Abstract
Glycans on human immunodeficiency virus (HIV) envelope protein play an important role in infection and evasion from host immune responses. To examine the role of specific glycans, we introduced single or multiple mutations into potential N-linked glycosylation sites in hypervariable regions (V1 to V3) of the env gene of HIV type 1 (HIV-1) 89.6. Three mutants tested showed enhanced sensitivity to soluble CD4. Mutant N7 (N197Q) in the carboxy-terminal stem of the V2 loop showed the most pronounced increase in sensitivity to broadly neutralizing antibodies (NtAbs), including those targeting the CD4-binding site (IgG1b12) and the V3 loop (447-52D). This mutant is also sensitive to CD4-induced NtAb 17b in the absence of CD4. Unlike the wild-type (WT) Env, mutant N7 mediates CD4-independent infection in U87-CXCR4 cells. To study the immunogenicity of mutant Env, we immunized pig-tailed macaques with recombinant vaccinia viruses, one expressing SIVmac239 Gag-Pol and the other expressing HIV-1 89.6 Env gp160 in WT or mutant forms. Animals were boosted 14 to 16 months later with simian immunodeficiency virus gag DNA and the cognate gp140 protein before intrarectal challenge with SHIV89.6P-MN. Day-of-challenge sera from animals immunized with mutant N7 Env had significantly higher and broader neutralizing activities than sera from WT Env-immunized animals. Neutralizing activity was observed against SHIV89.6, SHIV89.6P-MN, HIV-1 SF162, and a panel of subtype B primary isolates. Compared to control animals, immunized animals showed significant reduction of plasma viral load and increased survival after challenge, which correlated with prechallenge NtAb titers. These results indicate the potential advantages for glycan modification in vaccine design, although the role of specific glycans requires further examination.
The failure to induce broadly neutralizing antibodies (NtAbs) against primary isolates of human immunodeficiency virus (HIV) remains a major impediment to the development of effective vaccines against AIDS. Although HIV type 1 (HIV-1) envelope proteins have been targeted for vaccine development for over two decades, immunity induced by early vaccines has been effective only against laboratory-adapted isolates (10, 17, 32, 45) or chimeric viruses (SHIV) bearing the homologous env genes (54, 68). In these instances, protection has been correlated with high-titer NtAbs directed to the V3 hypervariable region of gp120. However, the neutralizing activities generated are largely isolate specific and are minimally active against most primary isolates of HIV-1 (8, 19, 53, 71). The failure of subunit gp120 vaccines in phase III clinical trials to protect against HIV-1 acquisition or to lower viral load in those who did become infected (41, 44) underscores the difficulty of the task.
Multiple mechanisms may contribute to the neutralization resistance of primary HIV-1. Studies in simian immunodeficiency virus (SIV) and other lentiviruses indicate that development of neutralization resistance is accompanied by cumulative changes in the hypervariable regions of the envelope antigens (21, 24, 31, 53, 60, 79, 80, 93, 97, 102, 110). Such changes not only alter the tropism and phenotype of the virus but often its pattern of glycosylation (14, 79, 93, 110). The envelope antigens of HIV-1, like those of other lentiviruses, are extensively glycosylated. The surface antigen gp120 contains both N-linked and O-linked glycans, contributing to nearly 50% of its molecular mass (11, 43, 51). These carbohydrates play an important role in the structure and function of the envelope glycoproteins, including virus assembly (49), receptor and coreceptor binding (63, 67, 73), and syncytium formation (52). In addition, multiple studies have shown that carbohydrate moieties on viral envelope modulate its antigenicity and the sensitivity of the virus to NtAbs (5, 28, 58, 64, 74, 92). However, such an effect could be enhancing or interfering, depending on the specific combination of antibody and glycan involved.
Despite considerable evidence indicating the role of glycosylation in modulating Env antigenicity, relatively few have addressed its potential role in influencing the immunogenicity of HIV-1 envelope proteins. Haigwood et al. (50) compared immune responses generated by gp120 produced in mammalian (native and glycosylated) versus those produced in yeast cells (denatured and nonglycosylated) and found that the native structure is superior in inducing broad-spectrum NtAbs in baboons. Benjouad et al. (9) analyzed antibodies raised against native or deglycosylated forms of gp120 generated by various enzymatic reactions and found that only the native or the desialylated form of gp160, but not the alpha-mannosidase-treated form, was able to induce NtAbs against the homologous lab-adapted virus HIV-1 LAI. Both investigators compared immunogens that have global differences in their glycosylation pattern and perhaps overall structure. Others have examined the effects of site-specific deglycosylations but have not observed any major difference between immune responses elicited by wild-type (WT) versus modified envelope proteins (15, 16, 20, 85). On the other hand, Desrosiers and coworkers (90, 91) reported a significant increase in antigenicity and immunogenicity of mutant forms of SIV depleted of N-linked glycans in the V1 region of its envelope proteins. Immunization with a multiply deglycosylated SIVmac239 Env, however, failed to protect against homologous virus challenge (77).
In the present study, we sought to extend earlier observations by focusing on three N-linked glycan mutants in the V2 and V3 regions of HIV-1 gp120 that showed increased neutralization sensitivity. We examined the effect of glycan modifications on viral infectivity, as well as the antigenicity and immunogenicity of the envelope glycoproteins. The results from these studies indicate that specific N-linked glycan modifications may have major effects on viral infectivity, sensitivity to NtAbs, and the ability of the envelope protein to elicit cross-reactive NtAb responses.
MATERIALS AND METHODS
Cells.
The U87 human astroglioma cell line stably transduced with human CXCR4 (34) was cultured in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/ml), streptomycin (100 μg/ml), glutamine (2 mM), and selected with 1 μg of puromycin/ml. The U87-CD4-CXCR4 cells obtained through the AIDS Research and Reference Reagent Program (ARRRP; catalog no. 4036) were contributed by HongKui Deng and Dan R. Littman (13) and were cultured in the same medium containing 15% of FBS and selected with puromycin (1 μg/ml) plus G418 (300 μg/ml). JC53-BL cells (referred to as TZM-bl cells here) contributed by John Kappes and Xiaoyun Wu (111) were also obtained from the National Institutes of Health (NIH) ARRRP (catalog no. 8129). Both TZM-bl and 293T (ATCC catalog no. 11268) cells were cultured in Dulbecco modified Eagle medium supplemented with 10% of FBS, penicillin (100 U/ml), streptomycin (100 μg/ml), and glutamine (2 mM). The CEMx174 cells (98) were maintained as a suspension culture in RPMI 1640 medium with 10% FBS plus penicillin, streptomycin, and glutamine.
Construction of N-linked glycosylation site mutants.
Plasmid pSL1180 containing the rev-env gene of HIV-1 89.6 was used as the template for site-directed mutagenesis with a QuikChange mutagenesis kit (Stratagene). A conservative substitution from asparagine (N) to glutamine (Q) was introduced in potential N-linked glycosylation sites (N-X-S/T) in variable regions V1/V2 and V3 of the env gene (Fig. 1). The primers used for the mutagenesis were as follows: for the N6 mutant, 5′-GTAGTACCAATAGAAAATACTCAGAATACTAAGTATAGG-3′ (forward) and 5′-CCTATACTTAGTATTCTGAGTATTTTCTATTGGTACTAC-3′ (reverse); for the N7 mutant, 5′-GGTTAATAAGTTGTCAGACCTCAGTCATTACACAGGCC-3′ (forward) and 5′-GGCCTGTGTAATGACTGAGGTCTGACAACTTATTAACC-3′ (reverse); and for the NV3 mutant, 5′-GTACAAGACCCAACCAGAATACAAGAAGAAGGTTATC-3′ (forward) and 5′-GATAACCTTCTTCTTGTATTCTGGTTGGGTCTTGTAC-3′ (reverse). The N67V3 mutation was made by inserting the BglII fragment (nucleotides 7164 to 7729) with the NV3 mutation into the N67 containing plasmid. The mutated rev-env genes were then subcloned into mammalian expression vector pCI-neo. The PCR cycling parameters for the mutagenesis included two steps: step 1 (95°C for 30 s) and step 2, 18 cycles of 95°C for 30 s followed by 52°C for 1 min, and then 68°C for 18 min. The results of site-directed mutagenesis were verified by DNA sequencing.
FIG. 1.
N-linked glycan mutants in HIV-1 Env. Potential N-linked glycosylation sites in HIV-1 89.6 surface antigen gp120 are indicated by “⋆” symbols (complex type) and “×” symbols (high-mannose type). The positions of mutants N1-N7 and NV3 relative to variable loops V1 to V5 are indicated at the top. Amino acid sequence of V2 and V3 loops and the specific sites changed in mutants N6, N7, and NV3 are indicated at the bottom.
Monoclonal antibodies.
The following monoclonal antibodies were obtained from NIH ARRRP: 4E10 (103), 2F5 (18, 83, 84), and 2G12 (18, 108) were from Kermann Katinger; IgG1b12 was from Dennis Burton and Carlos Barbas (6, 22, 23, 94); 447-52D (30, 46-48, 78, 118) was from Susan Zolla-Pazner; and 17b (65, 104, 106, 107, 112, 113) was from James E. Robinson. Recombinant soluble CD4-183, also obtained from the same source, contains the first two domains of human CD4 produced in Escherichia coli (42).
Coreceptor usage analysis.
A complementation assay with pseudotyped HIV-1 was performed as previously described (67, 74). Briefly, pseudotyped viruses carrying wild-type (WT) or mutant 89.6 envelope glycoproteins were produced by 293T cells after cotransfection with pCI-neo plasmid expressing the rev and the env gene of interest and an env-deficient HIV-1 backbone vector pNL4.3LucR−E− expressing the firefly luciferase gene (contributed by N. Landau and obtained from the ARRRP). Two days after transfection, culture supernatants containing pseudotyped virus were collected and filtered through a 0.45-μm-pore-size filter before use for infectivity assays in U87-CXCR4 or U87-CD4-CXCR4 cells. Cells were seeded at 104 cells per well in a 96-well plate overnight and treated with Polybrene (2 μg/ml) for 30 min prior to infection by pseudotyped viruses. Two hours after inoculation, the inoculum was replaced with 200 μl of fresh medium without any antibiotics, and infection continued for a total of 48 h. Infectivity was measured as relative luminescence units (RLU) in infected cell cultures quantified by the SteadyLite HTS luminescence reporter gene assay system (Perkin-Elmer). To compare the relative infectivity of WT and mutant 89.6 in U87-CXCR4 cells, we used inoculum containing the same infectivity (RLU = 2.0 × 105) as determined in U87-CD4-CXCR4 cells.
Infectivity and neutralization assay.
Infectivity of replication competent viruses, or with Env-pseudotyped viruses, was measured by luciferase reporter gene expression in TZM-bl cells. Neutralization as measured by reduction of luciferase gene expression was performed as previously described (64, 76). Briefly, indicator virus containing 200 50% tissue culture infective doses (TCID50) (unless otherwise indicated) was incubated either with a single dilution (1:15) or with serial threefold dilutions of serum samples in triplicate in a total volume of 150 μl for 1 h at 37°C in 96-well flat-bottom culture plates. Freshly trypsin-treated cells (10,000 cells in 100 μl of growth medium containing 75 μg of DEAE dextran/ml) were added to each well. One set of control wells received cells plus virus (virus control), and another set received cells only (background control). After a 48-h incubation, 100 μl of cells were transferred to a 96-well black solid plates (Costar) for assays of luciferase activities by using BrightGlo substrate solution as described by the supplier (Promega). Assay stocks of Env-pseudotyped viruses were prepared by transfection in 293T cells (66), whereas stocks of the replication-competent viruses SHIV 89.6, SHIV 89.6P-MN, and HIV-1 SF162 were prepared in human peripheral blood mononuclear cells. All assay stocks were titrated in TZM-bl cells as described previously (66). Neutralization activity was expressed either as the percent reduction of RLU for experiments with single (1:15) serum dilutions or as the serum dilution that resulted in 50% reduction of RLU in serial dilution experiments. Values obtained with preimmune sera were subtracted from those obtained with immune sera for each animal.
