optica Co.) (24).
Mucin staining.
We used laser scanning confocal microscopy to examine mucin by incubating colon tissue slices with 20 μg/ml of fluorescent tetramethyl rhodamine isocyanate conjugated to lectin from Ulex europaeus (Sigma Corp.) for 30 min at room temperature for mucin-like glycoprotein staining as described previously (17).
Preparation of mucin.
Mucin was prepared essentially as described by Chen et al. (11). In brief, mouse intestines were harvested, cut into wide sections, and washed 10 times with SHEM, and the mucin layer was scraped with a scalpel from the lumen and suspended in PBS. The turbid solution was vortexed for 10 min and then centrifuged at 27,000 × g for 25 min at 4°C. The upper clear supernatant was collected and frozen at −70°C.
Statistical analyses.
All data are presented as means ± standard deviations (SD). Statistical differences among groups were assessed with a two-tailed Student t test. P values of <0.05 were considered significant and are marked in the figures by asterisks.
RESULTS
Organ cultures from normal and malignant human colon tissues.
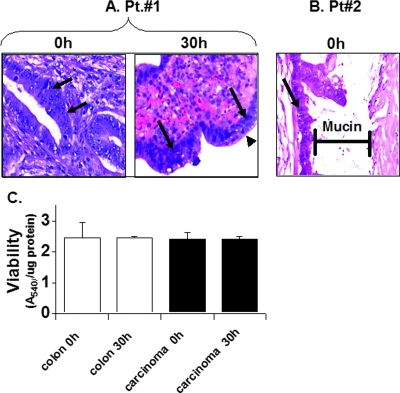
Organ cultures were prepared from human colon tissues, maintaining the interactions of the epithelial and mesenchymal layers in their native spatial organization. To ensure the viability of these organ cultures over the duration of the experiments, histology analysis was performed both immediately after preparation of organ cultures and after 30 h of ex vivo incubation (Fig. 1A). A similar morphological appearance of colon carcinoma tissue at zero time and after 30 h in culture was noted. Moreover, close examination of the epithelial layer revealed mitotic cells after 30 h of incubation ex vivo (Fig. 1A, arrowhead), indicating the viability of these cultures. The colon carcinoma tissue specimen presented in Fig. 1B is a mucin-secreting adenocarcinoma representative of about 10% of all human colon carcinomas, which secrete mucin like normal colon tissue (9). Mucin is evident by typical bluish gray staining of the acellular material. To further validate the viability of the organ cultures, the MTT assay was applied. The results shown in Fig. 1C demonstrate stable dehydrogenase enzyme-specific activity in both the carcinoma and normal human colon tissues, indicating tissue viability during the first 30 h of organ culture, a longer duration than that previously described for the mouse colon and colon carcinoma (22). Thus, colon organ culture of both human and mouse origin is a valid system with which to study ex vivo viral infection and gene transfer.
FIG. 1.
Viability of human colon tissues in organ culture. Human normal colon and colon carcinoma tissues were obtained from the surgery room in accordance with approval no. 20-01/08/03 of the institutional review board of Hadassah Hospital. Organ cultures were prepared as described in Materials and Methods and incubated for the indicated times. (A and B) Histological sections (5 μm) were prepared from organ cultures of human colon carcinoma tissues immediately after obtaining them from the surgery room and at 0 h and 30 h postculture. The slides were stained with hematoxylin and eosin. A mucin-producing human carcinoma is shown in panel B, displaying the mucin layer. Mucin is evident by typical bluish gray acellular material. Carcinoma cells are indicated by arrows, and mitotic cells are indicated by an arrowhead. (C) An MTT viability assay was carried out and corrected for the protein content of the human tissue as described in Materials and Methods. Pretreatment of the tissues with 70% ethanol resulted in a loss of viability and consequently eliminated MTT activity. Data are means ± SD (n = 6).
HSV-1 preferentially infects human and mouse colon carcinomas compared to normal colon tissues.
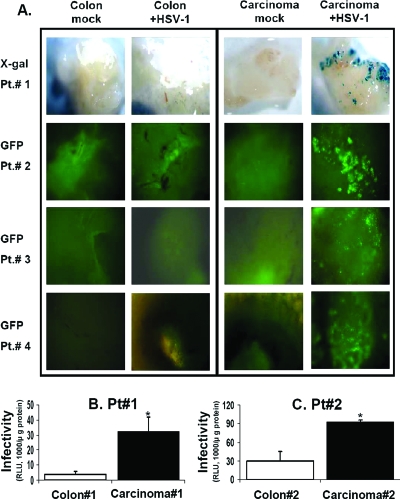
To compare HSV-1 tropism for the normal human colon with HSV-1 tropism for colon carcinomas, tissues were prepared for organ culture and infected with HSV-1 for 24 h. Two reporter genes, those encoding GFP and β-Gal, carried by the HSV-1 vector, were used simultaneously to observe and quantify infection. Since the human tumor tissues of different donors varied with respect to viability and the relative positions of the tumor and the adjacent normal tissue, we repeated the experiment with tissues from 18 patients. For 16 of the 18 patients, there was strong GFP and β-Gal expression in the tumor, while expression in the normal colon was minimal. Representative results of viral infection of tissues from 4 of the 16 patients (patients 1 to 4) are presented in Fig. 2A. It is clear that HSV-1 preferentially infected the colon carcinoma tissues compared to the normal colon. The qualitative observation was supported by a quantitative analysis of β-Gal expression, determined with crude extracts derived from the infected organ cultures (Fig. 2B and C). Reporter enzyme activity was corrected for the protein content of the individual tissue in order to reduce variability due to differences in the sizes of the organ culture slices. The specific activity of the β-Gal enzyme was significantly higher (3- to 10-fold) in extracts derived from carcinomas than in those derived from normal colon tissues from two representative patients. Histological analysis of the infected colon cancer tissue after X-Gal staining indicated infection of the carcinoma cells.
FIG. 2.
HSV-1 preferentially infects human colon carcinoma tissues compared to the normal colon. Organ cultures were prepared from human normal colon and colon carcinoma tissues of 18 patients as described in the legend to Fig. 1, and infection was carried out for 24 h with HSV-1 (2 × 106 IU/well), as described in Materials and Methods. (A) Organ cultures were taken at 24 h postinfection, examined for β-Gal expression by X-Gal staining in situ (patient 1) and for GFP expression (patients 2, 3, and 4), and photographed at a magnification of ×100. Results of a representative experiment with tissues from 4 of 16 patients examined are presented. (B and C) Specific activity of β-Gal enzyme in organ cultures at 24 h postinfection was measured using a Beta-Glo kit (Promega) and normalized for protein content as described in Materials and Methods. Data reflect means ± SD (n = 6 replicates).
