Abstract
We have performed a screen aimed at identifying human herpesvirus 6 (HHV-6)-encoded proteins that modulate immune recognition. Here we show that the U24 protein encoded by HHV-6 variant A downregulates cell surface expression of the T-cell receptor (TCR)/CD3 complex, a complex essential to T-cell activation and the generation of an immune adaptive response. In the presence of U24, the TCR/CD3 complex is endocytosed but is not recycled back to the plasma membrane. Instead, it accumulates in early and late endosomes. Interestingly, whereas CD3 downregulation from the cell surface is normally associated with T-cell activation, U24 downregulates CD3 independently of T-cell activation. Moreover, we found that U24-expressing T cells are resistant to activation by antigen-presenting cells. HHV-6 has evolved a unique mechanism of inhibition of T-cell activation that may impair the establishment of an adaptive immune response. Furthermore, lymphocyte activation creates an environment favorable to the reactivation and replication of lymphotropic herpesviruses. Thus, by inhibiting T-cell activation, HHV-6 might limit its reactivation and thus minimize immune recognition.
Human herpesvirus 6 (HHV-6) is a ubiquitous pathogen of the betaherpesvirus family, which includes cytomegalovirus and HHV-7, that primarily infects CD4+ T cells (26, 38). Like other herpesviruses, HHV-6 establishes latency after the initial productive infection (35) and thus is never cleared from its host. Two subtypes of HHV-6 have been identified: variants A and B. Although these two variants are similar in sequence and genome organization, they are associated with different pathologies (8, 10). HHV-6 variant B (HHV-6B) causes exanthem subitum (roseola infantum) in young children (43). It has also recently been associated with mesial temporal lobe epilepsy (11). HHV-6A has been implicated in the etiology of several other pathologies (4, 42), but this remains to be confirmed. Reports suggest a role of this variant in multiple sclerosis (36) as well as encephalitis (28, 39). Importantly, several lines of experimental and clinical evidence suggest that HHV-6A might accelerate AIDS progression (24). In particular, a recent study reported that HHV-6A triggered a faster progression of AIDS in simian immunodeficiency virus (SIV)-infected macaques (23). HHV-6 can reactivate in immune-compromised and transplant patients (31) and can be associated with severe encephalitis in these patients. Whether the reactivation of HHV-6 affects the immune competency of these patients or whether it is solely an innocuous marker of immune deficiency is not yet known.
The mechanisms by which HHV-6 establishes persistence in its host are not well understood. Whereas herpesviruses devote a large fraction of their coding potential to immune evasion (33, 41), only a few HHV-6 proteins involved in immune evasion have been characterized so far. The HHV-6 immediate-early protein IE1 inhibits beta interferon induction, potentially affecting the initiation of an innate immune response (17). HHV-6 encodes a functional chemokine, U83, which might have an impact on the cell type recruited to the site of infection (9, 46). Finally, a recent study showed that the HHV-6-encoded protein U21 downregulates major histocompatibility complex class I cell surface expression (14). The characterization of viral immune modulators not only sheds light on viral pathogenesis but can also have a profound impact in the understanding of immunology and cell biology (6, 22).
One key player in the generation of an adaptive immune response is the T-cell receptor (TCR)/CD3 complex signaling receptor present on CD4+ T cells, the main cell type infected by HHV-6. Lusso et al. reported that CD3 expression was altered in CD4+ T cells infected with HHV-6, suggesting that HHV-6 might encode a protein that modulates CD3 expression (26). Alternatively, this observation could be due to a bystander effect of HHV-6 replication in these cells. To distinguish between these two possibilities, we generated a library of HHV-6 candidate genes and tested whether one of these genes modulates the expression of the TCR/CD3 complex. Here we show that HHV-6 U24 protein inhibits CD3 recycling to the cell surface and, as a consequence, downregulates CD3 cell surface expression and prevents T-cell activation.
MATERIALS AND METHODS
Cell lines, transfections, and reagents.
Jurkat, Jurkat CD8+, JCaM, and P116 cell lines were generously provided by Arthur Weiss (University of California, San Francisco). Jurkat and BJAB cell lines were grown in RPMI 1640 with 5% fetal bovine serum and 100 μg/ml penicillin-streptomycin. For transfection, 16 × 106 cells were mixed with 30 μg of the appropriate vector and electroporated using a Bio-Rad Gene Pulser set at 960 μF and 250 V. Phorbol myristate acetate (PMA) and ionomycin (both from Sigma) were used at 50 ng/ml and 1 μg/ml, respectively. Phycoerythrin (PE)-conjugated antibodies against CD3ɛ (UCHT1) and αβ TCR (IP26) and antibodies against CD69, ICAM-1, and HLA-A, -B, and -C were purchased from BD Biosciences. PE-conjugated goat anti-mouse immunoglobulin G was purchased from Caltag. Antibodies against ULBP1, ULBP2, CD4, and CD8 were generously provided by David Raulet (University of California, Berkeley). A purified antibody against CD3 (OKT3) was a gift from Nilab Shastri (University of California, Berkeley). Staphylococcal enterotoxin E (SEE) was purchased from Toxin Technologies.
Plasmids and constructs.
