Abstract
The in vitro susceptibility of human- and bovine-origin Mycobacterium paratuberculosis to the thioupurine drugs 6-mercaptopurine (6-MP) and azathioprine (AZA) was established using conventional plate counting methods and the MGIT 960 ParaTB culture system. Both 6-MP and AZA had antibacterial activity against M. paratuberculosis; isolates from Crohn's disease patients tended to be more susceptible than were bovine-origin isolates. Isolates of Mycobacterium avium, used as controls, were generally resistant to both AZA and 6-MP, even at high concentrations (≥64.0 μg/ml). Among rapidly growing mycobacteria, Mycobacterium phlei was susceptible to 6-MP and AZA whereas Mycobacterium smegmatis strains were not. AZA and 6-MP limited the growth of, but did not kill, M. paratuberculosis in a dose-dependent manner. Anti-inflammatory drugs in the sulfonamide family (sulfapyridine, sulfasalazine, and 5-aminosalycilic acid [mesalamine]) had little or no antibacterial activity against M. paratuberculosis. The conventional antibiotics azithromycin and ciprofloxacin, used as control drugs, were bactericidal for M. paratuberculosis, exerting their killing effects on the organism relatively quickly. Simultaneous exposure of M. paratuberculosis to 6-MP and ciprofloxacin resulted in significantly higher CFU than use of ciprofloxacin alone. These data may partially explain the paradoxical response of Crohn's disease patients infected with M. paratuberculosis to treatment with immunosuppressive thiopurine drugs, i.e., they do not worsen with anti-inflammatory treatment as would be expected with a microbiological etiologic pathogen. These findings also should influence the design of therapeutic trials to evaluate antibiotic treatments of Crohn's disease: AZA drugs may confound interpretation of data on therapeutic responses for both antibiotic-treated and control groups.
The etiology of Crohn's disease remains elusive, but current consensus opinion is that Crohn's disease results from the interplay of host genetics and one or more environmental triggers (7). Genetic markers for susceptibility to Crohn's disease have been discovered, notably the CARD15 (15, 21, 28) gene and most recently the interleukin-23r (10) and ATG16L1 (16, 32) genes. The increased incidence seen in many industrialized countries supports the existence of an environmental trigger for Crohn's disease (11, 23, 37, 39, 43). Bacterial pathogens fit a pathobiology model involving abnormal host response to infection resulting from genetic defects in gastrointestinal antigen processing.
One trigger for Crohn's disease may be Mycobacterium paratuberculosis, also referred to as Mycobacterium avium subsp. paratuberculosis, with or without host genetic influences that increase infection susceptibility or alter response to infection (9, 40). M. paratuberculosis causes a type of chronic inflammatory bowel disease in a wide array of ruminant species, as well as in nonhuman primates (5, 18). The infection is prevalent in domestic agriculture ruminants, and it has been diagnosed in wildlife species as well. Human and animal M. paratuberculosis isolates share common genotypes (12, 29). Exposure of humans to M. paratuberculosis could occur by both direct and indirect contamination of food and water (3, 4, 44).
The thiopurine drugs azathioprine (AZA; Imuran) and its metabolite 6-mercaptopurine (6-MP) are among the most commonly used immunosuppressive drugs used to treat Crohn's disease symptoms (42). They both induce and maintain Crohn's disease remission. Compounds related to 5-aminosalicyclates (sulfasalazine [SS] and mesalamine) are other drugs used to suppress inflammation in Crohn's disease patients (17). One observation arguing against an involvement of M. paratuberculosis, or any other infectious agent, as a primary cause of Crohn's disease is that patients treated with immunosuppressive drugs do not clinically worsen (35).
Greenstein et al. demonstrated, however, that thiopurine drugs inhibit the growth of M. paratuberculosis (13). The goal of this study was to characterize 6-MP's effect on M. paratuberculosis growth in culture and contrast it with the antibacterial effects of conventional antimycobacterial antibiotics.
MATERIALS AND METHODS
Bacterial strains and inoculum preparation.
A total of 11 bovine- and human-origin M. paratuberculosis strains were used in this study (Table 1). All strains were initially cultured in 7H9 broth supplemented with 10% (vol/vol) oleic acid-albumin-dextrose-catalase (OADC; Becton Dickinson, Sparks, MD) and 2 μg/ml of mycobactin J (Allied Monitor, Fayette, MO) for 1 month at 37°C. Seven Mycobacterium avium strains were used for comparison. These were cultured in 7H9 broth supplemented with 10% OADC (Becton Dickinson, Sparks, MD) for 2 weeks to 1 month at 37°C (Table 1).
TABLE 1.
Bacterial strains tested in this study
| Bacterial strain | Isolate source |
|---|---|
| M. paratuberculosis | |
| ATCC 19698 | Bovine, clinical case of paratuberculosis, type strain |
| JTC303 | Bovine, clinical case of paratuberculosis, JTCa |
| UCF-3 | Human, Crohn's disease patient ileum, UCFb |
| UCF-4 | Human, Crohn's disease patient ileum, UCFb |
| UCF-5 | Human, Crohn's disease patient ileum, UCFb |
| UCF-7 | Human, Crohn's disease patient ileum, UCFb |
| UCF-8 | Human, Crohn's disease patient ileum, UCFb |
| B213 | Bovine, clinical case of paratuberculosis, UCFb |
| B236 | Bovine, clinical case of paratuberculosis, UCFb |
| B238 | Bovine, clinical case of paratuberculosis, UCFb |
| B244 | Bovine, clinical case of paratuberculosis, UCFb |
| M. avium | |
| ATCC 35712 | Chicken, TMC701,c serotype 2 |
| ATCC 25291 | Chicken, liver, TMC724, serotype 2, type strain |
| 104 | Human, AIDS patient, serotype 1d |
| JTC48627 | Bison, fecal sample |
| JTC981 | Bongo, fecal sample |
| EPA3 | Water, WSLHe |
| WSLH1544 | Water, WSLH |
| Rapidly growing mycobacteria | |
| M. phlei ATCC11758 | TMC1458, type strain |
| M. smegmatis ATCC14468 | TMC1546, suggested neotype |
| M. smegmatis mc2155f | Transformably competent isolate of mc26 |
| Other bacterial speciesg | |
| E. coli ATCC 25922 | Clinical isolate, FDA strain |
| E. faecalis ATCC 29212 | Human urine |
JTC, Johne's Testing Center, Madison, WI.
Saleh Naser, University of Central Florida (UCF), Orlando, FL.
Trudeau Mycobacterial Culture Collection.
Obtained from A. M. Talaat (University of Wisconsin-Madison; originally from Raul Barletta at the University of Nebraska).
WSHL, Wisconsin State Hygiene Laboratory.
Obtained from the American Type Culture Collection.
Quality control strains used in antibiotic susceptibility testing.