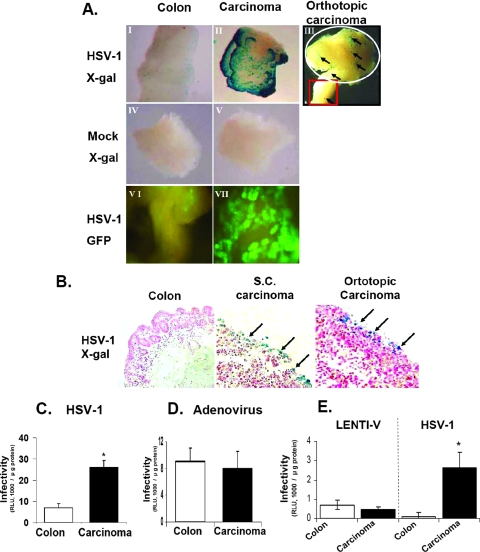
To elucidate the mechanisms responsible for the remarkable selectivity of HSV-1 for colon cancer, as shown in Fig. 2, an experimental system of higher consistency was required. To this end, we applied an organ culture system of mouse colon tissues. Colon carcinoma tissues were obtained after injection of CT26 mouse cells, either directly into the colon to create an orthotopic tumor or s.c. After tumors developed, organ cultures were prepared. Similar to the human setting, the results presented in Fig. 3A (panels I to III, VI, and VII) indicate a significantly higher level of infection of HSV-1 in the s.c. and orthotopic carcinoma organ cultures than in the normal colon organ cultures. Interestingly, infection of an organ culture encompassing the border of the orthotopic carcinoma and colon tissues resulted in enhanced expression of the β-Gal reporter gene in the area of the tumor (Fig. 3A, panel III, white circle).
FIG. 3.
HSV-1 preferentially infects mouse colon carcinoma tissue compared to the normal colon. Orthotopic and s.c. colon carcinoma tissues and normal colon tissues of BALB/c mice were prepared for organ culture as described in Materials and Methods. Tissues were infected for 24 h with HSV-1 (2 × 106 IU/well) as described in Materials and Methods. (A) (I to V) Mock-infected and infected organ cultures were taken for in situ examination of β-Gal expression by X-Gal staining. Blue spots represent infected cells and are indicated by arrows. (VI and VII) GFP expression in situ. Magnification, ×100. (B) HSV-1-infected organ cultures were stained with X-Gal and taken for paraffin sections. Histological sections (5 μm) were prepared from organ cultures of normal and tumor tissues and stained with nuclear fast red stain as described in Materials and Methods. Blue spots represent infected cells and are indicated by arrows. Magnification, ×100. (C, D, and E) Organ cultures were infected for 24 h with HSV-1 or adenovirus (orthotopic organ cultures) or with HSV-1 and lentivirus (s.c. organ cultures), all at 2 × 106 IU/well, as described in Materials and Methods. Infected organ cultures were analyzed for β-Gal enzyme specific activity by the Beta-Glo assay as described in Materials and Methods. Data reflect means ± SD (n = 6 replicates). Results are representative of three independent experiments.
Histological analysis of the infected tissues, with staining by X-Gal, further confirmed the finding that the normal colon, in contrast to carcinoma tissue, is resistant to HSV-1 infection (Fig. 3B).
It should be noted, however, that HSV-1 infection of the carcinoma tissue in organ cultures for 24 h was localized to the surface of the tissue, with poor penetration to the inner layers.
To further validate the preferential infection of HSV-1 in carcinomas in both murine and human tissues, we compared it with adenovirus, which is known to infect carcinomas and the normal colon equally well, and with lentivirus (22). Quantitative and qualitative analyses of β-Gal enzyme expression in the infected tissues recapitulated the unique tropism of HSV-1 for the malignant colon, in contrast with both adenovirus and lentivirus vector. While adenovirus and lentivirus infected the normal and carcinoma tissues of mice (Fig. 3D and E) and humans (data not shown) equally well, HSV-1 preferentially infected the carcinoma tissue (Fig. 2A and 3C and E). These observations indicate that HSV-1 is the preferential viral vector for infection of colon carcinomas compared to other examined viral vectors and that, in contrast to other vectors, it does not adversely affect the normal colon tissue.
Determinants responsible for resistance of normal colon to HSV-1 infection.
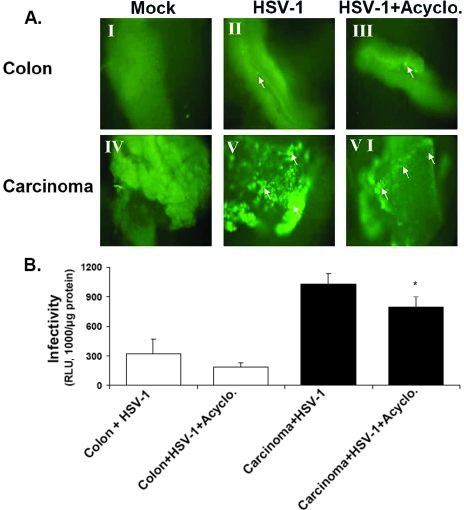
The relative resistance of normal colon tissues to HSV-1 infection could be a consequence of any of three restriction levels. First, a specific intracellular block to the replication cycle of the virus in the normal colon may exist. Second, different levels of a receptor(s) may facilitate viral entry. Third, extracellular restriction may exclude the virus from the cellular receptors. To examine the first possibility, we treated the colon and carcinoma tissues with acyclovir at a concentration (20 μM) that completely blocks HSV-1 DNA replication in both organ culture and cell lines (data not shown). Acyclovir does not affect tissue viability and does not interfere with β-Gal expression by the incoming parental DNA, as the RSV constitutive promoter acts independently of virus DNA replication (50). Both the qualitative and quantitative results indicated a relatively mild inhibition of β-Gal expression by acyclovir in the carcinoma tissue (Fig. 4A and B). It appears from these results that replication of viral DNA during the infection time (24 h) is minimal, suggesting that earlier steps of infection are likely to be involved in the preferential infection of carcinoma tissue.
FIG. 4.
