Abstract
Rifabutin (RFB) is administered for treatment of tuberculosis and Mycobacterium avium complex infection, including use for patients coinfected with human immunodeficiency virus (HIV). Increased systemic exposure to RFB and its equipotent active metabolite, 25-O-desacetyl-RFB (dAc-RFB), has been reported during concomitant administration of CYP3A4 inhibitors, including ritonavir (RTV), lopinavir, and amprenavir (APV); therefore, a reduction in the RFB dosage is recommended when it is coadministered with these protease inhibitors. Fosamprenavir (FPV), the phosphate ester prodrug of the HIV type 1 protease inhibitor APV, is administered either with or without RTV. A randomized, open-label, two-period, two-sequence, balanced, crossover drug interaction study was conducted with 22 healthy adult subjects to compare steady-state plasma RFB pharmacokinetic parameters during concomitant administration of FPV-RTV (700/100 mg twice a day [BID]) with a 75%-reduced RFB dose (150 mg every other day [QOD]) to the standard RFB regimen (300 mg once per day [QD]) by geometric least-squares mean ratios. Relative to results with RFB (300 mg QD), coadministration of dose-adjusted RFB with FPV-RTV resulted in an unchanged RFB area under the concentration-time curve for 0 to 48 h (AUC0-48) and a 14% decrease in the maximum concentration of drug in plasma (Cmax), whereas the AUC0-48 and Cmax of dAc-RFB were increased by 11- and 6-fold, respectively, resulting in a 64% increase in the total antimycobacterial AUC0-48. Relative to historical controls, the plasma APV AUC from 0 h to the end of the dosing interval (AUC0-τ) and Cmax were increased ∼35%, and the concentration at the end of the dosing interval at steady state was unchanged following coadministration of RFB with FPV-RTV. The safety profile of the combination of RFB and FPV-RTV was consistent with previously described events with RFB or FPV-RTV alone. Based on the results of this study, a reduction in the RFB dose by ≥75% (to 150 mg QOD or three times per week) is recommended when it is coadministered with FPV-RTV (700/100 mg BID).
Rifabutin (RFB) is a semisynthetic rifamycin antibiotic used commonly for the prophylaxis of Mycobacterium avium complex infection or the treatment of tuberculosis coinfection in human immunodeficiency virus (HIV)-infected patients (Mycobutin [rifabutin] product information, February 2002). RFB has five identified metabolites, including 25-O-desacetyl-RFB (dAc-RFB), which has antimycobacterial activity that is equipotent to that of the parent compound on a molar basis (1, 5; Mycobutin product information, February 2002). Following standard doses, the plasma dAc-RFB area under the concentration-time curve (AUC) is approximately 10% of that of RFB and therefore contributes to 10% of the total antimycobacterial activity of RFB (1; Mycobutin product information, February 2002).
Plasma exposure to RFB and dAc-RFB, both substrates of CYP3A4, has been increased significantly by coadministration with CYP3A4 inhibitors, including HIV protease inhibitors (PIs) (1, 5), which has the potential to increase the risk of RFB-associated adverse events, including neutropenia and uveitis (Mycobutin product information, February 2002). Concomitant administration of amprenavir (APV) (1,200 mg twice a day [BID]) and RFB (300 mg once per day [QD]) for 10 days increased plasma RFB AUC from 0 to 24 h (AUC0-24) and maximum concentration (Cmax) 2.9- and 2.2-fold, respectively, and increased plasma dAc-RFB AUC0-24, Cmax, and minimum concentration 13.3-, 7.4-, and 32.9-fold, respectively, relative to those of RFB (300 mg once a day [QD]) alone; a 50% reduction in the RFB dose was recommended for the combination of RFB and unboosted APV (5). Similarly, coadministration of lopinavir (LPV)-ritonavir (RTV) (400/100 mg BID) and a 50%-reduced RFB dose (150 mg QD) for 10 days increased plasma RFB AUC0-24 and Cmax 3.0- and 4.9-fold, respectively, increased the plasma dAc-RFB AUC0-24, Cmax, and minimum concentration 47.5-, 23.6- and 94.9-fold, respectively, and increased the total antimycobacterial AUC0-24 5.7-fold relative to those of RFB (300 mg QD) alone (Kaletra [lopinavir-ritonavir] product information, January 2006). A reduction in the RFB dosage of at least 75% is recommended during coadministration with LPV-RTV (Kaletra product information, January 2006). Also an inducer of CYP3A4, RFB (300 mg QD) has been associated with a 34% reduction in indinavir AUC (3) and a 15% reduction in APV AUC (5), which increases the risk to patients for subtherapeutic antiretroviral treatment; however, LPV AUC was unchanged following administration of LPV-RTV (400/100 mg BID) with RFB (150 mg QD) (Kaletra product information, January 2006).