Three representatives of rapidly growing mycobacteria were tested: Mycobacterium phlei ATCC 11758, Mycobacterium smegmatis ATCC 14468, and M. smegmatis mc2155. Two nonmycobacterial strains, Escherichia coli ATCC 25922 and Enterococcus faecalis ATCC 29212, were included as commonly used drug susceptibility control organisms (Table 1).
The identity of all mycobacteria was verified by multiplex PCR for insertion elements IS900, IS901, IS1311, and IS1245 (Johne's Testing Center, Madison, WI) as well as by high-pressure liquid chromatography (HPLC) of cell wall mycolic acids by a reference laboratory (Wisconsin State Laboratory of Hygiene, Madison, WI).
Single-cell suspensions of each strain were prepared as previously described with slight modifications (41). Tenfold serial dilutions from seed lots of each strain were plated on 7H10 agar, and single colonies were inoculated into MGIT ParaTB tubes (Becton Dickinson, Sparks, MD) to quantify the number of organisms per ml (38). Seed lots of each strain were then kept in small aliquots at −80°C until use. After plate counting, 104 to 106 CFU of each strain were inoculated to tubes of MGIT ParaTB medium to determine the average time to detection (TTD) in the absence of drugs. For M. paratuberculosis in MGIT ParaTB medium, the TTD is directly related to the number of organisms inoculated into each tube (38).
Drugs tested.
All test drugs were obtained from Sigma-Aldrich Co., St. Louis, MO, in a chemically pure form (purity > 99.0%). The drugs were SS, 5-aminosalicylic acid (5-ASA), sulfapyridine (SP), rifampin (RIF), nalidixic acid (NAL), 6-MP, AZA, azithromycin (AZM), and ciprofloxacin (CIP). Lyophilized drugs were dissolved in appropriate diluents according to the manufacturer's instructions. The drugs SS, 5-ASA, SP, 6-MP, and AZA were dissolved in 0.05 M NaOH in 10 mM phosphate-buffered saline (PBS), RIF and NAL were dissolved in 10 mM PBS (pH 7.2), AZM was dissolved in 50% ethanol, and CIP was dissolved in 0.5 N HCl in 10 mM PBS. All stock drug solutions were freshly prepared for each experiment and filter sterilized using a 0.22-μm polycarbonate syringe filter (Millipore Corp., Bedford, MA).
MGIT 960 drug susceptibility testing.
Methods published for M. tuberculosis and M. avium susceptibility testing (1, 2, 20, 22, 31, 34) were adapted as closely as possible; however, the significantly longer generation time of M. paratuberculosis compared to M. tuberculosis made this challenging. For this reason, we performed drug susceptibility studies in three phases. First, we evaluated our MGIT 960 antibacterial susceptibility testing method for M. paratuberculosis ATCC 19698 by comparing MGIT 960 results with those of conventional agar plate methods using drugs well characterized for antimycobacterial activity (phase I). Second, we evaluated the effects of thiopurine drugs and 5-aminosalicylates on M. paratuberculosis and M. avium growth in MGIT ParaTB medium in comparison to conventional bactericidal antibiotics (phase II). Next, we tested the reproducibility of the findings and expanded the study to include more strains and mycobacterial species (phase III). Lastly, we tested the effect of a combination of CIP and 6-MP over a range of achievable concentrations in tissue on the viability of M. paratuberculosis (phase IV). The methods for each of the four phases of study were as follows.
(i) Phase I.
A single type strain of M. paratuberculosis, ATCC 19698, was tested at three MGIT ParaTB medium inoculum levels (104, 105, and 106 CFU per MGIT ParaTB tube). The final concentrations of each drug tested ranged from 1.0 to 64.0 μg/ml.
(ii) Phase II.
Phase II, designed based on the results of phase I, used seven clinical strains of M. paratuberculosis of bovine or human origin and one M. avium strain, ATCC 35712, as a control. Some drugs to which M. paratuberculosis was not susceptible in phase I, specifically the SS drug family (SS, 5-ASA, and SP), were excluded, and AZA, a prodrug of 6-MP, was added to the trial (19, 27).
(iii) Phase III.
The reproducibility of findings in phase II was determined. Additional strains of M. paratuberculosis also were tested, along with three other species of mycobacteria. The thiopurine drug concentrations tested were the same as in phase II, but one higher concentration (128.0 μg/ml) was added for some strains (see Table 4).
TABLE 4.
Lowest concentrations of two thiopurine drugs to inhibita and killb strains of M. paratuberculosis, M. avium, M. smegmatis, and M. phlei
| Bacterial strain | Lowest concn of:
|
|||
|---|---|---|---|---|
| 6-MP for:
|
AZA for:
|
|||
| Inhibition | Killing | Inhibition | Killing | |
| M. paratuberculosis | ||||
| ATCC 19698 | 1.0 | 16.0 | 2.0 | 16.0 |
| UCF-3 | <1.0 | 8.0 | 2.0 | 32.0 |
| UCF-4 | 2.0 | >32.0 | 4.0 | >32.0 |
| UCF-5 | 1.0 | >32.0 | 2.0 | >32.0 |
| UCF-7 | <1.0 | 8.0 | 2.0 | 16.0 |
| UCF-8 | <1.0 | 16.0 | 2.0 | 32.0 |
| B213 | 2.0 | >32.0 | 4.0 | >32.0 |
| B236 | 4.0 | >32.0 | 4.0 | >32.0 |
| B238 | 2.0 | >32.0 | 4.0 | >32.0 |
| B244 | 4.0 | >32.0 | 8.0 | >32.0 |
| M. avium | ||||
| ATCC 35712 | >64.0 | >64.0 | >64.0 | >64.0 |
| ATCC 25291 | >64.0 | >64.0 | >64.0 | >64.0 |
| 104 | >64.0 | >64.0 | >64.0 | >64.0 |
| JTC4862 | 32.0 | >64.0 | 64.0 | >64.0 |
| JTC981 | >64.0 | >64.0 | >64.0 | >64.0 |
| EPA3 | 16.0 | >64.0 | 32.0 | >64.0 |
| WSLH1544 | 8.0 | >64.0 | 32.0 | >64.0 |
| Rapid growing mycobacteria | ||||
| M. phlei ATCC 11758 | 1.0 | 16.0 | 2.0 | 32.0 |
| M. smegmatis ATCC 14468 | >128.0 | >128.0 | >128.0 | >128.0 |
| M. smegmatis mc2155 | >128.0 | >128.0 | >128.0 | >128.0 |
| Nonmycobacterial controls | ||||
| E. coli ATCC 25922 | >128.0 | >128.0 | >128.0 | >128.0 |
| E. faecalis ATCC 29212 | >128.0 | >128.0 | >128.0 | >128.0 |
The lowest drug concentration producing a TTD greater than that for the 1:100 inoculum dilution control tube.
Signal negative in the BACTEC MGIT 960 instrument for up to 56 days postinoculation.
(iv) Phase IV.
The effect of combined CIP and 6-MP on M. paratuberculosis strain UCF-7 viability was determined over a range of concentrations (0, 2, 4, and 6 μg/ml) and drug exposure times (0, 3, 6, 9, and 12 days). The results for this drug combination for M. paratuberculosis counts were compared to those for each drug individually, the drug-free control, and the 1% (1:100 original inoculum dilution) drug-free control.