The preferential infection of colon carcinoma tissues by HSV-1 is not due to enhanced viral replication. Colon carcinomas (s.c.) and normal colons of BALB/c mice were prepared for organ culture and infected with HSV-1 as described in Materials and Methods. (A) GFP expression in situ. Organ cultures were infected for 24 h with HSV-1 (2 × 106 IU/well), without acyclovir (II and V) or with acyclovir (20 μM) (III and VI). Acyclovir at 20 μM was found to prevent HSV-1 replication and progeny virus in both Vero cells and colon tissues (data not shown). Green spots represent infected cells and are indicated by arrows. Magnification, ×100. Acyclo., acyclovir. (B) Quantitative analysis of infection. Organ cultures at 24 h postinfection were analyzed for β-Gal enzyme by using the Beta-Glo assay and normalized for protein content as described in Materials and Methods. Data reflect means ± SD (n = 6 replicates). Results are representative of three independent experiments. The asterisk indicates that infection of colon carcinomas treated with acyclovir was significantly enhanced (P < 0.05) compared to that of acyclovir-treated normal colons.
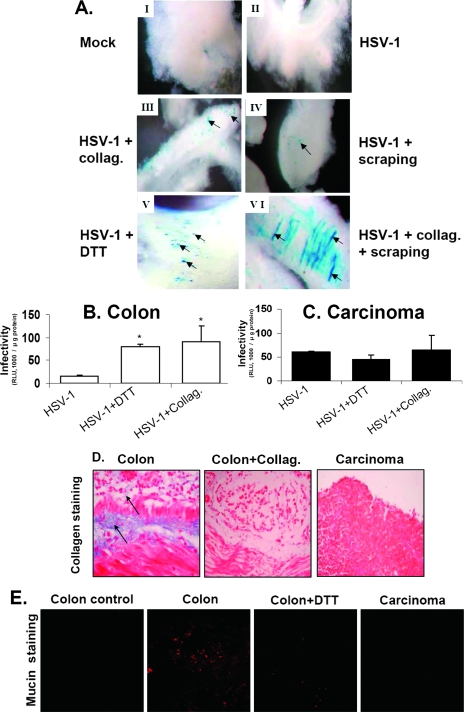
We next investigated extracellular barriers that may interfere with HSV-1 infectivity in normal colon tissue. We focused on collagen, a major constituent of the extracellular matrix, and on the mucin layer of colon epithelium, which consists mostly of the mucin-2 glycoprotein (2, 9, 27). It should be noted that the mucin layer is maintained in colon organ cultures, as shown directly by specific mucin staining in Fig. 5E. Pretreatment of normal colon tissue with collagenase significantly enhanced HSV-1 infectivity (Fig. 5A, panel III). Removal of the mucin layer by mechanical scraping of the epithelial aspect of the colon or by treatment of the tissue with a reducing agent (dithiothreitol [DTT]) known to disrupt the mucin layer (4) also increased HSV-1 infection (Fig. 5A, panels IV and V). Finally, partial digestion of the colon tissue with collagenase followed by scraping of the mucin layer appeared to be most effective at stimulating HSV-1 infection (Fig. 5A, panel VI). To discard the possibility that increased HSV-1 infectivity following DTT and scraping treatments of the normal colon was due to disruption of epithelial integrity, we treated the colon with the chelating agent EGTA, which is known to break epithelial cell-cell connections (25). No effect of EGTA was observed (data not shown).
FIG. 5.
Extracellular matrix components in colon tissue inhibit HSV-1 infection. (A) Organ cultures were prepared from normal colons of BALB/c mice as described in Materials and Methods. (I) Control, mock-infected normal colon. (II) Tissue infected with HSV-1. (III) Tissue slices were pretreated for 40 min at 37°C with collagenase 2 (50 μg/ml; Worthington Biochemical Corp.). (IV) Mucin layer was removed from the tissue by scraping with a scalpel. (V) Tissue slices were preincubated for 40 min at 37°C with DTT (1 mM) to remove the mucin layer (4). (VI) Mucin layer was removed by scraping as for panel IV and treated with collagenase 2. Next, tissues were infected for 24 h with HSV-1 at 2 × 106 IU/well as described in Materials and Methods. Tissues were stained for β-Gal expression by X-Gal as described in Materials and Methods and were photographed under a light binocular microscope at a magnification of ×50. Blue staining represents infected cells, which are indicated by arrows. Collag., collagenase 2. (B and C) Organ cultures of normal colons and colon carcinomas were infected with HSV-1 as described for panel A, and β-Gal enzyme was measured using a Beta-Glo kit (Promega) and normalized for protein content as described in Materials and Methods. Data reflect means ± SD (n = 6 replicates). Results shown are representative of three independent experiments. (D) Staining for collagen. Histological sections (5 μm) were prepared from organ cultures of BALB/c normal colons (with and without collagenase 2 treatment) and colon carcinoma tissues. Collagen (blue color; indicated by arrows) was examined by Masson Trichrome staining as described in Materials and Methods (24). (E) Staining for mucin. Confocal micrographs show the mucin polymer by red staining with tetramethyl rhodamine isocyanate conjugated to lectin from Ulex europaeus as described in Materials and Methods. Colon tissue without staining serves as a control. Colon slices were preincubated for 40 min at 37°C with DTT (1 mM) to remove the mucin layer. Note the decreased mucin stain (red) in the colon tissue treated with DTT and in the carcinoma tissue compared to that in the colon tissue.
Quantitative analysis of β-Gal expression in the normal colon tissue further supported the inhibitory effects of collagen and the mucin layer upon viral infection (Fig. 5B). Of special note, treatment of the carcinoma tissue in organ culture with collagenase or removal of the mucin by DTT had only a marginal effect on HSV-1 infectivity (Fig. 5C).
To directly test whether there is a difference in collagen content between the normal colon and carcinoma tissues, respective histological sections were stained with Masson Trichrome, by which collagen fibers are specifically stained blue (24). The slides presented in Fig. 5D show positive collagen stains in the muscle and mucosa layers. Note that partial digestion with collagenase, while maintaining the basic structure of the normal colon, practically eliminated collagen from the tissue. In contrast, the colon carcinoma tissue was hardly stained for collagen. To determine whether there is a difference in mucin secretion between the normal colon and carcinoma tissues, both colon and carcinoma tissues were stained for mucin (Fig. 5E) (17). Note the extensive red staining of the mucin layer in the normal colon organ, in contrast to weak staining after treatment with DTT, indicating partial removal of this mucin layer. In comparison, there was very weak staining of mucin in the carcinoma tissue.
Taken together, these data highlight the role of a number of extracellular components in the normal colon, but not in the carcinoma tissue, which lost these extracellular components, in protecting the tissue from HSV-1 infection.
