Abstract
To afford the greatest possible immune protection, candidate human immunodeficiency virus (HIV) vaccines must generate diverse and long-lasting CD8+ T lymphocyte responses. In the present study, we evaluate T-cell receptor Vβ (variable region beta) gene usage and a CDR3 (complementarity-determining region 3) sequence to assess the clonality of epitope-specific CD8+ T lymphocytes generated in rhesus monkeys following vaccination and simian-human immunodeficiency virus (SHIV) challenge. We found that vaccine-elicited epitope-specific CD8+ T lymphocytes have a clonal diversity comparable to those cells generated in response to SHIV infection. Moreover, we show that the clonal diversity of vaccine-elicited CD8+ T-lymphocyte responses is dictated by the epitope sequence and is not affected by the mode of antigen delivery to the immune system. Clonal CD8+ T-lymphocyte populations persisted following boosting with different vectors, and these clonal cell populations could be detected for as long as 4 years after SHIV challenge. Finally, we show that the breadth of these epitope-specific T lymphocytes transiently focuses in response to intense SHIV replication. These observations demonstrate the importance of the initial immune response to SHIV, induced by vaccination or generated during primary infection, in determining the clonal diversity of cell-mediated immune responses and highlight the focusing of this clonal diversity in the setting of high viral loads. Circumventing this restricted CD8+ T-lymphocyte clonal diversity may present a significant challenge in the development of an effective HIV vaccine strategy.
The induction of cytotoxic T lymphocytes (CTL) is an issue of central importance in human immunodeficiency virus type 1 (HIV-1) vaccine development (3, 9, 11, 27, 32, 39, 42, 46, 64). Candidate HIV-1 vaccines must generate not only functionally robust, but also clonally diverse and persistent, CD8+ T-lymphocyte responses. If a population of CTL recognizes multiple epitopes of HIV-1, the likelihood of viral mutation at a single epitope allowing this mutant virus to escape from recognition by effector T cells is limited (8, 53). Moreover, clonal diversity within populations of CTL that recognize the same epitope is also important, as the existence of CD8+ T lymphocytes with multiple T-cell receptors (TCRs) capable of recognizing a single HIV-1 epitope sequence will further minimize the chance that a viral epitope sequence variant will go unrecognized (17, 35, 36). In fact, broad, polyclonal CTL responses have been shown to confer immune protection and suppress viral replication in the setting of persistent viral infections (25, 51). Thus, in primate immunodeficiency virus infections, it is hypothesized that clonally diverse CD8+ T-lymphocyte responses can diminish chronic viral replication, further reducing the generation of mutant viruses (7).
In addition to inducing a clonally diverse CTL response, candidate HIV-1 vaccines must also elicit long-lasting CTL populations. However, generating such cell populations may ultimately prove challenging, as T-cell persistence in vivo is limited by many factors. Specifically, persistence is restricted by homeostatic mechanisms that limit T-lymphocyte division, clonal deletion of CD8+ T lymphocytes through activation-induced cell death, and clonal exhaustion leading to loss of T-cell function, all of which have been described in the setting of persistent antigenic stimulation (1, 5, 13, 18, 20, 23, 24, 38, 43, 50, 55, 60, 62, 65, 67, 69). HIV-1-specific CD8+ T lymphocytes, in particular, can be highly activated during the primary immune response and are consequently especially prone to apoptosis (6, 45). Since HIV-1-specific CD8+ T lymphocytes may rapidly disappear following early infection or may undergo clonal exhaustion during chronic infection, it is important to determine the extent to which vaccine-elicited CTL populations can expand and persist following primate immunodeficiency virus infection.
Although polyclonal, persistent CD8+ T-lymphocyte responses have a crucial role in suppressing viral replication and limiting HIV-1 pathogenicity, a dearth of information exists on the diversity and persistence of clonal populations of primate immunodeficiency virus epitope-specific CTL induced by vaccination. While vaccine studies have explored the epitopic breadth and magnitude of CD8+ T-lymphocyte responses elicited by vaccination with plasmid DNA, live recombinant viral vectors, and various prime/boost strategies, the clonality of vaccine-elicited epitope-specific T-lymphocyte populations has not been systematically evaluated (4, 10, 16, 49, 52-54, 63). In the present study, we examine the clonality of epitope-specific CD8+ T lymphocytes, first following vaccination and then following viral challenge in the rhesus monkey/simian-human immunodeficiency virus (SHIV) model. We demonstrate a remarkable persistence of clonal CD8+ T-lymphocyte populations after vaccination and infection, as well as transient clonal focusing associated with the period of intense antigenic stimulation during acute infection with a pathogenic SHIV, SHIV-89.6P.
MATERIALS AND METHODS
Animals.
The rhesus monkeys used in this study were maintained in accordance with the guidelines of the Institutional Animal Care and Use Committee for Harvard Medical School and the Guide for the Care and Use of Laboratory Animals (25a). Monkeys were screened for the presence of the Mamu-A*01 allele using a PCR-based technique as previously described (30, 32). DNA sequence analysis was performed on all potential positive samples to confirm identity with the established Mamu-A*01 sequence (37).
Immunizations and viruses.
All vaccinated monkeys described in this study were first immunized with plasmid DNA consisting of the minimal 9-amino-acid major histocompatibility complex class I restricted epitopes p11C (CTPYDINQM), p41A (YAPPISGQI), and p68A (STPPLVRLV) in tandem. Following a series of immunizations with this DNA plasmid, monkeys 90-98, 95-98, 128-97, and 135-97 were boosted with live recombinant modified vaccinia virus Ankara (rMVA) constructs and 6 months later were boosted again with live recombinant adenovirus (rAd) serotype 5 constructs. These vaccine constructs expressed simian immunodeficiency virus mac239 (SIVmac239) Gag-Pol and HIV-1 Env. Monkeys 196-97 and 132-97 were boosted only with live recombinant vaccinia virus (rVV) expressing SIVmac239 Gag-Pol and HIV-1 Env. Following these prime/boost immunizations, monkeys 90-98, 128-97, and 196-97 were challenged with nonpathogenic SHIV-89.6, while monkeys 95-98, 132-97, and 135-97 were challenged with pathogenic SHIV-89.6P (57, 58). The unvaccinated monkeys 153, 146, 144, and 134 were also infected with SHIV-89.6P (52).
Antibodies, tetramers, and peptides.
Antibodies used in this study were directly coupled to fluorescein isothiocyanate (FITC), phycoerythrin-Texas red (ECD), or allophycocyanin. The following monoclonal antibodies were used: ECD-conjugated anti-CD8α (clone 7PT3F9; Beckman-Coulter), ECD-conjugated anti-CD8αβ (Beckman-Coulter), FITC-conjugated anti-CD3 (clone FN18; BioSource International, Camarillo, CA), or FITC-conjugated anti-CD3 (clone SP34; BD PharMingen, San Diego, CA). Mamu-A*01/p11C/β2m (SIVmac Gag), Mamu-A*01/p41A/β2m (HIV-1 Env), and Mamu-A*01/p68A/β2m (SIVmac Pol) tetramer complexes were prepared as previously described (2, 21, 31, 40). Phycoerythrin-labeled ExtrAvidin (Sigma) was mixed stepwise with biotinylated Mamu-A*01-peptide complexes at a molar ratio of 1:4 to produce the tetrameric complexes. Gag p11C (CTPYDINQM), Env p41A (YAPPISGQI), and Pol p68A (STPPLVRLV) peptides were obtained from QCB/Biosource (Hopkinton, MA). Lyophilized peptides were dissolved in a minimum volume of dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO), diluted to a stock peptide concentration of 15 mg/ml in water containing 5 mM dithiothreitol (Sigma-Aldrich), and then frozen at −80°C in aliquots. Before use, the peptides were diluted to a working concentration in RPMI 1640 medium (Mediatech, Herndon, VA) supplemented with glutamine, 12% fetal calf serum, penicillin, streptomycin, and gentamicin.
Flow cytometry.
Peripheral blood mononuclear cells (PBMC) cultured in the presence of p11C, p41A, and p68A peptide and interleukin-2 were harvested on days 10 to 14 and separated over a Ficoll layer (Ficoll-Paque Plus; Amersham-Pharmacia Biotech, Uppsala, Sweden). The cultured cells were stained with Mamu-A*01/p11C/β2m, Mamu-A*01/p41A/β2m, or Mamu-A*01/p68A/β2m tetramer for 30 min at room temperature. The cells were then stained with a mixture of anti-CD3 and anti-CD8 monoclonal antibodies for 30 min. Samples were analyzed on a FACSCalibur flow cytometer (BD Biosciences). We have previously shown that the TCR repertoire of such in vitro-stimulated lymphocytes is indistinguishable from that of freshly isolated lymphocytes in both breadth and magnitude (35). Therefore, the clonality of these in vitro-expanded lymphocyte subpopulations is representative of the cells in vivo.
Generation of cDNA.