Fosamprenavir (FPV) (Lexiva or Telzir), the phosphate ester prodrug of the HIV type 1 PI APV, is approved for the treatment of HIV infection in adults and can be administered with or without RTV (Lexiva [fosamprenavir calcium] product information). This study was designed to compare steady-state plasma RFB, dAc-RFB, and total antimycobacterial pharmacokinetics (PK) during coadministration of 75%-dose-reduced RFB (150 mg every other day [QOD]) with FPV-RTV (700/100 mg BID) to those with RFB (300 mg QD) alone. In addition, plasma APV PK during concomitant administration of FPV-RTV with RFB was compared to historical controls of FPV-RTV 700/100-mg-BID dosing alone.
MATERIALS AND METHODS
Materials.
FPV tablets for oral administration were supplied by GlaxoSmithKline (Ware, Hertfordshire, United Kingdom). Each 700-mg FPV tablet contained approximately 600 mg APV molar equivalents. Norvir soft gelatin capsules for oral administration contained 100 mg of RTV and were manufactured by Abbott Laboratories (Abbott Park, IL). Mycobutin capsules for oral administration contained 150 mg of RFB and were manufactured by Pharmacia & Upjohn Company, a subsidiary of Pfizer, Inc. (Kalamazoo, MI).
Subjects.
All subjects underwent screening assessments within 30 days of dosing to determine their eligibility for enrollment in this study. Subjects eligible to participate were healthy males or females between 18 and 55 years of age with a minimum body weight of 50 kg (men) or 45 kg (women) and a body mass index between 18.5 and 29.9 kg/m2. Subjects were excluded for abnormal gastrointestinal anatomy or motility, hepatic and/or renal dysfunction, current or recent (within 30 days of the study) use of prescription or nonprescription drugs, a history of allergy to the study drugs or drugs of this class, a clinical history of or current illicit drug or excessive alcohol use, a history of hemophilia, pregnancy, or lactation, a positive test for HIV, tuberculosis, hepatitis B or C, alanine aminotransferase (ALT) or aspartate aminotransferase (AST) levels greater than the upper limit of normal for the local lab, or an absolute neutrophil count or platelets below the lower limit of normal. Subjects were withdrawn from the study for any clinically significant adverse events (AEs) requiring discontinuation of the investigational product, an ALT or AST value increased to three times the upper limit of normal for the local lab, an absolute neutrophil count decreased to <1,000 cells/ml, reported noncompliance with the study drug, a requirement of prohibited concurrent medications, pregnancy, or a positive screen for illicit drugs or alcohol.
Study design.