U24 was amplified from an HHV-6A (GS) genomic preparation provided by Don Ganem (University of California, San Francisco) and was inserted into pIRES2-EGFP (U24/EGFP). Luciferase constructs were provided by Jenny Shapiro (University of California, San Francisco). N-terminally enhanced green fluorescent protein (EGFP)-tagged Rab constructs were made by inserting a unique MluI restriction site just upstream of the NotI site C-terminal to the EGFP in pIRES2-EGFP. Rab proteins were amplified from a HeLa cDNA library and inserted in frame with EGFP at the MluI site in the modified pIRES2-EGFP vector.
Luciferase assays.
Jurkat cells (16 × 106) were transiently transfected with 0.1 μg Renilla luciferase plasmid, 5 μg firefly luciferase plasmids with T-cell activation promoters, and 25 μg of U24/EGFP or pIRES2-EGFP as a control vector. The Dual-Luciferase reporter assay system (Promega) was used to analyze the samples.
Internalization assay.
Cells transiently transfected with U24/EGFP or the EGFP vector control were collected after 24 h and incubated with a PE-conjugated anti-CD3 antibody in cold flow buffer (1% bovine serum albumin in phosphate-buffered saline) for 30 min. Cells were washed with flow buffer to remove unbound antibody and were allowed to internalize at 37°C in RPMI-5% fetal bovine serum (FBS). At each time point, cells were removed from the 37°C medium and incubated in cold flow buffer to stop the reaction. Cells at each time point were split into two fractions: one was washed and analyzed by flow cytometry, while the other was stripped of membrane-bound antibody with flow buffer at pH 1.5 and immediately quenched in neutral flow buffer. This procedure removes more than 98% of surface-bound antibodies. These cells were washed and analyzed by flow cytometry. The percentage of internalization at each time point was calculated as (Stx − St0)/(Tt0 − St0) × 100, where Stx is the mean fluorescence of cells stripped of antibody at each time point, St0 is the mean fluorescence of cells stripped at time zero, and Tt0 is the mean fluorescence of cells that were not stripped of antibody.
Recycling assay.
Cells transiently transfected with U24/EGFP or the EGFP vector control were collected after 24 h, incubated with a PE-conjugated anti-CD3 antibody in RPMI-5% FBS, and allowed to internalize for 30 min. Cells were then washed with cold flow buffer and stripped of extracellularly bound antibody by the method described above. Cells were resuspended in warm RPMI-5% FBS and incubated at 37°C. Cells were removed at various times and incubated in cold flow buffer. Cells were split into two fractions: one was washed and analyzed by flow cytometry, and the other was stripped of extracellular antibody, washed, and analyzed by flow cytometry. The percentage of CD3 recycled was calculated indirectly as (St0 − Stx)/St0 × 100. The mean fluorescence of cells not stripped did not change significantly.
Indirect immunofluorescence.
Cells were transfected either with plasmids containing U24 and Rab-EGFP fusion constructs in bicistronic transcripts or with control Rab-EGFP constructs. After 24 h, cells transfected with U24-Rab-EGFP constructs were stained with a PE-conjugated CD3 antibody and used to gate the area of EGFP expression that corresponded to cells with low surface expression of CD3. These cells were sorted, as well as the control Rab-EGFP transfected cells that showed the same level of EGFP. Cells were allowed to settle on a glass slide, fixed with 3% paraformaldehyde-0.03 M sucrose, washed with phosphate-buffered saline, and permeabilized in 0.02% Triton X-100 in 3% bovine serum albumin. Cells were stained with antibody OKT3 followed by a goat anti-mouse secondary antibody conjugated to rhodamine and were visualized with a Zeiss LSM 510/NLO META confocal microscope using a Plan-Neofluar 40× objective (1.3 numerical aperture; 0.17-mm working distance). Images were magnified six times and analyzed with Zeiss LSM software.
Superantigen coculture assay.
Jurkat cells were transiently transfected with pIRES2-EGFP or pIRES2-U24-EGFP. After 24 h, these cells were incubated for 6 h with SEE-pulsed BJAB cells at a ratio of 2:1 (BJAB to Jurkat cells); subsequently, the cells were stained for CD69 expression. BJAB cells were pulsed with SEE by incubation with 200 ng/ml of SEE for 30 min, and excess SEE was washed away.
RESULTS
The TCR/CD3 complex is downregulated in the presence of HHV-6 U24 protein.
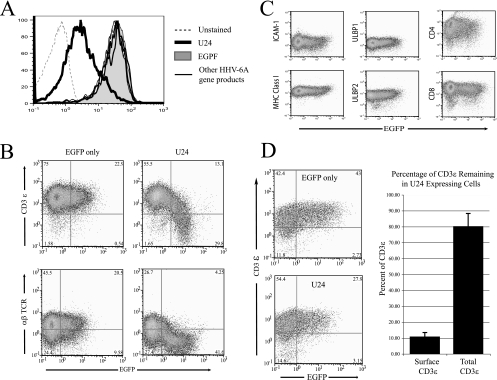
We generated a library of HHV-6 candidate genes and tested whether one of these genes modulates the expression of the TCR/CD3 complex. Our screen was limited to the open reading frames (ORFs) located at the left and right ends of the HHV-6 genome, regions that typically contain genes that are unique to each herpesvirus and are often involved in host-cell interactions. We cloned these ORFs into pIRES2-EGFP, a vector that expresses a bicistronic transcript encoding the gene candidate and EGFP. Each vector was transiently transfected into Jurkat cells, a human CD4+ T-cell line commonly used to study T-cell signaling. Surface expression of CD3ɛ and αβ TCR was measured by flow cytometry on EGFP-expressing cells at 24 h posttransfection. Among the different ORFs tested, only U24 significantly downregulated both CD3 and αβ TCR from the surfaces of Jurkat cells (Fig. 1A). Importantly, the expression of several other cell surface molecules involved in immune recognition was not affected by U24 expression (Fig. 1C), indicating that U24 does not massively modify the cell surface proteome.