RNA was extracted from Mamu-A*01/p11C/β2m, Mamu-A*01/p41A/β2m, and Mamu-A*01/p68A/β2m tetramer-binding CD8+ T-lymphocyte populations according to the instructions supplied with the RNeasy Mini Extraction kit from Qiagen (Valencia, CA). cDNA was then synthesized from the extracted RNA as outlined in the Super Smart PCR cDNA synthesis kit from Clontech Laboratories (Palo Alto, CA). Briefly, the single-stranded cDNA reaction was catalyzed using Moloney murine leukemia virus reverse transcriptase with the 3′ Smart CDS Primer II A and Smart II A oligonucleotide primers provided in the Super Smart cDNA synthesis kit. Preamplified double-stranded cDNA libraries were made using 10- to 25-cycle PCR amplification, utilizing the 5′ PCR Primer IIA primer and reagents also provided in the Clontech kit. The optimal number of cycles of preamplification was determined by performing a test run in the presence of SYBR Green to determine the maximum number of PCR cycles that could be performed in the log-linear amplification range (35).
Primers and sequencing.
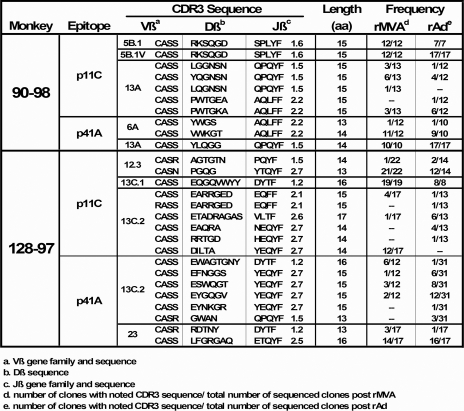
Primers used for the real-time PCR assay and for spectratyping (Table 1) were ordered through Biosource International, manufactured by Keystone Labs (Camarillo, CA), and purified by high-performance liquid chromatography. The real-time TaqMan probes were synthesized at Biosearch Technologies, Inc. (Novato, CA), and purified by high-performance liquid chromatography. Primers specific for the variable and constant regions of the TCR β chain were designed from rhesus monkey TCR sequences obtained from GenBank and generated by our own laboratory.
TABLE 1.
Real-time PCR assay and spectratyping primers
| Primera | 5′-3′ sequence |
|---|---|
| VB1Q(2) | GAGAGAGCAAAAGGAAACATTCTTG |
| VB2Q | CTCCACTCTGACAGTGACCAATG |
| VB3Q | AGGAGCGCTTCTCCCTGATT |
| VB4Q | CGCCCAAACCTAACATTCTCA |
| VB5(A)Q | TCTCAGGGCGCCAGTTCT |
| VB5(B.1)Q | CTGAATGTGAGCGCCTTGTTG |
| VB5(B.1var)Q | GCTGAATGTGAGTGCCTTGTTG |
| VB5(B.2)Q | CTATAGCTCTGAGCTGAATGTGAA |
| VB5(B.3)Q | GCCAGTTCCGTGACTATCATCA |
| VB6(A)Q | CCAGAGTTTCTGACTTACTTCAATTATC |
| VB6(B)Q | ACTTACTTCAGTTATGAAGCTCAACA |
| VB6(C)Q | CCTTTATTGGTACCGACGGACT |
| VB6(D)Q | TTACTTCCAGAATGATGCTCAACG |
| VB6(E)Q | GTGTGATCCAATTTCAGGTCATACC |
| VB6(F)Q | ATTTACTTCCAAGGCACGGGTA |
| VB7(A)Q | GTTAAGAAGCCGCCGGAGA |
| VB7(B)Q | AAGAACGGGCTGAAAACAACA |
| VB7(C)Q | CCGCCGGAGCTCATGTTTG |
| VB8Q(2) | AACAAGTCTCCGATAGATGATTCAG |
| VB9(A)Q | AGTTCCATATCGCTTCTCACCTAAG |
| VB9(B)Q(2) | GCTCATTTAAATCTTCACATCAAGTCTG |
| VB9(C)Q | TTTCAAATCGCTTCTCACCTGAC |
| VB10Q(2) | TGGAGATCCAGTCCACAGAGT |
| VB11Q | GAGATTTTTCCTCTGAGTCAACAGTCT |
| VB12.1Q | TTACTCATATGGTGTTGAAGACACTGA |
| VB12.2Q | CATTACTCATATGGTGTTCCAGACACTA |
| VB12.3Q | GAGAAGTCCCCGATGGCTATG |
| VB13(A)Q | CCCCGATGGCTACAATGTCA |
| VB13(C.1)Q | GTCCCCAATGGCTACAATGC |
| VB13(C.2)Q | ACAGTGTCTCCAGATTAAACAAAAC |
| VB13(C.3)Q | ATTCATTACTCAGCTACCGAGGA |
| VB14Q | CGTCTCTCGAAAAGAGAAGAGGAAT |
| VB15Q | ATACAGTGTCTCTCGACAGGAACAG |
| VB16Q | GAGTCCGGTATGCCCAACA |
| VB17Q | AATCCTTTCCTCTCACTGTGACATC |
| VB18Q(2) | GTTTTCTGCTGAATTTCCCAAAGA |
| VB19Q | CAAGAAACGGAGCTGCACAA |
| VB20Q | CCAGGACAGGCGGTTCAT |
| VB21.3Q | CTGCAGAGAGGCTCAAAGGAGTA |
| VB22(A.1)Q | TGAAATATTTGAAGATCGATTCTCAGTC |
| VB22(A.2)Q | CTCAGACAAGTCTGAAATGTTCGAT |
| VB22(B.1)Q | AGTTTCTGGTTTACTTCTATAATGGTGA |
| VB22(B.2)Q | CCTTCTATAATGGTAAGATCTCAGAGC |
| VB23Q | TCAGTGACTATCATTCTGAACTGAACA |
| VB24Q | TCCAGGAGGCCAAACACTTCT |
| VB25Q(2) | CCCCCCAAATTCACCCTGTAG |
| C Beta QProbe | TGTTCCCACCCAAGGTCGCTGTG |
| C Beta/R | GATCTCTGCTTCTGATGGCTCAA |
Primers specific for rhesus monkey TCRβ chain variable and constant regions.
Quantitative PCR.
cDNA derived from each sample was equally distributed into 48 individual PCR mixtures. Each reaction mixture contained a sense Vβ family-specific primer, an antisense Cβ-specific primer, and the TaqMan Cβ probe. PCRs were carried out using Sure-Start Taq (Stratagene). The real-time PCR was carried out for 50 cycles on an MX4000 QPCR machine (Stratagene) under the following conditions: 95°C for 10 min and 50 cycles of 95°C for 10 s, 58°C for 30 s, reading of fluorescence, and 72°C for 30 s. Background values for this Vβ quantitative PCR assay were, on average, 2.2% of the total number of copies in a series of reactions. Thus, we chose 5% as the threshold for considering a particular Vβ (variable region beta) gene family as contributing to the effective Vβ repertoire of an epitope-specific CD8+ T-cell response.
Spectratyping.
Identified Vβ families in each cDNA sample were assessed for CDR3 (complementarity-determining region 3) profiles through Genescan-based spectratyping (44, 68). cDNA generated for use in the quantitative PCR assay was used as a template for second-round PCRs utilizing individual Vβ primers and a 5′-6-carboxyfluorescein (FAM)-labeled Cβ primer (Biosource International Inc., Camarillo, CA). The cDNA was amplified for 30 cycles in a Perkin Elmer 9600 GeneAmp PCR system under the following conditions: 95°C for 10 s, 57°C for 30 s, and 68°C for 60 s, with a final 10-min extension at 68°C. Multiple dilutions of each reaction product were mixed with a ROX-500 size standard and run on an ABI 3730xl sequencer. Data were analyzed for size and fluorescence intensity by using Genemapper software, version 3.7 (Applied Biosystems). In conjunction with the CDR3 length display from the spectratype analysis, further cloning using the pGEM T-easy system (Promega) and sequencing using the Cβ antisense primer allowed the determination of CDR3 lengths and sequence. These CDR3 lengths were expressed as predicted numbers of amino acids spanning the portion of the variable, diverse, and joining segments.
RESULTS
TCR Vβ repertoires of epitope-specific CD8+ T lymphocytes in vaccinated and SHIV-infected Mamu-A*01+ rhesus monkeys.