A randomized, open-label, two-period, two-sequence, balanced, crossover drug interaction study was employed. Subjects received RFB (300 mg QD) for 13 days and a 75%-reduced RFB dose (150 mg QOD); dosing on days 1, 3, 5, 7, 9, 11, and 13) with FPV-RTV (700/100 mg BID) for 14 days in random order, with a 21- to 28-day washout period between treatments. Plasma trough samples were obtained prior to morning dosing on days 9 and 11. Serial plasma samples were obtained over the RFB dosing interval on the last day of each treatment (predose, 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, 10, 12, 16, and 24 h for RFB (300 mg QD) and also at 36 and 48 h for RFB (150 mg QOD) plus FPV-RTV (700/100 mg QD). Administration of FPV-RTV BID was continued throughout the PK sampling. Vital signs, physical exams, and clinical laboratory assessments were obtained periodically throughout the study. Subjects returned to the study center for a follow-up visit within 21 to 28 days after discontinuation of study drug(s).
Plasma analysis.
Whole blood samples were collected into 7-ml EDTA-containing evacuated blood collection tubes. Immediately after collection, the tube was inverted gently to mix the anticoagulant with the blood and the plasma was separated by centrifugation. The plasma samples were stored in cryotubes at −20°C prior to analysis.
Plasma APV, RFB, and dAc-RFB concentrations were quantified using liquid chromatography with tandem mass spectrometric detection following protein precipitation (APV) or liquid-liquid extraction (RFB and dAc-RFB). The lower limits of quantification were 10 ng/ml for APV and 2.00 ng/ml for RFB and dAc-RFB. Interassay precision (percent coefficient of variation) was ≤8.4% for RFB, ≤8.6% for dAc-RFB, and ≤4.6% for APV. Accuracy (percent bias) ranged between 2.8 to 4.4% for RFB, 1.4 to 3.5% for dAc-RFB, and −2.8 to 5.9% for APV. Total antimycobacterial concentrations were determined by summing RFB (molecular weight, 847.02) and dAc-RFB (molecular weight, 804.97) concentrations in molar (μM) units.
Data analysis.
PK analyses of APV, RFB, dAc-RFB, and total antimycobacterial plasma concentration-time data were conducted using noncompartmental Model 200 (for extravascular administration) of WinNonlin Professional software, version 4.1 (Pharsight Corporation, Mountain View, CA) using the linear (ascending) and logarithmic-linear (descending) trapezoidal rule. Actual elapsed time from dosing was used to estimate all individual plasma PK parameters for evaluable subjects.
The maximum observed Cmax in plasma represents the actual observed values. For subjects receiving RFB (300 mg QD) alone, RFB, dAc-RFB, and total antimycobacterial AUC0-24 values were doubled to estimate the AUC from 0 to 48 h (AUC0-48) for comparison with results for subjects concomitantly administered RFB (150 mg QOD) with FPV-RTV (700/100 mg BID). The average plasma concentration over the dosing interval at steady state (Cavg) was calculated as the AUC0-τ/τ. The plasma concentrations at the end of the dosing interval (τ) at steady state (Cτ) were calculated as the average of the predose concentrations on days 11 and 13.
Analysis of variance, considering the treatment, period, and sequence as fixed effects and the subject as a random effect, were performed to evaluate the impact of FPV-RTV coadministration on plasma RFB, dAc-RFB, and total antimycobacterial PK parameters using the SAS, version 8.2 (SAS, Cary, NC), mixed-linear-models procedure. The ratios of geometric least-squares (GLS) means and the associated 90% confidence intervals were estimated for the plasma AUC0-48, Cmax, and Cτ in each of the treatment comparisons. Based on known variability of the RFB AUC (5), a minimum of 20 subjects (10 subjects/sequence) were to be enrolled to achieve the 14 evaluable subjects required to provide 90% power for the 90% confidence interval (CI) of the treatment ratio to fall within the range 0.75 to 1.33 for each RFB treatment comparison.
APV PK parameters with concomitant RFB administration were compared to historical data pooled from six studies in which FPV-RTV (700/100 mg BID) was administered to 95 healthy volunteers (6, 9-12). Analysis of variance, considering treatment as a fixed effect, was used to evaluate the impact of RFB on plasma APV PK. The ratios of geometric least-squares means and the associated 90% CIs were estimated for the plasma APV Cmax, AUC0-τ, and Cτ for the treatment comparison.
