Abstract
The paramyxovirus simian virus 5 (SV5) establishes highly productive persistent infections of epithelial cells without inducing a global inhibition of translation. Here we show that an SV5 mutant (the P/V-CPI− mutant) with substitutions in the P subunit of the viral polymerase and the accessory V protein also establishes highly productive infections like wild-type (WT) SV5 but that cells infected with the P/V-CPI− mutant show an overall shutdown of both host and viral translation at late times postinfection. Reduced host and viral protein synthesis with the P/V-CPI− virus was not due to lower levels of mRNA or caspase-dependent apoptosis and correlated with phosphorylation of the translation initiation factor eIF-2α. WT SV5 was a poor activator of the eIF-2α kinase protein kinase R (PKR). By contrast, the P/V-CPI− mutant induced PKR phosphorylation, which correlated with the time course of translation inhibition but was independent of interferon signaling. In HeLa cells that expressed the PKR inhibitor influenza A virus NS1 or reovirus sigma3, the rate of host protein synthesis at late times after infection with the P/V-CPI− mutant was restored to ∼50% that of control HeLa cells. By contrast, the rates of P/V-CPI− viral protein synthesis in HeLa cells expressing NS1 or sigma3 were dramatically enhanced, between 5- and 20-fold, while levels of viral mRNA were increased only slightly (NS1-expressing cells) or remained constant (sigma3-expressing cells). Similar results were found using HeLa cells where PKR levels were reduced due to knockdown by small interfering RNA. Expression of either the WT P or the WT V protein from the genome of the P/V-CPI− mutant resulted in lower levels of PKR activation and rates of host and viral protein synthesis that closely matched those seen with WT SV5. Despite higher rates of translation, cells infected with the V- or P-complemented virus accumulated viral mRNAs to lower levels than that seen with the parental P/V-CPI− mutant. We present a model in which the paramyxovirus P/V gene products limit induction of PKR by limiting the synthesis of aberrant viral mRNAs and double-stranded RNA and thus prevent the shutdown of translation by a mechanism that differs from that of other PKR inhibitors such as NS1 and sigma3.
The inhibition of protein synthesis is an important antiviral mechanism by which the innate immune system controls viral replication (9, 16, 32, 55). Many cytopathic RNA viruses, such as vesicular stomatitis virus, poliovirus, and influenza virus, inhibit the translation of host mRNAs while selectively translating viral mRNAs, and it is thought that reduced host gene expression aids in the inhibition of antiviral responses (reviewed in reference 32). By contrast, some RNA viruses replicate very efficiently without inducing an overall inhibition of host cell gene expression. Examples of this include dengue virus (15), arenaviruses (4), some paramyxoviruses (e.g., human respiratory syncytial virus [11]), and some strains of reovirus (43).
Viral infections can trigger a shutdown of protein synthesis through the activation of the double-stranded RNA (dsRNA)-dependent protein kinase (PKR), a serine/threonine protein kinase that is constitutively expressed in mammalian tissues in a latent state (16, 17). During virus infection, PKR can become activated either by binding virus-derived dsRNA or under conditions of cellular stress by direct binding to the host cellular protein PACT (30). PKR activation involves homodimerization and autophosphorylation, which in turn can lead to phosphorylation of eIF-2α on serine 51 and an inhibition of translation (55). PKR expression can be upregulated by type I interferon (IFN) signaling, and this is one mechanism by which IFN is able to prime uninfected cells for a robust antiviral response (17). The importance of PKR in the antiviral response is evident from the diverse mechanisms viruses have evolved to counteract PKR functions (reviewed in references 16 and 29), including the expression of inhibitors that target the recognition of dsRNA (e.g., adenovirus VA RNA and reovirus sigma3), PKR dimerization (e.g., hepatitis C virus 5A protein), interactions of PKR with substrates (e.g., vaccinia virus K3L), and alteration of the levels (e.g., poliovirus) or subcellular location (cytomegalovirus TRS1 [21]) of PKR.
To date, there are no reports of a paramyxovirus gene product that specifically counteracts PKR activation. It has long been known that the paramyxovirus simian virus 5 (SV5) can establish a prolonged and robust replication cycle in epithelial cells without a global shutdown of host or viral protein synthesis (8, 37, 38), a finding that raises the question of how SV5 avoids activation of PKR. Here we describe a role for the SV5 P/V gene products in maintaining high-level viral gene expression without inducing a PKR-mediated shutdown of protein synthesis.
Paramyxoviruses have evolved mechanisms to counteract a number of host cell responses to infection. Many of these functions have been attributed to products of the P/V (or sometimes P/V/C) gene, which in the case of SV5 encodes both the phosphoprotein P and the accessory V protein (12, 18, 36, 39). Accurate transcription of the SV5 P/V gene results in an mRNA that codes for the V protein. The P mRNA is identical to the V mRNA except for the addition of two nontemplated G residues that are inserted by the viral polymerase at a precise location in the P/V transcript (48). Thus, the SV5 P and V proteins are identical for 164 amino-terminal residues (the shared P/V region) but have unique C terminal domains. The phosphoprotein P is an essential subunit of the viral RNA-dependent RNA polymerase (28). The V protein contains a highly conserved cysteine-rich zinc-binding domain that is required for many V-associated functions. V is thought to function in the regulation of viral RNA synthesis (24, 31). V protein has additional roles in counteracting host cell antiviral responses (reviewed in reference 19), including blocking of IFN signaling by targeting STAT1 for degradation (12) and inhibiting IFN gene expression (1, 7, 39).
The N-terminal region of the V protein, which is shared with the P protein, plays an important role in counteracting host cell antiviral pathways (6, 50). This is evident from the naturally occurring CPI− strain of SV5, which is defective in inducing STAT1 degradation and blocking type I IFN signaling (6). Mutational analyses have identified which of these six amino acid differences in the P/V region between wild-type (WT) recombinant SV5 (rSV5) and the CPI− strain are responsible for this loss of function in inducing STAT1 degradation (6). We have previously engineered an rSV5 mutant (rSV5 P/V-CPI−) to encode the same six CPI− P/V substitutions in the background of the WT rSV5 genome (50). This P/V mutant failed to target STAT1 for degradation, as expected. However, the mutant was also found (i) to be a potent inducer of IFN and proinflammatory cytokines (51, 56), (ii) to express viral RNA and proteins earlier and to higher levels than WT rSV5 (13, 50), and (iii) to induce extensive cell death by apoptosis that is not seen with WT SV5 (51, 52).
Here we report an additional phenotype of the P/V-CPI− mutant that is not seen with WT rSV5: human epithelial cells infected with the P/V-CPI− mutant show a dramatic shutoff of both host cell and viral protein synthesis at late times postinfection (p.i.). We have tested the hypotheses that WT and P/V mutant viruses differ in their activation of PKR and that PKR plays an important role in the shutdown of host and viral protein synthesis in P/V− mutant-infected cells. Altering the level or function of PKR in P/V-CPI− mutant-infected cells led to increased translation of host proteins and dramatically increased the rate of viral protein synthesis to levels greater than those seen with WT SV5-infected cells. Strikingly, expression of either WT V or WT P protein from the P/V-CPI− genome also increased the rates of both host cell and viral protein synthesis at late times p.i., but the higher translation rates differed from those seen with PKR inhibitors, since expression of WT V or P protein resulted in a decrease in viral mRNA levels in P/V-CPI− mutant-infected cells. We propose a model for the role of the SV5 P/V gene products in limiting the activation of PKR and maintaining a high level of viral gene expression without activating a global inhibition of translation.
MATERIALS AND METHODS
Cells and viruses.
Monolayer cultures of A549, HeLa, 2fTGH, and Jak-1 deficient U4C cells were grown in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS). 2fTGH and U4C cells were kind gifts from Curt Horvath (Northwestern University) and Ganes Sen (Cleveland Clinic), respectively. HeLa cells constitutively expressing the influenza virus PR8 NS1 protein and control neomycin-resistant HeLa cells were the kind gifts of Makoto Takeda (Kyushu University, Fukuoka, Japan) and were cultured as described previously (35). HeLa cells expressing the reovirus type 3 Dearing sigma3 protein were generated by transfection with pCXN-S4T3D (26) followed by selection in DMEM containing 0.5 mg/ml G418. Individual colonies were picked, expanded in medium containing G418, and screened by Western blotting with polyclonal rabbit serum against sigma3. The sigma3 plasmid and antiserum were kindly provided by T. Kobayashi and T. Dermody (Vanderbilt University School of Medicine).