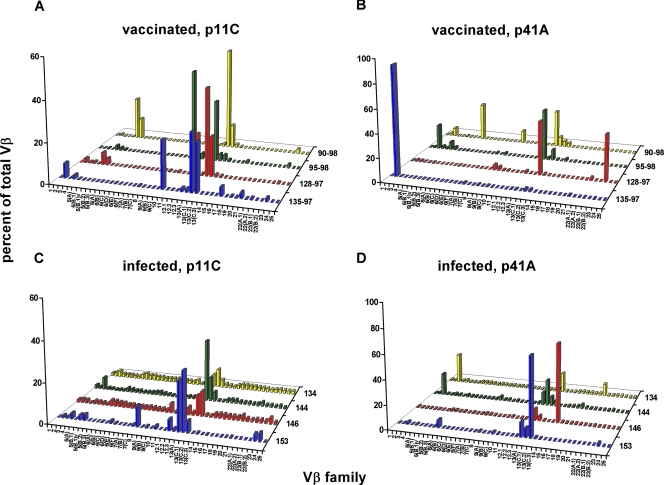
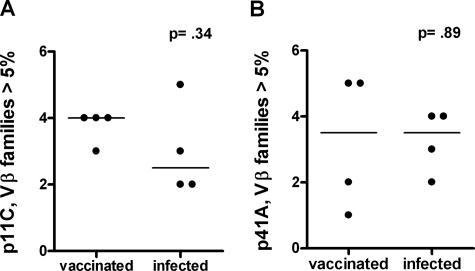
We were interested in exploring the clonal diversity of the virus-specific CD8+ T lymphocytes generated by either vaccination or infection with a pathogenic primate immunodeficiency virus. We initiated these studies by examining the TCR Vβ repertoires of Gag p11C and Env p41A epitope-specific CD8+ T-lymphocyte populations in four Mamu-A*01+ rhesus monkeys following plasmid DNA prime/rMVA boost vaccination and in four other Mamu-A*01+ rhesus monkeys chronically infected with SHIV-89.6P. p11C and p41A tetramer-binding CD8+ T-lymphocyte populations were sorted from the PBMC of vaccinated and infected monkeys to a purity of at least 98% and assessed for their expression of 46 Vβ gene families. The TCR Vβ repertoires of both the p11C and p41A epitope-specific CD8+ T-lymphocyte populations generated by vaccination and in response to SHIV-89.6P infection were quite diverse (Fig. 1). We compared the median number of Vβ families that represented more than 5% of the total Vβ repertoire within epitope-specific CD8+ T lymphocytes in vaccinated and infected monkeys using the Mann-Whitney test. The numbers of Vβ families contributing more than 5% of the total Vβ repertoire in p11C and p41A tetramer-sorted populations were not significantly different in vaccinated monkeys from those in infected monkeys (Fig. 2). A comparison of the numbers of Vβ families that contributed more than 10% of the total Vβ repertoire confirmed this result (data not shown). Thus, plasmid DNA/rMVA vaccination induced a SHIV epitope-specific CD8+ T-lymphocyte response with Vβ gene usage not significantly different in diversity from that generated in response to SHIV-89.6P infection.
FIG. 1.
TCR Vβ repertoires of epitope-specific CD8+ T lymphocytes generated by vaccination are as diverse as those induced in response to pathogenic-SHIV infection. PBMC were isolated from monkeys vaccinated with a plasmid DNA prime/rMVA boost regimen (135-97, 128-97, 95-98, and 90-98) and from monkeys infected with SHIV-89.6P (134, 144, 146, and 153). These cells were stimulated in vitro with p11C or p41A peptide and then stained and sorted with p11C or p41A tetramer, respectively. cDNAs synthesized from the RNAs extracted from these tetramer-binding CD8+ T-lymphocyte populations were used to determine Vβ repertoires. The Vβ repertoires of p11C (A) and p41A (B) tetramer-sorted CD8+ T lymphocytes from vaccinated monkeys and p11C (C) and p41A (D) tetramer-sorted CD8+ T lymphocytes from infected monkeys are shown.
FIG. 2.
The median number of Vβ families that contributed more than 5% of the total Vβ repertoire were compared using the Mann-Whitney test in p11C (A) and p41A (B) tetramer-sorted CD8+ T-lymphocyte populations from each of the vaccinated and infected monkeys illustrated in Fig. 1.
Moreover, we observed a similar bias toward Vβ 13 family usage (specifically, Vβ 13A, 13C.1, 13C.2, and 13C.3) in p11C tetramer-binding CD8+ T lymphocytes in both vaccinated and infected monkeys (Fig. 1A and C). Interestingly, Vβ 13 family members were also used by the p41A epitope-specific CD8+ T-lymphocyte populations, with three of four vaccinated monkeys and all four infected monkeys demonstrating Vβ 13 family expression in their TCR repertoires. Additionally, we observed Vβ 3 expression in p41A tetramer-binding CD8+ T-lymphocyte populations in two of four vaccinated and two of four infected monkeys (Fig. 1B and D). Taken together, these results suggest that plasmid DNA/rMVA vaccination induced a SHIV epitope-specific CD8+ T-lymphocyte response with a Vβ family gene usage comparable to that generated in response to a pathogenic SHIV infection.
Persistence of clonal p11C and p41A epitope-specific CD8+ T-lymphocyte populations.
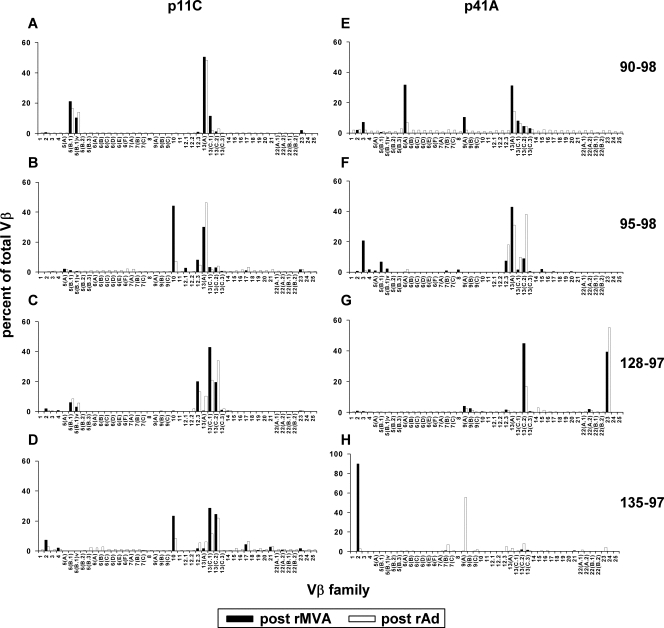
Having defined a portion of the virus-specific CD8+ T-lymphocyte TCR Vβ repertoire generated by DNA plasmid prime/rMVA boost vaccination, we sought to determine whether the clonal CD8+ T-lymphocyte populations generated by this vaccination persisted in these monkeys following a boost 6 months later with rAd immunogens. p11C and p41A tetramer-binding CD8+ T-lymphocyte populations were sorted from PBMC of the vaccinated monkeys 1 month after rMVA and 1 year after rAd vaccination and assessed for their expression of 46 Vβ gene families. The TCR Vβ repertoires of both the p11C and p41A epitope-specific CD8+ T cells were almost identical post-rMVA and post-rAd immunization, suggesting that most of the clonal populations of virus-specific CD8+ T lymphocytes persisted following the rAd boost (Fig. 3). In monkey 135-97, Vβ 7B, 9A, 13A, 13C.2, and 13C.3 were represented in the p41A epitope-specific CD8+ T lymphocytes but were overshadowed by the overwhelming use of Vβ 2 in that response (92% of the Vβ repertoire following rMVA immunization) (Fig. 3H). Moreover, the detection of these Vβ-expressing CD8+ T-lymphocyte populations for more than 2 years in these monkeys suggests that the CD8+ T-lymphocyte populations generated by this vaccination strategy persisted.
FIG. 3.
TCR Vβ repertoires of p11C and p41A epitope-specific CD8+ T-lymphocyte populations elicited by plasmid DNA/rMVA vaccination persist following rAd vaccination. PBMC isolated from vaccinated monkeys 90-98, 95-98, 128-97, and 135-97 1 month after rMVA and 1 year after rAd vaccination were stimulated in vitro with p11C and p41A peptides; stained with p11C or p41A tetramer, respectively; and then sorted. cDNAs synthesized from the RNAs extracted from these tetramer-binding CD8+ T-lymphocyte populations were used to evaluate Vβ repertoires. Shown are p11C-specific (A to D) and p41A-specific (E to H) CD8+ T lymphocytes.
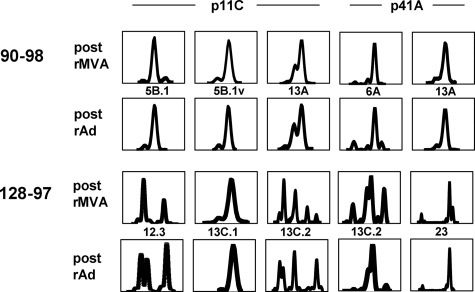
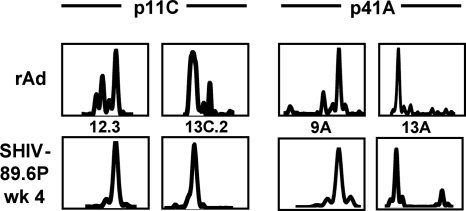
To further examine clonal persistence in these epitope-specific CD8+ T lymphocytes, we evaluated the clonality of epitope-specific CD8+ T lymphocytes from vaccinated monkeys 90-98 and 128-97 by examining the CDR3 regions of their Vβ regions using spectratyping and sequencing. The spectratype analysis revealed that the CDR3 lengths of all highly represented Vβ families employed by p11C and p41A epitope-specific CD8+ T-lymphocyte populations were indistinguishable following plasmid DNA prime/rMVA boost and following rAd vaccination (Fig. 4). Furthermore, cloning and sequencing of CDR3 regions revealed that there was identical Jβ family usage by these CD8+ T-lymphocyte populations following rMVA and following rAd immunizations (Fig. 5). D region sequences were also identical or nearly identical in the majority of cases. These data provide compelling evidence that clonal p11C and p41A epitope-specific CD8+ T-lymphocyte populations elicited by plasmid DNA/rMVA vaccination persisted following rAd vaccination.