Achievement of RFB and APV steady-state concentrations was assessed by calculating the 90% confidence interval of the slope of the linear regression using at least three log-transformed Cτ-versus-time values between days 9 and 13.
RESULTS
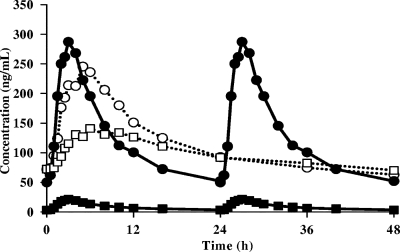
Subject disposition and demographics are summarized in Table 1. A total of 22 subjects were enrolled in the study, and 15 subjects completed all treatments. All subjects (17/17) completed the RFB (300 mg QD)-alone treatment, while 7 of 22 withdrew from the combined treatment of RFB (150 mg QOD) and FPV-RTV (700/100 mg BID). Median steady-state plasma concentrations of RFB and dAc-RFB are presented in Fig. 1. RFB, dAc-RFB, and total antimycobacterial PK parameters and treatment comparisons are summarized in Table 2. The RFB AUC0-48 and Cavg were unchanged following coadministration of RFB (150 mg QOD) and FPV-RTV (700/100 mg BID) relative to results with RFB (300 mg QD) alone; the RFB Cmax was decreased 14% (GLS mean ratio [90% CI], 0.861 [0.716, 1.04]) for the combination relative to results with RFB alone. In contrast, the plasma dAc-RFB AUC0-48 and Cmax GLS mean ratios (90% CI) were 11.2 (9.65, 13.0)-fold and 5.79 (4.79, 6.98)-fold, respectively, following coadministration of RFB (150 mg QOD) with FPV-RTV (700/100 mg BID) relative to results with RFB (300 mg QD) alone. The total antimycobacterial AUC0-48 was increased by 64% (GLS mean ratio [90% CI], 1.64 [1.46, 1.84]).
TABLE 1.
Subject disposition and demographics
| Characteristic | No. (%) of subjects in treatment group
|
Value for all subjects | |
|---|---|---|---|
| RFB (300 mg QD) | RFB (150 mg QOD) + FPV-RTV (700/100 mg BID) | ||
| Dosed | 17 | 22 | |
| Completed treatment | 17 | 15 | |
| Withdrawn (total) | 0 | 7 (32) | |
| Withdrawn due to AEs | 0 | 5 (23)a | |
| Withdrawn for other reasons | 0 | 2 (9) | |
| Demographics | |||
| Total | 22 | ||
| Females:males | 4:18 | ||
| Mean age, yr (SD) | 35.3 (9.5) | ||
| Mean wt, kg (SD) | 82.4 (12.4) | ||
| Race, Caucasian:African American | 18:4 | ||
Three subjects withdrew for rash, one for nausea, and one for myalgia/fever/increased white blood cell count.
FIG. 1.
Mean steady-state plasma concentration-versus-time profiles of RFB (circles) and dAc-RFB (squares) during dosing with RFB (300 mg QD) alone (filled symbols, full lines) or concomitant dosing of 75%-dosed-reduced RFB (150 mg QOD) with FPV-RTV (700/100 mg BID) (open symbols, dashed lines).
TABLE 2.