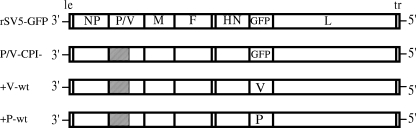
WT rSV5-green fluorescent protein (GFP) was recovered as described previously (50) from a cDNA plasmid (22) kindly provided by Robert Lamb (Northwestern University) and Biao He (Penn State University) and was grown in MDBK cells. The chimeric rSV5 P/V-CPI− mutant was grown in Vero cells as described previously (50). Stocks of the P/V-CPI− mutant were grown at low multiplicities of infection (MOI) and had infectivity-to-particle ratios (PFU-to-hemagglutination unit ratios) that were indistinguishable from those of rSV5-GFP preparations (6,100 ± 200 for rSV5-GFP versus 5,800 ± 300 for the P/V-CPI− mutant [data not shown]). The rSV5 P/V-CPI− viruses encoding an additional copy of the WT V or WT P gene inserted between the HN and L genes (the +V-wt and +P-wt viruses, respectively) are depicted schematically in Fig. 1 and have been described previously (13). Editing of the V and P genes in these viruses was disrupted through changes in the nucleotide sequence upstream of and including the editing site of the P/V gene such that only the V protein (+V-wt) or P protein (+P-wt) was expressed from the additional gene (13). Plaque assays and a beta interferon (IFN-β) enzyme-linked immunosorbent assay were carried out as described previously (13, 50).
FIG. 1.
Schematic diagram of SV5 variants described in this study. The genome structure of WT rSV5-GFP is shown schematically as negative-sense RNA, with an additional gene (GFP, WT V, or WT P) inserted between HN and L. Extra P or V genes have an altered editing site as described in Materials and Methods such that only the P or V protein is expressed. The cross-hatched box represents the shared N-terminal region of the P and V proteins, which contains the 6 CPI− amino acid substitutions. le, leader; tr, trailer.
Western blotting.
Dishes (diameter, 60 mm) of cells were either mock infected or infected at an MOI of 10 and incubated in DMEM containing 2% FBS. At the indicated time p.i., dishes were placed on ice, washed twice with ice-cold phosphate-buffered saline, and lysed in lysis buffer (50 mM Tris, 120 mM NaCl, 0.5% Nonidet P-40) containing 1 mM phenylmethylsulfonyl fluoride, 100 nM okadaic acid, 100 nM microcystin, and 100 nM NaF. Lysates were spun at 14,000 rpm and 4°C for 15 min, and supernatants were collected and stored at −80°C. Protein concentrations of cell lysates were determined by a bicinchoninic acid assay (Pierce Chemicals). Fifty to 70 μg of cell lysate was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and proteins were transferred to nitrocellulose membranes, blocked in 5% bovine serum albumin overnight, and probed with antibodies to either total PKR, PKR phosphorylated on threonine 446, total eIF-2α, or eIF-2α phosphorylated on serine 51 (all from Cell Signaling, Inc.). An anti-β-actin antibody (A5316) was purchased from Sigma. Samples were analyzed by horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence (Pierce Chemicals). Nitrocellulose membranes were stripped with 0.2 M glycine for 1 h before reprobing with a second antibody. As positive controls for PKR activation, mock-infected cells were treated 24 h before harvesting with human universal type IFN (1,000 U/ml; PBL InterferonSource) in DMEM containing 10% FBS. Two hours prior to harvesting, cells were transfected with 5 μg poly(I:C) using Lipofectamine 2000 (Invitrogen). For induction of eIF-2α phosphorylation, mock-infected cells were treated for 1 h with thapsigargin (1 μg/ml; Axxora) before harvesting as described above.
Metabolic labeling.
To analyze rates of protein synthesis, mock-infected or virus-infected cells were incubated for 30 min with DMEM lacking cysteine and methionine and then radiolabeled for 15 min with ∼100 μCi/ml Tran35S-label. Cells were then washed twice with warm phosphate-buffered saline, lysed in 1% SDS, and stored at −20°C. Four micrograms of lysate was resolved by SDS-PAGE, and radiolabeled proteins were visualized by exposing dried gels to film. Radioactivity in selected regions of interest was quantitated by phosphorimaging. The radioactivity in an identically sized area immediately adjacent to viral bands was quantitated and subtracted from the values in viral bands.
siRNA experiments.
HeLa cells in 24-well plates were transfected using Oligofectamine (Invitrogen) with short interfering RNA (siRNA) specific for PKR (P-002028-01-20) or GAPDH (D-001140-01-5) (both from Dharmacon; final concentration, 80 nM) according to the manufacturer's instructions. Twenty-four hours posttransfection, the medium was replaced with fresh DMEM containing 2% FBS. Forty-eight hours posttransfection, cells were infected as described above. At 24 h p.i., cells were either radiolabeled with Tran35S-label or harvested for Western blotting as described above.
Analysis of viral RNA.
The accumulation of viral mRNA by reverse transcription-real-time PCR was carried out as detailed previously (56). Briefly, total RNA was isolated from mock-infected or virus-infected cells at the indicated times postinfection using Trizol (Invitrogen). RNA was reverse transcribed with Moloney murine leukemia virus reverse transcriptase and an oligo(dT) primer, and a portion of the resulting cDNAs was used in a real-time PCR assay using an ABI Prism 7000 system with primer/probe sets designed to detect cellular actin or SV5 nucleocapsid protein mRNA as described previously (56).
RNase protection assays (RPAs) were performed using the Ambion RPAIII kit according to the manufacturer's instructions, using an RNase A/RNase T1 digestion step, followed by analysis on 6% polyacrylamide gels containing 9 M urea and autoradiography. Riboprobes were generated by in vitro transcription of a linearized pGem plasmid encoding negative-sense NP-specific sequences (genomic RNA bases 90 to 175) in the presence of [32P]CTP as described previously (13).
RESULTS
Reduced translation rate and increased eIF-2α phosphorylation at late times after infection with the P/V-CPI− mutant.
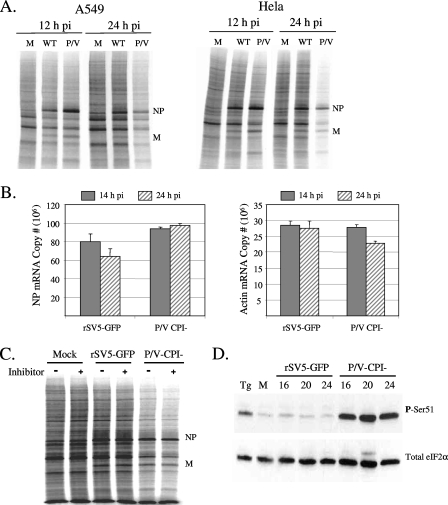
Radiolabeling experiments were carried out to examine the rate of protein synthesis following infection with WT rSV5 and the P/V-CPI− mutant. A WT rSV5 encoding GFP as an additional transcription unit between HN and L was used to control for the GFP encoded by the P/V mutant virus (Fig. 1), and this additional gene has been shown previously to have no detectable effect on rSV5 growth properties (22). HeLa and A549 cells were either mock infected or infected with rSV5-GFP or the P/V-CPI− mutant at an MOI of 10 to ensure that all cells in the population were infected. Cells were radiolabeled for 15 min with 35S-labeled amino acids at 12 and 24 h p.i., and equal amounts of cell lysate were analyzed by SDS-PAGE. As shown in Fig. 2A, radiolabeled viral proteins such as NP and M were abundantly synthesized at 12 h p.i., with viral protein synthesis being slightly higher for the P/V-CPI− virus than for rSV5-GFP, as described previously (50). In addition, there was little difference in the incorporation of radiolabel into host proteins between infected cells and control cells, as evident by the smear of radioactive proteins throughout the lanes. Likewise, by 24 h p.i., viral proteins in rSV5-infected cells continued to be synthesized at a high rate, and there was little difference in the synthesis of host proteins between infected and mock-infected cells. This result is consistent with previous reports that WT rSV5 does not induce a global shutdown of host protein synthesis (37, 38). However, in striking contrast to rSV5-GFP-infected cells, both A549 and HeLa cells infected with the P/V mutant showed reduced incorporation of radiolabel into host and viral proteins at 24 h p.i. (Fig. 2A).
FIG. 2.
Reduced rate of translation and increased eIF-2α phosphorylation following infection with the P/V-CPI− mutant but not WT rSV5. (A) Rates of protein synthesis. A549 or HeLa cells were either mock infected (lanes M) or infected at an MOI of 10 with WT rSV5-GFP (lanes WT) or the P/V-CPI− mutant (lanes P/V). At 12 or 24 h p.i., cells were radiolabeled for 15 min with Tran35S-label. Cells were lysed, and 4 μg of protein was analyzed by SDS-PAGE and autoradiography. (B) Levels of NP and actin mRNAs. Total RNA was extracted at 14 and 24 h p.i. from A549 cells that were infected at an MOI of 10 with rSV5-GFP or the P/V-CPI− mutant. Levels of NP or actin mRNA were quantitated by reverse transcription-real-time PCR as described in Materials and Methods. Values are means from three experiments. Error bars, standard deviations. (C) Effects of caspase inhibitors on rates of protein synthesis. HeLa cells infected with the indicated viruses were cultured with (+ lanes) or without (− lanes) 100 μM Z-VAD-fmk. At 24 h p.i., cells were radiolabeled and lysates analyzed as described for panel A. (D) eIF-2α phosphorylation. A549 cells were either mock infected or infected at an MOI of 10 with rSV5-GFP or the P/V-CPI− mutant. At 16, 20, and 24 h p.i., cell lysates were prepared and 70 μg of protein analyzed by Western blotting with antibodies specific for total eIF-2α or eIF-2α phosphorylated on serine 51 (P-Ser51). As a positive control, mock-infected cells were treated for 1 h with 1 μg/ml thapsigargin (Tg).