Immunogens.
Four recombinant vaccinia viruses were used in the present study: (i) recombinant v-ELgp160(89.6) expresses the full-length WT env gene of HIV-1 89.6 (29); (ii) v-ELgp160(89.6)N7 expresses the same env gene with a single glycan mutation N7; (iii) v-ELgp160(89.6)N67V3 expresses the env gene with triple glycan mutations N6, N7, and NV3; and (iv) recombinant v-ELgag/pol(SIVmac239) expresses the native gag-pol genes of SIVmac239. Expression of the transgene was under the control of a synthetic early-late promoter of vaccinia virus (25). The transgene was inserted into the thymidine kinase gene of the WR strain of vaccinia virus. Construction and propagation of these viruses was as previously described (55-57, 115). Expression of the transgene was verified by Western blot analysis. Plasmid DNA expressing codon-optimized SIVmac239 gag gene was kindly provided by J. Shiver (7, 39) and was prepared with EndoFree Maxiprep kit (Qiagen). Protein immunogens used in the present study were produced in African green monkey kidney cells (BSC-40) infected with recombinant vaccinia virus expressing SIVmac239 Gag-Pol particles, or HIV-1 89.6 Env gp140, in WT, N7, or N67V3 forms. SIVmac239 Gag-Pol particles were purified from infected cell supernatant by ultracentrifugation as described previously (56, 82). Recombinant vaccinia viruses expressing HIV-1 89.6 Env gp140 were constructed as described above, with the exception that two stop codons were introduced to substitute amino acids 680 and 681 (isoleucine and arginine) immediately upstream of the hydrophobic anchor sequence in gp41. WT or mutant gp140 was purified from infected BSC-40 cell supernatant by lentil lectin affinity and size exclusion chromatography as described previously (61).
Immunization.
Twenty-four juvenile pig-tailed macaques (M. nemestrina), all of which tested negative for simian type D retrovirus by serology and PCR, were used for the present study. Macaques in the experimental arm (total of 18, in three groups of 6 each) were inoculated by skin scarification at weeks 0 and 8 with 108 PFU of two recombinant viruses, one expressing SIVmac239 Gag-Pol and the other expressing one of three forms of HIV-1 89.6 Env (WT, N7, or N67V3). All animals were boosted with DNA plasmid expressing SIV Gag (39) and the cognate gp140 Env protein (WT, N7, or N67V3). DNA (5.0 mg/dose in phosphate-buffered saline) was administered by intramuscular injection at four sites. Env gp140 (100 μg/dose) was adjuvanted with 0.025% Alhydrogel and was also administered intramuscularly. Control animals (n = 6) were primed with parental vaccinia virus WR and boosted with vector plasmid and adjuvant only. To accommodate the large size of the study, we performed the booster immunization (and subsequent challenge) in two stages, separated by 2 months. Animals in groups 1 and 2 were rested for 16 months between the second vaccinia virus inoculation and the final immunization, whereas those in group 3 and the control group were rested for 14 months.
SHIV challenge.
Four weeks after the booster immunization, all macaques were challenged intrarectally with SHIV89.6P-MN. The challenge virus was derived from a macaque-passaged SHIV89.6P stock (89) (kindly provided by N. Letvin) after two passages in CD8+-depleted peripheral blood mononuclear cells from M. nemestrina (37). Intrarectal inoculation was performed atraumatically twice, 1 h apart, with 1 ml of the undiluted challenge virus containing 25 50% animal infectious doses. Animals were monitored for body weight, temperature, and general health, and tissue samples were collected periodically for in vitro analyses. Euthanasia was performed according to the following criteria: the presence of AIDS, termination of experiment, or deteriorating physical condition for reasons unrelated to the infection. Euthanasia was considered to be AIDS related if the animal exhibited peripheral blood CD4+ T-cell depletion (<200/mm3) and two or more of the following conditions: wasting, untreatable diarrhea, opportunistic infections, proliferative diseases (e.g., lymphoma), and abnormal hematology (e.g., anemia, thrombocytopenia, or leukopenia). All procedures involving live animals or tissues were performed with the approval of the Institutional Animal Care and Use Committee.
Viral load determination.
Plasma viral load was determined by real-time reverse transcription-PCR (RT-PCR) based on methods originally described by Suryanarayana et al. (105). Briefly, viral RNA was prepared from EDTA-anticoagulated, cell-free plasma by using a Gentra Puregene RNA isolation kit according to the manufacturer's instructions (Gentra Systems). RNA was precipitated in the presence of glycogen, resuspended in 50 μl of nuclease-free water, and analyzed immediately. Oligonucleotides were chosen within the SIV gag sequence. The primers GAG5f (5′-ACTTTCGGTCTTAGCTCCATTAGTG-3′) and GAG3r (5′-TTTTGCTTCCTCAGTGTGTTTCA-3) and the TaqMan probe GAG1tq (5′-TTCTCTTCTGCGTGAATGCACCAGATGA-3′) were all obtained from Applied Biosystems. In the probe the fluorescence reporter dye at the 5′ end was FAM (6-carboxyfluorescein), and the quencher dye at the 3′ end was TAMRA (6-carboxytetramethyl-rhodamine). A two-step RT-PCR using the TaqMan Gold RT-PCR kit (Applied Biosystems) was performed. The control template is an in vitro transcript containing a KpnI-BamHI SIV gag fragment from SIVmac239, prepared from the plasmid pSIV-BS6 kindly provided by Jeff Lifson. RNA transcripts were diluted in nuclease-free water and stored at −80°C in single-use aliquots. For each run, an RNA standard curve was generated from triplicate samples of purified pSIV-BS-derived in vitro transcript, ranging from 3 × 106 to nominal copy equivalents/reaction. For each test sample, four reactions were run. Triplicate aliquots were reverse transcribed and amplified, plus one aliquot that was processed without the addition of reverse transcriptase. The performance of the assay showed at least 5 log10 linear dynamic range, with typical R2 values for plots of threshold cycles versus log10 input copy number of >0.98.
Immunophenotype and hematologic analyses.
Absolute numbers of circulating CD3/4+ cells are determined via a two part process according to Centers for Disease Control and Prevention regulations for determining circulating CD3/4+ cells. Briefly, 1 ml of whole blood was treated with 14 ml of ammonium chloride lysis solution for 7 min, after which the mixture was centrifuged for 5 min at 700 × g, and the supernatant was discarded. The resultant cell pellet was then resuspended in 1 ml of staining medium (RPMI supplemented with 1% FBS and 0.02% NaN3), and 50-μl aliquots of the cell suspension were then tristained with fluorescein isothiocyanate-labeled anti-CD3 (clone SP34-2), PerCp-Cy5.5-labeled anti-CD4 (clone L200), and allophycocyanin-labeled anti-CD8 (clone SK1) and doubled stained with fluorescein isothiocyanate-labeled anti-CD2 (clone S5.2) and PerCP-Cy5.5-labeled anti-CD20 (clone L27); unstained cells were used as staining controls. All antibodies were purchased from Becton Dickinson Immunocytometry Systems. Cells were incubated in the dark for 20 to 30 min, washed with 200 μl of PBS, and then transferred to fluorescence-activated cell sorting tubes (Falcon) in a final volume of 230 μl of 1% paraformaldehyde and run on a four-color flow cytometer (FACSCalibur; Becton Dickinson Immunocytometry Systems), where a minimum of 10,000 gated lymphocyte events were collected. The absolute numbers of CD3+/4+ and other relevant T- and B-cell populations were determined via offline analysis using flow cytometry analysis software according to Centers for Disease Control and Prevention guidelines for T-cell determinations in HIV-infected individuals.
Statistical analysis.
SPSS version 10.0 (SPSS, Inc.), S-Plus 2000 (Insightful, Inc.), or Prism (GraphPad Software, Inc.) was employed for all statistical analyses, all using two-sided tests. The Mann-Whitney U test was used to compare NtAb responses in the three experimental groups to those in the control group. The independent sample t test was used to compare the log10 peak viral load, log10 set point viral load, and log10 CD4+ T-cell counts between the experimental and the control groups. Spearman correlation coefficients were used to assess the relationship between prechallenge NtAb levels and various markers of disease progression. Finally, Kaplan-Meier survival analysis, the log-rank test, and Cox proportional hazards regression were used to compare AIDS-free survival rates in all groups.
RESULTS
Neutralization sensitivity of glycan mutants of HIV-1 89.6.
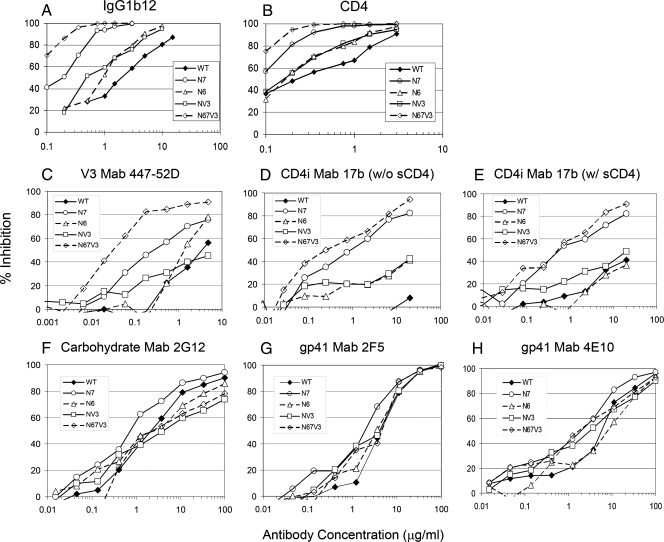
To examine the role of specific glycans in the antigenicity and immunogenicity of HIV-1 Env protein, we introduced mutations substituting the asparagine (N) residue with glutamine (Q) in potential N-linked glycosylation sites (N-X-T/S) in the envelope protein of HIV-1 89.6. Our initial effort included all N-linked glycans in the hypervariable V1, V2, and V3 regions of the surface antigen gp120. A detailed analysis of these mutants will be described elsewhere (Y. Li et al., unpublished data). For the present study, we focused on mutants that meet the following criteria: (i) mutant protein is expressed and processed normally; (ii) mutant protein retains overall functional integrity as indicated by successful rescue as viable virus; and (iii) mutant virus has enhanced susceptibility to broadly NtAbs. Three such mutants were identified: N6 (N187Q); N7 (N197Q), and NV3 (N301Q) (67). Compared to the WT 89.6, all three mutants showed enhanced sensitivity to a broadly neutralizing monoclonal antibody IgG1b12 (23) and to recombinant sCD4-Ig (1) (Fig. 2A and B). Among all of the mutants tested, N7 showed the most pronounced enhancement in neutralization sensitivity to IgG1b12 (∼30-fold) and sCD4-Ig (∼15-fold) (Table 1). Combinations of these mutations (N67V3) resulted in even greater sensitivity to these antibodies than single mutants (Fig. 2A and B and Table 1). We further characterized these mutants (three single mutants and one triple) against a panel of neutralizing monoclonal antibodies. As shown in Fig. 2C, virus pseudotyped with the N7 Env showed increased sensitivity to monoclonal antibody 447-52D, which recognizes the highly conserved sequence in the tip of the V3 loop. Neither the N6 nor the NV3 mutation affected the susceptibility to 447-52D. However, the triple mutant N67V3 showed even greater sensitivity than N7 to the same antibody, indicating the additive effect of the individual mutations (Table 1).