MGIT 960 methodology details.
Serial dilutions of single-cell suspensions of each mycobacterial strain were prepared, and 100 μl was inoculated into MGIT ParaTB medium (Becton Dickinson, Sparks, MD). Each tube contained 7 ml of medium and a fluorescent indicator embedded in silicone on the bottom of the tube. To each tube was added 800 μl of MGIT ParaTB supplement (Becton Dickinson, Sparks, MD), 500 μl of egg yolk suspension (Becton Dickinson, Sparks, MD), and 100 μl of test drug, resulting in final concentrations of 0.5 to 64.0 μg/ml. Tubes were incubated at 37°C in a MGIT 960 instrument and removed when the instrument signaled them as being positive. Samples from all signal-positive tubes were subcultured on Trypticase soy agar plates with 5% sheep blood (Becton Dickinson, Sparks, MD) to check for contamination. Acid-fast staining (Ziehl-Neelsen) was also performed on smears made from each signal-positive tube to confirm the presence of mycobacteria. For E. coli and E. faecalis, the standard broth microdilution method was used for susceptibility tests (8).
Interpretation of susceptibility results.
There are no interpretive criteria of MGIT 960 algorithms for antibacterial susceptibility test interpretation for mycobacteria other than M. tuberculosis and M. avium. We used a similar interpretation system with slight modifications (1, 2, 22, 33, 45). Briefly, positive control MGIT ParaTB tubes were inoculated with only the test organism and the relevant drug solvent (solvent control). For comparison, these drug-free solvent control vials were inoculated with a 1:100 dilution of the normal organism inoculum (designed to represent growth of 1% of the original bacterial population). All MGIT ParaTB medium tubes were inoculated in duplicate with specified numbers of test organisms. In phase I trials, three M. paratuberculosis inoculum levels were tested (104, 105, and 106 CFU), and in phases II, III, and IV only 105 to 106 CFU were inoculated to each MGIT ParaTB tube.
The bacterial growth rate in each MGIT ParaTB tube was defined by TTD, i.e., the number of days the MGIT ParaTB tubes were incubated until they were determined to be signal positive by the MGIT 960 instrument (38). The baseline for M. paratuberculosis inhibition was defined as the day that the 1:100 dilution inoculum control became signal positive. Thus the lowest concentration for each test drug that inhibited growth was the concentration that produced a TTD greater than that of the 1:100 dilution control tube. The minimum concentrations of each drug that completely suppressed growth of the test organism in the MGIT 960 system, i.e., the tubes were never signal positive by the end of the experiment at 56 days postinoculation, were also reported. For MGIT ParaTB tubes inoculated with 106 CFU M. paratuberculosis, this usually occurred between days 5 and 7 of incubation.
If the positive control tube became signal positive earlier than incubation day 4, the M. paratuberculosis inoculum was considered too high. Similarly, if the 1:100 dilution inoculum control tube did not become signal positive within 8 days after the positive control, the tube was considered underinoculated. If either control tube criterion was not met, the test was considered invalid and the test was repeated. An uninoculated MGIT ParaTB tube was used as the negative control for every trial.
Agar plate counting method.
Agar plate counting methods for drug susceptibility testing were performed with 7H10 medium supplemented with 10% OADC (Becton Dickinson, Sparks, MD) and 2 μg/ml of mycobactin J (Allied Monitor, Fayette, MO) for phase I, phase III, and phase IV trials (26). At selected times after exposure to drugs, surviving M. paratuberculosis cells were quantified by conventional plate counting for all controls and every drug concentration for the 106-CFU M. paratuberculosis inoculum in phase I. In phase III, plate counts were done for only two concentrations of each drug, 2.0 μg/ml (low concentration) and 16.0 μg/ml (high concentration).
Bacteria were mixed with each specific drug and drug concentration in MGIT ParaTB medium and incubated at 37°C. At 3 and 5 days postinoculation an aliquot (0.1 ml) was removed for plate count determinations (note that MGIT 960 instrument readings were not taken on these tubes; the tubes merely provided the medium in which the bacterium-drug interaction took place). Bacterial cells were harvested from MGIT tubes by centrifugation, resuspended in PBS, and homogenized to break up bacterial cell clumps by vortexing with glass beads (30). Then, 10-fold serial dilutions (100 to 10−6) were made in PBS, and 100 μl of each dilution was plated in quadruplicate on 7H10 agar supplemented 10% OADC (Becton Dickinson, Sparks, MD) and 2 μg/ml of mycobactin J (Allied Monitor, Fayette, MO). The numbers of CFU were determined by visual inspection after incubation of plates at 37°C for 8 weeks. The lowest concentration of each drug tested by the agar counting method that inhibited growth was defined as the lowest concentration of the drug that produced a 99% (2 log10) reduction in CFU. These concentrations were compared to those determined by MGIT 960 drug susceptibility testing.
Quality control.
M. paratuberculosis strain ATCC 19698 was included in all experiments and used to test the ability of each new lot of MGIT ParaTB medium, growth supplement, egg yolk, and mycobatin J to support M. paratuberculosis growth. Also, E. coli ATCC 25922 and E. faecalis ATCC 29212 were used as controls to assure drug efficacy, as these agents have predictable antibiotic susceptibility patterns. Solvent controls were used with each drug to distinguish anti-M. paratuberculosis activity due to solvents themselves from the activity of the drugs, and uninoculated MGIT ParaTB medium controls served to detect any contamination by extraneous organisms.
RESULTS
Phase I.
Inoculum size is a critical factor in drug susceptibility testing of slowly growing mycobacteria (31). Three M. paratuberculosis ATCC 19698 inocula initially tested were 104, 105, and 106 CFU/tube. The average TTDs for inoculum controls (drug solvent only) were 4.97, 7.89, and 10.76 days for 106, 105, and 104 CFU/tube, respectively. The 1:100 dilution inoculum controls became signal positive at 10.76, 14.32, 21.74 days on average, respectively. The drug solvents had no effect on TTD if >102 CFU M. paratuberculosis were inoculated (multiple comparison test, P = 0.83; data not shown). However, the 104-CFU inoculum was excluded from subsequent analyses because of the long incubation time required for the 1:100 dilution control to become signal positive, especially in the face of drug solvents, notably 50% ethanol.
When the M. paratuberculosis inoculum was ≥105 CFU/tube, there was good agreement between MGIT 960- and agar plate-determined drug effects, with previously reported mycobacterium MIC ranges for all drugs tested (Table 2). An M. paratuberculosis inoculum amount of 105 versus 106 CFU did not affect the drug effects for any compound with the exception of RIF (data not shown). While the concentration of RIF needed to inhibit M. paratuberculosis was low, the organism was able to grow to some extent even in the face of the highest RIF concentration tested, 64.0 μg/ml.
TABLE 2.