FIG. 4.
TCR CDR3 lengths of highly represented Vβ families of p11C and p41A epitope-specific CD8+ T-lymphocyte populations are the same following plasmid DNA/rMVA and rAd vaccinations. cDNAs generated for use in real-time PCR Vβ quantitation for monkeys 90-98 and 128-97 served as templates for second-round PCRs employing family-specific Vβ primers and a 5′-FAM-labeled Cβ primer. An aliquot of the reaction product was run on an ABI 3730xl, and CDR3 profiles were analyzed using Genemapper 3.7 software. x axes, CDR3 lengths (amino acids); y axes, relative fluorescence intensities.
FIG. 5.
TCR Jβ family usage is identical in p11C and p41A epitope-specific CD8+ T lymphocytes following rMVA and rAd vaccinations. Second-round PCRs used for spectratyping analysis of epitope-specific CD8+ T-lymphocyte CDR3 regions from monkeys 90-98 and 128-97 served as templates for a third-round PCR to provide material for cloning and sequencing. CDR3 sequencing was done using the Cβ reverse primer.
TCR Vβ repertoires of epitope-specific CD8+ T lymphocytes in vaccinated Mamu-A*01+ rhesus monkeys challenged with nonpathogenic and pathogenic SHIVs.
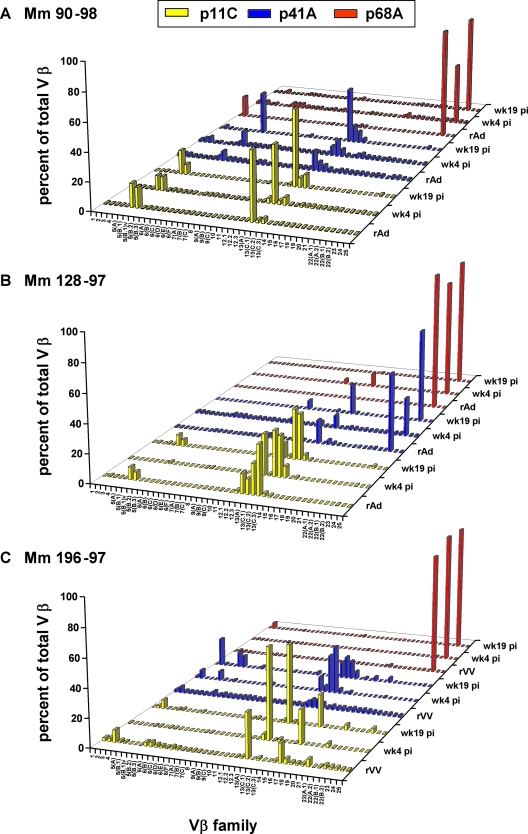
Having shown that clonal p11C and p41A epitope-specific CD8+ T-lymphocyte populations elicited by plasmid DNA/rMVA vaccination persist following rAd boost, we sought to determine whether these clonal vaccine-elicited CD8+ T lymphocytes would also persist following infection with SHIV. Moreover, we wanted to compare the effects of infection with a nonpathogenic and a pathogenic SHIV in shaping the TCR repertoires of vaccine-elicited CD8+ T lymphocytes. To accomplish this, we challenged three of the vaccinated monkeys (90-98, 128-97, and 196-97) with the nonpathogenic SHIV-89.6 and the other three vaccinated monkeys (95-98, 132-97, and 135-97) with the pathogenic SHIV-89.6P. p11C, p41A, and p68A tetramer-binding CD8+ T lymphocytes were sorted from PBMC isolated from each of these monkeys at 4 and 19 weeks following infection and assessed for their expression of 46 Vβ gene families. The Vβ repertoires of the vaccine-elicited, epitope-specific CD8+ T-lymphocyte populations were then compared to the repertoires generated following viral infection. Vβ families that represented more than 5% of the total Vβ repertoire within epitope-specific CD8+ T lymphocytes met the threshold for positivity for the presence of a Vβ population.
Strikingly, in the three monkeys infected with nonpathogenic SHIV, the Vβ repertoires of the p11C, p41A, and p68A epitope-specific CD8+ T lymphocytes generated following rAd (90-98 and 128-97) or rVV (196-97) boost remained, for the most part, the same after challenge at weeks 4 and 19 (Fig. 6). During the periods of acute and chronic SHIV-89.6 infection, p11C epitope-specific CD8+ T lymphocytes from monkey 90-98 expressed Vβ 5 and 13. In weeks 4 and 19, monkey 128-97 used predominantly Vβ families 12.3 and 13; Vβ5-specific populations were absent in week 4 but present again in week 19. Monkey 196-97 used Vβ families 3, 13A, and 16. At weeks 4 and 19 postchallenge, both monkeys 90-98 and 196-97 used members of the Vβ 13 family in their p41A epitope-specific CD8+ T lymphocytes, with monkey 90-98 additionally using Vβ 6A and monkey 196-97 also using Vβ 2 and 5. Monkey 128-97 used Vβ 13C.2 and 23 in its p41A epitope-specific CD8+ T-lymphocyte populations. All three monkeys exclusively used Vβ 23 in their p68A-specific CD8+ T lymphocytes following rAd or rVV vaccination and following infection with SHIV-89.6.
FIG. 6.
TCR Vβ repertoires of vaccine-elicited p11C, p41A, or p68A epitope-specific CD8+ T-lymphocyte populations do not change following infection with a nonpathogenic SHIV. PBMC from monkeys (Mm) 90-98 (A), 128-97 (B), and 196-97 (C) were isolated 4 (wk4 pi) and 19 (wk19 pi) weeks following SHIV-89.6 infection. The lymphocytes were cultured in vitro with p11C, p41A, or p68A peptide; stained with tetramers; and sorted. cDNA synthesized from the RNA extracted from these tetramer-binding CD8+ T-lymphocyte populations was used to evaluate Vβ repertoires.
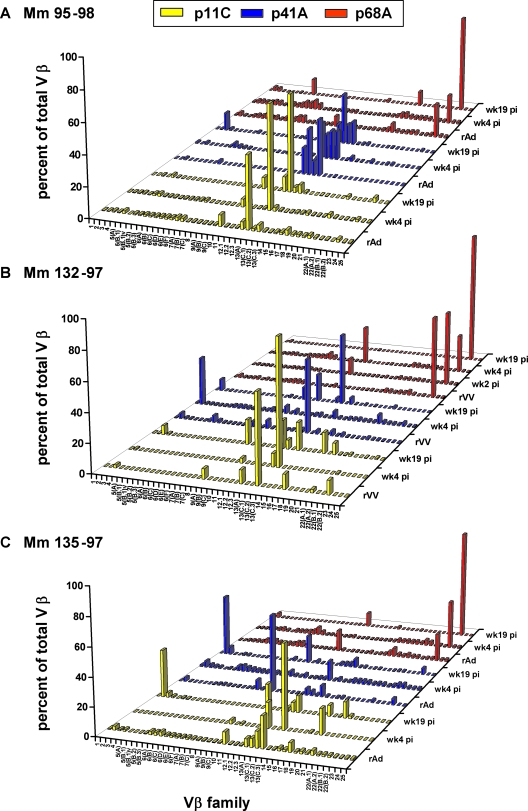
In contrast to these findings, TCR Vβ repertoire changes were observed in the vaccine-elicited epitope-specific CD8+ T lymphocytes in PBMC of monkeys following pathogenic-SHIV infection. After challenge with SHIV-89.6P, the clonal CD8+ T-lymphocyte response that was generated following rAd (95-98 and 135-97) or rVV (132-97) vaccination became more focused at week 4 and then more complex again at week 19. This transient clonal focusing was apparent in the TCR Vβ repertoires of p11C epitope-specific CD8+ T lymphocytes in all three evaluated monkeys and in the TCR Vβ repertoire of the p41A-specific CD8+ T lymphocytes in monkey 135-97 (Fig. 7).
FIG. 7.
TCR Vβ repertoires of p11C and p41A epitope-specific CD8+ T lymphocytes focus transiently following infection with a pathogenic SHIV. PBMC from monkeys (Mm) 95-98 (A), 132-97 (B), and 135-97 (C) were isolated 4 (wk4 pi) and 19 (wk19 pi) weeks following SHIV-89.6P infection. The lymphocytes were cultured in vitro with p11C, p41A, and p68A peptides; stained with tetramers; and sorted. cDNA synthesized from the RNA extracted from these tetramer-binding CD8+ T-lymphocyte populations was used to evaluate Vβ repertoires.