FIG. 2.
Neutralization sensitivity of WT HIV-1 89.6 and N-linked glycan mutants. Neutralization of pseudotyped virus generated with WT or mutant HIV-1 89.6 Env was performed as described in Materials and Methods. Neutralization was quantified as the percent inhibition of viral infectivity measured by RLU expressed in TZM-bl indicator cells. The data shown are representative results obtained from three individual experiments.
TABLE 1.
IC50 of a panel of monoclonal antibodies against HIV-1 89.6 pseudotyped with WT or mutant Env
| Env | IC50 (μg/ml)a
|
|||||||
|---|---|---|---|---|---|---|---|---|
| CD4b
|
V3 (447-52D) | CD4i
|
Mannan (2G12) | gp41 MPER
|
||||
| IgG1b12 | CD4-IgG | 17b without sCD4 | 17b with sCD4b | 2F5 | 4E10 | |||
| WT | 0.46 | 0.94 | 3.84 | >20.00 | >20.00 | 2.37 | 4.49 | 6.62 |
| N6 | 0.138 | 0.52 | 1.18 | >20.00 | >20.00 | 2.64 | 3.6 | 8.81 |
| N7 | 0.0163 | 0.065 | 0.31 | 1.01 | 0.63 | 0.75 | 2.26 | 2.22 |
| NV3 | 0.143 | 0.52 | >4.84 | >20.00 | >20.00 | 4.83 | 4.48 | 3.3 |
| N67V3 | 0.0069 | 0.047 | 0.037 | 0.24 | 0.59 | 1.61 | 5.2 | 1.97 |
Input virus was normalized by their infectivity at 100 TCID50 in TZM-bl cells. The IC50 is defined as the concentration at which 50% reduction of the viral infectivity was achieved. These results represent the average of two or more experiments. The column subheadings indicate the respective epitopes and monoclonal antibodies.
A subneutralizing concentration of sCD4 was used for these assays. Since the WT and mutant viruses exhibit different sensitivities to sCD4, the concentration of sCD4 used was determined empirically for each indicator virus: WT, 0.15 μg/ml; N6, 0.02 μg/ml; N7, 0.002 μg/ml; NV3, 0.02 μg/ml; and N67V3, 0.001 μg/ml.
Mutants similar to N7 have been introduced in HIV-1 ADA strain by Kolchinsky et al. (63, 64) and been found to have enhanced sensitivity to a number of neutralizing monoclonal antibodies, including those targeting CD4 binding site, V3, CD4-induced (CD4i), and gp41-specific membrane proximal ectodomain region (MPER) epitopes. We therefore examined mutants described here for their sensitivity to CD4i antibody 17b, carbohydrate-dependent antibody 2G12 (108), and MPER antibodies 2F5 and 4E10 (103). As shown in Fig. 2D, all four mutants tested showed increased sensitivity to 17b compared to WT 89.6 in the absence of sCD4, indicating accessibility of this epitope normally obscured in the unliganded form of gp120. Again, N7 and the triple mutant showed the most pronounced effect. The presence of sCD4 at subneutralizing concentrations augmented the neutralizing activities of 17b against the WT 89.6 but had minimal effect on the sensitivity of the glycan mutants (Fig. 2E). It is of interest that, even in the absence of sCD4, both the N7 and the triple mutants were >20-fold more sensitive to 17b than the WT or the N6 and NV3 mutants in the presence of sCD4 (Table 1), indicating that the N197 glycan plays a major role in controlling access to the epitope recognized by 17b.
The N7 mutant also showed a modest increase (∼3-fold) in neutralization sensitivity to monoclonal antibody 2G12 (Fig. 2F), which recognizes a mannan-dependent epitope in gp120. In contrast, the N6 mutant showed little effect, and the NV3 mutant showed a modest decrease (two- to threefold) in sensitivity to the same antibody, a finding consistent with the differential role of N-linked glycans in modulating the sensitivity to 2G12 (99). Together, the triple mutant N67V3 showed little change from WT in its sensitivity to 2G12, indicating the additive effect of these mutations. None of the mutants tested had any major effect on neutralization by 2F5 (Fig. 2G). However, the N7 and the triple mutants consistently showed a ∼3-fold increase in sensitivity to another MPER epitope in gp41, 4E10 (Fig. 2G and Table 1). The N7 mutant also showed enhanced neutralization sensitivity to polyclonal HIV-1 positive human sera but was not neutralized by monoclonal antibody directed to the V1-V2 loop of gp120 from heterologous isolates (data not shown). Together, these results indicate that all three N-linked glycans that we examined can modulate the neutralization sensitivity of HIV-1 89.6. However, the major effect was exerted by the N197 glycan located just outside of the carboxy terminus of the V2 loop.
CD4-independent infection by the N197Q mutant.
The observation that all three glycan mutants tested showed enhanced neutralization by 17b in the absence of sCD4 suggests that these mutants may have acquired a CD4-independent phenotype. This effect was previously reported by Kolchinsky et al. (63) for similar mutants in a CCR5-tropic strain ADA. In the present study, we tested all four glycan mutants described for their ability to mediate infection in the presence or absence of CD4. The infectivity of replication-competent SHIV 89.6 and marker-rescued Env mutant viruses was evaluated in TZM-bl (Fig. 3A). Mutants N6 and N7 retained WT levels of infectivity, as shown by the similar levels of reporter gene expression per unit of p27 input virus. The infectivity of mutants NV3 was reduced by 40%, a finding consistent with our previous observations (67). A significant reduction (80%) in infectivity was observed for the triple mutant N67V3 (Fig. 3A). Because the Env of 89.6 uses primarily CXCR4 as the coreceptor, we measured the infectivity of HIV-1 pseudotyped with WT or mutant envelope in U87-CXCR4 cells, using inocula normalized for their infectivity in isogenic CD4-expressing U87-CXCR4-CD4 cells. The results in Fig. 3B indicate that the relative infectivity of the N7 mutant was 10-fold higher than the WT virus in the absence of CD4, confirming the CD4-independent phenotype of the mutant Env. While the N6 mutant inside the V2 loop showed little effect on CD4-dependence, the NV3 mutant consistently showed a two- to threefold increase in infectivity in CD4-negative cells compared to WT. Combination of the N7 and the NV3 mutations (e.g., N67V3) resulted in further increases in infectivity in CD4-negative cells. These findings indicate that glycans at both the N7 and, to a lesser extent, the NV3 sites modulate the accessibility to the coreceptor binding site(s) on gp120 and that their effect appears to be additive.
FIG. 3.
(A) Infectivity of replication competent WT and mutant SHIV 89.6 in TZM-bl cells. The amount of input virus was normalized by the p27 level in the inoculum and was determined to be in the linear range of infectivity as measured by luciferase gene expression. Values on the y axis represent RLU (104). (B) CD4-independent infection by glycan mutants. The infectivity of HIV-1 pseudotyped with WT or mutant Env was measured in U87 cells expressing CXCR4. The inoculum used for each mutant virus was normalized by the infectivity in U87-CXCR4-CD4 cells measured at 200,000 RLU. Values on the y axis in this panel represent RLU (103). The results in both panels represent the average and standard deviation obtained from three individual experiments.
Immunogenicity of WT and mutant Env.
The observations described above suggested that removal of one or more N-linked glycans results in the unmasking of several important targets in the envelope protein. These include epitopes for broadly NtAbs and receptor-binding site(s). To test whether such glycan mutants can also affect the immunogenicity of HIV-1 89.6 Env protein, we immunized pig-tailed macaques (n = 6/group) with a prime-boost regimen. Since the most pronounced effects in neutralization sensitivity and CD4-independent infection were mediated by the N7 and the triple mutants, we chose to test these two together with WT Env. Animals were inoculated at weeks 0 and 8 with two recombinant vaccinia viruses, one expressing SIVmac239 Gag/Pol, and the other expressing HIV-1 89.6 Env gp160 in one of three forms: WT (group 1), single mutant N7 (group 2), or a triple mutant containing N6/N7/NV3 (group 3). Animals were boosted 14 or 16 months later (see Materials and Methods) with codon-optimized SIVmac239 gag DNA in saline and the cognate gp140 Env protein formulated in alum (Fig. 4). Control animals were primed with vaccinia virus and boosted with vector plasmid and adjuvant only. Immunization with recombinant vaccinia virus alone elicited low but persistent gp120-specific antibodies. After the final booster immunization, titers increased 30- to 50-fold to ∼105 in all three groups, indicating a long-lasting immune memory (Fig. 5). However, NtAb responses differed among the three groups. On the day of challenge, group 1 animals generated only low levels of NtAbs against SHIV89.6, SHIV89.6P-MN, and HIV-1SF162, with median titers of 70, 54, and 32, respectively, whereas group 2 animals had significantly higher neutralizing activities against the same viruses, with mean titers of 577, 920, and 1563, respectively (Fig. 6A). There was a significant correlation among the 50% neutralization titers against SHIV89.6, SHIV.6P-MN, and SF162 (P ≤ 0.0001; data not shown). Surprisingly, group 3 animals show only low NtAb against SHIV89.6 and SF162, with little or no activity against SHIV89.6P-MN.
FIG. 4.
Immunization and challenge scheme. See Materials and Methods for details.
FIG. 5.
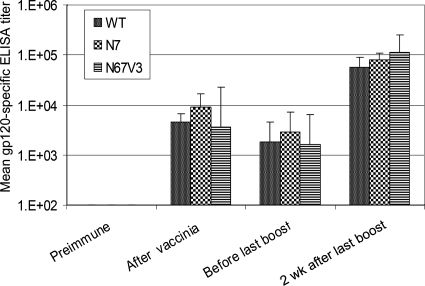
HIV-specific antibody responses after recombinant vaccinia and protein immunizations. HIV-1 SF162 gp120 (>95% pure, produced in stably transformed Chinese hamster ovary cells by Chiron Corp. and obtained from the NIH ARRRP, Division of AIDS, National Institute of Allergy and Infectious Disease, NIH) was used as the capturing antigen. Geometric mean endpoint titers of HIV-1 gp120-specific antibody response in immunized animals (n = 6/group) were measured before immunization (“Preimmune,” week 0), 2 weeks after the second recombinant vaccinia virus immunization (“After vaccinia,” week 10), on the day the animals received the last immunization (“Before last boost,: week 77 for groups 1 and 2 and week 66 for groups 3 and controls [see Materials and Methods]), and 2 weeks after the last immunization (“2 wk after last boost,” week 79 for groups 1 and 2 and week 68 for groups 3 and controls). Vertical bars indicate the standard deviations within each group.
FIG. 6.
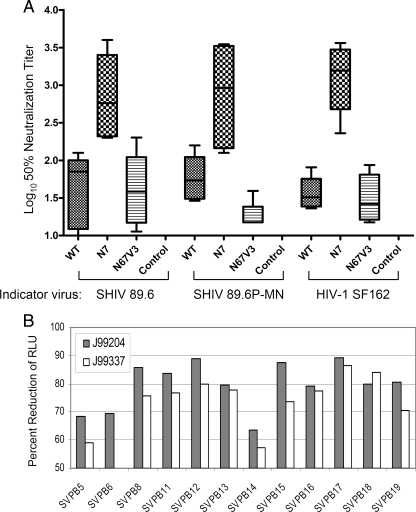
NtAb response on the day of challenge. (A) Neutralization titers were measured as the highest serum dilutions that resulted in 50% reduction of infectivity in the TZM-bl cell assay against replication-competent viruses: homologous WT virus HIV-1 89.6, challenge virus SHIV89.6P-MN, or a heterologous virus HIV-1 SF162. The boxplots indicate the median, as well as the range of responses, including the maximum, minimum, and the 75 and 25% quartiles as indicated. The P values for the differences in response between group 1 or group 2 and the control group were ≤0.004 and ≤0.002, respectively (Mann-Whitney U test). The P value for the differences between groups 1 and 2 was ≤0.002 by the same test. (B) Neutralization of viruses pseudotyped with Env from 12 primary HIV-1 subtype B isolates by day-of-challenge serum from two animals immunized with the N7 mutant Env (group 2). Neutralization was expressed as the percent reduction of RLU at a 1:15 serum dilution with the values from preimmune sera subtracted. The data shown represent results obtained from two (B) or three (A) independent experiments.