Tested drugs in phase I experiment and their effect on M. paratuberculosis ATCC 19698, as determined by MGIT 960a and agar plate counting methods
| Drug | Drug family/type | Lowest concn (μg/ml) producing inhibition in comparison with 1:100 dilution control by:
|
Minimum drug concn resulting in no detectable growth of M. paratuberculosisb | |
|---|---|---|---|---|
| MGIT 960 | Agar counting method | |||
| AZM | Macrolide | 4.0 | 4.0 | 16.0 |
| CIP | Quinolone | 1.0 | 1.0 | 32.0 |
| NAL | Quinolone | >64.0 | >64.0 | >64.0 |
| RIF | First-line tuberculosis drug | 2.0 | 2.0 | >64.0 |
| SP | Sulfonamide | >64.0 | >64.0 | >64.0 |
| SS | Sulfonamide | >64.0 | >64.0 | >64.0 |
| 5-ASA | Anti-inflammatory | >64.0 | >64.0 | >64.0 |
| 6-MP | Anti-inflammatory | 1.0 | 1.0 | 32.0 |
Each tube was inoculated with 106 CFU bacteria.
Signal negative by the BACTEC MGIT 960 instrument up to 56 days postinoculation.
Growth inhibition was seen at the lowest concentration with CIP (1.0 μg/ml). For AZM the lowest growth-inhibitory concentration was 4.0 μg/ml, and M. paratuberculosis was highly resistant to NAL (inhibitory concentration > 64.0 μg/ml) (Table 2).
Among the immunomodulatory drugs, the SS drug family (SS, 5-ASA, and SP) showed no significant anti-M. paratuberculosis activity even at high concentrations (64.0 μg/ml). Unexpectedly, 6-MP showed anti-M. paratuberculosis activity similar in potency to that of CIP (lowest inhibitory concentration = 1.0 μg/ml) by the MGIT 960 drug susceptibility test (Table 2).
Phase II.
The susceptibility of M. paratuberculosis to 6-MP was confirmed, and similar results were found for its precursor drug, AZA (Table 3). Across the seven M. paratuberculosis strains tested, growth inhibition for 6-MP was comparable to that found for CIP in concentrations ranging from 1.0 to 4.0 μg/ml. It required twofold-larger amounts of AZA to inhibit growth, with concentrations ranging from 2.0 to 8.0 μg/ml. Mycobacterium avium (ATCC 35712) was resistant to both thiopurine drugs, even at the 64.0 μg/ml (Table 3).
TABLE 3.
Lowest concentration to inhibita and concentration to killb seven M. paratuberculosis strains and one M. avium type strain by the MGIT 960 drug susceptibility test
| Drug | Concn (μg/ml) required to inhibit (kill)
|
|||||||
|---|---|---|---|---|---|---|---|---|
|
M. paratuberculosis strain:
|
M. avium ATCC 35712 | |||||||
| JTC303 | UCF-4 | UCF-5 | UCF-7 | B213 | B236 | B238 | ||
| 6-MP | 1.0 (32.0) | 2.0 (>32.0) | 1.0 (>32.0) | <1.0 (8.0) | 4.0 (>32.0) | 4.0 (>32.0) | 4.0 (>32.0) | >64.0 |
| AZA | 2.0 (>32.0) | 4.0 (>32.0) | 2.0 (>32.0) | 1.0 (16.0) | 8.0 (>32.0) | 8.0 (>32.0) | 16.0 (>32.0) | >64.0 |
| AZM | 1.0 (4.0) | 1.0 (2.0) | 0.5 (2.0) | 0.5 (1.0) | 1.0 (2.0) | 0.5 (4.0) | >0.5 (1.0) | 1.0 (16.0) |
| CIP | 2.0 (8.0) | 1.0 (4.0) | 2.0 (4.0) | 1.0 (4.0) | 4.0 (16.0) | 4.0 (16.0) | 4.0 (16.0) | 16.0 (64.0) |
The lowest drug concentration producing a TTD greater than that for the 1:100 inoculum dilution control tube.
Signal negative by the BACTEC MGIT 960 instrument for up to 56 days postinoculation.
In general, M. paratuberculosis isolates originating from Crohn's disease patients were more susceptible to thiopurine drugs than were bovine-origin isolates among tested strains. The patterns of drug susceptibility to CIP were the same regardless of isolate origin. One human isolate, UCF-7, failed to grow after 56 days of incubation in MGIT ParaTB medium containing 8.0 μg/ml 6-MP or 16.0 μg/ml AZA, while the growth of the other strains was eventually detected even in the presence of 32.0 μg/ml 6-MP or AZA.
Phase III.
Phase III verified the susceptibility of M. paratuberculosis to thiopurine drugs and showed that the effect was relatively specific for this mycobacterial species. Both 6-MP and AZA showed a stronger in vitro antimicrobial activity against 11 M. paratuberculosis strains than 7 M. avium strains; however growth inhibition patterns for thiopurine drugs against M. avium strains varied widely. The mean 6-MP concentrations for M. paratuberculosis growth inhibition ranged from <1.0 to 4.0 μg/ml, while those for M. avium ranged 8.0 to >128.0 μg/ml. Among M. avium strains, lower concentrations of the test compounds were needed to inhibit growth for isolates originating from water compared to clinical samples. The 6-MP initial inhibition range for human-origin M. paratuberculosis was <1.0 to 2.0 μg/ml, while for bovine-origin strains it ranged from 2.0 to 4.0 μg/ml. Again, 6-MP was found to inhibit M. paratuberculosis growth at one-half the concentration of AZA regardless of M. paratuberculosis strain origin. This finding was observed in other susceptible mycobacteria as well. No growth in MGIT ParaTB medium with 8.0 to 16.0 μg/ml of 6-MP was observed for three of the five Crohn's disease patient M. paratuberculosis isolates by 56 days, while growth of all M. avium strains was detected within 15 days at these same drug concentrations (Table 4).
The mycobacterial control M. phlei was as susceptible to thiopurine drugs as M. paratuberculosis, but M. smegmatis growth was completely resistant at 128 μg/ml of both thiopurine drugs. No antibacterial activity of thiopurine drugs against E. coli or E. faecalis was found, even at the highest concentration tested (128.0 μg/ml) (Table 4).
Comparison of drug actions against M. paratuberculosis.
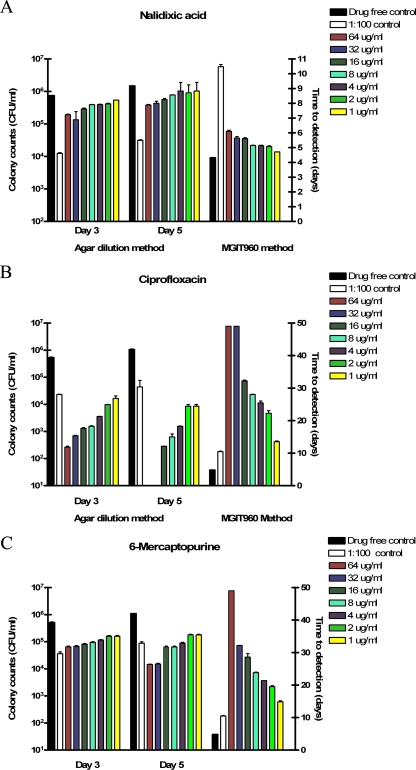
The drugs studied were either bacteriostatic or bactericidal or had no effect on M. paratuberculosis. Those with no effect, as evidenced by both plate count and MGIT 960 TTD data after either 3 or 5 days of bacterial exposure to the drugs, included the sulfonamide family (SS, SP, and 5-ASA; data not shown) and NAL (Fig. 1A).