FIG. 1.
Downregulation of TCR/CD3 from the surfaces of U24-expressing Jurkat cells. (A) Jurkat cells were transiently transfected with a bicistronic transcript encoding several HHV-6A gene products (U4, U21, U24, U61, U78) and EGFP. Twenty-four hours posttransfection, surface CD3ɛ in cells displaying GFP expression was analyzed by flow cytometry. (B) Jurkat cells were transiently transfected either with a bicistronic transcript of U24 and EGFP (U24/EGFP) or with EGFP alone. Cells were collected after 24 h, stained for surface CD3ɛ or αβ TCR, and analyzed by flow cytometry. (C) Jurkat cells were transiently transfected with U24/EGFP, collected after 24 h, and stained with antibodies for the indicated cell surface receptors. (D) Jurkat cells were transiently transfected with U24/EGFP or EGFP alone and collected after 24 h. (Left) Cells were fixed and permeabilized to stain for total CD3ɛ and were then analyzed by flow cytometry. (Right) Percentage of CD3 remaining in U24-expressing cells compared to that in EGFP controls. Data used in this analysis were from the mean fluorescence of cells stained with PE-conjugated anti-CD3ɛ antibodies gated on EGFP-expressing cells. Data compiled from at least three consecutive independent experiments were analyzed.
Intracellular levels of CD3ɛ were measured by flow cytometry of Jurkat cells transfected with a vector coexpressing U24 and EGFP. Levels of CD3ɛ in U24/EGFP- and control EGFP-expressing cells were similar (Fig. 1B), indicating that while the TCR complex is downregulated from the cell surface, it is not degraded but rather sequestered within the cell.
CD3 downregulation by U24 does not correlate with T-cell activation.
T-cell activation is initiated when the TCR encounters a specific major histocompatibility complex-peptide complex. The interaction triggers the activation of two protein kinases, lck and zap70, which in turn initiate a cascade of events leading to the nuclear translocation of specific transcription factors—NFAT, AP1, and NF-κB—and the upregulation of CD69 (1). T-cell activation is self-limited by a rapid internalization and degradation of the TCR/CD3 complex (13). We asked whether U24 activates T cells, subsequently leading to the downregulation of the TCR/CD3 complex.
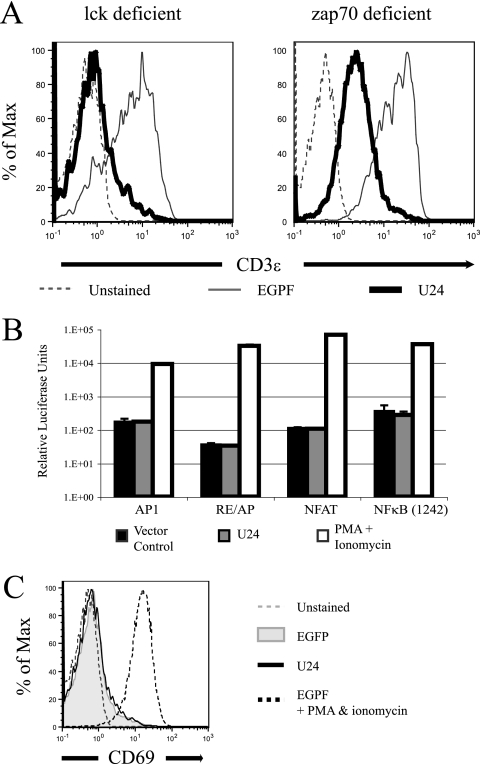
We first tested whether U24-mediated TCR/CD3 downregulation was dependent on the early mediators of TCR activation, lck and zap70. Jurkat cells deficient in either lck (JCaM cells) or zap70 (P116 cells) were transfected with a vector encoding U24, and CD3 surface expression was assessed by flow cytometry. As shown in Fig. 2A, U24 triggered CD3 downregulation in both cell lines, indicating that lck and zap70 are not involved in this process. Though zap70-deficient cells expressing U24 have higher levels of CD3 than lck-deficient cells, the percentages of downregulation are similar: 14.5% and 10.3% of total CD3, respectively.
FIG. 2.
U24 does not activate Jurkat T cells. (A) Jurkat cells deficient in either lck or zap70 were transiently transfected with U24/EGFP or EGFP alone. Twenty-four hours posttransfection, levels of surface CD3ɛ in cells displaying GFP expression were analyzed by flow cytometry. (B) Jurkat cells were cotransfected with firefly luciferase driven by promoters essential to T-cell activation and either U24 or vector alone. Experiments were performed in triplicate, and data were normalized with Renilla luciferase. (C) Jurkat cells were transfected with U24/EGFP or EGFP alone and were stained for CD69. Activated EGFP-transfected Jurkat cells were stimulated with PMA and ionomycin for 12 h before staining for CD69.