To assess the breadth of response, we tested prechallenge sera from all animals against a standard panel of subtype B primary isolates in a pseudotyped virus neutralization assay as described previously (67, 76). Viruses selected for this panel have neutralization sensitivity profiles typical of primary subtype B HIV-1 isolates (the so-called tier 2 isolates). We first tested sera from all animals at 1:15 dilution for their neutralization activity against seven primary isolates (Table 2). As expected, group 1 animals immunized with WT Env showed little or no neutralization activity. Only three of the six animals showed low or moderate levels (50 to 80% or 80 to 90% reduction in infectivity, respectively) of neutralization against three or more of the seven primary isolates tested, including a highly sensitive strain of primary virus (SS1196.1, SVPB9). In contrast, all six animals in group 2 immunized with mutant N7 Env neutralized one or more primary isolates in addition to SS1196.1. Consistent with results obtained in Fig. 6A, group 3 animals failed to generate any detectable NtAb against this primary isolate panel. Of the six control animals, two showed low levels of inhibitory activity in the pseudotyped virus assay. The nature of this activity is not clear. In subsequent studies, we focused on animals J99204 and J99337 from group 2 (N7 immunized) and evaluated their day-of-challenge sera against pseudotyped viruses representing 12 primary subtype B isolates. The results in Fig. 6B confirmed the breadth of the sera from J99204 and J99337, which neutralized 12 of 12 isolates and 11 of 12 isolates tested, respectively.
TABLE 2.
Neutralization of primary subtype B HIV-1 isolates by day-of-challenge sera from immunized and control animals
Reduction of viral load and maintenance of peripheral blood CD4+ T cells in immunized animals after SHIV89.6P-MN challenge.
To determine whether immune responses elicited by any of the vaccines described could protect macaques against primate lentivirus infection or disease, we challenged all animals 4 weeks after the last immunization with intrarectal inoculations of a chimeric virus SHIV89.6P-MN. Naive controls showed high levels of plasma viremia, with a mean peak viral load of 4.4 × 107 vRNA eq/ml at 2 weeks after infection (Fig. 7G and I). The mean plasma viral load at week 12 after infection was approximately 105 vRNA eq./ml, increasing to 3 × 106 vRNA eq/ml by 24 weeks after infection. Animals in groups 1 and 2 showed >30-fold reduction of peak viral load compared to control animals (P ≤ 0.007 and 0.014, respectively, for groups 1 and 2) (Fig. 7A, C, and I). Both groups also showed significant (>200-fold) reduction of mean post-acute plasma viral loads (P ≤ 0.01 for both groups compared to controls at week 16). There were no statistically significant differences between group 1 and 2 animals in their peak and setpoint viral loads after challenge. Although group 3 animals showed a lower mean plasma viral load compared to the control animals, the difference did not reach statistical significance (P ≤ 0.2). As is expected for SHIV89.6P-MN infection, five of the six control animals showed rapid and irreversible CD4+ T-cell loss within a month after challenge. In contrast, all immunized animals, with the exception of two animals (T00240 and Z00303) in group 3, maintained peripheral blood CD4+ T cells within the normal range for the first 10 months of the study.
FIG. 7.
Plasma viral load (left panels) and peripheral blood CD4+ T cells (right panels) in animals after SHIV89.6P-MN challenge. (A to H) Data for individual animals in each immunization or control group as indicated on the left margin. (I and J) Means and standard deviations of plasma viral load and peripheral blood CD4+ T cells, respectively, in control and experimental groups. The large “X” denotes the approximate date of euthanasia due to AIDS.
NtAb levels from all animals before and after challenge were examined for potential correlates of protective immunity. There is a significant correlation between the neutralization titer against the challenge virus SHIV89.6P-MN on the day of challenge and reduction in the peak (week 2) and setpoint (week 16) viral loads (P ≤ 0.005 and 0.010, respectively) (Fig. 8A and B). NtAb titers against SHIV89.6P-MN on day of challenge were also significantly correlated with the preservation of peripheral blood CD4+ T cells at week 28 (P ≤ 0.0001) (Fig. 8C). Taken together, these results indicate the potential importance of vaccine-induced NtAbs in the protection or control of infection. As expected from their differences in CD4+ T cells, animals in all three immunization groups had significantly greater AIDS-free survival compared to the unvaccinated controls (P ≤ 0.015). Proportional hazards analysis indicates that animals in groups 1 and 2 were significantly less likely to die than the control animals (89 and 90% reduction in risk of death for groups 1 and 2 versus controls, respectively; P ≤ 0.04 for both groups). There was a trend for animals in group 3 to be less likely to die than the control animals (75% reduction in risk), but the difference was not statistically significant (P ≤ 0.1).
FIG. 8.
Correlate of protection and survival curve of challenged animals. The log10-transformed 50% neutralization titer of each animal on the day of challenge against the challenge virus SHIV89.6P-MN was plotted against peak plasma viral load at week 2 (A), setpoint viral load at week 16 (B), or peripheral blood CD4+ T cells at week 28 (C). (D) Kaplan-Meier analysis of AIDS-free survival after challenge infection. Correlation coefficients (Spearman's r value) and P values (two-tailed) were calculated by using Prism 4 (GraphPad) software.
DISCUSSION
Glycan modification has been explored as an approach to enhance HIV NtAb responses. We report here that immunization with a mutant Env with a single glycan removed resulted in enhanced NtAb responses, not only against the mutant virus (data not shown) but also against the homologous WT virus and a panel of heterologous primary (“Tier 2”) isolates. This is not predicted by previous findings with glycan-modified envelope proteins. For instance, Bolmstedt et al. (16) compared gp160 and mutant envelope lacking three N-linked glycosylation sites in the V4-V5 region but only observed the preferred homologous NtAb response (i.e., sera from animals immunized with mutant gp160 preferentially neutralized the mutant but not the WT virus). These researchers also immunized mice with DNA lacking the N-terminal V3 (N306) glycan but failed to detect any difference between the WT and the mutant plasmid in their ability to elicit neutralization responses against either the wild-type or the mutant virus (15). Similarly, Quinones-Kochs et al. (85) reported that neither V1 nor V2 glycan mutant was any better than WT HIV-1 89.6 envelope at inducing NtAb. Burke et al. (20) reported an improved NtAb response elicited by HIV-1 SF162 Env gp140 with glycan modification at an N-terminal proximal site (residue 154) in V2 loop. However, the breadth of activities detected appears to be limited, neutralizing a heterologous subtype B virus (HIV-1 89.6) only at a 1:10 dilution. It should be noted that none of the investigators mentioned above examined the same glycan mutant (N197Q) as we did. The effects of glycan modification on Env immunogenicity therefore appear to be highly dependent on the specific glycans examined.
The basis for the enhanced NtAb response elicited by the N7 mutant remains unclear. As was observed by Kolchinsky et al. (63) with a CCR5-using strain ADA, removal of this V2 loop proximal glycan in the dualtropic 89.6 strain enables the mutant envelope to mediate CD4-independent infection. Similar observations have been made by Koch et al. (62) in HIV and Pikora et al. (81) in SIV, indicating that this highly conserved glycan may be important for virus survival by controlling access to the coreceptor binding sites. The results reported here further demonstrate that this mutation at this glycan site increases exposure not only for the coreceptor binding site but also for epitopes recognized by several key neutralizing monoclonal antibody, including those that target the CD4-binding site, the CD4-induced epitope, the tip of the V3 loop and, to a lesser extent, the mannan-dependent epitope defined by 2G12 and the MPER epitope defined by 4E10. Elucidation of the basis of these observations will likely require better understanding of the structure and function of the envelope glycoproteins. However, it is possible that the removal of the N197 glycan results in greater flexibility for the V2 loop, as suggested by Kolchinsky et al. (63). This flexibility could allow the envelope protein to stabilize in a conformation that is normally induced only upon binding to the CD4 receptor during the infection process. As a result, the CD4 binding site, the CD4-induced epitope, and the V3 loop may become more accessible in the mutant Env. In this regard, our observation lends support to approaches that aim to mimic the gp120-CD4 complex (35), to stabilize the envelope protein in the “liganded form” (27, 65, 112), or to utilize natural isolates that share similar properties with the glycan mutants described here (26, 33, 116, 117).
It is noteworthy that, although the triple-mutant virus (N67V3) showed greater neutralization sensitivity than the single-mutant N7, immunization with the triple-mutant Env elicited no better NtAb response than the WT protein. Thus, antigenicity does not predict immunogenicity. The basis for the difference is not clear. Virus bearing the triple mutant showed ∼80% reduction of infectivity per microgram of p27 in an infectivity assay compared to the WT or the other mutant viruses (Fig. 3A). The expression level and/or the stability of the triple mutant Env also appeared to be reduced compared to the WT Env in a transient-transfection assay (data not shown). However, these factors are unlikely to contribute to the reduced NtAb responses elicited by the triple-mutant Env. First, the gp120-specific responses, as measured by enzyme-linked immunosorbent assay (Fig. 5) and Western blot (data not shown) analyses, on sera obtained 2 weeks after the second vaccinia virus immunization showed no difference between the three experimental groups. Second, most of the antigen-specific responses were generated after the booster immunization, when equal quantities of the WT or mutant recombinant gp140 protein were used. Third, gp120-binding antibody titers after the booster immunization were similar among the three immunization groups (Fig. 5). Therefore, it is unlikely that the poor NtAb response elicited in N67V3-immunized animals was due to the lack of expression or overall immunogenicity of the mutant Env protein. Because of the additive effects of the glycan mutations on neutralization sensitivity (Fig. 2) and coreceptor usage (Fig. 3), it is possible that combination of these mutations may have cumulative effects on the overall structure of the Env protein, which in turn may influence its immunogenicity. However, until we have a better understanding of the effect of the glycan modifications on the structure of the Env protein and of the relationship between antigenicity and immunogenicity, it is difficult to predict the outcome of combining different glycan mutations. In this regard, it is noteworthy that previous failures to elicit better NtAb responses with glycan-modified Env proteins often utilized multiply deglycosylated proteins (16, 77, 85). It remains to be determined whether multiple changes may obscure the effects of single mutants (as was observed with the sensitivity to monoclonal antibody 2G12 when additional glycan mutations were introduced into the N7 mutant). Information on the structural differences between the single and triple glycan mutants described here and a better understanding of the nature of the NtAb responses generated by the N7 mutant proteins may provide further insights. In this regard, information generated by the work of Dhillon et al. (36) may prove important for further refinement of glycan modification as a vaccine design concept.