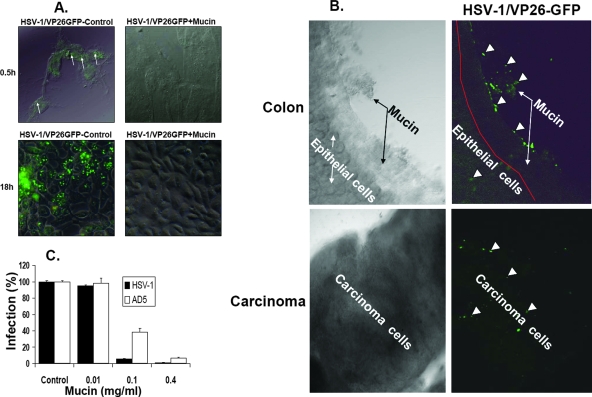
The mucin layer in the lumen of the colon may block HSV-1 infection either by serving as a physical barrier to protect the epithelial cell layer or via specific binding to viral particles. To differentiate between these two possibilities, crude mucin prepared from the mouse colon was mixed with a virus particle preparation. Next, viral adsorption and infectivity were tested. To examine the inhibition of virus adsorption to cells following mixing with mucin, we applied the HSV-1/VP26-GFP strain, whose virions are fluorescent due to the incorporation of the capsid fusion protein VP26-GFP (18). Adsorption of HSV-1/VP26-GFP particles to cells in culture at 4°C was detected with a fluorescence confocal microscope (Fig. 6A, 0.5 h, green spots). Pretreatment of the virus preparation with crude mucin effectively inhibited fluorescent viral particle adsorption to the cell layer compared with that of the control without mucin (Fig. 6A). To test the effect of mucin on viral infectivity, the experiment described above was repeated, but infected cell cultures were incubated at 37°C for 18 h to facilitate HSV-1/VP26-GFP viral replication. The results depicted in Fig. 6A clearly show a reduction of fluorescent VP26-GFP synthesis in cells infected with a virus preparation pretreated with mucin. The effect of mucin on HSV-1/VP26-GFP virion adsorption to colon tissue in organ culture was also tested (Fig. 6B). HSV-1/VP26-GFP was added to a colon organ culture for 1 h at 4°C, and adsorption of fluorescent virions to the colon tissue was observed with a fluorescence confocal microscope. The results indicated that most of the virus particles (green spots) were absorbed to the mucin layer. On the other hand, adding the virus to carcinoma tissue lacking the mucin layer resulted in scattered adsorption of the virus through all areas of the carcinoma tissue (Fig. 6B).
FIG. 6.
Mucin layer of the colon inhibits HSV-1 infectivity. (A) The mucin layer was isolated from normal colons (BALB/c mice) as described in Materials and Methods, and the protein suspension (3 mg/0.5 ml) was mixed with recombinant HSV-1 expressing the capsid protein VP26 as a fusion with GFP (HSV-1/VP26-GFP) (2 × 106 IU). As a control, HSV-1/VP26-GFP was mixed with bovine serum albumin (3 mg/0.5 ml). After 30 min at room temperature, the mixture was diluted with PBS and added to Vero cells (1 × 105 cells/well) for absorption for 30 min at 4°C. Cells were washed five times with PBS, treated with 3% paraformaldehyde for 5 min, examined directly for HSV-1/VP26-GFP absorption under a fluorescence confocal microscope, and photographed at a magnification of ×400. Green spots represent HSV-1/VP26-GFP viral particle adsorption to cells and are indicated by arrows. Cells were infected as described above and incubated for 18 h to observe HSV-1 replication by VP26-GFP protein synthesis. Results are representative of three independent experiments. (B) Adsorption of HSV-1/VP26-GFP to normal colon and carcinoma tissues. Organ cultures were prepared from normal and carcinoma tissues of BALB/c mice, incubated for 2 h with HSV-1/VP26-GFP (1 × 107 IU/1.5 ml), washed five times with PBS, and observed for HSV-1/VP26-GFP adsorption under a fluorescence confocal microscope. Magnification, ×100. The mucin layer is indicated by arrows. Green spots represent HSV-1/VP26-GFP viral particles adsorbed to the tissue, as indicated by the arrowheads. Results are representative of three independent experiments. (C) The mucin layer was isolated from normal colons (BALB/c mice) as described in Materials and Methods, and the indicated amounts of mucin protein were mixed with HSV-1 (105 IU) and adenovirus type 5 (105 IU) in a volume of 0.5 ml for 30 min at room temperature. Vero cells were infected with the suspensions, and residual viral infectivity was determined at 24 h postinfection, as described in Materials and Methods, using a Beta-Glo kit for determining β-Gal enzyme activity. Control virus, incubated in PBS containing bovine serum albumin (0.4 mg/ml), was taken as 100% infection. Data reflect means ± SD (n = 6 replicates). Results shown are representative of three independent experiments.
To estimate the potency of crude mucin in blocking HSV-1 infectivity, increasing amounts of mucin were added to the HSV-1 β-Gal virus preparation (Fig. 6C). Quantitative analysis of β-Gal expression indicated extensive inhibition of virus infectivity, even after mixing of virus with a low dose of mucin glycoprotein (0.1 mg/ml). We also found, by direct comparison, that HSV-1 is inhibited by mucin to a higher degree than is adenovirus (Fig. 6C).
In conclusion, these results indicate that the mucin layer in the normal colon adsorbs HSV-1, thereby interfering with viral-cell receptor interaction.
In view of the inhibitory effect of mucin molecules on HSV-1 infection (Fig. 5 and 6), we reexamined, in retrospect, the human colon carcinoma samples (Fig. 2) for mucin secretion and its consequence on viral infection. A pathologist blindly reexamined 10 of the human carcinoma specimens used in this work for mucin secretion and designated two of the specimens as mucin-secreting adenocarcinomas (Fig. 1). Interestingly, organ cultures from the same two tumors could not be infected by HSV-1.
HSV-1 inhibits the growth of colon carcinomas in vivo.
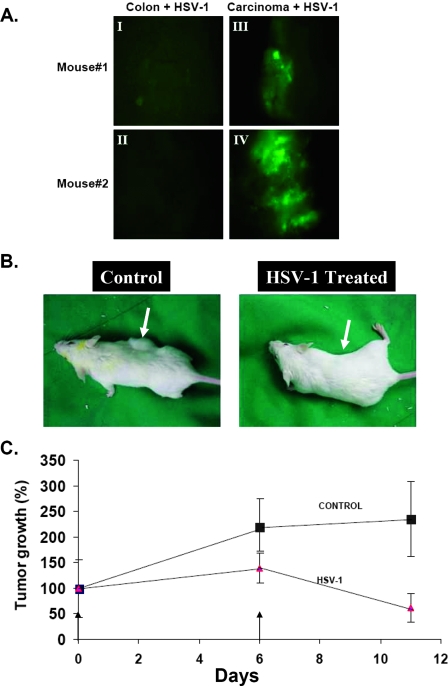
The ex vivo experiments described above indicated a selective adsorption and infection of HSV-1 in tumor tissues compared with the normal colon. Next, we examined whether, under in vivo conditions, colon carcinoma may also be infected preferentially. To this end, we injected HSV-1 (106 infectious units [IU]) directly into normal colons and s.c. colon carcinoma tissue in mice. After 48 h of infection, the tissues were harvested and rinsed, and the expression of the GFP reporter gene was examined immediately under a fluorescence microscope (Fig. 7A). As shown above for organ cultures, expression of GFP in vivo was much more intense in colon carcinomas than in normal colons.
