Abstract
Translocase I inhibitor compounds derived from capuramycin demonstrated rapid bactericidal activity against several different mycobacterial species. SQ641 was the most active of the compounds, with a MIC of 0.12 to 8 μg/ml, a postantibiotic effect of 55 h, and interesting synergistic effects with other antitubercular drugs.
Capuramycin (CM), a naturally occurring nucleoside antibiotic produced by Streptomyces griseus, inhibits bacterial translocase I, an enzyme essential in peptidoglycan (PG) biosynthesis of all bacteria. CM, however, has a narrow spectrum of activity and works best on mycobacteria (9, 10, 13). Earlier, chemical modifications and in vitro and in vivo evaluations had identified some promising CM analogues (7, 9, 10). In this report, we expand the in vitro analysis of the antimycobacterial activities of native CM (SQ997) and two of its most promising analogues in the series (SQ922 and SQ641) and demonstrate their potent activities against laboratory and clinical strains of several different mycobacteria.
CM and CM analogues for this study were synthesized and purified by Sankyo. Ethambutol (EMB), isoniazid (INH), rifampin (RIF), streptomycin (SM), clarithromycin (CLA), and amikacin (AMK) were obtained from Sigma Chemical Company (St. Louis, MO). SQ109 was manufactured according to Good Manufacturing Practices for clinical trials by the NCI, NIH, Bethesda, MD. Mycobacterium smegmatis MC2155, Mycobacterium tuberculosis H37Rv, M. tuberculosis H37Rv luciferase reporter strain pSMT1 (11), clinical isolates of M. tuberculosis, and human isolates of Mycobacterium avium complex forming smooth transparent colonies were from stock bacteria maintained at Sequella. Bacteria were grown in 7H9 broth supplemented with 10% albumin-dextrose-catalase (Becton Dickinson, Sparks, MD) and 0.05% Tween 80 (Sigma). MIC was determined by the Bactec radiometric method (3, 8) for M. tuberculosis and by microdilution (12) for M. avium complex and M. smegmatis. Minimal bactericidal concentration (MBC) was determined in 7H9 broth as described previously (3, 8). Rate of killing of M. tuberculosis was determined using M. tuberculosis pSMT1: numbers of viable bacteria at time zero and at different times thereafter were determined by counting the relative light units in a luminometer (11). The postantibiotic effect (PAE) of SQ641 and INH against log-phase cultures of M. tuberculosis was determined as described earlier (1). The synergistic activities of CM analogues in combination with other antitubercular (anti-TB) drugs were determined by checkerboard titration in 96-well microtiter plates. The fractional inhibitory concentrations (FICs) of the drugs and the FIC indices (ΣFIC) of the two-drug combinations were calculated as described previously (4).
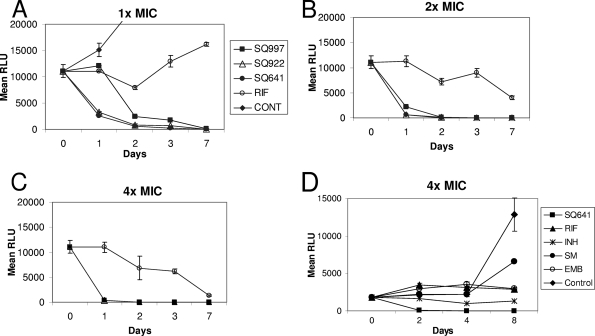
The MIC90 values for 20 M. tuberculosis clinical isolates were 4.0, 8.0, and 16.0 μg/ml for SQ641, SQ922, and SQ997, respectively. The hierarchy of activity against all three mycobacteria (M. tuberculosis, M. avium complex, and M. smegmatis) was SQ641 > SQ922 > SQ997. All three compounds were bactericidal against M. tuberculosis at their MICs (MBC/MIC ratio of 1). In time kill studies, at ≥2× MICs, all three compounds killed >50% of the bacteria within 24 h, >90% of the bacilli within 48 h, and virtually all the organisms by day 7 (Fig. 1). In fact, CM analogue SQ641 killed M. tuberculosis faster than other common anti-TB drugs (Fig. 1D) (P < 0.001 by Student's t test).