Specifically, in monkey 135-97, rAd vaccination generated p11C-specific CD8+ T lymphocytes with TCRs using Vβ 10, 12.3, 13A, 13C.1, 13C.2, and 17. Four weeks following challenge with SHIV-89.6P, only p11C epitope-specific CD8+ T lymphocytes using Vβ 12.3 and 13C.2 were still detected in this animal. A new SHIV-89.6P-induced p11C-specific CD8+ T-lymphocyte population using Vβ 19 was also present at week 4 in this monkey's PBMC. Interestingly, at week 19 following challenge, Vβ 2, 10, 13A, 13C.1, 17, and 20 expression was observed in the p11C-specific CD8+ T lymphocytes. However, no Vβ 13C.2 expression was detected in PBMC from this monkey at week 19 following challenge, although Vβ 13C.2 was the predominant Vβ gene employed by TCR of p11C-specific CD8+ T lymphocytes in week 4 PBMC from this monkey (Fig. 7C).
A focusing of TCR Vβ gene usage was also apparent in the p41A epitope-specific CD8+ T lymphocytes from monkey 135-97 following pathogenic-SHIV infection. Vaccine-elicited epitope-specific CD8+ T lymphocytes employing Vβ 7B, 9A, and 13C.2 focused to epitope-specific cells employing Vβ 13A at week 4 postinfection. At week 19, we observed the reemergence of p41A-specific CD8+ T lymphocytes using Vβ 9A and 13C.2 gene families seen following rAd immunization, as well as the emergence of CD8+ T lymphocytes using Vβ 2 and 3 (Fig. 7C).
Similarly, expression of Vβ 9A, 12.3, 13C.2, 17, and 22 in vaccine-elicited p11C-specific CD8+ T lymphocytes of monkey 132-97 dramatically focused to expression of primarily Vβ 13C.2 immediately following infection with SHIV-89.6P. This monkey also had p11C-specific CD8+ T lymphocytes using Vβ 13C.1 in its PBMC for the first time from week 4. By week 19, the analysis of p11C-specific CD8+ T lymphocytes expressing Vβ 9A, 12.3, 13C.2, and 17 reemerged, and new clones using Vβ 3, 13A, and 19 emerged as well.
However, there did not appear to be a focusing of Vβ gene family use by vaccine-elicited p41A epitope-specific CD8+ T lymphocytes in monkey 132-97 following SHIV-89.6P infection. Rather, a shift was seen from predominant usage of Vβ 13A following rVV boost to usage of Vβ 2 at week 4 following infection. By week 19, however, Vβ 2, 9A, 10, and 13A were expressed by p41A-specific CD8+ T lymphocytes, similar to what was generated following rVV immunization (Fig. 7B).
There was also a focusing of Vβ gene families used in p11C epitope-specific CD8+ T lymphocytes of monkey 95-98 following SHIV-89.6P challenge. Vβ 10-, 13A-, and 13C.2-expressing populations generated following rAd vaccination focused predominantly to Vβ 10- and 13A-expressing populations at week 4 following infection. Epitope-specific CD8+ T lymphocytes expressing Vβ 10, 12.3, 13A, and 13C.2 were detected at week 19 after challenge. p41A epitope-specific CD8+ T lymphocytes in monkey 95-98 did not exhibit focusing of Vβ gene family use at any time following infection, as Vβ 12.3, 13A, 13C.1, and 13C.2 were expressed following rAd vaccination and following infection (Fig. 7A).
TCR Vβ repertoires of p68A epitope-specific CD8+ T lymphocytes from all three monkeys infected with pathogenic virus showed predominantly Vβ 23 usage following rAd vaccination and at weeks 4 and 19 after SHIV-89.6P infection (Fig. 7).
After observing focusing at the level of TCR Vβ expression 4 weeks postinfection, we assessed whether focusing was also seen at the level of the TCR Vβ CDR3 length and sequence. We performed spectratyping to evaluate CDR3 lengths in highly represented Vβ families in both p11C and p41A epitope-specific CD8+ T lymphocytes from 4 weeks postinfection in the three Mamu-A*01+-vaccinated rhesus monkeys challenged with pathogenic SHIV-89.6P (monkeys 95-98, 132-97, and 135-97). In lymphocyte populations from monkey 135-97, focusing was observed at the level of CDR3 length in both p11C- and p41A-specific CD8+ T lymphocytes. Specifically, in p11C-specific CD8+ T lymphocytes, the three Vβ 12.3 CDR3 lengths generated following rAd immunization focused into a single CDR3 length at week 4 after infection, and the four Vβ 13C.2 CDR3 lengths generated following rAd also focused into a single CDR3 length. In p41A-specific CD8+ T lymphocytes, three Vβ 9A CDR3 lengths generated following rAd immunization focused into a single CDR3 length at week 4 after infection, while four Vβ 13A CDR3 lengths generated following rAd focused into two distinct CDR3 lengths (Fig. 8). Taken together, these data suggest that there can be a clonal focusing of vaccine-elicited, epitope-specific CD8+ T lymphocytes as assessed by TCR Vβ repertoires and CDR3 lengths following infection with SHIV-89.6P.
FIG. 8.
Focusing is apparent at the CDR3 level of p11C and p41A epitope-specific CD8+ T-lymphocyte populations following pathogenic-SHIV infection. cDNAs generated for use in real-time PCR Vβ quantitation for monkey 135-97 following SHIV-89.6P infection served as templates for second-round PCRs employing family-specific Vβ primers and a 5′-FAM-labeled Cβ primer. An aliquot of the reaction product was run on an ABI 3730xl, and CDR3 profiles were analyzed using Genemapper 3.7 software. x axes, CDR3 lengths (amino acids); y axes, relative fluorescence intensities.
DISCUSSION
In the present study, we show that vaccine-elicited epitope-specific CD8+ T lymphocytes are as diverse as those induced by SHIV-89.6P infection and that these clonal CD8+ T-lymphocyte populations persist. Moreover, our data indicate that the clonality of these epitope-specific T lymphocytes transiently focuses in response to intense SHIV-89.6P viral replication.
Interestingly, as shown in Fig. 1 and 2, CD8+ T-lymphocyte clonal diversity, as measured by the numbers of Vβ genes expressed within both p11C and p41A-specific CD8+ T-lymphocyte populations, was not significantly different in monkeys that were vaccinated versus those infected with SHIV-89.6P. This was surprising, as one might expect a chronic infection with a SHIV to elicit a greater breadth of TCR clonality within epitope-specific CD8+ T lymphocytes than would be generated by a prime with the Mamu-A*01-restricted epitopes p11C, p41A, and p68A. Thus, the vector in which the SHIV viral antigens were presented to the immune systems of these monkeys, either as a whole virus or as epitope sequences engineered into rMVA, rAd, or rVV vectors, did not affect the clonal diversity of their epitope-specific CD8+ T-lymphocyte responses. Furthermore, in the vaccinated monkey cohort, the clonal make-ups of epitope-specific CD8+ T-lymphocyte populations were almost identical in monkeys vaccinated with plasmid DNA/rMVA and in monkeys following vaccination with rAd (Fig. 3). It is, of course, formally possible that the initial exposure to these viral proteins limited the clonal constituents of subsequently stimulated epitope-specific CD8+ T-lymphocyte responses—a mechanism reminiscent of “original antigenic sin.” This mechanism could explain why the clonality of these responses following plasmid DNA immunization persisted after live recombinant vector boosting and subsequently after virus infection. However, the observation that the diversities of clones seen in these epitope-specific CD8+ T-lymphocyte populations were comparable in monkeys only vaccinated and in unvaccinated monkeys that were infected with SHIV-89.6P suggests that clonal diversity is dictated by the epitope sequence, not the mode of antigen delivery to the immune system.
Since a large body of investigative work supports the premise that persistent immune stimulation can lead to the exhaustion of clonal T-lymphocyte populations, it is of critical importance to understand whether CD8+ T-lymphocyte clones can still be detected in the setting of ongoing primate immunodeficiency replication. Others have shown that individual clonal populations of CTL are maintained in chronically HIV-1-infected individuals (15, 19, 28, 66); however, the frequency of clonal T-cell persistence has not been evaluated. The present study demonstrates that, as a rule, clonal CD8+ T-lymphocyte populations persist in the setting of ongoing primate immunodeficiency virus infection. In fact, in all monkeys that were vaccinated and subsequently infected, persistence of particular clonal populations of T lymphocytes was documented for greater than 4 years. The only major vaccine-elicited CD8+ T-lymphocyte clone that was present following the initial vaccination but did not persist at week 19 following infection was Vβ 13C.2 in monkey 135-97. Previous studies from our laboratory and others have shown that during HIV-1 infection, there can be death of CD8+ T-lymphocyte populations; thus, this lymphocyte population expressing Vβ 13C.2 may have undergone apoptosis between weeks 4 and 19 of infection (32). It is further assumed that vaccine-induced clonal populations of CD8+ T lymphocytes will expand rapidly on exposure to replicating virus to contain the spread of that virus. Previously, epitope-specific CD8+ T-lymphocyte populations generated through vaccination were shown to persist following challenge with primate immunodeficiency viruses, although the clonality of those responses was not evaluated. The results of the current study clearly show that vaccine-induced epitope-specific CD8+ T-lymphocyte clones expand and persist in the face of replicating virus. While we have not evaluated the functional integrity of these clonal T-lymphocyte populations in the present study, we have recently shown that prior vaccination protects the functional integrity of epitope-specific CD8+ T-lymphocyte populations following a pathogenic-SHIV challenge (1).