FIG. 7.
Oncolytic activity of HSV-1 in mice implanted with colon carcinoma cells. (A) BALB/c mice were injected s.c. with colon carcinoma CT26 cells (105) as described in Materials and Methods. Two weeks later, as tumors developed, three mice were injected with HSV-1 (1 × 106 IU in 50 μl PBS) directly into the normal colon (I and II) and into the tumor (III and IV). At 48 h postinfection, tissues were harvested and extensively washed in PBS, and the extent of infection was examined by GFP expression under a fluorescence microscope. Magnification, ×100. (B) BALB/c mice were injected s.c. with 1 × 105 colon carcinoma CT26 cells as described in Materials and Methods. Two weeks later, as tumors developed, all eight mice were injected (at day 0 and day 6, as indicated by the arrows) directly in the tumor with HSV-1 (5 × 106 IU/50 μl PBS) or with 50 μl PBS as a control. At 48 h postinfection, animals were treated by intraperitoneal injection of ganciclovir (10 mg/kg) twice daily. Pictures of two representative mice are presented. (C) Tumor volumes of the two groups (n = 8) were measured as described in Materials and Methods. Treatment was initiated when the tumor volume was about 25 (±10) mm3 (14 days after tumor inoculation). The tumor volume measured for each mouse on the first day of virus or PBS treatment was taken as 100% tumor volume. Data reflect means ± SD for four animals per group.
We then examined the potential of HSV-1 to inhibit colon carcinoma growth in vivo. The results shown in Fig. 7B and C indicate that injection of HSV-1 directly into mouse colon tumors, combined with ganciclovir treatment, significantly inhibits tumor development, causing a fourfold decrease in tumor volume compared to that of mock controls (PBS injected).
Taken together, the ex vivo and in vivo studies show a good correlation and indicate the potential of HSV-1 as a colon cancer-specific treatment, sparing the healthy colon.
DISCUSSION
Viral tropism in cultured cells is determined by receptor usage or the presence of intracellular restriction mechanisms. HSV-1, for example, infects many different cultured cells, derived from various species, via the ubiquitously expressed receptors herpesvirus entry mediator, nectin-1, nectin-2, and heparan sulfate molecules (10, 45). In addition, the replication of HSV-1 is subjected to the control of intracellular factors, such as the transcription factor Oct-2, and of cyclin-dependent kinases, especially in neuronal cells (43, 49). Yet the natural host of the virus is humans, and infection is usually restricted to epithelial cells around the lip and to the trigeminal ganglia. The different viral tropism in vivo and in cultured cells implies that while receptors are essential for viral infection, they are not the sole determinant of infection. Understanding HSV-1 tropism is important for applications of the virus as a vector for gene therapy and as an oncolytic virus against solid tumors (52). To overcome some of the problems associated with the study of viral tropism in animal models, we developed an ex vivo organ culture system from normal and malignant colon tissues and investigated the relative tropism of HSV-1 vectors for these tissues. A common entry site for many viruses is the epithelial tissue, which is usually covered with a mucous layer composed of specific mucin glycoproteins and various polysaccharides (38, 42, 48).
Our results indicate preferential infection of carcinoma tissues from both humans and mice over the corresponding normal tissues. Thus, the comparison between human and mouse colon tissues ex vivo demonstrated the validity of the mouse tumor model in studies related to HSV-1, which is naturally not a mouse virus.
A major finding of our study indicates that the preferential infection of colon carcinomas by HSV-1 is due to the extracellular matrix component. The evidence for this conclusion is as follows. (i) Enhanced infection was observed in the normal colon after removal of collagen and mucin extracellular molecules. Infection of the lumen-exposed normal colon was similar to the level observed for the carcinoma tissue. (ii) Direct inhibition of infection was shown upon preincubation of HSV-1 with the isolated mucin fraction. (iii) Direct observation of HSV-1/VP26-GFP viral particle interaction with the colon indicated that the virus binds to the luminal side of the mucin layer. (iv) The preferential infection of the carcinoma tissue was not due to enhanced replication of the virus in tumor cells, as similar differences were observed in the presence of acyclovir, which blocks HSV-1 DNA synthesis and subsequent replication. (v) Since most of the data reported here are based on reporter gene expression, it could be argued that promoter specificity is responsible for the apparent differential infection of the colon tissues. However, reporter genes expressed by either an RSV or CMV constitutive promoter gave identical results.
Two extracellular components, namely, collagen and mucin molecules, appear to be critical in the infection of normal colon tissue of mice and humans. The inhibitory effect of collagen was demonstrated when enhanced viral infection occurred upon partial removal of the collagen from colon tissue by collagenase. Collagenase pretreatment may enhance viral infection either by making the tissue more porous and thus facilitating HSV-1 penetration into the solid three-dimensional structure or by removing a dominant inhibitor from the tissue. Two lines of evidence support the former possibility, as follows. After collagenase digestion, histology analysis indicated that the structure of the tissue was more porous, and preincubation of HSV-1 with a purified collagen preparation did not show any inhibition of HSV-1 infection (data not shown).
A previous study showed that in vivo injection of adenovirus and HSV-1 vectors into melanomas and glioblastomas, respectively, resulted in localized infection of cells adjacent to the needle penetration site, with only limited diffusion to distant cells. Yet the addition of collagenase resulted in enhanced infection following virus injection (23, 28). Colon carcinoma tissues were found to produce much less collagen than the normal colon, and this could be one reason for the preferential infection of the carcinoma tissue. It should be noted that some human colonic carcinomas contain abundant collagen extracellular matrix (22), and in such cases, application of HSV-1 as an oncolytic virus may not be advisable.