To determine if changes in the rate of protein synthesis at 24 h p.i. correlated with changes in mRNA levels, A549 cells were infected at high MOI with rSV5-GFP or the P/V-CPI− mutant, and the accumulation of NP and actin mRNAs was determined by reverse transcription and real-time PCR. As shown in Fig. 2B, cells infected with rSV5-GFP showed a slight reduction in accumulated NP mRNA between 14 and 24 h p.i., while NP mRNA levels in P/V-CPI− mutant-infected cells were unchanged. Actin levels were unchanged in rSV5-GFP-infected cells and were reduced only slightly in P/V-CPI− mutant-infected cells (Fig. 2B), most likely due to the virus-induced cytopathic effect (51). Our findings that the P/V− CPI− mutant produces higher titers than rSV5-GFP under single-cycle growth conditions and produces higher-than-WT levels of viral protein, mRNA, and genomes (13, 50) are not consistent with the presence of contaminating defective-interfering particles (45, 57). Particle-to-PFU ratios were indistinguishable between rSV5-GFP and the P/V-CPI− mutant viral stocks (see Materials and Methods). Together, these data indicate that defective-interfering particles and changes in the accumulation of mRNAs cannot account for the differences in the rates of protein synthesis between cells infected with rSV5-GFP and cells infected with the P/V-CPI− mutant.
Induction of apoptosis can result in an inhibition of protein synthesis due to caspase-3-dependent cleavage of translation initiation factors (10). As shown in Fig. 2C, incubation of infected cells with the pan-caspase inhibitor Z-VAD-fmk had no effect on the reduced translation rates in P/V-CPI− mutant-infected cells, indicating that caspase-dependent apoptosis was not a major contributing factor to differences in translation rates for rSV5-GFP and the P/V mutant at 24 h p.i.
The translation initiation factor eIF-2α is a key regulator of translation and is inhibited by phosphorylation on serine residue 51 (42, 54). To determine if changes in the rate of protein synthesis correlated with eIF-2α phosphorylation, A549 cells were either mock infected or infected at an MOI of 10 with rSV5-GFP or the P/V-CPI− mutant. Lysates harvested at 16, 20, and 24 h p.i. were analyzed by immunoblotting with an antiserum specific for total eIF-2α or eIF-2α that is phosphorylated on serine 51 (phospho-eIF-2α). As shown in Fig. 2D, mock-infected samples showed low levels of phospho-eIF-2α, which were elevated by treatment with the endoplasmic reticulum stress-inducing compound thapsigargin. Cells infected with rSV5-GFP had phospho-eIF-2α levels very similar to those of mock-infected cells. By contrast, cells infected with the P/V-CPI− mutant showed elevated levels of phospho-eIF-2α at 16 h p.i., and this increase was sustained to 24 h p.i. (Fig. 2D). Very similar results were seen using HeLa cells (data not shown). Taken together, these data show that infection with the P/V-CPI− virus leads to an increase in eIF-2α phosphorylation and an overall reduction in the rate of both viral and host translation. These changes in translation rates cannot be explained by the induction of caspase-dependent apoptosis or by decreases in viral mRNA levels.
PKR phosphorylation following infection with the P/V-CPI− mutant but not rSV5-GFP.
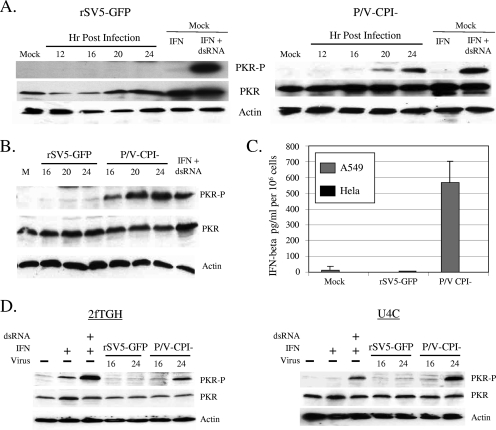
We hypothesized that eIF-2α phosphorylation and reduced translation in cells infected with the P/V mutant were the results of PKR activation. To test this hypothesis, A549 or HeLa cells were either mock infected or infected at high MOI with rSV5-GFP or the P/V-CPI− mutant. Lysates harvested at different times p.i. were analyzed by Western blotting for total PKR and PKR that had been phosphorylated on threonine 446 (phospho-PKR), a residue that has previously been shown to be critical for PKR kinase activity (reviewed in reference 17). As shown in Fig. 3A, high levels of phospho-PKR were detected in the positive-control samples of mock-infected A549 cells that had been treated with IFN and transfected dsRNA. Cells infected with rSV5-GFP showed very low levels of phospho-PKR (Fig. 3A, left), consistent with the low levels of phospho-eIF-2α shown in Fig. 2 above. By contrast, there was a time-dependent appearance of phospho-PKR in lysates from cells infected with the P/V-CPI− mutant (Fig. 3A, right), which was evident by 16 h p.i. and increased up to 24 h p.i. Similar results were seen for HeLa cells (Fig. 3B), in which phospho-PKR levels were detected by 16 h after infection with the P/V-CPI− mutant.
FIG. 3.
Increased PKR phosphorylation following infection with the P/V-CPI− mutant but not with WT rSV5-GFP. (A) PKR phosphorylation. A549 cells were either mock infected or infected at an MOI of 10 with rSV5-GFP (left) or the P/V-CPI− mutant (right). At the indicated times p.i., cell lysates were prepared and analyzed by Western blotting for actin, total PKR, or PKR phosphorylated on Thr446 (PKR-P). As a positive control, mock-infected cells were treated with IFN and transfected with dsRNA as described in Materials and Methods. (B) Time course of PKR phosphorylation in HeLa cells. Cell lysates were prepared and analyzed as described for panel A. (C) IFN-β induction does not correlate with activation of PKR or translation shutoff. HeLa or A549 cells were either mock infected or infected at an MOI of 10 with rSV5-GFP or the P/V-CPI− mutant. Media were collected at 24 h p.i. and assayed for levels of IFN-β. Data are means; error bars, standard deviations. (D) The P/V− mutant induces PKR phosphorylation in cells defective for IFN signaling. 2fTGH cells or IFN signaling-defective U4C cells were either mock infected (− lanes) or infected at an MOI of 10 with rSV5-GFP or the P/V-CPI− mutant. At 16 and 24 h p.i., cell lysates were prepared and analyzed by Western blotting for actin, total PKR, or PKR phosphorylated on Thr446. As a positive control, mock-infected cells were treated with IFN and transfected with dsRNA as described in Materials and Methods.
In contrast to WT rSV5, the P/V-CPI− mutant induces IFN and is defective in blocking IFN signaling (50), raising the hypothesis that differences in IFN induction are an important factor in the differential reduction in host and viral protein synthesis in cells infected with WT rSV5 versus the P/V mutant. To test this hypothesis, HeLa and A549 cells were infected with rSV5-GFP or the P/V-CPI− mutant, and the medium collected at 24 h p.i. was analyzed for levels of IFN-β. As shown in Fig. 3C, the P/V-CPI− mutant induced high levels of IFN-β in A549 cells that were not seen with rSV5-GFP infection. By contrast, HeLa cells infected with the P/V-CPI− mutant did not secrete detectable levels of IFN-β. Since both HeLa and A549 cells show P/V-CPI− mutant-mediated activation of PKR (Fig. 3A and B) and translation inhibition (Fig. 2A), these data show that induction of IFN-β is not an essential factor in the shutdown of translation by infection with the P/V-CPI− mutant.
To confirm that IFN signaling was not essential for activation of PKR by the P/V-CPI− mutant, we analyzed PKR activation in U4C cells, which are defective in IFN signaling, and the control parental 2fTGH cells (33, 44). As shown in Fig. 3D, dsRNA transfection resulted in the appearance of phospho-PKR in both cell lines, consistent with previous findings that U4C cells are capable of responding to dsRNA despite the defects in IFN signaling (46). In contrast to the results for rSV5-GFP, which did not alter phospho-PKR levels, phosphorylated PKR was detected in lysates from both cell lines after infection with the P/V-CPI− mutant. Taken together, these data demonstrate a correlation between activation of PKR and an inhibition of protein translation at late times p.i. by the P/V-CPI− mutant, but differences in PKR activation between WT and P/V mutant SV5 strains cannot be accounted for solely by differences in blocking of IFN signaling.