FIG. 1.
Inhibition of M. paratuberculosis ATCC 19698 growth by exposure to NAL, CIP, and 6-MP at 1 to 64 μg/ml for 3 or 5 days as determined by agar plate counts, and the impact of the same drug concentrations on TTD in MGIT ParaTB medium, as monitored by the BACTEC MGIT 960 instrument.
Antimicrobial drugs AZM, CIP, and RIF were bactericidal for M. paratuberculosis. The minimum concentration for each drug needed to kill the organism was defined as the drug concentration producing fewer CFU than the non-drug-containing 1:100 inoculum dilution control (Fig. 1A and B). Killing concentrations for AZM and CIP by plate counting and MGIT methods were in agreement. In the presence of RIF at lower doses, viable M. paratuberculosis numbers initially declined but then the organism resumed growth (Fig. 2C). Bovine- and human-origin M. paratuberculosis strains were similarly affected by the compounds; M. paratuberculosis was more susceptible to these antibiotics than was the control M. avium strain tested (Table 3). The concentration of CIP required for complete M. paratuberculosis growth inhibition after up to 56 days of incubation in MGIT ParaTB medium for human isolates was twofold lower than that for bovine isolates, i.e., 4.0 μg/ml and 16.0 μg/ml, respectively.
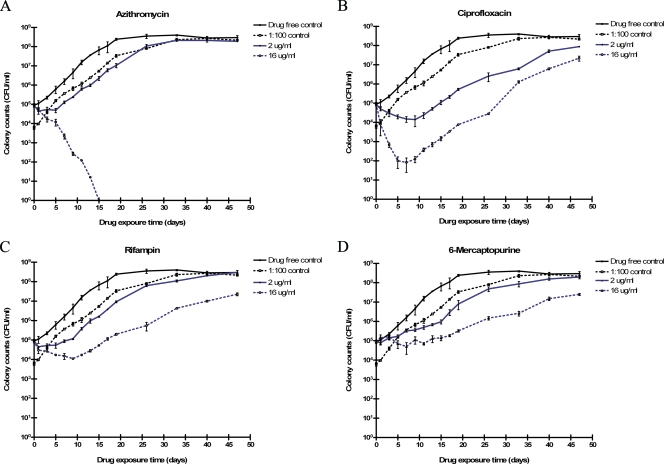
FIG. 2.
Effect of AZM (A), CIP (B), RIF (C), and 6-MP (D) on the viability of M. paratuberculosis ATCC 19698 over time (up to 56 days), as determined by standard plate counts (CFU).
Thiopurine drugs AZA and 6-MP inhibited M. paratuberculosis growth in a dose-dependent fashion. Plate count CFU were lower than the number obtained by the MGIT 960 culture counting method but generally higher than that for the 1:100 dilution control after both 3 and 5 days of M. paratuberculosis exposure to the drugs (Fig. 1C). The effect of these drugs appeared more profound by the MGIT 960 drug susceptibility method, where there was continuous contact of the drug with M. paratuberculosis in broth, i.e., the TTD was greater than that for the 1:100 inoculum dilution control for all concentrations of drug tested (≥1.0 μg/ml). MGIT 960 analysis also showed a direct relationship between AZA or 6-MP concentration and suppression of M. paratuberculosis growth, i.e., longer TTD.
Mycobacterial species specificity for thiopurine drugs.
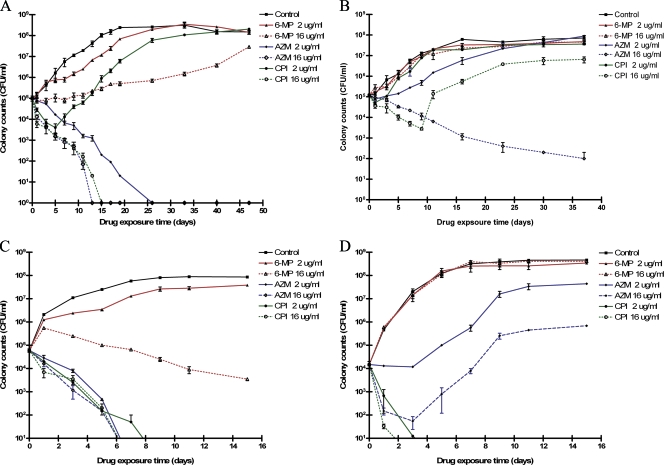
Not all mycobacterial species were affected by thiopurine drugs to the same extent: M. paratuberculosis and M. phlei were susceptible to growth inhibition effects, but M. avium and M. smegmatis were not (Fig. 3).
FIG. 3.
Effect of AZM, CIP, and 6-MP on the viability of M. paratuberculosis strain UCF-5 (A), M. avium ATCC 35712 (B), M. phlei ATCC 11758 (C), and M. smegmatis mc2155 (D) over time (up to 56 days), as determined by agar plate counts (CFU).
Phase IV.
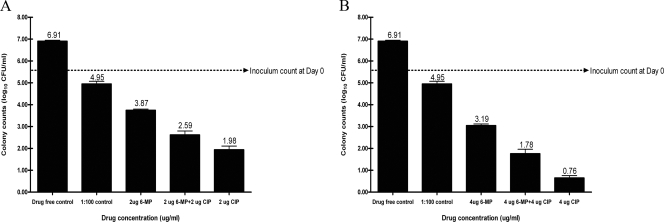
Exposure to ≥2 μg/ml 6-MP resulted in lower M. paratuberculosis CFU than the CFU in the 1:100 inoculum dilution control at every drug exposure time beyond 6 days. Simultaneous exposure of M. paratuberculosis to 6-MP and CIP at the same concentrations resulted in significantly higher CFU than use of CIP alone (Fig. 4).
FIG. 4.
Effect of CIP, 6-MP, and the combination of the two drugs both at 2 μg/ml (A) and 4 μg/ml (B) on the viability of M. paratuberculosis strain UCF-7 after 12 days of drug exposure as determined by MGIT 960 counting methods (38).
DISCUSSION
Results for the antimicrobial susceptibility of M. paratuberculosis to standard drugs were consistent between plate counting and MGIT 960 methods (Fig. 1). When growth-suppressive effects were observed, they were drug concentration dependent. Additionally, the results were consistent with those previously reported (31, 45).