Mucin is the second component that interferes with infection of the normal colon. The inhibitory effect of mucin was demonstrated both by removal of the mucous layer from the lumen of the colon and by direct inhibition of viral infection upon preincubation of the virus with an isolated mucin preparation. Moreover, direct observation of HSV-1/VP26-GFP particle interaction with the colon indicated binding to the mucosal luminal side of the colon. We therefore suggest that the mucous layer acts both as a direct inhibitor of the virus and as a physical barrier against infection of the epithelial cell layer. While most human colon carcinomas do not produce mucin, 10% of carcinomas are mucin positive. We analyzed carcinoma tissues derived from 18 patients in this study and observed that two of the tissue samples were poorly infected with HSV-1 in organ culture. Consequently, we obtained tissue slides of 10 samples used in the present work from the archive of the pathology department, and we observed that the two poorly infected tissues were indeed mucin positive. This analysis further validates the role of mucin in restricting HSV-1 infection in human colon tissues. In contrast to HSV-1, adenovirus did not exhibit preferential infection of colon carcinomas compared to the normal colon. Removal of the mucin layer from the colon did not enhance the infection of adenovirus. We suggest that adenovirus infection in the normal colon may be blocked at the level of CAR, which is known to be expressed on the basolateral side of the epithelium (53). The finding that adenovirus does not efficiently infect the carcinoma tissue may be explained by down-regulation of the CAR receptor in the tumor tissue (20).
Earlier research on the application of HSV-1 as an oncolytic virus employed engineered herpesvirus vectors with the ability to replicate selectively in cancer cells due to intracellular differences from normal cells. Several viral genes (i.e., the thymidine kinase, ICP6, ribonucleotide reductase, and γ34.5 genes) were mutated in order to facilitate preferential replication in tumor cells and to attenuate virus neurovirulence (12, 13, 26, 31, 52). In addition, viruses were engineered to express cytotoxic genes, cytokines, and viral genes under the control of a tumor-specific promoter (32, 37, 52, 54).
In this work, we describe a new principle of viral selectivity, i.e., extracellular differences between tumor and normal tissues lead to selective infectivity of the tumor tissue.
The combination of extracellular and intracellular differences between normal and carcinoma tissues can now be exploited to improve the efficacy and safety of oncolytic viruses.
The new insights derived from this work may be applied to the design of clinical trials for colon carcinoma therapy. While colorectal metastases to the liver constitute an advanced stage of the disease associated with a poor prognosis, we focused in this study on localized rectal cancer. Rectal carcinoma is a subtype of colon cancer and is one of the most common tumors in human subjects. It poses significant therapeutic challenges, primarily related to preserving anal sphincter anatomy and function, which are critical to maintenance of a normal lifestyle. These efforts include treating the patient with chemo- and radiotherapy prior to surgery. The goal is to decrease the tumor mass and allow a less radical and mutilating operation (40). However, chemo- and radiotherapies are time-consuming and are associated with severe side effects. These concerns and the ability to approach the tumor directly with relative ease make rectal carcinoma an ideal target for oncolytic viral therapy. Indeed, we showed that direct injection of HSV-1 into implanted colon carcinomas in mice, combined with ganciclovir treatment, effectively reduced tumor masses.
Some of the findings described here appear relevant to viral interactions with additional epithelial tissues, such as those in the lungs, kidneys, and skin. These tissues are also potential targets for virally mediated gene therapy and oncolysis.
Acknowledgments
This work was supported by a grant from the European Union under program 6, The Cinigene Network of Excellence, and by a grant from Philip-Morris US and an international external research program.
We thank H. Falk, N. Mador, and E. Piontek of The Hebrew University-Hadassah Medical School for their contributions.
Footnotes
Published ahead of print on 31 October 2007.
REFERENCES
- 1.Alian, A., D. Sela-Donenfeld, A. Panet, and A. Eldor. 2000. Avian hemangioma retrovirus induces cell proliferation via the envelope (env) gene. Virology 276161-168. [DOI] [PubMed] [Google Scholar]
- 2.Allen, A., D. A. Hutton, and J. P. Pearson. 1998. The MUC2 gene product: a human intestinal mucin. Int. J. Biochem. Cell. Biol. 30797-801. [DOI] [PubMed] [Google Scholar]
- 3.Bischoff, J. R., D. H. Kirn, A. Williams, C. Heise, S. Horn, M. Muna, L. Ng, J. A. Nye, A. Sampson-Johannes, A. Fattaey, and F. McCormick. 1996. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science 274373-376. [DOI] [PubMed] [Google Scholar]
- 4.Blau, S., N. Levin, B. Schwartz, and A. Rubinstein. 2001. Adsorption of cationized bovine serum albumin onto epithelial crypt fractions of the rat colon. J. Pharm. Sci. 901516-1522. [DOI] [PubMed] [Google Scholar]
- 5.Bourcier, T., P. Forgez, V. Borderie, S. Scheer, W. Rostene, and L. Laroche. 2000. Regulation of human corneal epithelial cell proliferation and apoptosis by dexamethasone. Investig. Ophthalmol. Vis. Sci. 414133-4141. [PubMed] [Google Scholar]
- 6.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72248-254. [DOI] [PubMed] [Google Scholar]
- 7.Braun, E., T. Zimmerman, T. B. Hur, E. Reinhartz, Y. Fellig, A. Panet, and I. Steiner. 2006. Neurotropism of herpes simplex virus type 1 in brain organ cultures. J. Gen. Virol. 872827-2837. [DOI] [PubMed] [Google Scholar]
- 8.Brill-Almon, E., B. Stern, D. Afik, J. Kaye, N. Langer, S. Bellomo, M. Shavit, A. Pearlman, Y. Lippin, A. Panet, and N. Shani. 2005. Ex vivo transduction of human dermal tissue structures for autologous implantation production and delivery of therapeutic proteins. Mol. Ther. 12274-282. [DOI] [PubMed] [Google Scholar]