Reducing levels of PKR by siRNA results in higher rates of viral and cellular protein synthesis in cells infected with the P/V-CPI− virus.
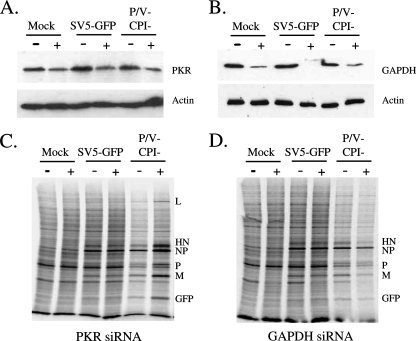
siRNA experiments were carried out to determine if knockdown of PKR levels affected the rate of protein synthesis at late times after infection with the P/V mutant virus. HeLa cells were transfected with siRNAs specific for PKR (Fig. 4A and C) or glyceraldehyde-3-phosphate dehydrogenase (GAPDH), used as a control (Fig. 4B and D), and then infected with either rSV5-GFP or the P/V-CPI− mutant. Western blotting showed that levels of PKR and GAPDH at 24 h p.i. were reduced by ∼50% and >80% in cells transfected with siRNAs specific for PKR and GAPDH, respectively (Fig. 4A and B). Virus infection had no detectable effect on the efficiency of siRNA-mediated knockdown. At 24 h p.i., cells were radiolabeled with 35S-labeled amino acids, and cell lysates were analyzed by SDS-PAGE. As shown in Fig. 4C, the rate of protein synthesis in mock-infected cells or in cells infected with rSV5-GFP was unaffected by transfection with a siRNA targeting PKR. However, in P/V-CPI− mutant-infected cells that had been treated with the PKR siRNA, host cell and viral translation rates were enhanced relative to those for control-treated cells. In control samples, transfection of cells with the control GAPDH siRNA did not enhance viral or cellular protein synthesis at late times after infection with the P/V-CPI− mutant (Fig. 4D). Taken together, these data indicate that even a modest reduction in the steady-state level of PKR by siRNA transfection results in enhanced rates of host and viral protein synthesis at a late time after infection with the P/V-CPI− mutant.
FIG. 4.
Reduction of levels of PKR by siRNA results in higher levels of viral and cellular protein synthesis in cells infected with the P/V-CPI− virus. (A and B) Levels of PKR and GAPDH. HeLa cells were transfected with siRNAs specific for PKR (A) or GAPDH (B) as described in Materials and Methods and were then infected at a high MOI with either WT rSV5-GFP or the P/V-CPI− mutant. At 24 h p.i., cell lysates were analyzed by Western blotting for levels of PKR, GAPDH, and actin. (C and D) Rates of protein synthesis. HeLa cells treated as described for panels A and B were radiolabeled with Tran35S-label, and lysates were analyzed as described in the legend to Fig. 2A. Plus and minus signs indicate transfection with or without the siRNA for PKR (C) or GAPDH (D).
Expression of either reovirus sigma3 protein or influenza A virus NS1 protein restores high-level protein synthesis and limits PKR phosphorylation in cells infected with the P/V-CPI− mutant.
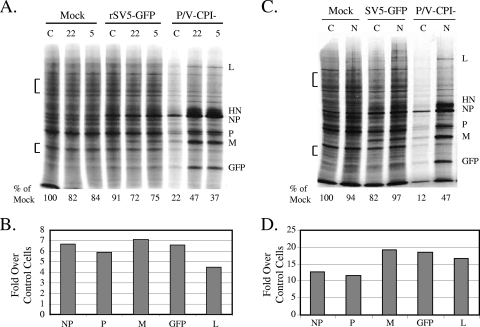
To test the role of dsRNA in the shutoff of protein synthesis by the P/V-CPI− mutant, HeLa cell lines (clones 22 and 5) were isolated that constitutively express the reovirus sigma3 protein, a viral polypeptide that inhibits PKR activation by sequestering dsRNA (43, 58). Control HeLa cells or sigma3-expressing cells were either mock infected or infected with rSV5-GFP or the P/V-CPI− mutant. Cells were radiolabeled at 24 h p.i., and lysates were analyzed by SDS-PAGE. As shown in Fig. 5A, the rates of host and viral protein synthesis at late times after infection with the P/V-CPI− virus were much higher in sigma3-expressing cells (lanes 22 and 5) than in control cells (lane C). In the representative experiment for which results are shown in Fig. 5A, host protein synthesis in sigma3-expressing cells at late times after infection with the P/V mutant was increased to ∼37 to 47% of that seen in mock-infected cells. Quantitation of translation rates from a representative experiment with cell line 22 showed that P/V-CPI− viral protein synthesis in the sigma3-expressing cells was increased an average of 7-, 6-, 7-, and 4.5-fold for NP, P, M, and L, respectively (Fig. 5B).
FIG. 5.
Effects of the reovirus sigma3 and influenza virus NS1 proteins on translation and PKR activation in cells infected with the P/V-CPI− mutant. (A) Rate of protein synthesis in sigma3-expressing cells. Control (lanes C) or sigma3-expressing (clone 22 or clone 5) HeLa cells were either mock infected or infected at an MOI of 10 with the indicated viruses. At 24 h p.i., cells were radiolabeled with Tran35S-label, and lysates were analyzed as described in the legend to Fig. 2. The amounts of radioactivity in host cell bands in two regions (bracketed) of the gel were quantitated and are expressed below the gel as percentages of the radioactivity found for mock-infected control cells. Results are representative of three experiments. (B) Viral protein synthesis in sigma3-expressing cells. Radioactivity in viral protein bands from infected sigma3-expressing cells was quantitated as described in Material and Methods and is expressed as the increase (n-fold) in viral protein synthesis over that in control cells. Results are representative of three experiments. (C) Rate of protein synthesis in NS1-expressing cells. Control or NS1-expressing (lanes N) HeLa cells were either mock infected or infected at an MOI of 10 with the indicated viruses. Cells were treated and analyzed as described for panel A. (D) Viral protein synthesis in NS1-expressing cells. Radioactivity in viral protein bands from infected NS1-expressing cells was quantitated as described for panel B.
Based on the reported role of influenza A virus NS1 protein in inhibiting PKR activity (2, 30), we also tested the hypothesis that expression of the NS1 protein would maintain high-level translation in P/V-CPI− mutant-infected cells. Control neomycin-resistant HeLa cells or HeLa cells constitutively expressing the NS1 protein from the PR8 strain of influenza A virus (35) were either mock infected or infected with rSV5-GFP or the P/V-CPI− mutant. Cells were radiolabeled at 24 h p.i., and lysates were analyzed by SDS-PAGE. As shown in Fig. 5C, the rates of both host and viral translation in P/V-CPI− mutant-infected HeLa cells that express NS1 (lanes N) were much greater than those in control cells lacking NS1 (lanes C). The level of host protein synthesis in control P/V-CPI− mutant-infected cells at 24 h p.i. was ∼12% of that in mock-infected samples (Fig. 5C, bottom), but in NS1-expressing P/V-CPI− mutant-infected cells, this level was elevated to ∼47% of that in mock-infected samples. Remarkably, P/V-CPI− viral protein translation rates were greatly increased by NS1 expression: rates of NP, P, M, GFP, and L translation in a representative experiment were enhanced ∼12-, 11-, 19-, 19-, and 16-fold, respectively, over levels in control HeLa cells (Fig. 5D). Virus yields following infection at a high MOI with the P/V-CPI− mutant were only two- to threefold higher in NS-1-expressing cells than in control cells (data not shown).