Despite the improved NtAb responses generated by the N7 Env, there were no statistically significant differences in peak and setpoint viral loads between WT- and N7-immunized animals after challenge. Several reasons could account for this apparent discrepancy. First, the level of prechallenge NtAb may still be too low to make a significant difference in the outcome of challenge. This is supported by the finding that none of the vaccines tested here was able to afford protection against infection after challenge with SHIV89.6P-MN. Second, NtAb responses measured in the serum compartment may not accurately reflect responses required to protect against a mucosal challenge. Third, anamnestic response after challenge may minimize the differences in prechallenge vaccine-induced NtAb responses. In support of this notion, animals in the three experimental groups generated similar titers of NtAb against the homologous virus 8 weeks after challenge (data not shown), despite the significant differences between them before challenge. Fourth, it is possible that cellular immunity or humoral responses other than NtAb also contributed to the control of infection. Finally, the lack of a statistically significant difference in viral load in group 1 and 2 animals despite their differences in prechallenge NtAb responses may simply be due to the small sample sizes. Consistent with this notion is the observation that when data from all of the animals in the present study were included in the analysis as shown in Fig. 8, there was a significant correlation between the day-of-challenge NtAb titers against the challenge virus and the reduction of viral load (P ≤ 0.005 and 0.010 for peak and setpoint viral loads, respectively) and preservation of peripheral blood CD4+ T cells after challenge (P ≤ 0.0001).
Reduction of viral load and protection against CD4+ T-cell loss resulting from SHIV89.6P infection has been achieved by a number of vaccines and immunization approaches (2-4, 12, 37, 38, 40, 59, 69, 70, 72, 75, 86, 87, 95, 96, 100, 101, 109). Reduction of viral load or “partial protection” was commonly associated with vaccine-elicited CD8+ T-cell-mediated responses, most notably against the Gag, Pol, Tat, and Nef antigens. With few exceptions (73, 88), NtAbs against the challenge virus were not elicited by vaccination nor present at the time of challenge but were induced after challenge and were believed to play a role in the control of infection (37, 38, 40, 83, 87, 96, 114). With the N7 mutant Env, we were able to elicit broadly NtAb responses, not only against the challenge virus but also a panel of primary subtype B (“Tier 2”) isolates. Although the potency of the response was insufficient to prevent infection, vaccine-induced NtAb responses are likely to play an important role in the control of infection, since a significant correlation was observed between prechallenge NtAb and the reduction of viral load and the protection against peripheral blood CD4+ T-cell loss after SHIV89.6P-MN challenge. Experiments with greater sample sizes are likely to be needed to confirm the validity of these observations. It should also be noted that the vaccine was based on 89.6 Env, whereas the challenge virus was based on the macaque-passaged SHIV89.6P, which most likely contains variants that escape immune responses elicited by SHIV89.6. This observation supports the potential breadth of the protective immunity elicited by the vaccines described. It therefore appears warranted that further improvements be made to the N7 Env to optimize antigen presentation and immunization regimen, including the use of more potent adjuvants.
We have not examined in depth the role of non-NtAbs nor the cellular immunity in the present study. It is of interest that even though animals in group 3 had little or no NtAb response at the time of challenge, four of six animals in this group, compared to only one of six in the control group, were able to recover or maintain normal levels of peripheral blood CD4+ T cells after acute infection (Fig. 7F to H). Although the difference does not achieve statistical significance, this result indicates a strong trend (P ≤ 0.1) toward a role for vaccine-induced immunity other than prechallenge NtAb in the preservation and recovery of peripheral blood CD4+ T cells. As indicated above, cellular immunity and non-NtAbs (e.g., antibody-dependent cellular cytotoxicity) prior to challenge, as well as anamnestic responses after challenge, may have contributed. Furthermore, NtAb present in anatomic sites where virus replicates (e.g., lymph nodes) may also contribute to the control of virus replication in group 3 animals.
The induction of potent and broadly NtAb against HIV remains a major goal of current AIDS vaccine research. The observation that envelope proteins with a single glycan removed can induce improved NtAb response and control of virus infection indicates a potential role for glycan modification in vaccine design. However, our findings also indicate that the effect may be dependent on the specific glycan modifications studied and that antigenicity per se does not predict immunogenicity. Therefore, the basis for the enhanced immunogenicity of the N197 glycan mutant envelope and the general applicability of glycan modification as a vaccine approach need to be further examined.
Acknowledgments
We thank Heather Mack, Modou Mbowe, Leon Flanary, Jane Moon, Jennifer McKenna, and Yvonne Stevens for expert technical assistance; the NIH AIDS Research and Reference Reagent Program for providing monoclonal antibodies, HIV-1 SF162 gp120, and indicator cell lines; John Mascola and Nancy Haigwood for helpful discussions; Leonidas Stamatatos for critical reading of the manuscript; and Barbara Droker and Janeth Talty for editorial assistance.
This study was supported in part by NIH grants R21 AI 042720 and P01 AI 054564 and AmFAR grant 2574.
Footnotes
Published ahead of print on 24 October 2007.
REFERENCES
- 1.Allaway, G. P., K. L. Davis-Bruno, G. A. Beaudry, E. B. Garcia, E. L. Wong, A. M. Ryder, K. W. Hasel, M.-C. Gauduin, R. A. Koup, J. S. McDougal, and P. J. Maddon. 1995. Expression and characterization of CD4-IgG2, a novel heterotetramer that neutralizes primary HIV type 1 isolates. AIDS Res. Hum. Retrovir. 11533-539. [DOI] [PubMed] [Google Scholar]
- 2.Amara, R. R., C. Ibegbu, F. Villinger, D. C. Montefiori, S. Sharma, P. Nigam, Y. Xu, H. M. McClure, and H. L. Robinson. 2005. Studies using a viral challenge and CD8 T-cell depletions on the roles of cellular and humoral immunity in the control of an SHIV-89.6P challenge in DNA/MVA-vaccinated macaques. Virology 343246-255. [DOI] [PubMed] [Google Scholar]
- 3.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 29269-74. [DOI] [PubMed] [Google Scholar]
- 4.Amara, R. R., F. Villinger, S. I. Staprans, J. D. Altman, D. C. Montefiori, N. L. Kozyr, Y. Xu, L. S. Wyatt, P. L. Earl, J. G. Herndon, H. M. McClure, B. Moss, and H. L. Robinson. 2002. Different patterns of immune responses but similar control of a simian-human immunodeficiency virus 89.6P mucosal challenge by modified vaccinia virus Ankara (MVA) and DNA/MVA vaccines. J. Virol. 767625-7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Back, N. K., L. Smit, J. J. De Jong, W. Keulen, M. Schutten, J. Goudsmit, and M. Tersmette. 1994. An N-glycan within the human immunodeficiency virus type 1 gp120 V3 loop affects virus neutralization. Virology 199431-438. [DOI] [PubMed] [Google Scholar]
- 6.Barbas, C. F., III, E. Bjorling, F. Chiodi, N. Dunlop, D. Cababa, T. M. Jones, S. L. Zebedee, M. A. Persson, P. L. Nara, E. Norrby, and D. R. Burton. 1992. Recombinant human Fab fragments neutralize human type 1 immunodeficiency virus in vitro. Proc. Natl. Acad. Sci. USA 899339-9343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barouch, D. H., A. Craiu, M. J. Kuroda, J. E. Schmitz, X. X. Zheng, S. Santra, J. D. Frost, G. R. Krivulka, M. A. Lifton, C. L. Crabbs, G. Heidecker, H. C. Perry, M. E. Davies, H. Xie, C. E. Nickerson, T. D. Steenbeke, C. I. Lord, D. C. Montefiori, T. B. Strom, J. W. Shiver, M. G. Lewis, and N. L. Letvin. 2000. Augmentation of immune responses to HIV-1 and simian immunodeficiency virus DNA vaccines by IL-2/Ig plasmid administration in rhesus monkeys. Proc. Natl. Acad. Sci. USA 974192-4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beddows, S., S. Lister, R. Cheingsong, C. Bruck, and J. Weber. 1999. Comparison of the antibody repertoire generated in healthy volunteers following immunization with a monomeric recombinant gp120 construct derived from a CCR5/CXCR4-using human immunodeficiency virus type 1 isolate with sera from naturally infected individuals. J. Virol. 731740-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benjouad, A., J. C. Gluckman, H. Rochat, L. Montagnier, and E. Bahraoui. 1992. Influence of carbohydrate moieties on the immunogenicity of human immunodeficiency virus type 1 recombinant gp160. J. Virol. 662473-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berman, P. W., T. J. Gregory, L. Riddle, G. R. Nakamura, M. A. Champe, J. P. Porter, F. M. Wurm, R. D. Hershberg, E. K. Cobb, and J. W. Eichberg. 1990. Protection of chimpanzees from infection by HIV-1 after vaccination with recombinant glycoprotein gp120 but not gp160. Nature 345622-625. [DOI] [PubMed] [Google Scholar]
- 11.Bernstein, H. B., S. P. Tucker, E. Hunter, J. S. Schutzbach, and R. W. Compans. 1994. Human immunodeficiency virus type 1 envelope glycoprotein is modified by O-linked oligosaccharides. J. Virol. 68463-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertley, F. M. N., P. A. Kozlowski, S. W. Wang, J. Chappelle, J. Patel, O. Sonuyi, G. Mazzara, D. Montefiori, A. Carville, K. G. Mansfield, and A. Aldovini. 2004. Control of simian/human immunodeficiency virus viremia and disease progression after IL-2-augmented DNA-modified vaccinia virus Ankara nasal vaccination in nonhuman primates. J. Immunol. 1723745-3757. [DOI] [PubMed] [Google Scholar]
- 13.Björndal, A., H. Deng, M. Jansson, J. R. Fiore, C. Colognesi, A. Karlsson, J. Albert, G. Scarlatti, D. R. Littman, and E. M. Fenyo. 1997. Coreceptor usage of primary human immunodeficiency virus type 1 isolates varies according to biological phenotype. J. Virol. 717478-7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blay, W. M., S. Gnanakaran, B. Foley, N. A. Doria-Rose, B. T. Korber, and N. L. Haigwood. 2006. Consistent patterns of change during the divergence of human immunodeficiency virus type 1 envelope from that of the inoculated virus in simian/human immunodeficiency virus-infected macaques. J. Virol. 80999-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bolmstedt, A., J. Hinkula, E. Rowcliffe, M. Biller, B. Wahren, and S. Olofsson. 2001. Enhanced immunogenicity of a human immunodeficiency virus type 1 env DNA vaccine by manipulating N glycosylation signals: effects of elimination of the V3 N306 glycan. Vaccine 20397-405. [DOI] [PubMed] [Google Scholar]