PK parameters and treatment comparisons for RFB, dAc-RFB, and total antimycobacterial (RFB plus dAc-RFB) plasma concentrationsa
| Drug and parameter | Value for treatment group
|
Treatment comparison | |
|---|---|---|---|
| RFB (300 mg QD) (n = 15) | RFB (150 mg QOD + FPV-RTV 700/100 mg BID) (n = 15) | ||
| RFB | |||
| AUC0-48 (μg·h/ml) | 6.11 (5.33-7.01) | 5.81 (5.04-6.68) | 0.951 (0.843-1.07) |
| Cmax (μg/ml) | 0.313 (0.267-0.366) | 0.268 (0.227-0.316) | 0.861 (0.716-1.04) |
| Cavg (μg/ml) | 0.127 (0.111-0.146) | 0.121 (0.105-0.139) | 0.951 (0.843-1.07) |
| dAc-RFB | |||
| AUC0-48 (μg·h/ml) | 0.411 (0.343-0.493) | 4.60 (4.17-5.06) | 11.2 (9.65-13.0) |
| Cmax (μg/ml) | 0.024 (0.019-0.030) | 0.139 (0.124-0.156) | 5.79 (4.79-6.98) |
| Cavg (μg/ml) | 0.009 (0.007-0.010) | 0.096 (0.087-0.106) | 11.2 (9.65-13.0) |
| Total antimycobacterial agents | |||
| AUC0-48 (μM·h) | 7.74 (6.77-8.86) | 12.7 (11.5-14.0) | 1.64 (1.46-1.84) |
Parameters are reported as geometric means (95% confidence interval); treatment comparisons are expressed as GLS mean ratios (90% confidence interval), comparing the combination to the treatment with RFB alone. Calculations were performed as described in Materials and Methods.
A comparison of plasma APV PK following coadministration of FPV-RTV (700/100 mg BID) with RFB (150 mg QOD) to historical data for FPV-RTV (700/100 mg BID) alone is summarized in Table 3. Plasma APV AUC0-τ and Cmax were increased 35% and 36%, respectively, during FPV-RTV coadministration with RFB. In contrast, the APV Cτ was not significantly changed in this study relative to historical controls.
TABLE 3.
Summary of PK parameters and treatment comparisons for APVa
| Parameter | Value for treatment group
|
Treatment comparison | |
|---|---|---|---|
| FPV-RTV (700/100 mg BID) (historical control) (n = 95) | RFB (150 mg QOD) + FPV-RTV (700/100 mg BID) (n = 15) | ||
| AUC(0-τ) (μg·h/ml) | 34.8 (32.6-37.2) | 47.1 (40.2-55.1) | 1.35 (1.17-1.56) |
| Cmax (μg/ml) | 5.38 (5.06-5.73) | 7.29 (6.43-8.27) | 1.36 (1.18-1.55) |
| Cτ (μg/ml) | 1.97 (1.83-2.13) | 2.32 (1.98-2.72) | 1.17 (0.995-1.39) |
Parameters are reported as geometric means (95% confidence interval); treatment comparisons are expressed as geometric least-squares mean ratios (90% confidence interval), comparing the combination to the FPV-RTV treatment alone. Calculations were performed as described in Materials and Methods.
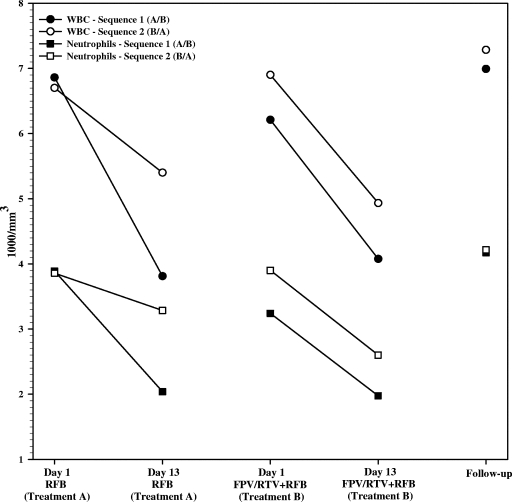
Drug-related AEs are summarized by treatment in Table 4. The most frequently occurring AEs were chromaturia, headache, and diarrhea. Overall, a higher percentage (86%) of subjects receiving the FPV-RTV-containing treatment reported AEs than those receiving RFB alone (71%). All AEs were mild to moderate in severity. Five subjects withdrew from the study due to AEs: three due to moderate rash and one due to mild nausea, which the investigator felt were related to the study drugs, and one due to myalgia, neutrophil percentage increase, and pyrexia, which the investigator felt were not related to the study drugs. One