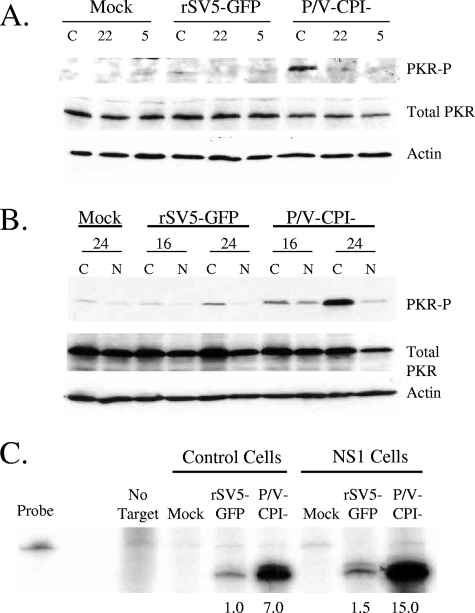
To determine if expression of sigma3 reduced PKR activation in P/V-CPI− mutant-infected cells, control HeLa cells or sigma3-expressing cells (clone 22 or 5) were either mock infected or infected with rSV5-GFP or the P/V-CPI− mutant. Cell lysates were analyzed by Western blotting at 24 h p.i. for PKR phosphorylation. As shown in Fig. 6A, P /V− CPI− mutant infection of control HeLa cells (lanes C) resulted in phosphorylation of PKR, which was not seen for rSV5-GFP-infected cells. Most importantly, sigma3-expressing cells showed much lower PKR activation following infection with the P/V-CPI− mutant (Fig. 6A, lanes 22 and 5). Similar results were seen for NS1-expressing cells. As shown in Fig. 6B, only very low levels of PKR phosphorylation were detected in control cells (lanes C) or in NS1-expressing cells (lanes N) following infection with rSV5-GFP. Control cells infected with the P/V mutant showed abundant levels of phosphorylated PKR. Most importantly, in the NS1-expressing cells, levels of phosphorylated PKR following P/V-CPI− mutant infection were greatly reduced (Fig. 6B, lanes N).
FIG. 6.
Effects of the reovirus sigma3 and influenza virus NS1 proteins on PKR activation in cells infected with the P/V-CPI− mutant. (A) PKR phosphorylation in sigma3-expressing cells. HeLa control cells (lanes C) and HeLa-sigma3 cell clones (clones 22 and 5) were infected at an MOI of 10 with the indicated viruses. Cell lysates were prepared at 24 h p.i. and analyzed by Western blotting for actin, total PKR, or PKR phosphorylated on Thr446 (PKR-P). (B) PKR phosphorylation in NS1-expressing cells. HeLa control cells and NS1-expressing cells (lanes N) were infected with the indicated viruses and analyzed for levels of phosphorylated PKR as described for panel A. (C) mRNA levels. Control or NS1-expressing HeLa cells were either mock infected or infected at an MOI of 10 with the indicated viruses. RNA harvested at 24 h p.i. was analyzed by RPA using a probe specific for NP mRNA. The probe-only lane represents 1/50 of the input probe for each sample. Numbers below the gel are the increases (n-fold) in mRNA levels over those for control rSV5-GFP-infected cells (set at 1). Results are representative of two independent experiments.
Levels of NP mRNA were analyzed by an RPA to determine if the enhanced rate of P/V-CPI− mutant protein synthesis correlated with increased levels of viral mRNA relative to those in control cells. As shown in Fig. 6C and as reported previously (13, 50), levels of NP mRNA accumulation at 24 h p.i. were higher in NS1-expressing cells infected with the P/V-CPI− mutant than in NS1-expressing cells infected with rSV5-GFP. Most importantly, the level of NP mRNA in NS1-expressing cells after infection with the P/V-CPI− mutant was only ∼2-fold higher than that in control P/V-CPI− mutant-infected cells, and this difference could not account for the ∼12-fold increase in the NP translation rate (see Fig. 5D). Sigma3-expressing cells infected with the P/V-CPI− mutant did not have higher levels of NP mRNA than control cells (data not shown). Taken together, these data indicate that cells expressing PKR inhibitors have lower activation of PKR following infection with the P/V-CPI− mutant and an enhanced rate of viral protein synthesis at late times p.i., but this increase cannot be explained simply by an increase in viral mRNA levels in cells expressing PKR inhibitors.
Expression of the WT V protein or WT P protein from the P/V-CPI− genome restores high translation rates and limits PKR activation.
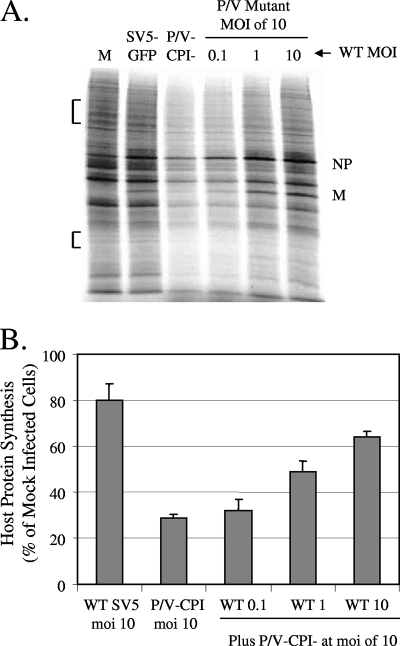
Our previous coinfection experiments have shown that suppression of some host cell responses by WT rSV5 is dominant over the activation of these responses by the P/V-CPI− mutant (51). To test the hypothesis that coinfection with WT rSV5 can restore translation in P/V-CPI− mutant-infected cells, A549 cells were either mock infected or infected with rSV5-GFP or the P/V-CPI− mutant at an MOI of 10. In addition, separate cell cultures were coinfected with the P/V-CPI− mutant at a constant MOI of 10 and increasing amounts of rSV5-GFP (MOI of 0.1, 1, and 10). We have previously shown that under these conditions, A549 cells are efficiently infected by both viruses, with no evidence of viral exclusion (51). Cells were then metabolically labeled with 35S-labeled amino acids at 24 h p.i., and cell lysates were analyzed by SDS-PAGE. Figure 7A shows the results of a representative experiment, and Fig. 7B shows the quantitation of 35S incorporation from three experiments, expressed as a percentage of that found for mock-infected cells. Cells infected with rSV5-GFP showed a small reduction in radiolabel incorporation (down to ∼80% of that seen for mock-infected cells), similar to that seen in the quantitations in Fig. 5 above. As expected, cells infected with the P/V-CPI− mutant showed a dramatic reduction in host cell translation, down to ∼30% that of control cells. However, in cells that were coinfected with WT and mutant viruses, there was a dose-dependent increase in translation rates with increasing MOI of rSV5-GFP. These results are consistent with the hypothesis that WT gene products supplied in trans can maintain high levels of protein synthesis at late times after infection with the P/V-CPI− mutant.
FIG. 7.
Coinfection with WT rSV5-GFP restores high-level protein synthesis to cells infected with the P/V-CPI− mutant. (A) Rate of protein synthesis. A549 cells were either mock infected (lane M), infected at an MOI of 10 with the P/V-CPI− mutant or rSV5-GFP only, or coinfected with the P/V-CPI− mutant at an MOI of 10 and WT rSV5-GFP at an MOI of 0.1, 1, or 10. At 24 h p.i., cells were were radiolabeled with Tran35S-label, and lysates were analyzed as described in the legend to Fig. 2A. (B) Quantitation of radioactivity incorporation. The amounts of radioactivity in two regions of the SDS-PAGE gels (bracketed in panel A) were quantitated by phosphorimager analysis. Results from three independent experiments are expressed as the mean percentage of the value for mock-infected cells. Error bars, standard deviations.
The differential abilities of rSV5-GFP and the P/V-CPI− mutant to activate PKR and reduce translation rates could be due to the CPI− substitutions in the V protein, the P protein, or both the V and P proteins. We have previously described two variants of the P/V mutant that have been engineered to individually express either the WT V protein (+V-wt) or the WT P protein (+P-wt) (Fig. 1) from an additional nonedited gene inserted between the HN and L genes (13). Cells infected with either the +V-wt or the +P-wt virus showed reduced IFN-β secretion and decreased apoptosis relative to those for the parental P/V-CPI− virus (13).
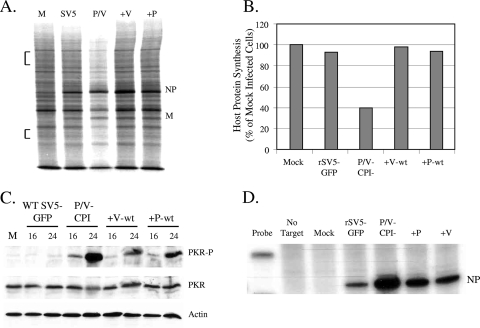
To determine the effect of WT V or WT P protein expression on translation rates in P/V-CPI− mutant-infected cells, HeLa cells were either mock infected or infected at high MOI with rSV5-GFP, the P/V-CPI− mutant, or the +V-wt or +P-wt virus. Cells were radiolabeled with 35S-labeled amino acids at 24 h p.i., and lysates were analyzed by SDS-PAGE. As shown in Fig. 8A, cells infected with the P/V-CPI− mutant had reduced translation rates for viral and host proteins at 24 h p.i. that were not seen in rSV5-GFP-infected cells. By contrast, cells infected with either the +V-wt or the +P-wt virus had rates of host cell translation that were very similar to that seen during infection with rSV5-GFP (Fig. 8B). Viral protein synthesis in cells infected with either the +V-wt or the +P-wt virus was very similar to that in rSV5-GFP-infected cells but was not elevated to the same extent as that for infected NS1- or sigma3-expressing cells. Very similar results were seen for A549 cells infected with the +V-wt or +P-wt virus (data not shown).