The clonal focusing observed in epitope-specific CD8+ T lymphocytes in monkeys infected with pathogenic SHIV-89.6P and the absence of this focusing in those monkeys infected with nonpathogenic SHIV-89.6 may reflect the markedly different properties of these two viruses. First, SHIV-89.6 infection does not lead to CD4+ T-lymphocyte loss, while SHIV-89.6P infection can lead to profound loss of naïve CD4+ T lymphocytes. Since CD4+ T lymphocytes are needed for the generation and persistence of antigen-experienced CD8+ T lymphocytes (26, 33, 56, 59, 61), it is possible that the loss of CD4+ T lymphocytes in the SHIV-89.6P-infected monkeys may have led to a diminution of CD8+ epitope-specific T-lymphocyte populations during the first weeks following infection. Second, the SHIV-89.6P-infected monkeys had significantly higher viral loads than the SHIV-89.6-infected monkeys. In SHIV-89.6P-infected monkeys, peak viral loads were 3 log units higher and set point viral loads were 1 log unit higher than in the SHIV-89.6-infected monkeys. High viral loads have been implicated in the ablation of CD8+ T-lymphocyte populations (22, 29). Apoptosis of CTL can increase with increasing antigen load, and this CD8+ lymphocyte death occurs shortly after initial infection. Thus, high viral loads can contribute to the deletion of virus-specific CTL during the primary viral infection in the presence of high viral burden (14, 23, 60). Moreover, consistent with the observations of the present study, a more clonally restricted epitope-specific cell population has been observed in CD8+ T lymphocytes responding to pathogenic HIV-1 than in those responding to the less pathogenic HIV-2 (12, 34, 47). Thus, taken together, these data support our observations in the SHIV-89.6/89.6P model and suggest that differences in primate immunodeficiency virus infections can affect the clonal breadth of CD8+ T-lymphocyte responses to the viruses.
The clonal focusing within the epitope-specific CD8+ T-lymphocyte populations may have important implications for the control of pathogenic primate immunodeficiency viruses during primary infection. In considering that viral escape from CTL has been described in the SIV/rhesus monkey system as early as 4 weeks postchallenge (41), particularly high levels of viral replication in association with clonal CD8+ T-lymphocyte focusing during this early period may increase the likelihood of viral escape from CTL (48). Overcoming this limitation of the number of clonal CD8+ T lymphocytes available to control viral replication early in infection may be a significant challenge in designing an effective cell-mediated HIV-1 vaccine.
Acknowledgments
We thank Ediane Dutra for her assistance with the spectratyping analysis.
This research was supported by the Harvard University Center for AIDS Research (CFAR), NIH grant P30 AI060354 (N.L.L.), NIH grant AIO20729 (N.L.L.), and a Howard Hughes Medical Institute Research Training Fellowship (P.S.).
Footnotes
Published ahead of print on 31 October 2007.
REFERENCES
- 1.Acierno, P. M., J. E. Schmitz, D. A. Gorgone, Y. Sun, S. Santra, M. S. Seaman, M. H. Newberg, J. R. Mascola, G. J. Nabel, D. Panicali, and N. L. Letvin. 2006. Preservation of functional virus-specific memory CD8+ T lymphocytes in vaccinated, simian human immunodeficiency virus-infected rhesus monkeys. J. Immunol. 1765338-5345. [DOI] [PubMed] [Google Scholar]
- 2.Allen, T. M., J. Sidney, M. F. del Guercio, R. L. Glickman, G. L. Lensmeyer, D. A. Wiebe, R. DeMars, C. D. Pauza, R. P. Johnson, A. Sette, and D. I. Watkins. 1998. Characterization of the peptide binding motif of a rhesus MHC class I molecule (Mamu-A*01) that binds an immunodominant CTL epitope from simian immunodeficiency virus. J. Immunol. 1606062-6071. [PubMed] [Google Scholar]
- 3.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 29269-74. [DOI] [PubMed] [Google Scholar]
- 4.Amara, R. R., F. Villinger, S. I. Staprans, J. D. Altman, D. C. Montefiori, N. L. Kozyr, Y. Xu, L. S. Wyatt, P. L. Earl, J. G. Herndon, H. M. McClure, B. Moss, and H. L. Robinson. 2002. Different patterns of immune responses but similar control of a simian-human immunodeficiency virus 89.6P mucosal challenge by modified vaccinia virus Ankara (MVA) and DNA/MVA vaccines. J. Virol. 767625-7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Appay, V., D. F. Nixon, S. M. Donahoe, G. M. Gillespie, T. Dong, A. King, G. S. Ogg, H. M. Spiegel, C. Conlon, C. A. Spina, D. V. Havlir, D. D. Richman, A. Waters, P. Easterbrook, A. J. McMichael, and S. L. Rowland-Jones. 2000. HIV-specific CD8+ T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 19263-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Appay, V., L. Papagno, C. A. Spina, P. Hansasuta, A. King, L. Jones, G. S. Ogg, S. Little, A. J. McMichael, D. D. Richman, and S. L. Rowland-Jones. 2002. Dynamics of T cell responses in HIV infection. J. Immunol. 1683660-3666. [DOI] [PubMed] [Google Scholar]
- 7.Barouch, D. H., J. Kunstman, J. Glowczwskie, K. J. Kunstman, M. A. Egan, F. W. Peyerl, S. Santra, M. J. Kuroda, J. E. Schmitz, K. Beaudry, G. R. Krivulka, M. A. Lifton, D. A. Gorgone, S. M. Wolinsky, and N. L. Letvin. 2003. Viral escape from dominant simian immunodeficiency virus epitope-specific cytotoxic T lymphocytes in DNA-vaccinated rhesus monkeys. J. Virol. 777367-7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barouch, D. H., J. Kunstman, M. J. Kuroda, J. E. Schmitz, S. Santra, F. W. Peyerl, G. R. Krivulka, K. Beaudry, M. A. Lifton, D. A. Gorgone, D. C. Montefiori, M. G. Lewis, S. M. Wolinsky, and N. L. Letvin. 2002. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature 415335-339. [DOI] [PubMed] [Google Scholar]
- 9.Barouch, D. H., and N. L. Letvin. 2004. HIV escape from cytotoxic T lymphocytes: a potential hurdle for vaccines? Lancet 36410-11. [DOI] [PubMed] [Google Scholar]
- 10.Barouch, D. H., S. Santra, M. J. Kuroda, J. E. Schmitz, R. Plishka, A. Buckler-White, A. E. Gaitan, R. Zin, J. H. Nam, L. S. Wyatt, M. A. Lifton, C. E. Nickerson, B. Moss, D. C. Montefiori, V. M. Hirsch, and N. L. Letvin. 2001. Reduction of simian-human immunodeficiency virus 89.6P viremia in rhesus monkeys by recombinant modified vaccinia virus Ankara vaccination. J. Virol. 755151-5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, E. A. Emini, J. W. Shiver, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290486-492. [DOI] [PubMed] [Google Scholar]
- 12.Berry, N., K. Ariyoshi, S. Jaffar, S. Sabally, T. Corrah, R. Tedder, and H. Whittle. 1998. Low peripheral blood viral HIV-2 RNA in individuals with high CD4 percentage differentiates HIV-2 from HIV-1 infection. J. Hum. Virol. 1457-468. [PubMed] [Google Scholar]
- 13.Bestilny, L. J., M. J. Gill, C. H. Mody, and K. T. Riabowol. 2000. Accelerated replicative senescence of the peripheral immune system induced by HIV infection. AIDS 14771-780. [DOI] [PubMed] [Google Scholar]
- 14.Betts, M. R., D. A. Price, J. M. Brenchley, K. Lore, F. J. Guenaga, A. Smed-Sorensen, D. R. Ambrozak, S. A. Migueles, M. Connors, M. Roederer, D. C. Douek, and R. A. Koup. 2004. The functional profile of primary human antiviral CD8+ T cell effector activity is dictated by cognate peptide concentration. J. Immunol. 1726407-6417. [DOI] [PubMed] [Google Scholar]
- 15.Brander, C., P. J. Goulder, K. Luzuriaga, O. O. Yang, K. E. Hartman, N. G. Jones, B. D. Walker, and S. A. Kalams. 1999. Persistent HIV-1-specific CTL clonal expansion despite high viral burden post in utero HIV-1 infection. J. Immunol. 1624796-4800. [PubMed] [Google Scholar]