We then tested the ability of U24 to activate NFAT, AP1, or NF-κB in Jurkat cells expressing luciferase reporter constructs. While PMA and ionomycin induced a strong stimulation of luciferase expression, U24 had no effect, showing that the viral protein does not activate these transcription factors (Fig. 2B). Finally, we observed that CD69, an early marker of T-cell activation, was not upregulated in cells expressing U24. By contrast, PMA and ionomycin induced strong upregulation of CD69 expression at the cell surface (Fig. 2C). Taken together, these results indicate that U24 downregulates the CD3/TCR complex independently of the T-cell activation pathway.
CD3 downregulation is mediated by a block in receptor recycling back to the cell surface.
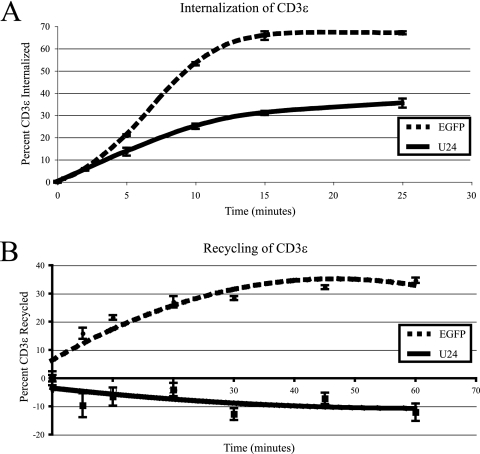
As with ligand-mediated endocytosis, U24 may be acting to enhance CD3 internalization from the cell surface. To measure CD3 internalization, cells coexpressing U24 and EGFP, or expressing EGFP alone, were incubated with a PE-conjugated anti-CD3ɛ antibody at 37°C for various times to allow for antibody-mediated endocytosis of CD3ɛ. Surface-associated antibody was removed by acid stripping, and internalized antibody levels were measured by flow cytometry. Surprisingly, cells expressing U24 showed decreased rates of CD3 internalization compared to that for the EGFP control (Fig. 3A). How could a lower rate of endocytosis result in downregulation from the cell surface? One possibility is that upon U24 expression, the CD3/TCR complex is blocked from recycling back to the plasma membrane, whereas the complex normally recycles constitutively in resting cells (18, 20). A block in CD3/TCR recycling back to the plasma membrane may also sequester factors necessary for the internalization of CD3ɛ, which would explain the lower rate of endocytosis observed in the presence of U24. In order to test this model, cells expressing either U24/EGFP or EGFP alone were incubated with a PE-conjugated anti-CD3ɛ antibody at 37°C to allow for antibody-mediated internalization. After 30 min, the antibody remaining at the cell surface was removed by acid stripping, and the internalized antibody was allowed to recycle back to the cell surface for various times. Cells were again subjected to acid stripping, and the amount of bound antibody retained within the cells was measured by flow cytometry. As shown in Fig. 3B, cells expressing U24 retained CD3 within the cells in contrast to cells expressing EGFP, indicating that U24 causes a block in CD3 recycling to the cell surface.
FIG. 3.
U24 impairs antibody-mediated internalization of CD3ɛ and inhibits recycling. (A) Jurkat cells were transfected with U24/EGFP or EGFP alone. After 24 h, cells were stained at 4°C with PE-conjugated anti-CD3ɛ antibodies, and unbound antibodies were washed away. Cells were then placed at 37°C and allowed to internalize for various times. Surface antibodies were removed with an acid wash, and mean fluorescence was determined by flow cytometry. (B) Jurkat cells were transfected with U24/EGFP or EGFP alone. After 24 h, cells were incubated with PE-conjugated anti-CD3ɛ antibodies at 37°C to allow for internalization. Surface-bound antibodies were stripped with an acid solution, and the cells were again incubated at 37°C. Samples were taken at various time points over a period of 1 h. Cells were again stripped, and the mean fluorescence was determined by flow cytometry.
U24 causes the exclusion of CD3 from recycling endosomes.
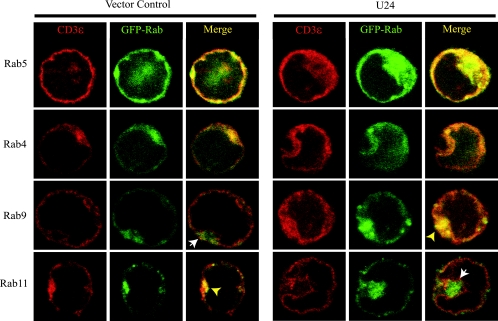
Rab proteins are small GTPases that are used as markers of endosomal compartments (45). Endosomal recycling involves the sorting of cargo to and from distinct organelles containing specific and distinct Rab proteins (27). Rab5 is associated with clathrin-coated pits and with early endosomes (15, 29). These Rab5-containing endosomes fuse with Rab4-containing sorting endosomes. If the receptors are to be recycled, the sorting endosomes then fuse with Rab11-containing recycling endosomes (37). If not, receptors are trafficked to Rab9-containing late endosomes (2, 21).