FIG. 8.
Expression of WT V protein or WT P protein from the P/V-CPI− strain genome restores high-level synthesis of viral and cellular proteins. (A) Effects of WT V and P on protein synthesis. HeLa cells were either mock infected (M) or infected at an MOI of 10 with rSV5-GFP (SV5), the P/V-CPI− mutant (P/V), or a P/V-CPI− mutant that was engineered to express the WT V protein (+V) or WT P protein (+P). At 24 h p.i., cells were radiolabeled with Tran35S-label, and lysates were analyzed as described in the legend to Fig. 2. (B) Quantitation of radioactivity incorporation. The amounts of radioactivity in two regions of SDS-PAGE gels (bracketed in panel A) were quantitated by phosphorimager analysis. Results are representative of three independent experiments and are expressed as the percentage of the value for mock-infected cells. (C) Effects of WT V and P proteins on PKR phosphorylation. A549 cells were either mock infected or infected at an MOI of 10 with the P/V-CPI−, +V-wt, or +P-wt virus. At 16 and 24 h p.i., cell lysates were prepared and analyzed by Western blotting for actin, total PKR, or PKR phosphorylated on Thr446 (PKR-P). (D) Accumulation of viral mRNA. A549 cells were infected with the indicated viruses, and at 14 h p.i., total RNA was analyzed for the presence of positive-sense NP-specific RNA by RPA.
The high rate of protein synthesis at late times after infection with the +V-wt or +P-wt virus correlated with reduced phosphorylation of PKR. This is evident in the Western blot in Fig. 8C, where levels of phospho-PKR in cells infected with the +V-wt or +P-wt virus are greatly reduced relative to those in cells infected with the parental P/V-CPI− virus. Low-level activation of PKR phosphorylation was detected by 16 h after infection with the +V-wt or +P-wt virus, but levels of phospho-PKR did not increase dramatically to the levels seen at later times after infection with the P/V-CPI− mutant.
Our published data have shown that steady-state levels of viral mRNA in cells infected with the +V-wt or +P-wt virus are much lower than those seen for the P/V-CPI− mutant and are similar to those seen for rSV5-GFP (13). As shown in Fig. 8D, the steady-state levels of NP mRNA in cells infected with the +V-wt or +P-wt virus were actually lower than the elevated levels seen in P/V-CPI− mutant-infected cells. Thus, expression of WT V or WT P protein enhances the rate of viral protein synthesis over that with the P/V-CPI− mutant, but unlike the situation with NS1- or sigma3-expressing cells, this increased translation correlates with a decrease in the accumulation of viral mRNA.
DISCUSSION
Many viruses have evolved mechanisms to prevent PKR activation (reviewed in references 16, 17, 29, and 55). However to our knowledge, no paramyxovirus gene product has been shown to specifically inhibit PKR activity. The finding that SV5 does not induce a global shutdown of host or viral protein synthesis (8, 37, 38) raises the question of how SV5 avoids activation of PKR during high-level replication. We have found that infection of epithelial cells with the P/V-CPI− mutant results in PKR activation, phosphorylation of eIF-2α, and a dramatic shutdown of both host and viral protein synthesis at late times p.i. We have used cell lines engineered to express the influenza A virus NS1 protein or the reovirus sigma3 protein, as well as targeting PKR by siRNA transfection, to demonstrate that PKR is a key cellular factor involved in the differential shutoff of translation in cells infected with rSV5-GFP or the P/V-CPI− mutant. Thus, because the P/V-CPI− virus contains alterations to the P/V gene only, our data suggest that P/V gene products contribute to the ability of WT rSV5 to maintain high levels of viral gene expression without inhibition of translation. In support of this conclusion, the most striking finding from our work is that expression of either the WT V protein or the WT P protein in the context of infection with the P/V-CPI− mutant results in lower PKR activation and a restoration of host and viral translation to the levels seen with WT rSV5 infection.
The influenza A virus NS1 protein binds to PKR and inhibits its activation (30). In NS1-expressing cells, the rate of host protein synthesis at late times after infection with the P/V mutant was ∼5-fold higher than that in control cells, but this was still restored only to ∼50% of the rate in mock-infected cells. By contrast, NS1 had a much greater effect on viral translation in P/V mutant-infected cells, since the rate of viral protein synthesis was enhanced ∼12- to 20-fold in NS1-expressing cells over that in control cells, depending on the individual viral protein analyzed. This enhanced translation of viral protein could not be accounted for by increased levels of viral mRNA. In addition to inhibiting PKR, the multifunctional NS1 protein is proposed to bind dsRNA (14); inhibit the activation of RIG-I, IRF-3, NF-κB, and 2′,5′-oligo(A) synthetase/RNase L systems (20, 27, 34, 47, 49); and enhance viral protein synthesis by binding to translation initiation factors (3, 41). It is possible that these NS1 functions may also significantly contribute to maintaining translation of viral mRNA in P/V-CPI− mutant-infected cells.
The only known mechanism by which the reovirus sigma3 protein inhibits PKR activation is through sequestering dsRNA (25, 43, 58). In sigma3-expressing cells, infection with the P/V mutant did not result in significant activation of PKR, suggesting that dsRNA produced during replication of the P/V-CPI− mutant serves as a major signal for activating antiviral responses and shutting down translation. It is reported that negative-strand RNA viruses do not generate dsRNA to levels that can be detected by a monoclonal antibody (53); however, mutant viruses were not analyzed in this previous study. We propose that rSV5-GFP and the P/V-CPI− mutant show differential activation of PKR because the P/V mutant produces higher levels of dsRNA than WT rSV5. As is the case with the NS1-expressing cells, host protein synthesis in P/V− mutant-infected sigma3-expressing cells was restored to ∼50% of the level seen in mock-infected cells, while viral protein synthesis was enhanced 4.5- to 7-fold over that for control cells.
Our results suggest that the overall levels of PKR in an infected cell can be important for inhibition of host versus viral translation. This is evident from our studies where siRNA transfection was found capable of reducing PKR levels by only ∼50% (Fig. 6A). Despite this modest change in PKR levels, translation of P/V-CPI− proteins in PKR knockdown cells was substantially enhanced over that in control-transfected cells, while host protein synthesis also increased to a lesser extent. Thus, there may be a critical threshold level of PKR that regulates the translation of viral and host mRNAs.
Since PKR is an IFN-stimulated gene, an important question was whether IFN signaling was essential for differential activation of PKR by rSV5-GFP and the P/V-CPI− mutant. WT rSV5 is a poor inducer of IFN-β synthesis and targets STAT1 for degradation (12, 23, 39, 50), whereas the P/V-CPI− mutant is a potent inducer of IFN-β and induces IFN-stimulated genes because it cannot block STAT1 activity (6, 50). Our data demonstrate that IFN signaling is not essential for translation shutoff by the P/V-CPI− mutant. This is evident from the findings that the P/V-CPI− mutant activates PKR and inhibition of translation in HeLa cells, which did not produce IFN in response to infection, and in U3A cells, which are defective in IFN signaling due to genetic defects. Interestingly, in our previous microarray studies (52), P/V-CPI− mutant-infected A549 cells showed only ∼3-fold upregulation of PKR mRNA over the level for mock-infected cells. This induction of PKR was much lower than the 98-fold induction of 2′,5′-oligo(A) synthetase-1 or the 66-fold induction of MX1 (52). The Western blot experiments for which results are shown here confirmed that PKR was not upregulated to a large extent in P/V-CPI− mutant-infected cells (Fig. 3A). Despite this marginal upregulation, PKR was efficiently phosphorylated following infection of A549 cells with the P/V-CPI− mutant. Together, these data indicate that constitutive basal levels of PKR are sufficient for cells to inhibit translation in response to infection with the P/V-CPI− mutant.
It should be emphasized that our P/V-CPI− virus contains six CPI− strain-derived amino acid substitutions in the context of the remaining W3A (WT) strain sequence. Our P/V-CPI− mutant shares some properties with the native CPI− virus that was the origin of the P/V amino acid substitutions (6), but the chimeric and native viruses also appear to differ with regard to shutoff of host and viral translation. Both the native CPI− strain and the chimeric P/V-CPI− mutant induce IFN synthesis, and both viruses are unable to block IFN signaling (5, 6, 50). As with the P/V-CPI− mutant described here, infection of HEp-2 cells with the native CPI− virus activates eIF-2α phosphorylation and results in inhibition of viral translation by 18 h p.i. (5). Unlike our results, however, the reduction in CPI− viral protein synthesis was most dramatic for proteins encoded downstream of the P/V gene, while synthesis of NP and P was decreased only slightly (5). In addition, HEp-2 cell lines expressing inhibitors of PKR could limit eIF-2α phosphorylation by the CPI− virus but did not show a significant increase in viral protein synthesis. Finally, IFN signaling played an important role in alterations to translation by the CPI− virus (5), whereas IFN signaling is not an absolute requirement for the translation inhibition seen during infection with the P/V-CPI− mutant. The effect of infection with the native CPI− virus on host translation was not addressed previously. Thus, we propose that introduction of the CPI− P/V gene substitutions into the context of the W3A backbone results in a chimeric virus (the P/V-CPI− virus) that is different from the native CPI− strain in terms of shutoff of host and viral translation.