Immunosuppressive drugs in the sulfonamide family, SS, 5-AZA, and SP, had no effect on M. paratuberculosis growth, even at 64 μg/ml (Table 2). NAL was tolerated by M. paratuberculosis up to the maximum concentration tested, 64.0 μg/ml, supporting its use in primary M. paratuberculosis culture media for suppression of contaminating microflora (Table 1 and Fig. 1).
AZM was bactericidal for M. paratuberculosis at concentrations of 4.0 to 16.0 μg/ml (Fig. 2A). These findings are in agreement with reports of improved clinical status of Crohn's disease patients treated with macrolides (14). CIP, by contrast, killed 1 to 3 log10 of M. paratuberculosis ATCC 19698 cells over the first 5 to 8 days, and then a seemingly drug-resistant population of cells resumed growth at rates comparable to the that for the drug-free controls (Fig. 2B). (An alternative explanation for these results is that by day 8 the CIP was no longer active, and future studies should evaluate residual drug activity.) Human-origin strains were more susceptible to CIP, with complete killing of 105 CFU M. paratuberculosis in 12 to 26 days (Fig. 3A). RIF produced an M. paratuberculosis growth pattern similar to that produced by CIP, i.e., transitory decline in CFU followed by regrowth (Fig. 2B and C).
AZA drugs killed M. paratuberculosis. The thiopurine drug 6-MP suppressed its growth more than did AZA at the same concentrations (μg/ml). If MGIT drug susceptibility standards for M. tuberculosis were used to interpret the data for 6-MP versus M. paratuberculosis, growth was suppressed at ≥2.0 μg/ml (potentially the MIC) compared with the 1:100 drug-free growth control (Fig. 2D). However, growth of M. paratuberculosis was not completely stopped, even at 16 μg/ml 6-MP, but was simply slowed in comparison to that of drug-free controls. Human-origin (Crohn's disease patient) isolates of M. paratuberculosis tended to be more susceptible to 6-MP than were bovine-origin isolates (Table 4). These findings are comparable to those reported by Greenstein et al. using the BACTEC 460 system for M. paratuberculosis in in vitro susceptibility studies (13, 45).
The growth-suppressive effects of 6-MP differed by mycobacterial species. These data suggest a possible mycobacterial-species-specific mechanism by which 6-MP interferes with replication or metabolism.
The antimycobacterial effect of thiopurine drugs is a novel and unexpected observation. The implications of the present study for the possible etiologic role of M. paratuberculosis in Crohn's disease and approaches to Crohn's disease therapy are important. Some investigators describe the failure of M. paratuberculosis to multiply in the face of immunosuppressive therapy with thiopurine drugs as evidence that Crohn's disease cannot be caused by M. paratuberculosis (35). Perhaps this pathogen, found in resected bowel tissue and peripheral blood leukocytes of some Crohn's disease patients (6, 25, 36), is held in check by thiopurine therapy since it has both immunosuppressive and anti-M. paratuberculosis activity.
These data also offer another perspective on data from clinical trials with Crohn's disease patients using antimycobacterial drugs. Since patients in the “control” group are maintained on standard therapy (which commonly employs thiopurine drugs), both the treatment group (antimycobacterial drugs) and control group (no antimycobacterial drugs but continued thiopurine drugs) are exposed to compounds with anti-M. paratuberculosis activity. If M. paratuberculosis is integral to Crohn's disease, then the opportunity to observe clear-cut therapeutic differences between the treatment and control groups in these trials is limited.
Antimycobacterial and thiopurine drugs used in concert may produce an interactive effect. The apparently bacteriostatic effects of 6-MP on M. paratuberculosis rendered the organism less susceptible to the bactericidal effects of CIP. This further complicates interpretation of many of the prior clinical trials with Crohn's disease patients that employed antimicrobials in addition to immunosuppressive drugs. Given the potential side effects of these medications, pursuit of therapeutic trials with patients in the absence of sound in vitro data is both premature and inappropriate.
A major challenge for antimicrobial susceptibility studies of M. paratuberculosis is its long generation time, i.e., roughly 2 days (24), and uncertainty about the stability of the test drugs in MGIT ParaTB medium at 37°C over the course of incubation. Without accepted standards for M. paratuberculosis antimicrobial susceptibility testing, it was vital that multiple methods and controls be employed and that results be descriptive and interpreted in relative rather than absolute terms such as “susceptible” or “resistant.” Multiple mycobacterial species as well as nonmycobacterial species were needed as antibiotic susceptibility quality control standards. We also assessed whether drugs had an inhibitory or lethal effect on target organisms by subculture to drug-free media. More-extensive in vitro drug susceptibility trials with M. paratuberculosis are required to establish which drugs are most efficacious and which drugs, when used in combination, have a modulated effect on M. paratuberculosis. While the results of this research are provocative, expanded studies should include an assessment of the stability and activity of antimicrobial drugs in MGIT ParaTB medium at 37°C over the extended incubation period required for M. paratuberculosis drug susceptibility testing.
This work is hypothesis generating, not definitive. Methodological issues, in particular, that the mycobacteria were tested in an extracellular location, and the limited number of strains of each mycobacterial species tested are among just some of the caveats regarding extension of these findings to the clinical situation. However, this work highlights the complexity of studying M. paratuberculosis interactions with antibiotics and the possibility that anti-inflammatory drugs may exert antibacterial effects directly on this organism and/or have negative interactions with conventional antimicrobial drugs. These caveats argue against simple adoption of drug susceptibility testing methods used for M. tuberculosis or M. avium and for more-comprehensive characterization of the bactericidal or growth-inhibitory effects of antimicrobial and anti-inflammatory drugs alone and in combination on M. paratuberculosis.
Acknowledgments
This work was funded by the Johne's Testing Center, School of Veterinary Medicine, University of Wisconsin-Madison.
We are grateful for the donation of both human and bovine strains of M. paratuberculosis by S. Naser, University of Central Florida, the technical help of Seth Kramer, and the manuscript editorial assistance of E. Manning. We also acknowledge the stimulus of Robert Greenstein for our pursuit of this line of investigation.
Footnotes
Published ahead of print on 10 December 2007.