subject was withdrawn due to abnormal creatinine phosphokinase prior to study drug administration, and one subject decided to withdraw for reasons unrelated to safety. Declines in the mean white blood cell count and absolute neutrophil count were noted over the course of 13 days of RFB treatment; these declines were similar with and without FPV-RTV coadministration and resolved upon discontinuation of the study medication (Fig. 2). An absolute neutrophil count <1,000/mm3 was observed for one subject receiving RFB with FPV-RTV (800/mm3, from a predose baseline of 3,100/mm3), which resolved when the subject was off the study medication. Five ALT elevations were reported as AEs for the combination of RFB and FPV-RTV (Table 4). All ALT abnormalities were less than twofold the upper limit of normal for the local lab (range, 62 to 93 IU/liter; normal, 5 to 60 IU/liter). Four subjects had elevated ALT at the follow-up visit (n = 3) or at the check-in for period 2 (n = 1), following RFB and FPV-RTV dosing. One subject had an ALT of 70 IU/liter reported as an AE on period 1, day 7 (RFB and FPV-RTV), which resolved by period 2, day 1. An ALT of 123 IU/liter was noted on laboratory values on day 7 of period 2 (RFB alone) for this same subject but was not reported as an AE by the investigator. All ALT abnormalities resolved prior to the subjects' discharge from the study. No other consistent trends in laboratory abnormalities were noted, and no relationships between AEs and RFB or dAc-RFB PK parameters were identified upon graphical inspection of the data.
TABLE 4.
Most commonly reported drug-related AEs (occurring in at least two subjects in any treatment)
| AE reported | No. (%) of subjects in treatment group
|
|
|---|---|---|
| RFB (300 mg QD) (n = 17) | RFB (150 mg QOD) + FPV-RTV (700/100 mg BID) (n = 22) | |
| Any | 12 (71) | 19 (86) |
| Nervous system disorders (any event) | 6 (35) | 12 (55) |
| Headache | 6 (35) | 10 (45) |
| Renal and urinary disorders (any event) | 10 (59) | 13 (59) |
| Chromaturia | 10 (59) | 11 (50) |
| Pollakiuria | 0 | 4 (18) |
| Gastrointestinal disorders (any event) | 6 (35) | 12 (55) |
| Diarrhea | 2 (12) | 7 (32) |
| Nausea | 0 | 4 (18) |
| Investigations (any event) | 6 (35) | 9 (41) |
| Neutrophil count decreased | 3 (18) | 3 (14) |
| ALT increased | 0 | 5 (23) |
| Neutrophil count increased | 0 | 3 (14) |
| General disorders and administration site conditions (any event) | 3 (18) | 7 (32) |
| Fatigue | 0 | 4 (18) |
| Chills | 0 | 3 (14) |
| Pain | 2 (12) | 1 (5) |
| Musculoskeletal and connective tissue disorders (any event) | 2 (12) | 5 (23) |
| Myalgia | 0 | 4 (18) |
| Skin and subcutaneous tissue disorders (any event) | 0 | 6 (27) |
| Rash generalized | 0 | 2 (9) |
| Blood and lymphatic system disorders (any event) | 3 (18) | 3 (14) |
| Neutropenia | 2 (12) | 1 (5) |
| Thrombocytopenia | 0 | 3 (14) |
| Psychiatric disorders (any event) | 5 (29) | 1 (5) |
| Insomnia | 4 (24) | 1 (5) |
FIG. 2.
Summary of mean white blood cell (WBC) and neutrophil counts over time in various phases of the study.