Why did prevention of PKR activation by expression of NS1 or sigma3 lead to a large increase in viral translation? The key finding is that PKR is not activated, but viral mRNA levels remain high (Fig. 6). Thus, inhibition of PKR leads to elevated viral protein synthesis compared to that for control cells, because the high levels of viral mRNA are efficiently translated. By contrast, viral mRNA levels were decreased for the +V-wt and +P-wt viruses, and viral translation was not as striking as that seen for the P/V mutant when PKR inhibitors were present (Fig. 8).
How do the V accessory protein and the P subunit of the viral polymerase contribute to limiting PKR activation and maintaining high rates of protein synthesis during a WT SV5 infection? One possibility is that both WT P and WT V proteins directly inhibit PKR and that the CPI− versions of these proteins are defective in this function. However, a key finding on V and P was our previously published results that cells infected with either the +V-wt or the +P-wt virus accumulate lower levels of viral mRNA than the P/V-CPI− parental virus (13). Based on their known roles in controlling viral RNA synthesis, we propose that V and P limit PKR activation by a mechanism that differs from those of the sigma3 and NS1 proteins, which directly bind dsRNA or PKR. In our model, both WT V and P proteins decrease the activity and increase the fidelity of the viral polymerase, thereby limiting the synthesis of aberrant products such as dsRNA (45, 57) and indirectly preventing PKR activation. By contrast, the CPI− V and P proteins are defective in modulating these key polymerase functions. By limiting dsRNA production, WT SV5 is able to synthesize lower levels of mRNA than the P/V-CPI− mutant but still maintain a higher rate of translation at late times p.i. because PKR is not activated. Consistent with this proposal, P/V-CPI− mutant translation rates were increased in cells expressing direct inhibitors of PKR, but mRNA levels were largely unchanged from those in control cells. We plan to test this hypothesis in future work using P proteins that are defective in RNA synthesis. This proposal is also consistent with the findings that the measles virus C protein can regulate viral RNA synthesis (40) and that a recombinant measles virus lacking C protein expression induces eIF-2α phosphorylation and reduced viral translation (35).
Acknowledgments
We thank members of the Parks lab and Doug Lyles for comments on the manuscript. We are grateful to Curt Horvath (Northwestern University), Ganes Sen (Cleveland Clinic Foundation), and Makoto Takeda (Tokyo University) for the kind gifts of cell lines and to Takeshi Kobayashi and Terry Dermody (Vanderbilt University) for the kind gifts of sigma3 plasmid and antiserum.
This work was supported by NIH grant AI42023.
Footnotes
Published ahead of print on 31 October 2007.
REFERENCES
- 1.Andrejeva, J., K. S. Childs, D. F. Young, R. S. Carlos, N. Stock, S. Goodbourn, and R. E. Randall. 2004. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-β promoter. Proc. Natl. Acad. Sci. USA 10117264-17269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergmann, M., A. Garcia-Sastre, E. Carnero, H. Pehamberger, K. Wolff, P. Palese, and T. Muster. 2000. Influenza virus NS1 protein counteracts PKR-mediated inhibition of replication. J. Virol. 746203-6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burgui, I., T. Aragon, J. Ortin, and A Nieto. 2003. PABP1 and eIF4GI associate with influenza A virus NS1 protein in viral mRNA translation initiation complexes. J. Gen. Virol. 843263-3274. [DOI] [PubMed] [Google Scholar]
- 4.Campbell-Dwyer, E. J., H. Lai, R. C. MacDonald, M. S. Salvato, and K. L. Borden. 2000. The LCMV ring protein Z associates with eukaryotic initiation factor 4E and selectively represses translation in a ring-dependent manner. J. Virol. 743293-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlos, T. S., D. Young, S. Stertz, G. Kochs, and R. E. Randall. 2007. Interferon-induced inhibition of parainfluenza virus type 5; the roles of MxA, PKR and oligo A synthetase/RNAse L. Virology 363166-173. [DOI] [PubMed] [Google Scholar]
- 6.Chatziandreou, N., D. Young, J. Andrejeva, S. Goodbourn, and R. E. Randall. 2002. Differences in interferon sensitivity and biological properties of two related isolates of simian virus 5; a model for virus persistence. Virology 293234-242. [DOI] [PubMed] [Google Scholar]
- 7.Childs, K. S., N. Stock, C. Ross, J. Andrejeva, L. Hilton, M. Skinner, R. E. Randall, and S. Goodbourn. 2007. mda-5, but not RIG-I, is a common target for paramyxovirus V proteins. Virology 359190-200. [DOI] [PubMed] [Google Scholar]
- 8.Choppin, P. W. 1964. Multiplication of a myxovirus (SV5) with minimal cytopathic effects and without interference. Virology 23224-233. [DOI] [PubMed] [Google Scholar]
- 9.Clemens, M. J. 2005. Translation control in virus-infected cells: models for cellular stress responses. Semin. Cell Dev. Biol. 1613-20. [DOI] [PubMed] [Google Scholar]
- 10.Clemens, M. J., M. Bushell, I. W. Jeffrey, V. M. Pain, and S. J. Morley. 2000. Translation initiation factor modifications and the regulation of protein synthesis in apoptotic cells. Cell Death Differ. 7603-615. [DOI] [PubMed] [Google Scholar]
- 11.Collins, P. L., and J. E. Crowe. 2007. Respiratory syncytial virus and metapneumovirus, p. 1601-1646. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 12.Didcock, L., D. F. Young, S. Goodbourn, and R. E. Randall. 1999. The V protein of SV5 inhibits interferon signaling by targeting STAT1 for proteasome-mediated degradation. J. Virol. 739928-9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dillon, P. J., and G. D. Parks. 2007. A role for the P subunit of the paramyxovirus polymerase in limiting host cell antiviral responses. J. Virol. 8111116-11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donelan, N. R., C. F. Basler, and A. Garcia-Sastre. 2003. A recombinant influenza A virus expressing an RNA-binding-defective NS1 protein induces high levels of beta interferon and is attenuated in mice. J. Virol. 7713257-13266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edgil, D., C. Polacek, and E. Harris. 2006. Dengue virus utilizes a novel strategy for translation initiation when cap-dependent translation is inhibited. J. Virol. 802976-2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gale, M., and M. G. Katze. 1998. Molecular mechanisms of interferon resistance mediated by viral-directed inhibition of PKR, the interferon-induced protein kinase. Pharmacol. Ther. 7829-46. [DOI] [PubMed] [Google Scholar]
- 17.García, M. A., J. Gil, I. Ventoso, S. Guerra, E. Domingo, C. Rivas, and M. Esteban. 2006. Impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action. Microbiol. Mol. Biol. Rev. 701032-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcin, D., P. Latorre, and D. Kolakofsky. 1999. Sendai virus C proteins counteract the interferon-mediated induction of an antiviral state. J. Virol. 736559-6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodbourn, S., L. Didcock, and R. E. Randall. 2000. Interferons: cell signaling, immune modulation, antiviral responses and virus countermeasures. J. Gen. Virol. 812341-2364. [DOI] [PubMed] [Google Scholar]
- 20.Guo, Z., L. M. Chen, H. Zeng, J. A. Gomez, J. Plowden, T. Fujita, J. M. Katz, R. O. Donis, and S. Sambhara. 2007. NS1 protein of influenza A virus inhibits the function of intracytoplasmic pathogen sensor, RIG-I. Am. J. Respir. Cell Mol. Biol. 36263-269. [DOI] [PubMed] [Google Scholar]
- 21.Hakki, M., E. E. Marshall, K. L. De Niro, and A. P. Geballe. 2006. Binding and nuclear relocalization of PKR by human cytomegalovirus TRS1. J. Virol. 8011817-11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He, B., R. G. Paterson, C. D. Ward, and R. A. Lamb. 1997. Recovery of infectious SV5 from cloned DNA and expression of a foreign gene. Virology 237249-260. [DOI] [PubMed] [Google Scholar]
- 23.He, B., R. G. Paterson, N. Stock, J. E. Durbin, R. K. Durbin, S. Goodbourn, R. E. Randall, and R. A. Lamb. 2002. Recovery of paramyxovirus simian virus 5 with a V protein lacking the conserved cysteine-rich domain: the multifunctional V protein blocks both interferon-β induction and interferon signaling. Virology 30315-32. [DOI] [PubMed] [Google Scholar]