FIG. 1.
Rates of killing of M. tuberculosis (H37Rv-pSMT1) following exposure to 1× (A), 2× (B), and 4× (C) MICs of CM, CM analogues, and RIF and to 4× MICs of SQ641 and other anti-TB drugs (D). RLU, relative light units; CONT, control.
PAE determines the time required for organisms to recover following exposure to an antimicrobial agent for a short duration. Exposure of M. tuberculosis to 4 μg/ml SQ641 induced a prolonged PAE of 55 h, compared to 17 h for INH at a similar concentration.
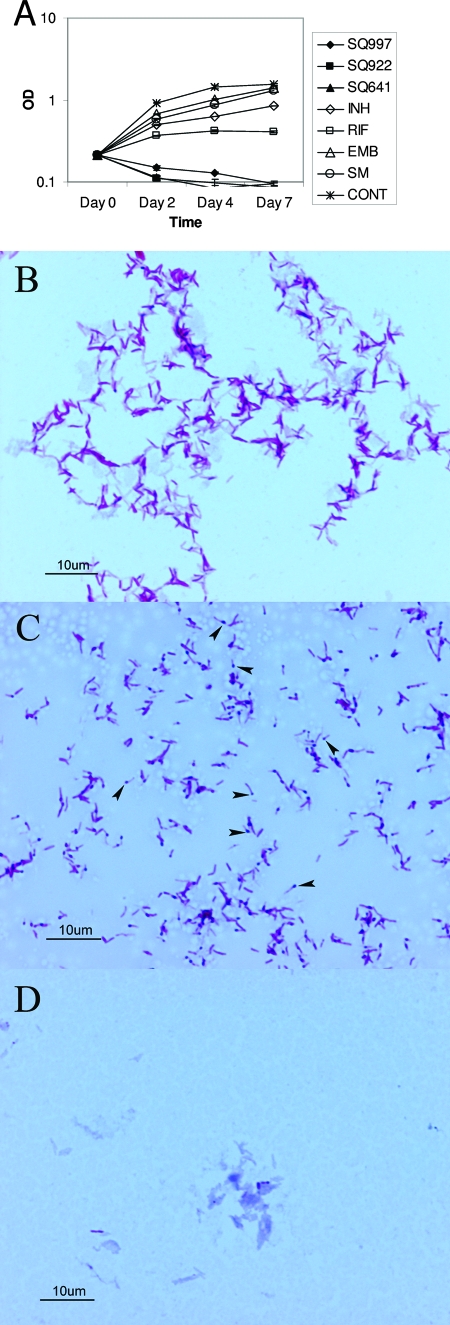
The turbidities of cultures exposed to all three compounds decreased over time, suggesting that these drugs induce bacteriolysis (Fig. 2A). Unlike INH and EMB (Fig. 2D), CM analogues did not affect acid-fast staining of M. tuberculosis, although they each markedly altered the morphology of M. tuberculosis. Most M. tuberculosis bacilli exposed to CM analogues showed deeply stained swollen ends (Fig. 2C).
FIG. 2.
Effect of exposure to CM and CM analogues on the morphology and integrity of M. tuberculosis. (A) Bacterial disintegration and fall in turbidity of M. tuberculosis culture following exposure to CM analogues. OD, optical density; CONT, control. (B) M. tuberculosis stained with Ziehl-Neelsen stain showing acid-fast bacilli (control). (C) Morphological changes in M. tuberculosis following exposure to SQ641 (Ziehl-Neelsen stain). Arrows point to swollen ends of tubercle bacilli. (D) M. tuberculosis exposed to INH. Ziehl-Neelsen staining showed loss of acid fastness (stained blue) of most bacilli.