- 9.Byrd, J. C., and R. S. Bresalier. 2004. Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev. 2377-99. [DOI] [PubMed] [Google Scholar]
- 10.Campadelli-Fiume, G., F. Cocchi, L. Menotti, and M. Lopez. 2000. The novel receptors that mediate the entry of herpes simplex viruses and animal alphaherpesviruses into cells. Rev. Med. Virol. 10305-319. [DOI] [PubMed] [Google Scholar]
- 11.Chen, C. C., M. Baylor, and D. M. Bass. 1993. Murine intestinal mucins inhibit rotavirus infection. Gastroenterology 10584-92. [DOI] [PubMed] [Google Scholar]
- 12.Dambach, M. J., J. Trecki, N. Martin, and N. S. Markovitz. 2006. Oncolytic viruses derived from the gamma34.5-deleted herpes simplex virus recombinant R3616 encode a truncated UL3 protein. Mol. Ther. 13891-898. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein, D. J., and S. K. Weller. 1988. Herpes simplex virus type 1-induced ribonucleotide reductase activity is dispensable for virus growth and DNA synthesis: isolation and characterization of an ICP6 lacZ insertion mutant. J. Virol. 62196-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hasson, E., Y. Slovatizky, Y. Shimoni, H. Falk, A. Panet, and E. Mitrani. 2005. Solid tissues can be manipulated ex vivo and used as vehicles for gene therapy. J. Gene Med. 7926-935. [DOI] [PubMed] [Google Scholar]
- 15.Haviv, Y. S., J. L. Blackwell, A. Kanerva, P. Nagi, V. Krasnykh, I. Dmitriev, M. Wang, S. Naito, X. Lei, A. Hemminki, D. Carey, and D. T. Curiel. 2002. Adenoviral gene therapy for renal cancer requires retargeting to alternative cellular receptors. Cancer Res. 624273-4281. [PubMed] [Google Scholar]
- 16.Hemminki, A., A. Kanerva, B. Liu, M. Wang, R. D. Alvarez, G. P. Siegal, and D. T. Curiel. 2003. Modulation of coxsackie-adenovirus receptor expression for increased adenoviral transgene expression. Cancer Res. 63847-853. [PubMed] [Google Scholar]
- 17.Henke, M. O., A. Renner, R. M. Huber, M. C. Seeds, and B. K. Rubin. 2004. MUC5AC and MUC5B mucins are decreased in cystic fibrosis airway secretions. Am. J. Respir. Cell Mol. Biol. 3186-91. [DOI] [PubMed] [Google Scholar]
- 18.Hoppe, S., M. Schelhaas, V. Jaeger, T. Liebig, P. Petermann, and D. Knebel-Morsdorf. 2006. Early herpes simplex virus type 1 infection is dependent on regulated Rac1/Cdc42 signalling in epithelial MDCKII cells. J. Gen. Virol. 873483-3494. [DOI] [PubMed] [Google Scholar]
- 19.Jass, J. R., and M. D. Walsh. 2001. Altered mucin expression in the gastrointestinal tract: a review. J. Cell Mol. Med. 5327-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanerva, A., M. Wang, G. J. Bauerschmitz, J. T. Lam, R. A. Desmond, S. M. Bhoola, M. N. Barnes, R. D. Alvarez, G. P. Siegal, D. T. Curiel, and A. Hemminki. 2002. Gene transfer to ovarian cancer versus normal tissues with fiber-modified adenoviruses. Mol. Ther. 5695-704. [DOI] [PubMed] [Google Scholar]
- 21.Kirby, T. O., A. Rivera, D. Rein, M. Wang, I. Ulasov, M. Breidenbach, M. Kataram, J. L. Contreras, C. Krumdieck, M. Yamamoto, M. G. Rots, H. J. Haisma, R. D. Alvarez, P. J. Mahasreshti, and D. T. Curiel. 2004. A novel ex vivo model system for evaluation of conditionally replicative adenoviruses therapeutic efficacy and toxicity. Clin. Cancer Res. 108697-8703. [DOI] [PubMed] [Google Scholar]
- 22.Kolodkin-Gal, D., G. Zamir, E. Pikarski, A. Pikarski, N. Shimony, H. Wu, Y. S. Haviv, and A. Panet. 2007. A novel system to study adenovirus tropism to normal and malignant colon tissues. Virology 35791-101. [DOI] [PubMed] [Google Scholar]
- 23.Kuriyama, N., H. Kuriyama, C. M. Julin, K. R. Lamborn, and M. A. Israel. 2001. Protease pretreatment increases the efficacy of adenovirus-mediated gene therapy for the treatment of an experimental glioblastoma model. Cancer Res. 611805-1809. [PubMed] [Google Scholar]
- 24.Larkin, L. M., S. Calve, T. Y. Kostrominova, and E. M. Arruda. 2006. Structure and functional evaluation of tendon-skeletal muscle constructs engineered in vitro. Tissue Eng. 123149-3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marozin, S., U. Prank, and B. Sodeik. 2004. Herpes simplex virus type 1 infection of polarized epithelial cells requires microtubules and access to receptors present at cell-cell contact sites. J. Gen. Virol. 85775-786. [DOI] [PubMed] [Google Scholar]
- 26.Martuza, R. L., A. Malick, J. M. Markert, K. L. Ruffner, and D. M. Coen. 1991. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science 252854-856. [DOI] [PubMed] [Google Scholar]
- 27.Matsuda, K., T. Masaki, T. Watanabe, J. Kitayama, H. Nagawa, T. Muto, and Y. Ajioka. 2000. Clinical significance of MUC1 and MUC2 mucin and p53 protein expression in colorectal carcinoma. Jpn. J. Clin. Oncol. 3089-94. [DOI] [PubMed] [Google Scholar]
- 28.McKee, T. D., P. Grandi, W. Mok, G. Alexandrakis, N. Insin, J. P. Zimmer, M. G. Bawendi, Y. Boucher, X. O. Breakefield, and R. K. Jain. 2006. Degradation of fibrillar collagen in a human melanoma xenograft improves the efficacy of an oncolytic herpes simplex virus vector. Cancer Res. 662509-2513. [DOI] [PubMed] [Google Scholar]
- 29.McKenzie, S. P., S. L. Barnes, and R. W. Schwartz. 2005. An update on the surgical management of rectal cancer. Curr. Surg. 62407-411. [DOI] [PubMed] [Google Scholar]
- 30.Meier, O., and U. F. Greber. 2004. Adenovirus endocytosis. J. Gene Med. 6(Suppl. 1)S152-S163. [DOI] [PubMed] [Google Scholar]
- 31.Mineta, T., S. D. Rabkin, T. Yazaki, W. D. Hunter, and R. L. Martuza. 1995. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat. Med. 1938-943. [DOI] [PubMed] [Google Scholar]
- 32.Mullen, J. T., H. Kasuya, S. S. Yoon, N. M. Carroll, T. M. Pawlik, S. Chandrasekhar, H. Nakamura, J. M. Donahue, and K. K. Tanabe. 2002. Regulation of herpes simplex virus 1 replication using tumor-associated promoters. Ann. Surg. 236502-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura, T., K. W. Peng, S. Vongpunsawad, M. Harvey, H. Mizuguchi, T. Hayakawa, R. Cattaneo, and S. J. Russell. 2004. Antibody-targeted cell fusion. Nat. Biotechnol. 22331-336. [DOI] [PubMed] [Google Scholar]