- 24.Horikami, S. M., S. Smallwood, and S. A. Moyer. 1996. The Sendai virus V protein interacts with the NP protein to regulate viral genome RNA replication. Virology 222383-390. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs, B. L., and J. O. Langland. 1998. Reovirus sigma 3 protein: dsRNA binding and inhibition of RNA-activated protein kinase. Curr. Top. Microbiol. Immunol. 233185-196. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi, T., A. A. R. Antar, K. W. Boehme, P. Danthi, E. A. Eby, K. M. Gugliemi, G. H. Holm, E. M. Johnson, M. S. Maginnis, S. Naik, W. B. Skelton, J. D. Wetzel, G. J. Wilson, J. D. Chapell, and T. S. Dermody. 2007. A plasmid-based reverse genetics system for animal double-stranded RNA viruses. Cell Host Microbe 1147-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kochs, G., A. Garcia-Sastre, and L. Martinez-Sobrido. 2007. Multiple anti-interferon actions of the influenza A virus NS1 protein. J. Virol. 817011-7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lamb, R. A., and G. Parks. 2007. Paramyxoviridae: the viruses and their replication, p. 1449-1496. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 29.Langland, J. O., J. M. Cameron, M. C. Heck, J. K. Jancovich, and B. L. Jacobs. 2006. Inhibition of PKR by RNA and DNA viruses. Virus Res. 119100-110. [DOI] [PubMed] [Google Scholar]
- 30.Li, S., J. Y. Min, R. M. Krug, and G. C. Sen. 2006. Binding of the influenza A virus NS1 protein to PKR mediates the inhibition of its activation by either PACT or double-stranded RNA. Virology 34913-21. [DOI] [PubMed] [Google Scholar]
- 31.Lin, Y., F. Horvath, J. A. Aligo, R. Wilson, and B. He. 2005. The role of simian virus 5 V protein on viral RNA synthesis. Virology 338270-280. [DOI] [PubMed] [Google Scholar]
- 32.Lyles, D. S. 2000. Cytopathogenesis and inhibition of host gene expression by RNA viruses. Microbiol. Mol. Biol. Rev. 64709-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKendry, R., J. John, D. Flavell, M. Muller, I. M. Kerr, and G. R. Stark. 1991. High-frequency mutagenesis of human cells and characterization of a mutant unresponsive to both alpha and gamma interferons. Proc. Natl. Acad. Sci. USA 8811455-11459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Min, J. Y., and R. M. Krug. 2006. The primary function of RNA binding by the influenza A virus NS1 protein in infected cells: inhibiting the 2′-5′ oligo(A) synthetase/RNase L pathway. Proc. Natl. Acad. Sci. USA 1037100-7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakatsu, Y., M. Takeda, S. Ohno, R. Koga, and Y. Yanagi. 2006. Translational inhibition and increased interferon induction in cells infected with C protein-deficient measles virus. J. Virol. 8011861-11867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parisien, J. P., J. F. Lau, J. J. Rodriguez, B. M. Sullivan, A. Moscona, G. D. Parks, R. A. Lamb, and C. M. Horvath. 2001. The V protein of human parainfluenza virus 2 antagonizes type I interferon responses by destabilizing signal transducer and activator of transcription 2. Virology 283230-239. [DOI] [PubMed] [Google Scholar]
- 37.Peluso, R. W., R. A. Lamb, and P. W. Choppin. 1977. Polypeptide synthesis in simian virus 5-infected cells. J. Virol. 23177-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peluso, R. W., R. A. Lamb, and P. W. Choppin. 1978. Infection with paramyxoviruses stimulates synthesis of cellular polypeptides that are also stimulated in cells transformed by Rous sarcoma virus or deprived of glucose. Proc. Natl. Acad. Sci. USA 756120-6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poole, E., B. He, R. A. Lamb, R. E. Randall, and S. Goodbourn. 2002. The V proteins of simian virus 5 and other paramyxoviruses inhibit induction of interferon-beta. Virology 30333-46. [DOI] [PubMed] [Google Scholar]
- 40.Reutter, G. L., C. Cortese-Grogan, J. Wilson, and S. A. Moyer. 2001. Mutations in the measles virus C protein that up regulate viral RNA synthesis. Virology 285100-109. [DOI] [PubMed] [Google Scholar]
- 41.Salvatore, M., C. F. Basler, J. P. Parisien, C. M. Horvath, S. Bourmakina, H. Zhen, T. Muster, P. Palese, and A. Garcia-Sastre. 2002. Effects of influenza A virus NS1 protein on protein expression: the NS1 protein enhances translation and is not required for shutoff of host protein synthesis. J. Virol. 761206-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Samuel, C. E. 1993. The eIF-2α protein kinases, regulators of translation in eukaryotes from yeast to humans. J. Biol. Chem. 2687603-7606. [PubMed] [Google Scholar]
- 43.Schmechel, S., M. Chute, P. Skinner, R. Anderson, and L. Schiff. 1997. Preferential translation of reovirus mRNA by a sigma3-dependent mechanism. Virology 23262-73. [DOI] [PubMed] [Google Scholar]
- 44.Stark, G. R. 1997. Genetic analysis of interferon and other mammalian signaling pathways. Harvey Lect. 931-16. [PubMed] [Google Scholar]
- 45.Strahle, L., D. Garcin, and D. Kolakofsky. 2006. Sendai virus defective-interfering genomes and the activation of interferon-beta. Virology 351101-111. [DOI] [PubMed] [Google Scholar]
- 46.Sun, Y., and D. W. Leaman. 2004. Ectopic expression of toll-like receptor-3 (TLR-3) overcomes the double-stranded RNA (dsRNA) signaling defects of P2.1 cells. J. Interferon Cytokine Res. 24350-361. [DOI] [PubMed] [Google Scholar]
- 47.Talon, J., C. M. Horvath, R. Polley, C. F. Basler, T. Muster, P. Palese, and A. Garcia-Sastre. 2000. Activation of interferon regulatory factor 3 is inhibited by the influenza A virus NS1 protein. J. Virol. 747989-7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas, S. M., R. A. Lamb, and R. G. Paterson. 1988. Two mRNAs that differ by two nontemplated nucleotides encode the amino co-terminal proteins P and V of paramyxovirus SV5. Cell 54891-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang, X., M. Li, H. Zheng, T. Muster, P. Palese, A. A. Beg, and A. Garcia-Sastre. 2000. Influenza A virus NS1 protein prevents the activation of NF-κB and induction of type I interferon. J. Virol. 7411566-11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wansley, E. K., and G. D. Parks. 2002. Naturally occurring substitutions in the P/V gene convert the noncytopathic paramyxovirus simian virus 5 into a virus that induces alpha/beta interferon synthesis and cell death. J. Virol. 7610109-10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wansley, E. K., J. M. Grayson, and G. D. Parks. 2003. Apoptosis induction and interferon signaling but not IFN-β promoter induction by an SV5 P/V mutant are rescued by coinfection with wild-type SV5. Virology 31641-54. [DOI] [PubMed] [Google Scholar]
- 52.Wansley, E. K., P. J. Dillon, M. D. Gainey, J. Tam, S. D. Cramer, and G. D. Parks. 2005. Growth sensitivity of a recombinant simian virus 5 P/V mutant to type I interferon differs between tumor cell lines and normal primary cells. Virology 335131-144. [DOI] [PubMed] [Google Scholar]
- 53.Weber, F., V. Wagner, S. B. Rasmussen, R. Hartmann, and S. R. Paludan. 2006. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J. Virol. 805059-5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wek, R. C. 1994. eIF-2 kinases: regulators of general and gene-specific translation initiation. Trends Biochem. Sci. 19491-496. [DOI] [PubMed] [Google Scholar]
- 55.Williams, B. R. 1999. PKR, a sentinel kinase for cellular stress. Oncogene 186112-6120. [DOI] [PubMed] [Google Scholar]
- 56.Young, V. A., P. J. Dillon, and G. D. Parks. 2006. Variants of the paramyxovirus simian virus 5 with accelerated or delayed viral gene expression activate proinflammatory cytokine synthesis. Virology 35090-102. [DOI] [PubMed] [Google Scholar]
- 57.Yount, J. S., T. A. Krause, C. M. Horvath, T. M. Moran, and C. B. Lopez. 2006. A novel role for viral-defective interfering particles in enhancing dendritic cell maturation. J. Immunol. 1774503-4513. [DOI] [PubMed] [Google Scholar]
- 58.Yue, Z., and A. J. Shatkin. 1997. Double-stranded RNA-dependent protein kinase (PKR) is regulated by reovirus structural proteins. Virology 234364-371. [DOI] [PubMed] [Google Scholar]