DISCUSSION
This study was designed to evaluate the effect of coadministering FPV-RTV with RFB on parent RFB, active metabolite, dAc-RFB, and total antimycobacterial (RFB plus dAC-RFB) PK. Plasma RFB AUC0-48 was unchanged following coadministration of dose-adjusted RFB (150 mg QOD) with FPV-RTV (700/100 mg BID) compared to standard doses of RFB (300 mg QD). In contrast, the plasma dAc-RFB AUC0-48 was increased 11.2-fold, from less than 10% of the parent value to approximately 80% of the parent AUC0-48. As a result of the significant increase in dAc-RFB, which is equipotent to RFB on a molar basis (1, 5; Mycobutin product information, February 2002), the total antimycobacterial AUC0-48 was increased by approximately 64% for the combination of RFB (150 mg QOD) and FPV-RTV (700/100 mg BID) relative to that for RFB (300 mg QD) alone. As noted previously, RFB and dAc-RFB AUC0-24 values were increased 3.0- and 47.5-fold, respectively, following coadministration of LPV-RTV (400/100 mg BID) and RFB at a 50% reduced dose (150 mg QD) compared to results for standard-dose RFB (300 mg QD) alone, and a 75% RFB dose reduction currently is recommended in the LPV-RTV product labeling (Kaletra product information, January 2006). Adjusting for the different RFB dosing intervals to allow comparison of the LPV-RTV and FPV-RTV data, it is predicted that coadministration of LPV-RTV with RFB (150 mg QOD) would increase the RFB and dAc-RFB AUC0-48 values by approximately 1.5- and 24-fold, respectively, greater than was observed with FPV-RTV, where there was no change in RFB AUC0-48 and an 11.2-fold increase in dAC-RFB AUC0-48.
Unlike the 15% decrease in the APV AUC following coadministration of RFB (300 mg QD) with APV (1,200 mg BID) (5), coadministration of dose-adjusted RFB with FPV-RTV (700/100 mg BID) in this study increased plasma APV AUC0-τ by 35% and Cmax by 36% and had no effect on Cτ compared to pooled historical APV data following FPV-RTV BID. Boosting with RTV may alter the inductive effect of RFB on PIs, since LPV was unchanged to slightly increased following coadministration of 50%-dose reduced RFB with LPV-RTV (Kaletra product information, January 2006). In addition, this small increase in APV AUC may reflect differences in study populations. The true effect of RFB (QOD) on APV PK is unclear without intrasubject comparisons, but the effect appears to be relatively minor.
The combination of RFB (150 mg QOD) and FPV-RTV (700/100 mg BID) for 14 days had a side effect profile similar to previously described events with RFB or FPV-RTV alone (1, 2). Neutropenia (absolute neutrophil count of <750/mm3) has been found in 25% of subjects receiving RFB in efficacy studies (Mycobutin product information, February 2002) but has not been reported for healthy subjects receiving FPV with or without RTV (4, 6-9). Therefore, it is likely that the neutropenia observed during this study was related to RFB administration. A rash occurs more frequently with a higher withdrawal rate in healthy subjects receiving FPV-RTV (7-9) than in HIV-infected patients receiving FPV-RTV (Lexiva [fosamprenavir calcium] product information, June 2006), which may in part reflect a different risk-benefit profile for patients and a true potential for less-frequent rashes in HIV-infected patients. Safety findings were also similar to those in other studies of protease inhibitors coadministered with RFB (2, 3, 5). Therefore, the coadministration of RFB and FPV-RTV does not appear to cause safety concerns in addition to those of either treatment alone.
When coadministered with FPV-RTV (700/100 mg BID), RFB (150 mg QOD) achieves a comparable RFB exposure and acceptable increases in dAc-RFB and total antimycobacterial exposures relative to those of the standard RFB (300 mg QD) regimen. The safety profile of this combination therapy of RFB and FPV-RTV is similar to the safety profile for either treatment alone. A reduction in the RFB dose of at least 75% (to 150 mg QOD or three times weekly) is recommended when it is coadministered with FPV-RTV (700/100 mg BID); the safety and efficacy of the combination, however, have not been confirmed for tuberculosis-infected HIV-positive patients.