CM analogues were evaluated for interactions with a variety of antimycobacterial drugs (Table 1). In these interaction studies, a ΣFIC of ≤0.5 indicated synergistic activity (4). SQ997 and SQ922 failed to show synergy with any anti-TB drugs. In contrast, SQ641 demonstrated synergy with EMB for all three mycobacteria, INH for M. smegmatis and M. tuberculosis, and SQ109 for M. tuberculosis (Table 1). Studies on PAE, time kill, synergy, lytic activity, and effect on morphology were repeated at least twice each, with results consistent with data reported here.
TABLE 1.
Interaction of SQ641 with other antimycobacterial drugs against M. tuberculosis, M. smegmatis, and M. avium complex strains
| Organism and drug combination | MIC (μg/ml)
|
ΣFICa | |
|---|---|---|---|
| Alone | In combination | ||
| M. tuberculosis | |||
| SQ641 | 0.5 | 0.25 | 1.0 |
| INH | 0.06 | 0.03 | |
| SQ641 | 0.5 | 0.015 | 0.156 |
| EMB | 1.0 | 0.125 | |
| SQ641 | 0.5 | 0.125 | 0.5 |
| SM | 0.25 | 0.062 | |
| SQ641 | 0.5 | 0.031 | 0.187 |
| SQ109 | 0.25 | 0.031 | |
| M. smegmatis | |||
| SQ641 | 2.0 | 0.25 | 0.25 |
| INH | 8.0 | 1.0 | |
| SQ641 | 2.0 | 1.0 | 0.75 |
| RIF | 8.0 | 2.0 | |
| SQ641 | 2.0 | 0.25 | 0.375 |
| EMB | 0.5 | 0.12 | |
| SQ641 | 2.0 | 1.0 | 1.0 |
| SM | 0.5 | 0.25 | |
| M. avium | |||
| SQ641 | 0.25 | 0.062 | 0.313 |
| EMB | 1.0 | 0.062 | |
| SQ641 | 0.25 | 0.125 | 0.75 |
| CLA | 0.125 | 0.031 | |
| SQ641 | 0.25 | 0.125 | 0.75 |
| AMK | 2.0 | 0.5 | |
ΣFIC is synergistic (≤0.5), additive (>0.5 to 2.0), or antagonistic (>4.0).
Since PG is unique to bacterial cell walls, it is an ideal target for the development of new antimicrobial agents. CM inhibits PG synthesis by interfering with the enzyme phospho-N-acetylmuramyl-pentapeptide translocase (translocase I) (6). In vitro, SQ641 was the CM analogue most active against all three mycobacterial species. Structurally, SQ641 differs from its parent, SQ997, by having a lipophilic decanoyl side chain attached to the sugar moiety (7). This side chain likely contributes to its low solubility, increased antibacterial activity, and synergistic interactions with EMB, another cell wall-active antibiotic.
CM analogues all caused phenotypic changes in M. tuberculosis that were different from effects induced by exposure to INH or EMB (Fig. 2) and caused bacterial disintegration and loss of culture turbidity. The exact mechanism of such antibiotic-induced bacteriolysis is unclear at present. Cytoplasmic turgor due to blockage of PG synthesis and expansion of murein sacculus could result in swelling and subsequent lysis at the weak ends of the nascent bacilli. It is also possible that CM compounds directly or indirectly activate mycobacterial autolysins to cause bacterial disintegration. Autolysins associated with mycobacterial PG have been demonstrated (5); recently, one of the M. tuberculosis autolysins has been cloned and characterized (2). However, their role in mycobacterial division and death is not known.
All the CM compounds were bactericidal and killed M. tuberculosis much faster than other first-line anti-TB drugs, and a single exposure to SQ641 caused a long-lived effect on the recovery of M. tuberculosis bacilli compared with exposure to INH. These striking features, if effective in animal models of TB, could reduce the time frame for effective anti-TB chemotherapy. Koga et al. (7) demonstrated in vivo efficacies of CM analogues delivered intranasally against M. tuberculosis and M. intracellulare in mouse models of infection. In their current form, however, none of the translocase I inhibitors are absorbed well during oral administration (unpublished results). We are evaluating alternative treatment routes and drug delivery vehicles in experimental animal models of TB to harness the exceptional in vitro activities of SQ641.