We used immunofluorescence to determine the endosomal compartment to which CD3ɛ is sequestered. EGFP-tagged Rab constructs were expressed in bicistronic transcripts with U24 (U24-Rab4-EGFP, U24-Rab5-EGFP, U24-Rab9-EGFP, and U24-Rab11-EGFP). U24 was functional in these constructs, as assessed by CD3 downregulation in transfected Jurkat cells (data not shown). Jurkat cells were transfected either with the bicistronic U24-Rab-EGFP constructs or with vectors encoding Rab-EGFP alone. Cells expressing high levels of EGFP were isolated by fluorescence-activated cell sorting. These cells were then fixed, permeabilized, stained with an anti-CD3ɛ antibody, and analyzed by confocal microscopy. As expected, cells that did not express U24 showed CD3ɛ in Rab5 and Rab4 early endosomes, as well as in the Rab11 recycling compartment, but were excluded from Rab9-containing late endosomes (Fig. 4, left panels). By contrast, in cells expressing U24, CD3ɛ was completely excluded from Rab11 recycling endosomes in more than three-fourths of cells observed (Fig. 4, right panels), while none of the cells in GFP controls showed this phenotype. CD3 was found mostly in Rab5- and Rab4-containing early endosomes and to a lesser extent in Rab9-containing late endosomes. These data indicate that U24 prevents the CD3 complex from entering recycling endosomes, likely producing decreased levels of CD3 at the cell surface.
FIG. 4.
U24 blocks CD3ɛ access to Rab11 recycling endosomes. Cells expressing GFP-Rab proteins and either U24/EGFP (right panels) or EGFP alone (left panels) were sorted for high GFP expression that corresponded to low surface CD3 expression. These cells were then fixed, permeabilized, and stained with an anti-CD3ɛ antibody (OKT3) and a rhodamine-conjugated secondary antibody. Cells were visualized using a Zeiss 510 META/NLO confocal microscope with a 63× objective, and images were magnified six times. Rab5 and Rab4 are markers of early and sorting endosomes, respectively. Rab9 is a marker of late endosomes, and Rab11 is a marker of recycling endosomes. Yellow arrows indicate areas of colocalization, while white arrows indicate areas of noncolocalization.
U24 inhibits T-cell activation in vitro.
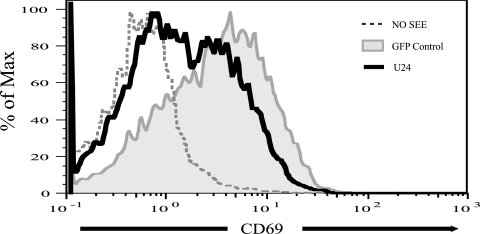
We next tested the functional significance of CD3 downregulation in T-cell stimulation accompanying antigen presentation. We used a model system that employs cultured human T- and B-cell lines and replaces conventional antigens with the superantigen SEE (7, 12). Our assay measures the ability of the BJAB B-cell line to stimulate Jurkat cells in the presence of SEE. Jurkat cells transiently transfected with either U24/EGFP or EGFP alone were cocultured with BJAB cells pulsed with SEE. Jurkat cell activation was assessed by measuring surface CD69 expression by flow cytometry in EGFP-positive cells. Jurkat cells expressing U24 (low level of CD3) showed low levels of CD69, whereas control cells (transfected with an EGFP-only vector) had strongly upregulated surface CD69 (Fig. 5). This result indicates that the level of CD3 downregulation induced by U24 results in a defect in T-cell function.
FIG. 5.
Upregulation of an early T-cell activation marker, CD69, is blocked in U24-expressing cells. Jurkat T cells expressing U24/EGFP or EGFP alone were incubated with SEE-pulsed or nonpulsed BJAB B cells for 6 h. EGFP-positive populations were analyzed for CD69 upregulation by flow cytometry. Max, maximum.
DISCUSSION
We have identified an ORF encoded by HHV-6 that causes downregulation of the TCR/CD3 complex by inhibiting its constitutive recycling. Interestingly, Lusso and colleagues found that in patients with active HHV-6 infection, virally infected T cells lacked CD3 at the cell surface (26). Upon closer inspection, abundant CD3 was found sequestered within these cells, matching the phenotype of U24 expression described in this paper. CD3 downregulation has also been seen in other cases of active HHV-6 infection in vivo (5) as well as in vitro (16). Lusso and colleagues showed that transcripts of various CD3 chains were downregulated upon HHV-6 infection (25). While transcriptional downregulation may play an important role in keeping TCR levels down, it cannot account for the full downregulation of surface CD3, since these molecules are abundantly found within infected cells. Furthermore, transcriptional downregulation of TCR alone would be a slow process, since the half-life of the TCR complex has been shown to be approximately 10.5 h (40). U24-mediated downregulation of the TCR is concomitant with U24 expression (5 h following transfection [data not shown]), consistent with an effect at the protein level. Thus, TCR downregulation by HHV-6A may be mediated by two separate mechanisms: a downregulation of protein from the cell surface and a block in new CD3 synthesis. However, the fast downregulation induced by U24 is more likely to be effective in infected cells during the limited time interval between infection and virus-mediated lysis.
The HHV-6A U24 ORF encodes a small protein with no known similarity to any cellular proteins. A string of hydrophobic residues indicates there may be a transmembrane region at the C terminus; however, we have not yet found any sequence similarity to any known domain structures in the N terminus. HHV-6B encodes a highly homologous protein (84% amino acid identity) and is therefore likely to behave similarly to the U24 in variant A, since both viral variants have been shown to downregulate the TCR complex (16). HHV-7 encodes a positional homologue with a predicted C-terminal transmembrane region, but it has only 28% identity with HHV-6A U24 protein. No conclusive evidence exists, as yet, as to whether HHV-7 downregulates TCRs. Both HHV-6 variants and HHV-7 primarily infect CD4+ T cells, and further study of the role of U24 in roseolavirus infection is warranted.