REFERENCES
- 1.Adjers-Koskela, K., and M. L. Katila. 2003. Susceptibility testing with the manual mycobacteria growth indicator tube (MGIT) and the MGIT 960 system provides rapid and reliable verification of multidrug-resistant tuberculosis. J. Clin. Microbiol. 41:1235-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ardito, F., B. Posteraro, M. Sanguinetti, S. Zanetti, and G. Fadda. 2001. Evaluation of BACTEC mycobacteria growth indicator tube (MGIT 960) automated system for drug susceptibility testing of Mycobacterium tuberculosis. J. Clin. Microbiol. 39:4440-4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayele, W. Y., P. Svastova, P. Roubal, M. Bartos, and I. Pavlik. 2005. Mycobacterium avium subspecies paratuberculosis cultured from locally and commercially pasteurized cow's milk in the Czech Republic. Appl. Environ. Microbiol. 71:1210-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bannantine, J. P., R. G. Barletta, J. R. Stabel, M. L. Paustian, and V. Kapur. 2004. Application of the genome sequence to address concerns that Mycobacterium avium subspecies paratuberculosis might be a foodborne pathogen. Foodborne Pathog. Dis. 1:3-15. [DOI] [PubMed] [Google Scholar]
- 5.Biet, F., M. L. Boschiroli, M. F. Thorel, and L. A. Guilloteau. 2005. Zoonotic aspects of Mycobacterium bovis and Mycobacterium avium-intracellulare complex (MAC). Vet. Res. 36:411-436. [DOI] [PubMed] [Google Scholar]
- 6.Bull., T. J., E. J. McMinn, K. Sidi-Boumedine, A. Skull, D. Durkin, P. Neild, G. Rhodes, R. Pickup, and J. Hermon-Taylor. 2003. Detection and verification of Mycobacterium avium subsp. paratuberculosis in fresh ileocolonic mucosal biopsy specimens from individuals with and without Crohn's disease. J. Clin. Microbiol. 41:2915-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chamberlin, W. M., and S. A. Naser. 2006. Integrating theories of the etiology of Crohn's disease. On the etiology of Crohn's disease: questioning the hypotheses. Med. Sci. Monit. 12:RA27-RA33. [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial susceptibility testing; 15th informational supplement. Document M100-S15. Clinical and Laboratory Standards Institute, Wayne, PA.
- 9.Collins, M. T., G. Lisby, C. Moser, D. Chicks, S. Christensen, M. Reichelderfer, N. Høiby, B. A. Harms, O. O. Thomsen, U. Skibsted, and V. Binder. 2000. Results of multiple diagnostic tests for Mycobacterium avium subsp. paratuberculosis in patients with inflammatory bowel disease and in controls. J. Clin. Microbiol. 38:4373-4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duerr, R. H., K. D. Taylor, S. R. Brant, J. D. Rioux, M. S. Silverberg, M. J. Daly, A. H. Steinhart, C. Abraham, M. Regueiro, A. Griffiths, T. Dassopoulos, A. Bitton, H. Yang, S. Targan, L. W. Datta, E. O. Kistner, L. P. Schumm, A. T. Lee, P. K. Gregersen, M. M. Barmada, J. I. Rotter, D. L. Nicolae, and J. H. Cho. 2006. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 314:1461-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fireman, Z., A. Grossman, P. Lilos, Y. Eshchar, E. Theodor, and T. Gilat. 1989. Epidemiology of Crohn's disease in the Jewish population of central Israel, 1970-1980. Am. J. Gastroenterol. 84:255-258. [PubMed] [Google Scholar]
- 12.Ghadiali, A. H., M. Strother, S. A. Naser, E. J. Manning, and S. Sreevatsan. 2004. Mycobacterium avium subsp. paratuberculosis strains isolated from Crohn's disease patients and animal species exhibit similar polymorphic locus patterns. J. Clin. Microbiol. 42:5345-5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenstein, R. J., L. Su, V. Haroutunian, A. Shahidi, and S. T. Brown. 2007. On the action of methotrexate and 6-mercaptopurine on M. avium subspecies paratuberculosis. PLoS ONE 2:e161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gui, G. P., P. R. Thomas, M. L. Tizard, J. Lake, J. D. Sanderson, and J. Hermon-Taylor. 1997. Two-year-outcomes analysis of Crohn's disease treated with rifabutin and macrolide antibiotics. J. Antimicrob. Chemother. 39:393-400. [DOI] [PubMed] [Google Scholar]
- 15.Hampe, J., A. Cuthbert, P. J. Croucher, M. M. Mirza, S. Mascheretti, S. Fisher, H. Frenzel, K. King, A. Hasselmeyer, A. J. MacPherson, S. Bridger, S. van Devanter, A. Forbes, S. Nikolaus, J. E. Lennard-Jones, U. R. Foelsch, M. Krawczak, C. Lewis, S. Schreiber, and C. G. Mathew. 2001. Association between insertion mutation in NOD2 gene and Crohn's disease in German and British populations. Lancet 357:1925-1928. [DOI] [PubMed] [Google Scholar]
- 16.Hampe, J., A. Franke, P. Rosenstiel, A. Till, M. Teuber, K. Huse, M. Albrecht, G. Mayr, L. De, V., J. Briggs, S. Gunther, N. J. Prescott, C. M. Onnie, R. Hasler, B. Sipos, U. R. Folsch, T. Lengauer, M. Platzer, C. G. Mathew, M. Krawczak, and S. Schreiber. 2007. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat. Genet. 39:207-211. [DOI] [PubMed] [Google Scholar]
- 17.Hanauer, S. B. 2005. The case for using 5-aminosalicyclates in Crohn's disease: pro. Inflamm. Bowel Dis. 11:609-612. [DOI] [PubMed] [Google Scholar]
- 18.Harris, N. B., and R. G. Barletta. 2001. Mycobacterium avium subsp. paratuberculosis in veterinary medicine. Clin. Microbiol. Rev. 14:489-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hindorf, U., M. Lindqvist, C. Peterson, P. Soderkvist, M. Strom, H. Hjortswang, A. Pousette, and S. Almer. 2006. Pharmacogenetics during standardised initiation of thiopurine treatment in inflammatory bowel disease. Gut 55:1423-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang, T. S., H. Z. Tu, S. S. Lee, W. K. Huang, and Y. C. Liu. 2002. Antimicrobial susceptibility testing of Mycobacterium tuberculosis to first-line drugs: comparisons of the MGIT 960 and BACTEC 460 systems. Ann. Clin. Lab. Sci. 32:142-147. [PubMed] [Google Scholar]
- 21.Hugot, J. P., M. Chamaillard, H. Zouali, S. Lesage, J. P. Cezard, J. Belaiche, S. Almer, C. Tysk, C. A. O'Morain, M. Gassull, V. Binder, Y. Finkel, A. Cortot, R. Modigliani, P. Laurent-Puig, C. Gower-Rousseau, J. Macry, J. F. Colombel, M. Sahbatou, and G. Thomas. 2001. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature 411:599-603. [DOI] [PubMed] [Google Scholar]
- 22.Krüüner, A., M. D. Yates, and F. A. Drobniewski. 2006. Evaluation of MGIT 960-based antimicrobial testing and determination of critical concentrations of first- and second-line antimicrobial drugs with drug-resistant clinical strains of Mycobacterium tuberculosis. J. Clin. Microbiol. 44:811-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kugathasan, S., R. H. Judd, R. G. Hoffmann, J. Heikenen, G. Telega, F. Khan, S. Weisdorf-Schindele, P. W. San, Jr., J. Perrault, R. Park, M. Yaffe, C. Brown, M. T. Rivera-Bennett, I. Halabi, A. Martinez, E. Blank, S. L. Werlin, C. D. Rudolph, and D. G. Binion. 2003. Epidemiologic and clinical characteristics of children with newly diagnosed inflammatory bowel disease in Wisconsin: a statewide population-based study. J. Pediatr. 143:525-531. [DOI] [PubMed] [Google Scholar]