- 16.Casimiro, D. R., L. Chen, T. M. Fu, R. K. Evans, M. J. Caulfield, M. E. Davies, A. Tang, M. Chen, L. Huang, V. Harris, D. C. Freed, K. A. Wilson, S. Dubey, D. M. Zhu, D. Nawrocki, H. Mach, R. Troutman, L. Isopi, D. Williams, W. Hurni, Z. Xu, J. G. Smith, S. Wang, X. Liu, L. Guan, R. Long, W. Trigona, G. J. Heidecker, H. C. Perry, N. Persaud, T. J. Toner, Q. Su, X. Liang, R. Youil, M. Chastain, A. J. Bett, D. B. Volkin, E. A. Emini, and J. W. Shiver. 2003. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 776305-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charini, W. A., M. J. Kuroda, J. E. Schmitz, K. R. Beaudry, W. Lin, M. A. Lifton, G. R. Krivulka, A. Necker, and N. L. Letvin. 2001. Clonally diverse CTL response to a dominant viral epitope recognizes potential epitope variants. J. Immunol. 1674996-5003. [DOI] [PubMed] [Google Scholar]
- 18.Dagarag, M., H. Ng, R. Lubong, R. B. Effros, and O. O. Yang. 2003. Differential impairment of lytic and cytokine functions in senescent human immunodeficiency virus type 1-specific cytotoxic T lymphocytes. J. Virol. 773077-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong, T., G. Stewart-Jones, N. Chen, P. Easterbrook, X. Xu, L. Papagno, V. Appay, M. Weekes, C. Conlon, C. Spina, S. Little, G. Screaton, A. van der Merwe, D. D. Richman, A. J. McMichael, E. Y. Jones, and S. L. Rowland-Jones. 2004. HIV-specific cytotoxic T cells from long-term survivors select a unique T cell receptor. J. Exp. Med. 2001547-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Effros, R. B., and G. Pawelec. 1997. Replicative senescence of T cells: does the Hayflick Limit lead to immune exhaustion? Immunol. Today 18450-454. [DOI] [PubMed] [Google Scholar]
- 21.Egan, M. A., M. J. Kuroda, G. Voss, J. E. Schmitz, W. A. Charini, C. I. Lord, M. A. Forman, and N. L. Letvin. 1999. Use of major histocompatibility complex class I/peptide/β2M tetramers to quantitate CD8+ cytotoxic T lymphocytes specific for dominant and nondominant viral epitopes in simian-human immunodeficiency virus-infected rhesus monkeys. J. Virol. 735466-5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuller, M. J., and A. J. Zajac. 2003. Ablation of CD8 and CD4 T cell responses by high viral loads. J. Immunol. 170477-486. [DOI] [PubMed] [Google Scholar]
- 23.Gallimore, A., A. Glithero, A. Godkin, A. C. Tissot, A. Pluckthun, T. Elliott, H. Hengartner, and R. Zinkernagel. 1998. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J. Exp. Med. 1871383-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goepfert, P. A., A. Bansal, B. H. Edwards, G. D. Ritter, Jr., I. Tellez, S. A. McPherson, S. Sabbaj, and M. J. Mulligan. 2000. A significant number of human immunodeficiency virus epitope-specific cytotoxic T lymphocytes detected by tetramer binding do not produce gamma interferon. J. Virol. 7410249-10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hadrup, S. R., J. Strindhall, T. Kollgaard, T. Seremet, B. Johansson, G. Pawelec, P. thor Straten, and A. Wikby. 2006. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J. Immunol. 1762645-2653. [DOI] [PubMed] [Google Scholar]
- 25a.Institute of Laboratory Animal Research. 1996. Guide for the care and use of laboratory animals. National Academies Press, Washington, DC.
- 26.Janssen, E. M., E. E. Lemmens, T. Wolfe, U. Christen, M. G. von Herrath, and S. P. Schoenberger. 2003. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421852-856. [DOI] [PubMed] [Google Scholar]
- 27.Jones, N. A., X. Wei, D. R. Flower, M. Wong, F. Michor, M. S. Saag, B. H. Hahn, M. A. Nowak, G. M. Shaw, and P. Borrow. 2004. Determinants of human immunodeficiency virus type 1 escape from the primary CD8+ cytotoxic T lymphocyte response. J. Exp. Med. 2001243-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalams, S. A., R. P. Johnson, A. K. Trocha, M. J. Dynan, H. S. Ngo, R. T. D'Aquila, J. T. Kurnick, and B. D. Walker. 1994. Longitudinal analysis of T cell receptor (TCR) gene usage by human immunodeficiency virus 1 envelope-specific cytotoxic T lymphocyte clones reveals a limited TCR repertoire. J. Exp. Med. 1791261-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim, M., H. B. Moon, K. Kim, and K. Y. Lee. 2006. Antigen dose governs the shaping of CTL repertoires in vitro and in vivo. Int. Immunol. 18435-444. [DOI] [PubMed] [Google Scholar]
- 30.Knapp, L. A., E. Lehmann, M. S. Piekarczyk, J. A. Urvater, and D. I. Watkins. 1997. A high frequency of Mamu-A*01 in the rhesus macaque detected by polymerase chain reaction with sequence-specific primers and direct sequencing. Tissue Antigens 50657-661. [DOI] [PubMed] [Google Scholar]
- 31.Kuroda, M. J., J. E. Schmitz, D. H. Barouch, A. Craiu, T. M. Allen, A. Sette, D. I. Watkins, M. A. Forman, and N. L. Letvin. 1998. Analysis of Gag-specific cytotoxic T lymphocytes in simian immunodeficiency virus-infected rhesus monkeys by cell staining with a tetrameric major histocompatibility complex class I-peptide complex. J. Exp. Med. 1871373-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuroda, M. J., J. E. Schmitz, W. A. Charini, C. E. Nickerson, M. A. Lifton, C. I. Lord, M. A. Forman, and N. L. Letvin. 1999. Emergence of CTL coincides with clearance of virus during primary simian immunodeficiency virus infection in rhesus monkeys. J. Immunol. 1625127-5133. [PubMed] [Google Scholar]
- 33.Lichterfeld, M., D. E. Kaufmann, X. G. Yu, S. K. Mui, M. M. Addo, M. N. Johnston, D. Cohen, G. K. Robbins, E. Pae, G. Alter, A. Wurcel, D. Stone, E. S. Rosenberg, B. D. Walker, and M. Altfeld. 2004. Loss of HIV-1-specific CD8+ T cell proliferation after acute HIV-1 infection and restoration by vaccine-induced HIV-1-specific CD4+ T cells. J. Exp. Med. 200701-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopes, A. R., A. Jaye, L. Dorrell, S. Sabally, A. Alabi, N. A. Jones, D. R. Flower, A. De Groot, P. Newton, R. M. Lascar, I. Williams, H. Whittle, A. Bertoletti, P. Borrow, and M. K. Maini. 2003. Greater CD8+ TCR heterogeneity and functional flexibility in HIV-2 compared to HIV-1 infection. J. Immunol. 171307-316. [DOI] [PubMed] [Google Scholar]
- 35.Manuel, E. R., W. A. Charini, P. Sen, F. W. Peyerl, M. J. Kuroda, J. E. Schmitz, P. Autissier, D. A. Sheeter, B. E. Torbett, and N. L. Letvin. 2006. Contribution of TCR repertoire breadth to the dominance of epitope-specific CD8+ T lymphocyte responses. J. Virol. 8012032-12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McMichael, A., and T. Hanke. 2002. The quest for an AIDS vaccine: is the CD8+ T-cell approach feasible? Nat. Rev. Immunol. 2283-291. [DOI] [PubMed] [Google Scholar]
- 37.Miller, M. D., H. Yamamoto, A. L. Hughes, D. I. Watkins, and N. L. Letvin. 1991. Definition of an epitope and MHC class I molecule recognized by gag-specific cytotoxic T lymphocytes in SIVmac-infected rhesus monkeys. J. Immunol. 147320-329. [PubMed] [Google Scholar]
- 38.Moskophidis, D., E. Laine, and R. M. Zinkernagel. 1993. Peripheral clonal deletion of antiviral memory CD8+ T cells. Eur. J. Immunol. 233306-3311. [DOI] [PubMed] [Google Scholar]
- 39.Musey, L., J. Hughes, T. Schacker, T. Shea, L. Corey, and M. J. McElrath. 1997. Cytotoxic-T-cell responses, viral load, and disease progression in early human immunodeficiency virus type 1 infection. N. Engl. J. Med. 3371267-1274. [DOI] [PubMed] [Google Scholar]
- 40.Newberg, M. H., M. J. Kuroda, W. A. Charini, A. Miura, C. I. Lord, J. E. Schmitz, D. A. Gorgone, M. A. Lifton, K. Kuus-Reichel, and N. L. Letvin. 2002. A simian immunodeficiency virus nef peptide is a dominant cytotoxic T lymphocyte epitope in Indian-origin rhesus monkeys expressing the common MHC class I allele mamu-A*02. Virology 301365-373. [DOI] [PubMed] [Google Scholar]
- 41.O'Connor, D. H., T. M. Allen, T. U. Vogel, P. Jing, I. P. DeSouza, E. Dodds, E. J. Dunphy, C. Melsaether, B. Mothe, H. Yamamoto, H. Horton, N. Wilson, A. L. Hughes, and D. I. Watkins. 2002. Acute phase cytotoxic T lymphocyte escape is a hallmark of simian immunodeficiency virus infection. Nat. Med. 8493-499. [DOI] [PubMed] [Google Scholar]