Several herpesviruses are able to modulate antigen receptor signaling. K1, encoded by Karposi's sarcoma-associated herpesvirus, has been shown to downregulate cell surface B-cell receptor BCR expression by blocking its transport to the cell surface (19). LMP2A, encoded by Epstein-Barr virus, blocks BCR signaling by inhibiting downstream tyrosine kinases (30). Herpesvirus saimiri, a gammaherpesvirus of New World monkeys, encodes Tip, which downregulates the TCR and CD4 coreceptor (32). However, K1, LMP2A, and Tip have also been shown to selectively activate lymphocyte signaling pathways (3). U24-mediated downregulation of CD3 is unique in the sense that it is not associated with T-cell activation. Moreover, the cells become refractory to activation upon stimulation by antigen-presenting cells. CD3 downregulation by U24 might thus represent a novel mechanism of immune evasion by blocking T-cell activation. Inhibiting T-cell activation will affect the release of cytokines and potentially dampen the generation of an adaptive immune response. In addition, lymphocyte activation is often associated with herpesvirus reactivation. By controlling the level of CD3, HHV-6 might prevent its own reactivation, thereby keeping HHV-6 in a latent state, which is less prone to immune recognition.
Consistent with this idea, Roffman and Frenkel have shown that pretreatment of cells with an anti-CD3 antibody that induces T-cell activation greatly increases viral replication (34). As a result of viral evolution, U24 may serve to keep HHV-6 titers low enough that they do not cause massive immune activation. Massive immune activation would cause viral titers to remain high, resulting in a positive feedback loop where the virus would activate the immune system and the active immune system would increase viral production. While HHV-6 does produce high titers in the initial infection, the virus has reached an equilibrium with its host by remaining persistent and relatively nonpathogenic in immune-competent individuals. CD3 downregulation might be an important aspect of this equilibrium. Microarray experiments performed by Yao et al. (44) showing that U24 is expressed after 8 h of a lytic infection are consistent with this hypothesis.
Interestingly, Schindler et al. found a correlation between the pathogenicity of SIV strains and their ability to downregulate the TCR (35a). The severity of CD4+ T-cell lymphopenia in sooty mangabeys infected with SIV strongly correlated with the strain's ability to downregulate the TCR. Sooty mangabeys with low CD4+ T-cell counts were found to be infected with SIV strains with decreased abilities to downregulate TCR, whereas animals with normal CD4+ T-cell counts were found to be infected with SIV strains that robustly downregulate the TCR. Furthermore, the ability to downregulate the TCR by different nef alleles from SIV and human immunodeficiency virus strains correlated with resistance to activation-induced cell death. Whether this is a common viral strategy between human immunodeficiency virus/SIV and HHV-6, both CD4+ T-tropic viruses, remains to be determined.
Acknowledgments
The authors gratefully acknowledge the Weiss lab for generously providing reagents used in this study as well as Jeroen Roose for his technical expertise. We are also grateful to Nadine Jarousse for critical discussions of the data and manuscript. Data for this study were acquired and analyzed at the CRL Molecular Imaging Center as well as the Flow Cytometry Facility at UC Berkeley.
Footnotes
Published ahead of print on 31 October 2007.
REFERENCES
- 1.Abraham, R. T., and A. Weiss. 2004. Jurkat T cells and development of the T-cell receptor signalling paradigm. Nat. Rev. Immunol. 4301-308. [DOI] [PubMed] [Google Scholar]
- 2.Barbero, P., L. Bittova, and S. R. Pfeffer. 2002. Visualization of Rab9-mediated vesicle transport from endosomes to the trans-Golgi in living cells. J. Cell Biol. 156511-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brinkmann, M. M., and T. F. Schulz. 2006. Regulation of intracellular signalling by the terminal membrane proteins of members of the Gammaherpesvirinae. J. Gen. Virol. 871047-1074. [DOI] [PubMed] [Google Scholar]
- 4.Campadelli-Fiume, G., P. Mirandola, and L. Menotti. 1999. Human herpesvirus 6: an emerging pathogen. Emerg. Infect. Dis. 5353-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Comar, M., P. D'Agaro, D. Horejsh, M. Galvan, S. Fiorentini, M. Andolina, A. Caruso, D. Di Luca, and C. Campello. 2005. Long-lasting CD3+ T-cell deficiency after cord blood stem cell transplantation in a human herpesvirus 6-infected child. J. Clin. Microbiol. 432002-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coscoy, L. 2007. Immune evasion by Kaposi's sarcoma-associated herpesvirus. Nat. Rev. Immunol. 7391-401. [DOI] [PubMed] [Google Scholar]