- 16.Bolmstedt, A., S. Sjolander, J. E. Hansen, L. Akerblom, A. Hemming, S. L. Hu, B. Morein, and S. Olofsson. 1996. Influence of N-linked glycans in V4-V5 region of human immunodeficiency virus type 1 glycoprotein gp160 on induction of a virus-neutralizing humoral response. J. Acquir. Immune. Defic. Syndr. Hum. Retrovirol. 12213-220. [DOI] [PubMed] [Google Scholar]
- 17.Bruck, C., C. Thiriart, L. Fabry, M. Francotte, P. Pala, O. Van Opstal, J. Culp, M. Rosenberg, M. De Wilde, and P. Heidt. 1994. HIV-1 envelope-elicited neutralizing antibody titres correlate with protection and virus load in chimpanzees. Vaccine 121141-1148. [DOI] [PubMed] [Google Scholar]
- 18.Buchacher, A., R. Predl, K. Strutzenberger, W. Steinfellner, A. Trkola, M. Purtscher, G. Gruber, C. Tauer, F. Steindl, A. Jungbauer, and H. Katinger. 1994. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res. Hum. Retrovir. 10359-369. [DOI] [PubMed] [Google Scholar]
- 19.Bures, R., A. Gaitan, T. Zhu, C. Graziosi, K. M. McGrath, J. Tartaglia, P. Caudrelier, H. R. El, M. Klein, A. Lazzarin, D. M. Stablein, M. Deers, L. Corey, M. L. Greenberg, D. H. Schwartz, and D. C. Montefiori. 2000. Immunization with recombinant canarypox vectors expressing membrane-anchored glycoprotein 120 followed by glycoprotein 160 boosting fails to generate antibodies that neutralize R5 primary isolates of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 162019-2035. [DOI] [PubMed] [Google Scholar]
- 20.Burke, B., N. R. Derby, Z. Kraft, C. J. Saunders, C. Dai, N. Llewellyn, I. Zharkikh, L. Vojtech, T. Zhu, and I. K. Srivastava. 2006. Viral evolution in macaques coinfected with CCR5- and CXCR4-tropic SHIVs in the presence or absence of vaccine-elicited anti-CCR5 SHIV neutralizing antibodies. Virology 355138-151. [DOI] [PubMed] [Google Scholar]
- 21.Burns, D. P., and R. C. Desrosiers. 1994. Envelope sequence variation, neutralizing antibodies, and primate lentivirus persistence. Curr. Top. Microbiol. Immunol. 188185-219. [DOI] [PubMed] [Google Scholar]
- 22.Burton, D. R., C. F. Barbas III, M. A. Persson, S. Koenig, R. M. Chanock, and R. A. Lerner. 1991. A large array of human monoclonal antibodies to type 1 human immunodeficiency virus from combinatorial libraries of asymptomatic seropositive individuals. Proc. Natl. Acad. Sci. USA 8810134-10137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burton, D. R., J. Pyati, R. Koduri, S. J. Sharp, G. B. Thornton, P. W. Parren, L. S. Sawyer, R. M. Hendry, N. Dunlop, P. L. Nara, M. Lamacchia, E. Garratti, E. R. Stiehm, Y. J. Bryson, Y. Cao, J. P. Moore, D. D. Ho, and C. F. Barbas III. 1994. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 2661024-1027. [DOI] [PubMed] [Google Scholar]
- 24.Chackerian, B., L. M. Rudensey, and J. Overbaugh. 1997. Specific N-linked and O-linked glycosylation modifications in the envelope V1 domain of simian immunodeficiency virus variants that evolve in the host alter recognition by neutralizing antibodies. J. Virol. 717719-7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chakrabarti, S., J. R. Sisler, and B. Moss. 1997. Compact, synthetic, vaccinia virus early/late promoter for protein expression. BioTechniques 231094-1097. [DOI] [PubMed] [Google Scholar]
- 26.Cham, F., P. F. Zhang, L. Heyndrickx, P. Bouma, P. Zhong, H. Katinger, J. Robinson, G. van der Groen, and G. V. Quinnan, Jr. 2006. Neutralization and infectivity characteristics of envelope glycoproteins from human immunodeficiency virus type 1 infected donors whose sera exhibit broadly cross-reactive neutralizing activity. Virology 34736-51. [DOI] [PubMed] [Google Scholar]
- 27.Chen, B., E. M. Vogan, H. Gong, J. J. Skehel, D. C. Wiley, and S. C. Harrison. 2005. Structure of an unliganded simian immunodeficiency virus gp120 core. Nature 433834-841. [DOI] [PubMed] [Google Scholar]
- 28.Clevestig, P., L. Pramanik, T. Leitner, and A. Ehrnst. 2006. CCR5 use by human immunodeficiency virus type 1 is associated closely with the gp120 V3 loop N-linked glycosylation site. J. Gen. Virol. 87607-612. [DOI] [PubMed] [Google Scholar]
- 29.Collman, R., J. W. Balliet, S. A. Gregory, H. Friedman, D. L. Kolson, N. Nathanson, and A. Srinivasan. 1992. An infectious molecular clone of an unusual macrophage-tropic and highly cytopathic strain of human immunodeficiency virus type 1. J. Virol. 667517-7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conley, A. J., M. K. Gorny, J. A. Kessler, L. J. Boots, M. Ossorio-Castro, S. Koenig, D. W. Lineberger, E. A. Emini, C. Williams, and S. Zolla-Pazner. 1994. Neutralization of primary human immunodeficiency virus type 1 isolates by the broadly reactive anti-V3 monoclonal antibody, 447-52D. J. Virol. 686994-7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cook, R. F., S. L. Berger, K. E. Rushlow, J. M. McManus, S. J. Cook, S. Harrold, M. L. Raabe, R. C. Montelaro, and C. J. Issel. 1995. Enhanced sensitivity to neutralizing antibodies in a variant of equine infectious anemia virus is linked to amino acid substitutions in the surface unit envelope glycoprotein. J. Virol. 691493-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooney, E. L., M. J. McElrath, L. Corey, S.-L. Hu, A. C. Collier, D. Arditti, M. Hoffman, R. W. Coombs, G. E. Smith, and P. D. Greenberg. 1993. Enhanced immunity to human immunodeficiency virus (HIV) envelope elicited by a combined vaccine regimen consisting of priming with a vaccinia recombinant expressing HIV envelope and boosting with gp160 protein. Proc. Natl. Acad. Sci. USA 901882-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Decker, J. M., F. Bibollet-Ruche, X. Wei, S. Wang, D. N. Levy, W. Wang, E. Delaporte, M. Peeters, C. A. Derdeyn, S. Allen, E. Hunter, M. S. Saag, J. A. Hoxie, B. H. Hahn, P. D. Kwong, J. E. Robinson, and G. M. Shaw. 2005. Antigenic conservation and immunogenicity of the HIV coreceptor binding site. J. Exp. Med. 2011407-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng, H., R. Liu, W. Ellmeier, S. Choe, D. Unutmaz, M. Burkhart, M. P. Di, S. Marmon, R. E. Sutton, C. M. Hill, C. B. Davis, S. C. Peiper, T. J. Schall, D. R. Littman, and N. R. Landau. 1996. Identification of a major coreceptor for primary isolates of HIV-1. Nature 381661-666. [DOI] [PubMed] [Google Scholar]
- 35.DeVico, A., A. Silver, A. M. Thronton, M. G. Sarngadharan, and R. Pal. 1996. Covalently cross-linked complexes of human immunodeficiency virus type 1 (HIV-1) gp120 and CD4 receptor elicit a neutralizing immune response that includes antibodies selective for primary virus isolates. Virology 218258-263. [DOI] [PubMed] [Google Scholar]
- 36.Dhillon, A. K., H. Donners, R. Pantophlet, W. E. Johnson, J. M. Decker, G. M. Shaw, F. H. Lee, D. D. Richman, R. W. Doms, G. Vanham, and D. R. Burton. 2007. Dissecting the neutralizing antibody specificities of broadly neutralizing sera from human immunodeficiency virus type 1-infected donors. J. Virol. 816548-6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doria-Rose, N. A., C. Ohlen, P. Polacino, C. C. Pierce, M. T. Hensel, L. Kuller, T. Mulvania, D. Anderson, P. D. Greenberg, S. L. Hu, and N. L. Haigwood. 2003. Multigene DNA priming-boosting vaccines protect macaques from acute CD4+-T-cell depletion after simian-human immunodeficiency virus SHIV89.6P mucosal challenge. J. Virol. 7711563-11577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Earl, P. L., L. S. Wyatt, D. C. Montefiori, M. Bilska, R. Woodward, P. D. Markham, J. D. Malley, T. U. Vogel, T. M. Allen, D. I. Watkins, N. Miller, and B. Moss. 2002. Comparison of vaccine strategies using recombinant env-gag-pol MVA with or without an oligomeric Env protein boost in the SHIV rhesus macaque model. Virology 294270-281. [DOI] [PubMed] [Google Scholar]
- 39.Egan, M. A., W. A. Charini, M. J. Kuroda, J. E. Schmitz, P. Racz, K. Tenner-Racz, K. Manson, M. Wyand, M. A. Lifton, C. E. Nickerson, T. Fu, J. W. Shiver, and N. L. Letvin. 2000. Simian immunodeficiency virus (SIV) gag DNA-vaccinated rhesus monkeys develop secondary cytotoxic T-lymphocyte responses and control viral replication after pathogenic SIV infection. J. Virol. 747485-7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Egan, M. A., S. Y. Chong, N. F. Rose, S. Megathi, K. J. Lopez, E. B. Schadeck, J. E. Johnson, A. Masood, P. Piacente, R. E. Druilhet, P. W. Barras, D. L. Hasselschwert, P. Reilly, E. M. Mishkin, D. C. Montefiori, M. G. Lewis, D. K. Clarke, R. M. Hendry, P. A. Marx, J. H. Eldridge, S. A. Udem, Z. R. Israel, and J. K. Rose. 2004. Immunogenicity of attenuated vesicular stomatitis virus vectors expressing HIV type 1 Env and SIV Gag proteins: comparison of intranasal and intramuscular vaccination routes. AIDS Res. Hum. Retrovir. 20989-1004. [DOI] [PubMed] [Google Scholar]
- 41.Flynn, N. M., D. N. Forthal, C. D. Harro, F. N. Judson, K. H. Mayer, and M. F. Para. 2005. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J. Infect. Dis. 191654-665. [DOI] [PubMed] [Google Scholar]
- 42.Garlick, R. L., R. J. Kirschner, F. M. Eckenrode, W. G. Tarpley, and C. S. Tomich. 1990. Escherichia coli expression, purification, and biological activity of a truncated soluble CD4. AIDS Res. Hum. Retrovir. 6465-479. [DOI] [PubMed] [Google Scholar]
- 43.Geyer, H., C. Holschbach, G. Hunsmann, and J. Schneider. 1988. Carbohydrates of human immunodeficiency virus. Structures of oligosaccharides linked to the envelope glycoprotein 120. J. Biol. Chem. 26311760-11767. [PubMed] [Google Scholar]
- 44.Gilbert, P. B., M. L. Peterson, D. Follmann, M. G. Hudgens, D. P. Francis, M. Gurwith, W. L. Heyward, D. V. Jobes, V. Popovic, S. G. Self, F. Sinangil, D. Burke, and P. W. Berman. 2005. Correlation between immunologic responses to a recombinant glycoprotein 120 vaccine and incidence of HIV-1 infection in a phase 3 HIV-1 preventive vaccine trial. J. Infect. Dis. 191666-677. [DOI] [PubMed] [Google Scholar]
- 45.Girard, M., M. P. Kieny, A. Pinter, F. Barre-Sinoussi, P. Nara, H. Kolbe, K. Kusumi, A. Chaput, T. Reinhart, and E. Muchmore. 1991. Immunization of chimpanzees confers protection against challenge with human immunodeficiency virus. Proc. Natl. Acad. Sci. USA 88542-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gorny, M. K., A. J. Conley, S. Karwowska, A. Buchbinder, J. Y. Xu, E. A. Emini, S. Koenig, and S. Zolla-Pazner. 1992. Neutralization of diverse human immunodeficiency virus type 1 variants by an anti-V3 human monoclonal antibody. J. Virol. 667538-7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gorny, M. K., T. C. VanCott, C. Hioe, Z. R. Israel, N. L. Michael, A. J. Conley, C. Williams, J. A. Kessler, P. Chigurupati, S. Burda, and S. Zolla-Pazner. 1997. Human monoclonal antibodies to the V3 loop of HIV-1 with intra- and interclade cross-reactivity. J. Immunol. 1595114-5122. [PubMed] [Google Scholar]