- 42.Ogg, G. S., X. Jin, S. Bonhoeffer, P. R. Dunbar, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, V. Cerundolo, A. Hurley, M. Markowitz, D. D. Ho, D. F. Nixon, and A. J. McMichael. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 2792103-2106. [DOI] [PubMed] [Google Scholar]
- 43.Opferman, J. T., B. T. Ober, R. Narayanan, and P. G. Ashton-Rickardt. 2001. Suicide induced by cytolytic activity controls the differentiation of memory CD8+ T lymphocytes. Int. Immunol. 13411-419. [DOI] [PubMed] [Google Scholar]
- 44.Pannetier, C., J. Even, and P. Kourilsky. 1995. T-cell repertoire diversity and clonal expansions in normal and clinical samples. Immunol. Today 16176-181. [DOI] [PubMed] [Google Scholar]
- 45.Pantaleo, G., H. Soudeyns, J. F. Demarest, M. Vaccarezza, C. Graziosi, S. Paolucci, M. Daucher, O. J. Cohen, F. Denis, W. E. Biddison, R. P. Sekaly, and A. S. Fauci. 1997. Evidence for rapid disappearance of initially expanded HIV-specific CD8+ T cell clones during primary HIV infection. Proc. Natl. Acad. Sci. USA 949848-9853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peyerl, F. W., H. S. Bazick, M. H. Newberg, D. H. Barouch, J. Sodroski, and N. L. Letvin. 2004. Fitness costs limit viral escape from cytotoxic T lymphocytes at a structurally constrained epitope. J. Virol. 7813901-13910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Popper, S. J., A. D. Sarr, A. Gueye-Ndiaye, S. Mboup, M. E. Essex, and P. J. Kanki. 2000. Low plasma human immunodeficiency virus type 2 viral load is independent of proviral load: low virus production in vivo. J. Virol. 741554-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Price, D. A., S. M. West, M. R. Betts, L. E. Ruff, J. M. Brenchley, D. R. Ambrozak, Y. Edghill-Smith, M. J. Kuroda, D. Bogdan, K. Kunstman, N. L. Letvin, G. Franchini, S. M. Wolinsky, R. A. Koup, and D. C. Douek. 2004. T cell receptor recognition motifs govern immune escape patterns in acute SIV infection. Immunity 21793-803. [DOI] [PubMed] [Google Scholar]
- 49.Publicover, J., E. Ramsburg, and J. K. Rose. 2005. A single-cycle vaccine vector based on vesicular stomatitis virus can induce immune responses comparable to those generated by a replication-competent vector. J. Virol. 7913231-13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rocha, B., N. Dautigny, and P. Pereira. 1989. Peripheral T lymphocytes: expansion potential and homeostatic regulation of pool sizes and CD4/CD8 ratios in vivo. Eur. J. Immunol. 19905-911. [DOI] [PubMed] [Google Scholar]
- 51.Sacre, K., G. Carcelain, N. Cassoux, A. M. Fillet, D. Costagliola, D. Vittecoq, D. Salmon, Z. Amoura, C. Katlama, and B. Autran. 2005. Repertoire, diversity, and differentiation of specific CD8 T cells are associated with immune protection against human cytomegalovirus disease. J. Exp. Med. 2011999-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santra, S., D. H. Barouch, B. Korioth-Schmitz, C. I. Lord, G. R. Krivulka, F. Yu, M. H. Beddall, D. A. Gorgone, M. A. Lifton, A. Miura, V. Philippon, K. Manson, P. D. Markham, J. Parrish, M. J. Kuroda, J. E. Schmitz, R. S. Gelman, J. W. Shiver, D. C. Montefiori, D. Panicali, and N. L. Letvin. 2004. Recombinant poxvirus boosting of DNA-primed rhesus monkeys augments peak but not memory T lymphocyte responses. Proc. Natl. Acad. Sci. USA 10111088-11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Santra, S., D. H. Barouch, M. J. Kuroda, J. E. Schmitz, G. R. Krivulka, K. Beaudry, C. I. Lord, M. A. Lifton, L. S. Wyatt, B. Moss, V. M. Hirsch, and N. L. Letvin. 2002. Prior vaccination increases the epitopic breadth of the cytotoxic T-lymphocyte response that evolves in rhesus monkeys following a simian-human immunodeficiency virus infection. J. Virol. 766376-6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sette, A., and J. Fikes. 2003. Epitope-based vaccines: an update on epitope identification, vaccine design and delivery. Curr. Opin. Immunol. 15461-470. [DOI] [PubMed] [Google Scholar]
- 55.Shankar, P., M. Russo, B. Harnisch, M. Patterson, P. Skolnik, and J. Lieberman. 2000. Impaired function of circulating HIV-specific CD8+ T cells in chronic human immunodeficiency virus infection. Blood 963094-3101. [PubMed] [Google Scholar]
- 56.Shedlock, D. J., and H. Shen. 2003. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 300337-339. [DOI] [PubMed] [Google Scholar]
- 57.Subbramanian, R. A., W. A. Charini, M. J. Kuroda, M. Seaman, H. Chhay, M. A. Lifton, D. A. Gorgone, J. E. Schmitz, A. Carville, and N. L. Letvin. 2006. Expansion after epitope peptide exposure in vitro predicts cytotoxic T lymphocyte epitope dominance hierarchy in lymphocytes of vaccinated Mamu-A*01+ rhesus monkeys. AIDS Res. Hum. Retrovir. 22445-452. [DOI] [PubMed] [Google Scholar]
- 58.Subbramanian, R. A., M. J. Kuroda, W. A. Charini, D. H. Barouch, C. Costantino, S. Santra, J. E. Schmitz, K. L. Martin, M. A. Lifton, D. A. Gorgone, J. W. Shiver, and N. L. Letvin. 2003. Magnitude and diversity of cytotoxic-T-lymphocyte responses elicited by multiepitope DNA vaccination in rhesus monkeys. J. Virol. 7710113-10118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun, J. C., and M. J. Bevan. 2003. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 300339-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takahashi, M., E. Osono, Y. Nakagawa, J. Wang, J. A. Berzofsky, D. H. Margulies, and H. Takahashi. 2002. Rapid induction of apoptosis in CD8+ HIV-1 envelope-specific murine CTLs by short exposure to antigenic peptide. J. Immunol. 1696588-6593. [DOI] [PubMed] [Google Scholar]
- 61.Tanchot, C., F. A. Lemonnier, B. Perarnau, A. A. Freitas, and B. Rocha. 1997. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science 2762057-2062. [DOI] [PubMed] [Google Scholar]
- 62.von Boehmer, H., and K. Hafen. 1993. The life span of naive alpha/beta T cells in secondary lymphoid organs. J. Exp. Med. 177891-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vuola, J. M., S. Keating, D. P. Webster, T. Berthoud, S. Dunachie, S. C. Gilbert, and A. V. Hill. 2005. Differential immunogenicity of various heterologous prime-boost vaccine regimens using DNA and viral vectors in healthy volunteers. J. Immunol. 174449-455. [DOI] [PubMed] [Google Scholar]
- 64.Walker, C. M., D. J. Moody, D. P. Stites, and J. A. Levy. 1986. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science 2341563-1566. [DOI] [PubMed] [Google Scholar]
- 65.Wei, C. H., H. Yagita, M. G. Masucci, and V. Levitsky. 2001. Different programs of activation-induced cell death are triggered in mature activated CTL by immunogenic and partially agonistic peptide ligands. J. Immunol. 166989-995. [DOI] [PubMed] [Google Scholar]
- 66.Wilson, J. D., M. Cranage, N. Cook, S. Leech, A. J. McMichael, and M. F. Callan. 1998. Evidence for the persistence of monoclonal expansions of CD8+ T cells following primary simian immunodeficiency virus infection. Eur. J. Immunol. 281172-1180. [DOI] [PubMed] [Google Scholar]
- 67.Zajac, A. J., J. N. Blattman, K. Murali-Krishna, D. J. Sourdive, M. Suresh, J. D. Altman, and R. Ahmed. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 1882205-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou, D., Y. Shen, L. Chalifoux, D. Lee-Parritz, M. Simon, P. K. Sehgal, L. Zheng, M. Halloran, and Z. W. Chen. 1999. Mycobacterium bovis bacille Calmette-Guerin enhances pathogenicity of simian immunodeficiency virus infection and accelerates progression to AIDS in macaques: a role of persistent T cell activation in AIDS pathogenesis. J. Immunol. 1622204-2216. [PubMed] [Google Scholar]
- 69.Zhou, S., R. Ou, L. Huang, G. E. Price, and D. Moskophidis. 2004. Differential tissue-specific regulation of antiviral CD8+ T-cell immune responses during chronic viral infection. J. Virol. 783578-3600. [DOI] [PMC free article] [PubMed] [Google Scholar]