- 7.Coscoy, L., and D. Ganem. 2001. A viral protein that selectively downregulates ICAM-1 and B7-2 and modulates T cell costimulation. J. Clin. Investig. 1071599-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Bolle, L., L. Naesens, and E. De Clercq. 2005. Update on human herpesvirus 6 biology, clinical features, and therapy. Clin. Microbiol. Rev. 18217-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dewin, D. R., J. Catusse, and U. A. Gompels. 2006. Identification and characterization of U83A viral chemokine, a broad and potent beta-chemokine agonist for human CCRs with unique selectivity and inhibition by spliced isoform. J. Immunol. 176544-556. [DOI] [PubMed] [Google Scholar]
- 10.Dockrell, D. H. 2003. Human herpesvirus 6: molecular biology and clinical features. J. Med. Microbiol. 525-18. [DOI] [PubMed] [Google Scholar]
- 11.Fotheringham, J., D. Donati, N. Akhyani, A. Fogdell-Hahn, A. Vortmeyer, J. D. Heiss, E. Williams, S. Weinstein, D. A. Bruce, W. D. Gaillard, S. Sato, W. H. Theodore, and S. Jacobson. 2007. Association of human herpesvirus-6B with mesial temporal lobe epilepsy. PLoS Med. 4e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraser, J. D., M. E. Newton, and A. Weiss. 1992. CD28 and T cell antigen receptor signal transduction coordinately regulate interleukin 2 gene expression in response to superantigen stimulation. J. Exp. Med. 1751131-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geisler, C. 2004. TCR trafficking in resting and stimulated T cells. Crit. Rev. Immunol. 2467-86. [DOI] [PubMed] [Google Scholar]
- 14.Glosson, N. L., and A. W. Hudson. 2007. Human herpesvirus-6A and -6B encode viral immunoevasins that downregulate class I MHC molecules. Virology 365125-135. [DOI] [PubMed] [Google Scholar]
- 15.Gorvel, J. P., P. Chavrier, M. Zerial, and J. Gruenberg. 1991. rab5 controls early endosome fusion in vitro. Cell 64915-925. [DOI] [PubMed] [Google Scholar]
- 16.Grivel, J. C., F. Santoro, S. Chen, G. Faga, M. S. Malnati, Y. Ito, L. Margolis, and P. Lusso. 2003. Pathogenic effects of human herpesvirus 6 in human lymphoid tissue ex vivo. J. Virol. 778280-8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaworska, J., A. Gravel, K. Fink, N. Grandvaux, and L. Flamand. 2007. Inhibition of transcription of the beta interferon gene by the human herpesvirus 6 immediate-early 1 protein. J. Virol. 815737-5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krangel, M. S. 1987. Endocytosis and recycling of the T3-T cell receptor complex. The role of T3 phosphorylation. J. Exp. Med. 1651141-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee, B. S., X. Alvarez, S. Ishido, A. A. Lackner, and J. U. Jung. 2000. Inhibition of intracellular transport of B cell antigen receptor complexes by Kaposi's sarcoma-associated herpesvirus K1. J. Exp. Med. 19211-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu, H., M. Rhodes, D. L. Wiest, and D. A. Vignali. 2000. On the dynamics of TCR:CD3 complex cell surface expression and downmodulation. Immunity 13665-675. [DOI] [PubMed] [Google Scholar]
- 21.Lombardi, D., T. Soldati, M. A. Riederer, Y. Goda, M. Zerial, and S. R. Pfeffer. 1993. Rab9 functions in transport between late endosomes and the trans Golgi network. EMBO J. 12677-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loureiro, J., and H. L. Ploegh. 2006. Antigen presentation and the ubiquitin-proteasome system in host-pathogen interactions. Adv. Immunol. 92225-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lusso, P., R. W. Crowley, M. S. Malnati, C. Di Serio, M. Ponzoni, A. Biancotto, P. D. Markham, and R. C. Gallo. 2007. Human herpesvirus 6A accelerates AIDS progression in macaques. Proc. Natl. Acad. Sci. USA 1045067-5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lusso, P., and R. C. Gallo. 1995. Human herpesvirus 6 in AIDS. Immunol. Today 1667-71. [DOI] [PubMed] [Google Scholar]
- 25.Lusso, P., M. Malnati, A. De Maria, C. Balotta, S. E. DeRocco, P. D. Markham, and R. C. Gallo. 1991. Productive infection of CD4+ and CD8+ mature human T cell populations and clones by human herpesvirus 6. Transcriptional down-regulation of CD3. J. Immunol. 147685-691. [PubMed] [Google Scholar]
- 26.Lusso, P., P. D. Markham, E. Tschachler, F. di Marzo Veronese, S. Z. Salahuddin, D. V. Ablashi, S. Pahwa, K. Krohn, and R. C. Gallo. 1988. In vitro cellular tropism of human B-lymphotropic virus (human herpesvirus-6). J. Exp. Med. 1671659-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maxfield, F. R., and T. E. McGraw. 2004. Endocytic recycling. Nat. Rev. Mol. Cell Biol. 5121-132. [DOI] [PubMed] [Google Scholar]