- 48.Gorny, M. K., J. Y. Xu, S. Karwowska, A. Buchbinder, and S. Zolla-Pazner. 1993. Repertoire of neutralizing human monoclonal antibodies specific for the V3 domain of HIV-1 gp120. J. Immunol. 150635-643. [PubMed] [Google Scholar]
- 49.Haggerty, S., M. P. Dempsey, M. I. Bukrinsky, L. Guo, and M. Stevenson. 1991. Posttranslational modifications within the HIV-1 envelope glycoprotein which restrict virus assembly and CD4-dependent infection. AIDS Res. Hum. Retrovir. 7501-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haigwood, N. L., P. L. Nara, E. Brooks, G. A. Van Nest, G. Ott, K. W. Higgins, N. Dunlop, C. J. Scandella, J. W. Eichberg, and K. S. Steimer. 1992. Native but not denatured recombinant human immunodeficiency virus type 1 gp120 generates broad-spectrum neutralizing antibodies in baboons. J. Virol. 66172-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hansen, J. E., H. Clausen, S. L. Hu, J. O. Nielsen, and S. Olofsson. 1992. An O-linked carbohydrate neutralization epitope of HIV-1 gp 120 is expressed by HIV-1 env gene recombinant vaccinia virus. Arch. Virol. 12611-20. [DOI] [PubMed] [Google Scholar]
- 52.Hansen, J. E., H. Clausen, C. Nielsen, L. S. Teglbjaerg, L. L. Hansen, C. M. Nielsen, E. Dabelsteen, L. Mathiesen, S. I. Hakomori, and J. O. Nielsen. 1990. Inhibition of human immunodeficiency virus (HIV) infection in vitro by anticarbohydrate monoclonal antibodies: peripheral glycosylation of HIV envelope glycoprotein gp120 may be a target for virus neutralization. J. Virol. 642833-2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hanson, C. V. 1994. Measuring vaccine-induced HIV neutralization: report of a workshop. AIDS Res. Hum. Retrovir. 10645-648. [DOI] [PubMed] [Google Scholar]
- 54.Hu, S.-L., J. E. Klaniecki, B. M. Travis, T. Wrey, S. Pennathur, D. C. Montefiori, J. L. Thompson, M. B. Agy, L. Kuller, and W. R. Morton. 1997. Immunization with HIV-1 gp160 by the “prime and boost” regimen protects macaques against SHIV HXBc2 challenge, p. 291-298. In F. Brown, D. Burton, P. Doherty, J. Mekalanos, and E. Norrby (ed.), Vaccines 97. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 55.Hu, S.-L., S. G. Kosowski, and J. M. Dalrymple. 1986. Expression of AIDS virus envelope gene in recombinant vaccinia viruses. Nature 320537-540. [DOI] [PubMed] [Google Scholar]
- 56.Hu, S.-L., B. M. Travis, J. Garrigues, J. M. Zarling, P. Sridhar, T. Dykers, J. W. Eichberg, and C. Alpers. 1990. Processing, assembly, and immunogenicity of human immunodeficiency virus core antigens expressed by recombinant vaccinia virus. Virology 179321-329. [DOI] [PubMed] [Google Scholar]
- 57.Hu, S.-L., B. M. Travis, V. Stallard, K. Abrams, L. Misher, P. Moran, J. M. Zarling, A. J. Langlois, L. Kuller, and W. R. Morton. 1992. Immune responses to SIVmne envelope glycoproteins protect macaques from homologous SIV infection. AIDS Res. Hum. Retrovir. 81489-1494. [DOI] [PubMed] [Google Scholar]
- 58.Kang, S. M., F. Shi Quan, C. Huang, L. Guo, L. Ye, C. Yang, and R. W. Compans. 2005. Modified HIV envelope proteins with enhanced binding to neutralizing monoclonal antibodies. Virology 33120-32. [DOI] [PubMed] [Google Scholar]
- 59.Kim, J. J., J. S. Yang, L. K. Nottingham, D. J. Lee, M. Lee, K. H. Manson, M. S. Wyand, J. D. Boyer, K. E. Ugen, and D. B. Weiner. 2001. Protection from immunodeficiency virus challenges in rhesus macaques by multicomponent DNA immunization. Virology 285204-217. [DOI] [PubMed] [Google Scholar]
- 60.Kinsey, N. E., M. G. Anderson, T. J. Unangst, S. V. Joag, O. Narayan, M. C. Zink, and J. E. Clements. 1996. Antigenic variation of SIV: mutations in V4 alter the neutralization profile. Virology 22114-21. [DOI] [PubMed] [Google Scholar]
- 61.Klaniecki, J., T. Dykers, B. Travis, R. Schmitt, M. Wain, A. Watson, P. Sridhar, J. McClure, B. Morein, and J. T. Ulrich. 1991. Cross-neutralizing antibodies in rabbits immunized with HIV-1 gp160 purified from simian cells infected with a recombinant vaccinia virus. AIDS Res. Hum. Retrovir. 7791-798. [DOI] [PubMed] [Google Scholar]
- 62.Koch, M., M. Pancera, P. D. Kwong, P. Kolchinsky, C. Grundner, L. Wang, W. A. Hendrickson, J. Sodroski, and R. Wyatt. 2003. Structure-based, targeted deglycosylation of HIV-1 gp120 and effects on neutralization sensitivity and antibody recognition. Virology 313387-400. [DOI] [PubMed] [Google Scholar]
- 63.Kolchinsky, P., E. Kiprilov, P. Bartley, R. Rubinstein, and J. Sodroski. 2001. Loss of a single N-linked glycan allows CD4-independent human immunodeficiency virus type 1 infection by altering the position of the gp120 V1/V2 variable loops. J. Virol. 753435-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kolchinsky, P., E. Kiprilov, and J. Sodroski. 2001. Increased neutralization sensitivity of CD4-independent human immunodeficiency virus variants. J. Virol. 752041-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kwong, P. D., R. Wyatt, J. Robinson, R. W. Sweet, J. Sodroski, and W. A. Hendrickson. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393648-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li, M., F. Gao, J. R. Mascola, L. Stamatatos, V. R. Polonis, M. Koutsoukos, G. Voss, P. Goepfert, P. Gilbert, K. M. Greene, M. Bilska, D. L. Kothe, J. F. Salazar-Gonzalez, X. Wei, J. M. Decker, B. H. Hahn, and D. C. Montefiori. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 7910108-10125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li, Y., M. A. Rey-Cuille, and S. L. Hu. 2001. N-linked glycosylation in the V3 region of HIV type 1 surface antigen modulates coreceptor usage in viral infection. AIDS Res. Hum. Retrovir. 171473-1479. [DOI] [PubMed] [Google Scholar]
- 68.Liu, M. A. 1997. The immunologist's grail: vaccines that generate cellular immunity. Proc. Natl. Acad. Sci. USA 9410496-10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu, Z., D. K. Singh, D. Sheffer, M. S. Smith, S. Dhillon, Y. Chebloune, R. Hegde, S. Buch, and O. Narayan. 2006. Immunoprophylaxis against AIDS in macaques with a lentiviral DNA vaccine. Virology 351444-454. [DOI] [PubMed] [Google Scholar]
- 70.Maggiorella, M. T., S. Baroncelli, Z. Michelini, E. Fanales-Belasio, S. Moretti, L. Sernicola, A. Cara, D. R. Negri, S. Butto, V. Fiorelli, A. Tripiciano, A. Scoglio, A. Caputo, A. Borsetti, B. Ridolfi, R. Bona, P. ten Haaft, I. Macchia, P. Leone, M. R. Pavone-Cossut, F. Nappi, M. Ciccozzi, J. Heeney, F. Titti, A. Cafaro, and B. Ensoli. 2004. Long-term protection against SHIV89.6P replication in HIV-1 Tat vaccinated cynomolgus monkeys. Vaccine 223258-3269. [DOI] [PubMed] [Google Scholar]
- 71.Mascola, J. R., S. W. Snyder, O. S. Weislow, S. M. Belay, R. B. Belshe, D. H. Schwartz, M. L. Clements, R. Dolin, B. S. Graham, G. J. Gorse, M. C. Keefer, M. J. McElrath, M. C. Walker, K. F. Wagner, J. G. McNeil, F. E. McCutchan, D. S. Burke, et al. 1996. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. J. Infect. Dis. 173340-348. [DOI] [PubMed] [Google Scholar]
- 72.Mascola, J. R., A. Sambor, K. Beaudry, S. Santra, B. Welcher, M. K. Louder, T. C. VanCott, Y. Huang, B. K. Chakrabarti, W. P. Kong, Z. Y. Yang, L. Xu, D. C. Montefiori, G. J. Nabel, and N. L. Letvin. 2005. Neutralizing antibodies elicited by immunization of monkeys with DNA plasmids and recombinant adenoviral vectors expressing human immunodeficiency virus type 1 proteins. J. Virol. 79771-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Matthews, T. J., K. J. Weinhold, H. K. Lyerly, A. J. Langlois, H. Wigzell, and D. P. Bolognesi. 1987. Interaction between the human T-cell lymphotropic virus type IIIB envelope glycoprotein gp120 and the surface antigen CD4: role of carbohydrate in binding and cell fusion. Proc. Natl. Acad. Sci. USA 845424-5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McCaffrey, R. A., C. Saunders, M. Hensel, and L. Stamatatos. 2004. N-linked glycosylation of the V3 loop and the immunologically silent face of gp120 protects human immunodeficiency virus type 1 SF162 from neutralization by anti-gp120 and anti-gp41 antibodies. J. Virol. 783279-3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McKenna, P. M., P. P. Aye, B. Dietzschold, D. C. Montefiori, L. N. Martin, P. A. Marx, R. J. Pomerantz, A. Lackner, and M. J. Schnell. 2004. Immunogenicity study of glycoprotein-deficient rabies virus expressing simian/human immunodeficiency virus SHIV89.6P envelope in a rhesus macaque. J. Virol. 7813455-13459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Montefiori, D. C. 2004. Evaluating neutralizing antibodies against HIV, SIV and SHIV in luciferase reporter gene assays, p.12.11.1-12.11.15. In J. E. Coligan, A. M. Kruisbeek, D. H. Margulies, E. M. Shevach, W. Strober, and R. Coico (ed.), Current protocols in immunology. John Wiley & Sons, Inc., New York, NY. [DOI] [PubMed]
- 77.Mori, K., C. Sugimoto, S. Ohgimoto, E. E. Nakayama, T. Shioda, S. Kusagawa, Y. Takebe, M. Kano, T. Matano, T. Yuasa, D. Kitaguchi, M. Miyazawa, Y. Takahashi, M. Yasunami, A. Kimura, N. Yamamoto, Y. Suzuki, and Y. Nagai. 2005. Influence of glycosylation on the efficacy of an Env-based vaccine against simian immunodeficiency virus SIVmac239 in a macaque AIDS model. J. Virol. 7910386-10396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nyambi, P. N., M. K. Gorny, L. Bastiani, G. van der Groen, C. Williams, and S. Zolla-Pazner. 1998. Mapping of epitopes exposed on intact human immunodeficiency virus type 1 (HIV-1) virions: a new strategy for studying the immunologic relatedness of HIV-1. J. Virol. 729384-9391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Overbaugh, J., and L. M. Rudensey. 1992. Alterations in potential sites for glycosylation predominate during evolution of the simian immunodeficiency virus envelope gene in macaques. J. Virol. 665937-5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Overbaugh, J., L. M. Rudensey, M. D. Papenhausen, R. E. Benveniste, and W. R. Morton. 1991. Variation in simian immunodeficiency virus env is confined to V1 and V4 during progression to simian AIDS. J. Virol. 657025-7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pikora, C., C. Wittish, and R. C. Desrosiers. 2005. Identification of two N-linked glycosylation sites within the core of the simian immunodeficiency virus glycoprotein whose removal enhances sensitivity to soluble CD4. J. Virol. 7912575-12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Polacino, P. S., V. Stallard, J. E. Klaniecki, S. Pennathur, D. C. Montefiori, A. J. Langlois, B. A. Richardson, W. R. Morton, R. E. Benveniste, and S. L. Hu. 1999. Role of immune responses against the envelope and the core antigens of simian immunodeficiency virus SIVmne in protection against homologous cloned and uncloned virus challenge in Macaques. J. Virol. 738201-8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Purtscher, M., A. Trkola, A. Grassauer, P. M. Schulz, A. Klima, S. Döpper, G. Gruber, A. Buchacher, T. Muster, and H. Katinger. 1996. Restricted antigenic variability of the epitope recognized by the neutralizing gp41 antibody 2F5. AIDS 10587-593. [DOI] [PubMed] [Google Scholar]