- 24.Lambrecht, R. S., J. F. Carriere, and M. T. Collins. 1988. A model for analyzing growth kinetics of a slowly growing Mycobacterium spp. Appl. Environ. Microbiol. 54:910-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naser, S. A., G. Ghobrial, C. Romero, and J. F. Valentine. 2004. Culture of Mycobacterium avium subspecies paratuberculosis from the blood of patients with Crohn's disease. Lancet 364:1039-1044. [DOI] [PubMed] [Google Scholar]
- 26.National Committee for Clinical Laboratory Standards. 2003. Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes; approved standard. Document M24-A. National Committee for Clinical Laboratory Standards, Wayne, PA. [PubMed]
- 27.Nielsen, O. H., B. Vainer, and J. Rask-Madsen. 2001. Review article: the treatment of inflammatory bowel disease with 6-mercaptopurine or azathioprine. Aliment. Pharmacol. Ther. 15:1699-1708. [DOI] [PubMed] [Google Scholar]
- 28.Ogura, Y., D. K. Bonen, N. Inohara, D. L. Nicolae, F. F. Chen, R. Ramos, H. Britton, T. Moran, R. Karaliuskas, R. H. Duerr, J. P. Achkar, S. R. Brant, T. M. Bayless, B. S. Kirschner, S. B. Hanauer, G. Nunez, and J. H. Cho. 2001. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 411:603-606. [DOI] [PubMed] [Google Scholar]
- 29.Overduin, P., L. Schouls, P. Roholl, A. van der Zanden, N. Mahmmod, A. Herrewegh, and D. van Soolingen. 2004. Use of multilocus variable-number tandem-repeat analysis for typing Mycobacterium avium subsp. paratuberculosis. J. Clin. Microbiol. 42:5022-5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parrish, N. M., C. G. Ko, J. D. Dick, P. B. Jones, and J. L. Ellingson. 2004. Growth, Congo Red agar colony morphotypes and antibiotic susceptibility testing of Mycobacterium avium subspecies paratuberculosis. Clin. Med. Res. 2:107-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piersimoni, C., D. Nista, S. Bornigia, and G. De Sio. 1998. Evaluation of a new method for rapid drug susceptibility testing of Mycobacterium avium complex isolates by using the mycobacteria growth indicator tube. J. Clin. Microbiol. 36:64-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rioux, J. D., R. J. Xavier, K. D. Taylor, M. S. Silverberg, P. Goyette, A. Huett, T. Green, P. Kuballa, M. M. Barmada, L. W. Datta, Y. Y. Shugart, A. M. Griffiths, S. R. Targan, A. F. Ippoliti, E. J. Bernard, L. Mei, D. L. Nicolae, M. Regueiro, L. P. Schumm, A. H. Steinhart, J. I. Rotter, R. H. Duerr, J. H. Cho, M. J. Daly, and S. R. Brant. 2007. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat. Genet. 39:596-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rusch-Gerdes, S., G. E. Pfyffer, M. Casal, M. Chadwick, and S. Siddiqi. 2006. Multicenter laboratory validation of the BACTEC MGIT 960 technique for testing susceptibilities of Mycobacterium tuberculosis to classical second-line drugs and newer antimicrobials. J. Clin. Microbiol. 44:688-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanders, C. A., R. R. Nieda, and E. P. Desmond. 2004. Validation of the use of Middlebrook 7H10 agar, BACTEC MGIT 960, and BACTEC 460 12B media for testing the susceptibility of Mycobacterium tuberculosis to levofloxacin. J. Clin. Microbiol. 42:5225-5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sartor, R. B. 2005. Does Mycobacterium avium subspecies paratuberculosis cause Crohn's disease? Gut 54:896-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sechi, L. A., A. M. Scanu, P. Molicotti, S. Cannas, M. Mura, G. Dettori, G. Fadda, and S. Zanetti. 2005. Detection and isolation of Mycobacterium avium subspecies paratuberculosis from intestinal mucosal biopsies of patients with and without Crohn's disease in Sardinia. Am. J. Gastroenterol. 100:1529-1536. [DOI] [PubMed] [Google Scholar]
- 37.Sedlack, R. E., J. Whisnant, L. R. Elveback, and L. T. Kurland. 1980. Incidence of Crohn's disease in Olmsted County, Minnesota, 1935-1975. Am. J. Epidemiol. 112:759-763. [DOI] [PubMed] [Google Scholar]
- 38.Shin, S. J., J. H. Han, E. J. Manning, and M. T. Collins. 2007. Rapid and reliable method for quantification of Mycobacterium paratuberculosis by use of the BACTEC MGIT 960 system. J. Clin. Microbiol. 45:1941-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shoda, R., K. Matsueda, S. Yamato, and N. Umeda. 1996. Epidemiologic analysis of Crohn's disease in Japan: increased dietary intake of n-6 polyunsaturated fatty acids and animal protein relates to the increased incidence of Crohn disease in Japan. Am. J. Clin. Nutr. 63:741-745. [DOI] [PubMed] [Google Scholar]
- 40.Sieswerda, L. E., and R. M. Bannatyne. 2006. Mapping the effects of genetic susceptibility and Mycobacterium avium subsp. paratuberculosis infection on Crohn's disease: strong but independent. J. Clin. Microbiol. 44:1204-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sung, N., and M. T. Collins. 1998. Thermal tolerance of Mycobacterium paratuberculosis. Appl. Environ. Microbiol. 64:999-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Travis, S. P., E. F. Stange, M. Lemann, T. Oresland, Y. Chowers, A. Forbes, G. D'Haens, G. Kitis, A. Cortot, C. Prantera, P. Marteau, J. F. Colombel, P. Gionchetti, Y. Bouhnik, E. Tiret, J. Kroesen, M. Starlinger, and N. J. Mortensen. 2006. European evidence based consensus on the diagnosis and management of Crohn's disease: current management. Gut 55(Suppl. 1):i16-i35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsironi, E., R. M. Feakins, C. S. Probert, D. S. Rampton, and D. Phil. 2004. Incidence of inflammatory bowel disease is rising and abdominal tuberculosis is falling in Bangladeshis in East London, United Kingdom. Am. J. Gastroenterol. 99:1749-1755. [DOI] [PubMed] [Google Scholar]
- 44.Whan, L., H. J. Ball, I. R. Grant, and M. T. Rowe. 2005. Occurrence of Mycobacterium avium subsp. paratuberculosis in untreated water in Northern Ireland. Appl. Environ. Microbiol. 71:7107-7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zanetti, S., P. Molicotti, S. Cannas, S. Ortu, N. Ahmed, and L. A. Sechi. 2006. “In vitro” activities of antimycobacterial agents against Mycobacterium avium subsp. paratuberculosis linked to Crohn's disease and paratuberculosis. Ann. Clin. Microbiol. Antimicrob. 5:27. [DOI] [PMC free article] [PubMed] [Google Scholar]