- 34.Naldini, L., U. Blomer, P. Gallay, D. Ory, R. Mulligan, F. H. Gage, I. M. Verma, and D. Trono. 1996. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272263-267. [DOI] [PubMed] [Google Scholar]
- 35.Plotnikov, A., T. Tichler, R. Korenstein, and Y. Keisari. 2005. Involvement of the immune response in the cure of metastatic murine CT-26 colon carcinoma by low electric field-enhanced chemotherapy. Int. J. Cancer 117816-824. [DOI] [PubMed] [Google Scholar]
- 36.Prevec, L., B. S. Christie, K. E. Laurie, M. M. Bailey, F. L. Graham, and K. L. Rosenthal. 1991. Immune response to HIV-1 gag antigens induced by recombinant adenovirus vectors in mice and rhesus macaque monkeys. J. Acquir. Immune Defic. Syndr. 4568-576. [PubMed] [Google Scholar]
- 37.Reinblatt, M., R. H. Pin, and Y. Fong. 2004. Carcinoembryonic antigen directed herpes viral oncolysis improves selectivity and activity in colorectal cancer. Surgery 136579-584. [DOI] [PubMed] [Google Scholar]
- 38.Rhodes, J. M. 1997. Mucins and inflammatory bowel disease. QJM 9079-82. [DOI] [PubMed] [Google Scholar]
- 39.Rivera, A. A., J. Davydova, S. Schierer, M. Wang, V. Krasnykh, M. Yamamoto, D. T. Curiel, and D. M. Nettelbeck. 2004. Combining high selectivity of replication with fiber chimerism for effective adenoviral oncolysis of CAR-negative melanoma cells. Gene Ther. 111694-1702. [DOI] [PubMed] [Google Scholar]
- 40.Ross, H. M., N. Mahmoud, and R. D. Fry. 2005. The current management of rectal cancer. Curr. Probl. Surg. 4272-131. [DOI] [PubMed] [Google Scholar]
- 41.Rots, M. G., M. G. Elferink, W. M. Gommans, D. Oosterhuis, J. A. Schalk, D. T. Curiel, P. Olinga, H. J. Haisma, and G. M. Groothuis. 2006. An ex vivo human model system to evaluate specificity of replicating and non-replicating gene therapy agents. J. Gene Med. 835-41. [DOI] [PubMed] [Google Scholar]
- 42.Sansonetti, P. J. 2004. War and peace at mucosal surfaces. Nat. Rev. Immunol. 4953-964. [DOI] [PubMed] [Google Scholar]
- 43.Schang, L. M., A. Bantly, and P. A. Schaffer. 2002. Explant-induced reactivation of herpes simplex virus occurs in neurons expressing nuclear cdk2 and cdk4. J. Virol. 767724-7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shayakhmetov, D. M., Z. Y. Li, S. Ni, and A. Lieber. 2002. Targeting of adenovirus vectors to tumor cells does not enable efficient transduction of breast cancer metastases. Cancer Res. 621063-1068. [PubMed] [Google Scholar]
- 45.Spear, P. G. 2004. Herpes simplex virus: receptors and ligands for cell entry. Cell. Microbiol. 6401-410. [DOI] [PubMed] [Google Scholar]
- 46.Stoff-Khalili, M. A., A. A. Rivera, L. P. Le, A. Stoff, M. Everts, J. L. Contreras, D. Chen, L. Teng, M. G. Rots, H. J. Haisma, R. P. Rocconi, G. J. Bauerschmitz, D. T. Rein, M. Yamamoto, G. P. Siegal, P. Dall, J. M. Mathis, and D. T. Curiel. 2006. Employment of liver tissue slice analysis to assay hepatotoxicity linked to replicative and nonreplicative adenoviral agents. Cancer Gene Ther. 13606-618. [DOI] [PubMed] [Google Scholar]
- 47.Stoff-Khalili, M. A., A. Stoff, A. A. Rivera, J. M. Mathis, M. Everts, M. Wang, Y. Kawakami, R. Waehler, Q. L. Mathews, M. Yamamoto, R. P. Rocconi, G. P. Siegal, D. F. Richter, P. Dall, Z. B. Zhu, and D. T. Curiel. 2005. Gene transfer to carcinoma of the breast with fiber-modified adenoviral vectors in a tissue slice model system. Cancer Biol. Ther. 41203-1210. [DOI] [PubMed] [Google Scholar]
- 48.Stonebraker, J. R., D. Wagner, R. W. Lefensty, K. Burns, S. J. Gendler, J. M. Bergelson, R. C. Boucher, W. K. O'Neal, and R. J. Pickles. 2004. Glycocalyx restricts adenoviral vector access to apical receptors expressed on respiratory epithelium in vitro and in vivo: role for tethered mucins as barriers to luminal infection. J. Virol. 7813755-13768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas, S., R. S. Coffin, P. Watts, G. Gough, and D. S. Latchman. 1998. The TAATGARAT motif in the herpes simplex virus immediate-early gene promoters can confer both positive and negative responses to cellular octamer-binding proteins when it is located within the viral genome. J. Virol. 723495-3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas, S. K., G. Gough, D. S. Latchman, and R. S. Coffin. 1999. Herpes simplex virus latency-associated transcript encodes a protein which greatly enhances virus growth, can compensate for deficiencies in immediate-early gene expression, and is likely to function during reactivation from virus latency. J. Virol. 736618-6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Beusechem, V. W., J. Grill, D. C. Mastenbroek, T. J. Wickham, P. W. Roelvink, H. J. Haisma, M. L. Lamfers, C. M. Dirven, H. M. Pinedo, and W. R. Gerritsen. 2002. Efficient and selective gene transfer into primary human brain tumors by using single-chain antibody-targeted adenoviral vectors with native tropism abolished. J. Virol. 762753-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Varghese, S., and S. D. Rabkin. 2002. Oncolytic herpes simplex virus vectors for cancer virotherapy. Cancer Gene Ther. 9967-978. [DOI] [PubMed] [Google Scholar]
- 53.Walters, R. W., T. Grunst, J. M. Bergelson, R. W. Finberg, M. J. Welsh, and J. Zabner. 1999. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J. Biol. Chem. 27410219-10226. [DOI] [PubMed] [Google Scholar]
- 54.Yamamura, H., M. Hashio, M. Noguchi, Y. Sugenoya, M. Osakada, N. Hirano, Y. Sasaki, T. Yoden, N. Awata, N. Araki, M. Tatsuta, S. I. Miyatake, and K. Takahashi. 2001. Identification of the transcriptional regulatory sequences of human calponin promoter and their use in targeting a conditionally replicating herpes vector to malignant human soft tissue and bone tumors. Cancer Res. 613969-3977. [PubMed] [Google Scholar]
- 55.Zhang, J. G., S. Ghosh, C. D. Ockleford, and M. Galinanes. 2000. Characterization of an in vitro model for the study of the short and prolonged effects of myocardial ischaemia and reperfusion in man. Clin. Sci. (London) 99443-453. [PubMed] [Google Scholar]