- 84.Purtscher, M., A. Trkola, G. Gruber, A. Buchacher, R. Predl, F. Steindl, C. Tauer, R. Berger, N. Barrett, A. Jungbauer, and H. Katinger. 1994. A broadly neutralizing human monoclonal antibody against gp41 of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 101651-1658. [DOI] [PubMed] [Google Scholar]
- 85.Quinones-Kochs, M. I., L. Buonocore, and J. K. Rose. 2002. Role of N-linked glycans in a human immunodeficiency virus envelope glycoprotein: effects on protein function and the neutralizing antibody response. J. Virol. 764199-4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ramsburg, E., N. F. Rose, P. A. Marx, M. Mefford, D. F. Nixon, W. J. Moretto, D. Montefiori, P. Earl, B. Moss, and J. K. Rose. 2004. Highly effective control of an AIDS virus challenge in macaques by using vesicular stomatitis virus and modified vaccinia virus Ankara vaccine vectors in a single-boost protocol. J. Virol. 783930-3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rasmussen, R. A., R. Hofmann-Lehman, D. C. Montefiori, P. L. Li, V. Liska, J. Vlasak, T. W. Baba, J. E. Schmitz, M. J. Kuroda, H. L. Robinson, H. M. McClure, S. Lu, S. L. Hu, T. A. Rizvi, and R. M. Ruprecht. 2002. DNA prime/protein boost vaccine strategy in neonatal macaques against simian human immunodeficiency virus. J. Med. Primatol. 3140-60. [DOI] [PubMed] [Google Scholar]
- 88.Rasmussen, R. A., R. Hofmann-Lehmann, P. L. Li, J. Vlasak, J. E. Schmitz, K. A. Reimann, M. J. Kuroda, N. L. Letvin, D. C. Montefiori, H. M. McClure, and R. M. Ruprecht. 2002. Neutralizing antibodies as a potential secondary protective mechanism during chronic SHIV infection in CD8+ T-cell-depleted macaques. AIDS 16829-838. [DOI] [PubMed] [Google Scholar]
- 89.Reimann, K. A., J. T. Li, G. Voss, C. Lekutis, K. Tenner-Racz, P. Racz, W. Lin, D. C. Montefiori, D. E. Lee-Parritz, Y. Lu, R. G. Collman, J. Sodroski, and N. L. Letvin. 1996. An env gene derived from a primary human immunodeficiency virus type 1 isolate confers high in vivo replicative capacity to a chimeric simian/human immunodeficiency virus in rhesus monkeys. J. Virol. 703198-3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reitter, J. N., and R. C. Desrosiers. 1998. Identification of replication-competent strains of simian immunodeficiency virus lacking multiple attachment sites for N-linked carbohydrates in variable regions 1 and 2 of the surface envelope protein. J. Virol. 725399-5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reitter, J. N., R. E. Means, and R. C. Desrosiers. 1998. A role for carbohydrates in immune evasion in AIDS. Nat. Med. 4679-684. [DOI] [PubMed] [Google Scholar]
- 92.Reynard, F., A. Fatmi, B. Verrier, and F. Bedin. 2004. HIV-1 acute infection env glycomutants designed from 3D model: effects on processing, antigenicity, and neutralization sensitivity. Virology 32490-102. [DOI] [PubMed] [Google Scholar]
- 93.Richman, D. D., T. Wrin, S. J. Little, and C. J. Petropoulos. 2003. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. USA 1004144-4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Roben, P., J. P. Moore, M. Thali, J. Sodroski, C. F. Barbas, III, and D. R. Burton. 1994. Recognition properties of a panel of human recombinant Fab fragments to the CD4 binding site of gp120 that show differing abilities to neutralize human immunodeficiency virus type 1. J. Virol. 684821-4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Robinson, H. L., D. C. Montefiori, F. Villinger, J. E. Robinson, S. Sharma, L. S. Wyatt, P. L. Earl, H. M. McClure, B. Moss, and R. R. Amara. 2006. Studies on GM-CSF DNA as an adjuvant for neutralizing Ab elicited by a DNA/MVA immunodeficiency virus vaccine. Virology 352285-294. [DOI] [PubMed] [Google Scholar]
- 96.Rose, N. F., P. A. Marx, A. Luckay, D. F. Nixon, W. J. Moretto, S. M. Donahoe, D. Montefiori, A. Roberts, L. Buonocore, and J. K. Rose. 2001. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106539-549. [DOI] [PubMed] [Google Scholar]
- 97.Rwambo, P. M., C. J. Issel, K. A. Hussain, and R. C. Montelaro. 1990. In vitro isolation of a neutralization escape mutant of equine infectious anemia virus (EIAV). Arch. Virol. 111275-280. [DOI] [PubMed] [Google Scholar]
- 98.Salter, R. D., D. N. Howell, and P. Cresswell. 1985. Genes regulating HLA class I antigen expression in T-B lymphoblast hybrids. Immunogenetics 21235-246. [DOI] [PubMed] [Google Scholar]
- 99.Sanders, R. W., M. Venturi, L. Schiffner, R. Kalyanaraman, H. Katinger, K. O. Lloyd, P. D. Kwong, and J. P. Moore. 2002. The mannose-dependent epitope for neutralizing antibody 2G12 on human immunodeficiency virus type 1 glycoprotein gp120. J. Virol. 767293-7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415331-335. [DOI] [PubMed] [Google Scholar]
- 101.Singh, D. K., Z. Liu, D. Sheffer, G. A. Mackay, M. Smith, S. Dhillon, R. Hegde, F. Jia, I. Adany, and O. Narayan. 2005. A noninfectious simian/human immunodeficiency virus DNA vaccine that protects macaques against AIDS. J. Virol. 793419-3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stephens, E. B., S. Mukherjee, M. Sahni, W. Zhuge, R. Raghavan, D. K. Singh, K. Leung, B. Atkinson, Z. Li, S. V. Joag, Z. Q. Liu, and O. Narayan. 1997. A cell-free stock of simian-human immunodeficiency virus that causes AIDS in pig-tailed macaques has a limited number of amino acid substitutions in both SIVmac and HIV-1 regions of the genome and has offered cytotropism. Virology 231313-321. [DOI] [PubMed] [Google Scholar]
- 103.Stiegler, G., R. Kunert, M. Purtscher, S. Wolbank, R. Voglauer, F. Steindl, and H. Katinger. 2001. A potent cross-clade neutralizing human monoclonal antibody against a novel epitope on gp41 of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 171757-1765. [DOI] [PubMed] [Google Scholar]
- 104.Sullivan, N., Y. Sun, Q. Sattentau, M. Thali, D. Wu, G. Denisova, J. Gershoni, J. Robinson, J. Moore, and J. Sodroski. 1998. CD4-induced conformational changes in the human immunodeficiency virus type 1 gp120 glycoprotein: consequences for virus entry and neutralization. J. Virol. 724694-4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Suryanarayana, K., T. A. Wiltrout, G. M. Vasquez, V. M. Hirsch, and J. D. Lifson. 1998. Plasma SIV RNA viral load determination by real-time quantification of product generation in reverse transcriptase-polymerase chain reaction. AIDS Res. Hum. Retrovir. 14183-189. [DOI] [PubMed] [Google Scholar]
- 106.Thali, M., J. P. Moore, C. Furman, M. Charles, D. D. Ho, J. Robinson, and J. Sodroski. 1993. Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J. Virol. 673978-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Trkola, A., T. Dragic, J. Arthos, J. M. Binley, W. C. Olson, G. P. Allaway, C. Cheng-Mayer, J. Robinson, P. J. Maddon, and J. P. Moore. 1996. CD4-dependent, antibody-sensitive interactions between HIV-1 and its coreceptor CCR-5. Nature 384184-187. [DOI] [PubMed] [Google Scholar]
- 108.Trkola, A., M. Purtscher, T. Muster, C. Ballaun, A. Buchacher, N. Sullivan, K. Srinivasan, J. Sodroski, J. P. Moore, and H. Katinger. 1996. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J. Virol. 701100-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang, S. W., F. M. N. Bertley, P. A. Kozlowski, L. Herrmann, K. Manson, G. Mazzara, M. Piatak, R. P. Johnson, A. Carville, K. Mansfield, and A. Aldovini. 2004. An SHIV DNA/MVA rectal vaccination in macaques provides systemic and mucosal virus-specific responses and protection against AIDS. AIDS Res. Hum. Retrovir. 20846-859. [DOI] [PubMed] [Google Scholar]
- 110.Wei, X., J. M. Decker, S. Wang, H. Hui, J. C. Kappes, X. Wu, J. F. Salazar-Gonzalez, M. G. Salazar, J. M. Kilby, M. S. Saag, N. L. Komarova, M. A. Nowak, B. H. Hahn, P. D. Kwong, and G. M. Shaw. 2003. Antibody neutralization and escape by HIV-1. Nature 422307-312. [DOI] [PubMed] [Google Scholar]
- 111.Wei, X., J. M. Decker, H. Liu, Z. Zhang, R. B. Arani, J. M. Kilby, M. S. Saag, X. Wu, G. M. Shaw, and J. C. Kappes. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 461896-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wyatt, R., P. D. Kwong, E. Desjardins, R. W. Sweet, J. Robinson, W. A. Hendrickson, and J. G. Sodroski. 1998. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature 393705-711. [DOI] [PubMed] [Google Scholar]
- 113.Wyatt, R., J. Moore, M. Accola, E. Desjardin, J. Robinson, and J. Sodroski. 1995. Involvement of the V1/V2 variable loop structure in the exposure of human immunodeficiency virus type 1 gp120 epitopes induced by receptor binding. J. Virol. 695723-5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xu, R., I. K. Srivastava, L. Kuller, I. Zarkikh, Z. Kraft, Z. Fagrouch, N. L. Letvin, J. L. Heeney, S. W. Barnett, and L. Stamatatos. 2006. Immunization with HIV-1 SF162-derived Envelope gp140 proteins does not protect macaques from heterologous simian-human immunodeficiency virus SHIV89.6P infection. Virology 349276-289. [DOI] [PubMed] [Google Scholar]
- 115.Zarling, J. M., W. Morton, P. A. Moran, J. McClure, S. G. Kosowski, and S.-L. Hu. 1986. T-cell responses to human AIDS virus in macaques immunized with recombinant vaccinia viruses. Nature 323344-346. [DOI] [PubMed] [Google Scholar]
- 116.Zhang, P. F., P. Bouma, E. J. Park, J. B. Margolick, J. E. Robinson, S. Zolla-Pazner, M. N. Flora, and G. V. Quinnan, Jr. 2002. A variable region 3 (V3) mutation determines a global neutralization phenotype and CD4-independent infectivity of a human immunodeficiency virus type 1 envelope associated with a broadly cross-reactive, primary virus-neutralizing antibody response. J. Virol. 76644-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang P. F., F. Cham, M. Dong, A. Choudhary, P. Bouma, Z. Zhang, Y. Shao, Y. R. Feng, L. Wang, N. Mathy, G. Voss, C. C. Broder, and G. V. Quinnan, Jr. 2007. Extensively cross-reactive anti-HIV-1 neutralizing antibodies induced by gp140 immunization. Proc. Natl. Acad. Sci. USA 10410193-10198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zolla-Pazner, S., J. O'Leary, S. Burda, M. K. Gorny, M. Kim, J. Mascola, and F. McCutchan. 1995. Serotyping of primary human immunodeficiency virus type 1 isolates from diverse geographic locations by flow cytometry. J. Virol. 693807-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]