- 28.McCullers, J. A., F. D. Lakeman, and R. J. Whitley. 1995. Human herpesvirus 6 is associated with focal encephalitis. Clin. Infect. Dis. 21571-576. [DOI] [PubMed] [Google Scholar]
- 29.McLauchlan, H., J. Newell, N. Morrice, A. Osborne, M. West, and E. Smythe. 1998. A novel role for Rab5-GDI in ligand sequestration into clathrin-coated pits. Curr. Biol. 834-45. [DOI] [PubMed] [Google Scholar]
- 30.Miller, C. L., J. H. Lee, E. Kieff, A. L. Burkhardt, J. B. Bolen, and R. Longnecker. 1994. Epstein-Barr virus protein LMP2A regulates reactivation from latency by negatively regulating tyrosine kinases involved in sIg-mediated signal transduction. Infect. Agents Dis. 3128-136. [PubMed] [Google Scholar]
- 31.Mookerjee, B. P., and G. Vogelsang. 1997. Human herpesvirus-6 encephalitis after bone marrow transplantation: successful treatment with ganciclovir. Bone Marrow Transplant. 20905-906. [DOI] [PubMed] [Google Scholar]
- 32.Park, J., B. S. Lee, J. K. Choi, R. E. Means, J. Choe, and J. U. Jung. 2002. Herpesviral protein targets a cellular WD repeat endosomal protein to downregulate T lymphocyte receptor expression. Immunity 17221-233. [DOI] [PubMed] [Google Scholar]
- 33.Rezaee, S. A., C. Cunningham, A. J. Davison, and D. J. Blackbourn. 2006. Kaposi's sarcoma-associated herpesvirus immune modulation: an overview. J. Gen. Virol. 871781-1804. [DOI] [PubMed] [Google Scholar]
- 34.Roffman, E., and N. Frenkel. 1991. Replication of human herpesvirus-6 in thymocytes activated by anti-CD3 antibody. J. Infect. Dis. 164617-618. [DOI] [PubMed] [Google Scholar]
- 35.Sandhoff, T., J. P. Kleim, and K. E. Schneweis. 1991. Latent human herpesvirus-6 DNA is sparsely distributed in peripheral blood lymphocytes of healthy adults and patients with lymphocytic disorders. Med. Microbiol. Immunol. 180127-134. [DOI] [PubMed] [Google Scholar]
- 35a.Schindler, M., J. Munch, O. Kutsch, H. Li, M. L. Santiago, F. Bibollet-Ruche, M. C. Muller-Trutwin, F. J. Novembre, M. Peeters, V. Courgnaud, E. Bailes, P. Roques, D. L. Sodora, G. Silvestri, P. M. Sharp, B. H. Hahn, and F. Kirchhoff. 2006. Nef-mediated suppression of T cell activation was lost in a lentiviral lineage that gave rise to HIV-1. Cell. 1251055-1067. [DOI] [PubMed] [Google Scholar]
- 36.Soldan, S. S., R. Berti, N. Salem, P. Secchiero, L. Flamand, P. A. Calabresi, M. B. Brennan, H. W. Maloni, H. F. McFarland, H. C. Lin, M. Patnaik, and S. Jacobson. 1997. Association of human herpesvirus 6 (HHV-6) with multiple sclerosis: increased IgM response to HHV-6 early antigen and detection of serum HHV-6 DNA. Nat. Med. 31394-1397. [DOI] [PubMed] [Google Scholar]
- 37.Sönnichsen, B., S. De Renzis, E. Nielsen, J. Rietdorf, and M. Zerial. 2000. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J. Cell Biol. 149901-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi, K., S. Sonoda, K. Higashi, T. Kondo, H. Takahashi, M. Takahashi, and K. Yamanishi. 1989. Predominant CD4 T-lymphocyte tropism of human herpesvirus 6-related virus. J. Virol. 633161-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torre, D., F. Speranza, R. Martegani, P. Ferrante, E. Omodeo-Zorini, R. Mancuso, and G. P. Fiori. 1998. Meningoencephalitis caused by human herpesvirus-6 in an immunocompetent adult patient: case report and review of the literature. Infection 26402-404. [DOI] [PubMed] [Google Scholar]
- 40.von Essen, M., C. M. Bonefeld, V. Siersma, A. B. Rasmussen, J. P. Lauritsen, B. L. Nielsen, and C. Geisler. 2004. Constitutive and ligand-induced TCR degradation. J. Immunol. 173384-393. [DOI] [PubMed] [Google Scholar]
- 41.Vossen, M. T., E. M. Westerhout, C. Soderberg-Naucler, and E. J. Wiertz. 2002. Viral immune evasion: a masterpiece of evolution. Immunogenetics 54527-542. [DOI] [PubMed] [Google Scholar]
- 42.Ward, K. N. 2005. Human herpesviruses-6 and -7 infections. Curr. Opin. Infect. Dis. 18247-252. [DOI] [PubMed] [Google Scholar]
- 43.Yamanishi, K., T. Okuno, K. Shiraki, M. Takahashi, T. Kondo, Y. Asano, and T. Kurata. 1988. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet i1065-1067. [DOI] [PubMed] [Google Scholar]
- 44.Yao, K., M. Mandel, N. Akyani, K. Maynard, N. Sengamalay, J. Fotheringham, E. Ghedin, F. Kashanchi, and S. Jacobson. 2006. Differential HHV-6A gene expression in T cells and primary human astrocytes based on multi-virus array analysis. Glia 53789-798. [DOI] [PubMed] [Google Scholar]
- 45.Zerial, M., and H. McBride. 2001. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2107-117. [DOI] [PubMed] [Google Scholar]
- 46.Zou, P., Y. Isegawa, K. Nakano, M. Haque, Y. Horiguchi, and K. Yamanishi. 1999. Human herpesvirus 6 open reading frame U83 encodes a functional chemokine. J. Virol. 735926-5933. [DOI] [PMC free article] [PubMed] [Google Scholar]