Acknowledgments
Maciej J. Zamek-Gliszczynski is acknowledged for his medical writing contributions to the manuscript.
Footnotes
Published ahead of print on 3 December 2007.
REFERENCES
- 1.Cato, A., III, J. Cavanaugh, H. Shi, A. Hsu, J. Leonard, and R. Granneman. 1998. The effect of multiple doses of ritonavir on the pharmacokinetics of rifabutin. Clin. Pharmacol. Ther. 63:414-421. [DOI] [PubMed] [Google Scholar]
- 2.Hamzeh, F. M., C. Benson, J. Gerber, J. Currier, J. McCrea, P. Deutsch, P. Ruan, H. Wu, J. Lee, and C. Flexner. 2003. Steady-state pharmacokinetic interaction of modified-dose indinavir and rifabutin. Clin. Pharmacol. Ther. 73:159-169. [DOI] [PubMed] [Google Scholar]
- 3.Kraft, W. K., J. B. McCrea, G. A. Winchell, A. Carides, R. Lowry, E. J. Woolf, S. E. Kusma, P. J. Deutsch, H. E. Greenberg, and S. A. Waldman. 2004. Indinavir and rifabutin drug interactions in healthy volunteers. J. Clin. Pharmacol. 44:305-313. [DOI] [PubMed] [Google Scholar]
- 4.Kurowski, M., R. K. Walli, A. Breske, G. Kruse, H. Stocker, N. Banik, H. Richter, and D. Mazur. 2007. Fosamprenavir/ritonavir plus tenofovir does not affect amprenavir pharmacokinetics: no effect of tenofovir. AIDS 21:1368-1370. [DOI] [PubMed] [Google Scholar]
- 5.Polk, R. E., D. F. Brophy, D. S. Israel, R. Patron, B. M. Sadler, G. E. Chittick, W. T. Symonds, Y. Lou, D. Kristoff, and D. S. Stein. 2001. Pharmacokinetic Interaction between amprenavir and rifabutin or rifampin in healthy males. Antimicrob. Agents Chemother. 45:502-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shelton, M. J., S. L. Ford, J. Borland, Y. Lou, M. B. Wire, S. S. Min, Z. G. Xue, and G. Yuen. 2006. Coadministration of esomeprazole with fosamprenavir has no impact on steady-state plasma amprenavir pharmacokinetics. J. Acquir. Immune Defic. Syndr. 42:61-67. [DOI] [PubMed] [Google Scholar]
- 7.Shelton, M. J., M. B. Wire, Y. Lou, B. Adamkiewicz, and S. S. Min. 2006. Pharmacokinetic and safety evaluation of high-dose combinations of fosamprenavir and ritonavir. Antimicrob. Agents Chemother. 50:928-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wire, M. B., C. Ballow, S. L. Preston, C. W. Hendrix, P. J. Piliero, Y. Lou, and D. S. Stein. 2004. Pharmacokinetics and safety of GW433908 and ritonavir, with and without efavirenz, in healthy volunteers. AIDS 18:897-907. [DOI] [PubMed] [Google Scholar]
- 9.Wire, M. B., K. L. Baker, L. S. Jones, M. J. Shelton, Y. Lou, G. J. Thomas, and M. M. Berrey. 2006. Ritonavir increases plasma amprenavir (APV) exposure to a similar extent when coadministered with either fosamprenavir or APV. Antimicrob. Agents Chemother. 50:1578-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wire, M. B., et al. 2002. Abstr. Prog. Conf. Retrovir. Opport. Infect., abstr. 431W.
- 11.Wire, M. B., et al. 2004. Abstr. Prog. 11th Conf. Retrovir. Opportun. Infect., abstr. 612.
- 12.Wire, M. B., et al. 2003. Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-1622.