Acknowledgments
This work was supported by grant 1R43AI066442-01A1 from the National Institutes of Health.
Footnotes
Published ahead of print on 10 December 2007.
REFERENCES
- 1.Chan, C. Y., C. Au-Yeang, W. W. Yew, C. C. Leung, and A. F. B. Cheng. 2004. In vitro postantibiotic effects of rifapentine, isoniazid, and moxifloxacin against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 48:340-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deng, L. L., D. E. Humphries, R. D. Arbeit, L. E. Carlton, S. C. Smole, and J. D. Carroll. 2005. Identification of a novel peptidoglycan hydrolase CwlM in Mycobacterium tuberculosis. Biochim. Biophys. Acta 1747:57-66. [DOI] [PubMed] [Google Scholar]
- 3.Heifets, L. 1998. Drug susceptibility tests in the management of chemotherapy of tuberculosis, p. 89-115. In L. B. Heifets (ed.), Drug susceptibility in the chemotherapy of mycobacterial infections. CRC Press, Boca Raton, FL.
- 4.Heifets, L. 1998. Drug combinations, p. 180-197. In L. B. Heifets (ed.), Drug susceptibility in the chemotherapy of mycobacterial infections. CRC Press, Boca Raton, FL.
- 5.Kilburn, J. O., and G. K. Best. 1977. Characterization of autolysins from Mycobacterium smegmatis. J. Bacteriol. 29:750-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimura, K., and T. D. H. Bugg. 2003. Recent advances in antimicrobial nucleoside antibiotics targeting cell wall biosynthesis. Nat. Prod. Rep. 20:252-273. [DOI] [PubMed] [Google Scholar]
- 7.Koga, T., T. Fukuoka, N. Doi, T. Harasaki, H. Inoue, H. Hotoda, M. Kakuta, Y. Muramatsu, N. Yamamura, M. Hoshi, and T. Hirota. 2004. Activity of capuramycin analogues against Mycobacterium tuberculosis, Mycobacterium avium and Mycobacterium intracellulare in vitro and in vivo. J. Antimicrob. Chemother. 54:755-760. [DOI] [PubMed] [Google Scholar]
- 8.Luna-Herrera, J., M. V. Reddy, and P. R. J. Gangadharam. 1995. In vitro activity of the benzoxazinorifamycin KRM-1648 against drug-susceptible and multidrug-resistant tubercle bacilli. Antimicrob. Agents Chemother. 39:440-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muramatsu, Y., A. Muramatsu, T. Ohnuki, M. Miyazawa Ishi, M. Kizuka, R. Enokita, S. Tsutsumi, M. Arai, Y. Goawa, T. Suzuki, T. Takatsu, and M. Inukai. 2003. Studies on novel bacterial translocase I inhibitors, A-500359s. I. Taxonomy, fermentation, isolation, physiochemical properties and structure elucidation of A-500359 A, C, D and G. J. Antibiot. 56:243-252. [DOI] [PubMed] [Google Scholar]
- 10.Muramatsu, Y., M. M. Ishii, and M. Inukai. 2003. Studies on novel bacterial translocase I inhibitors, A-500359s. II. Biological activities of A-500539 A, C, D and G. J. Antibiot. 56:253-258. [DOI] [PubMed] [Google Scholar]
- 11.Snewin, V. A., M. P. Gares, P. O. Gaora, Z. Hasan, I. N. Brown, and D. B. Young. 1999. Assessment of immunity to mycobacterial infection with luciferase reporter constructs. Infect. Immun. 67:4586-4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallace, R. J., D. R. Nash, L. C. Steel, and V. Steingrube. 1986. Susceptibility testing of slowly growing mycobacteria by a microdilution MIC method with 7H9 broth. J. Clin. Microbiol. 24:976-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamaguchi, H., S. Sato, S. Yoshida, K. Takada, and M. Itoh. 1986. Capuramycin, a new nucleoside antibiotic. Taxonomy, fermentation, isolation and characterization. J. Antibiot. 39:1047-1053. [DOI] [PubMed] [Google Scholar]