Abstract
Some pathogenic bacteria produce factors that have evolved a capacity to neutralize competing microbes. The cupredoxin family protein azurin, produced by Pseudomonas aeruginosa, exhibits a remarkable ability to impede invasion of a number of diverse intracellular pathogens, including the human AIDS virus human immunodeficiency virus type 1 and the protozoan parasite Plasmodium falciparum (which causes malaria). Here we report that azurin and an azurin-like protein (Laz) from gonococci/meningococci have activity against Toxoplasma, an apicomplexan parasite that causes opportunistic infection in immunocompromised individuals. We demonstrate that the mechanism of action for Laz involves interfering with the ability of Toxoplasma to adhere to host cells. Computer structural analysis reveals that azurin shares structural features with the predominant surface antigen SAG1, which is known to play an important role in parasite attachment. Interestingly, azurin also has structural similarities to a monoclonal antibody to SAG1. Surface plasmon resonance binding studies validate that SAG1 interacts strongly with Laz and, to lesser extent, azurin. Moreover, Toxoplasma mutants lacking SAG1 are not as susceptible to the growth-inhibitory effects of Laz. Collectively, our data show that Toxoplasma adhesion can be significantly impaired by Laz, and to some extent by azurin, via interactions with SAG1. These observations indicate that Laz can serve as an important tool in the study of host-pathogen interactions and is worthy of further study for development into potential therapeutic agents.
Apicomplexan protozoa include several members that present significant challenges to human health, such as Plasmodium (which causes malaria), Cryptosporidium (which causes cryptosporidiosis), and Toxoplasma gondii (which causes toxoplasmosis). Toxoplasma can cause congenital birth defects in newborns of infected mothers and remains a serious complication in AIDS patients and other immunocompromised individuals (7, 9, 27). Additionally, Toxoplasma is classified as a category B priority pathogen, relevant to biodefense research, by the National Institute for Allergy and Infectious Diseases (NIAID) (24). The toxicity associated with the primary treatment for recurring toxoplasmosis (pyrimethamine plus sulfadiazine) underscores the urgent need for novel strategies to combat Toxoplasma.
Apicomplexans are obligate intracellular parasites; thus, how they adhere to and invade the host cell is fundamental to disease progression. Toxoplasma tachyzoites, the invasive form of the parasite that produces the acute stage of infection, are covered with glycosylphosphatidylinositol-anchored surface antigen (SAG) proteins or SAG1-related sequence (SRS) proteins. The SRS proteins constitute a superfamily with at least 160 members, some of which are developmentally regulated (18). The large number of variant SRS proteins may explain why Toxoplasma is capable of entering nearly any type of nucleated cell (13). With respect to the mechanism of host cell attachment, SAG3 has been demonstrated to bind with high affinity to sulfated proteoglycans (16). Crystallography studies of SAG1 (p30) show that a positively charged groove forms at the homodimer interface, the dimensions of which could accommodate negatively charged proteoglycans (13). Following attachment to the host cell, the parasite sequentially releases contents from specialized organelles (micronemes, rhoptries, and dense granules) that trigger invasion and establishment of the parasitophorous vacuole (3).
SAG1, one of the more predominant SRS proteins on the parasite's surface, is involved in host cell attachment and is a key virulence factor (19, 22). Antibodies to SAG1 also interfere with the parasite's ability to invade host cells in vitro (20). SAG1 has been shown to be highly immunogenic, and anti-SAG1 antibodies protect mice infected with Toxoplasma (17). Thus, therapies designed to target SAG1 in vivo are likely to have a significant benefit in controlling toxoplasmosis.
Azurin (also referred to as “Paz”) is a 128-amino-acid periplasmic protein produced by the bacterium Pseudomonas aeruginosa that has antimicrobial activity (4). Azurin is a redox protein initially believed only to serve as an electron donor to nitrite reductase during anaerobic respiration, but no such obligatory role has been confirmed (26). Neisseria meningitidis and other members of the gonococci/meningococci produce an azurin-like protein called Laz on their surface. Laz has an additional 39-amino-acid peptide at its N terminus called an H.8 epitope (15). Azurin and Laz are collectively referred to as “azurin proteins” in this report. Azurin proteins are members of a family of copper-containing proteins known as cupredoxins, which are involved in electron transfer. Recently it was documented that portions of azurin proteins share structural similarities with a wide variety of other proteins, including variable domains of immunoglobulins and mammalian cell surface receptors and/or ligands. For example, azurin shows structural similarity to the Fab fragment that binds the Plasmodium falciparum merozoite surface protein I (MSP1) and binds to CD4, which acts as a receptor for the human immudeficiency virus type 1 (HIV-1) envelope glycoprotein gp120. Consequently, azurin proteins interfere with the invasion of HIV-1 and reduce parasitemia of Plasmodium falciparum by binding to envelope or surface proteins, respectively (4). This phenomenon has led to a hypothesis that azurin proteins may play dual roles in pathogenic bacteria, one of which is to serve as a versatile weapon against competing microbes attempting to invade the same host (8).
In this study, we report the effects of azurin proteins on Toxoplasma. Our data demonstrate that Laz is a potent inhibitor of Toxoplasma and that the mechanism of action involves interference with SAG1-mediated attachment to host cells. These results are supported by structural data, binding assays, and the observation that parasites lacking SAG1 are more resistant to the inhibitory effects of Laz. Significantly, these studies further illustrate that azurin proteins produced by bacteria may serve to inhibit invasion and growth of a diverse range of intracellular pathogens, from viruses to invasive protozoa. Such broad-spectrum inhibitors may have important implications in the design of novel therapeutics against infectious disease.
MATERIALS AND METHODS
Materials.
The hyperexpression and purification of azurin and Laz were performed as previously described (11, 15, 28). SAG1 knockout and complemented SAG1 knockout parasites were created by Michael Grigg in the laboratory of John Boothroyd (Stanford University) and supplied by Ira Blader (University of Oklahoma) (1, 22). Anti-Toxoplasma antibody was supplied by Andrew Hemphill (University of Bern, Bern, Switzerland). Anti-SAG1 conjugated to fluorescein isothiocyanate (FITC) was obtained from Biodesign.
Surface plasmon resonance analysis.
Direct protein-protein interactions between azurin proteins or their glutathione S-transferase (GST)-peptide fusions with GST-SAG1 were determined using a Biacore X biosensor system (Biacore AB), which is based on surface plasmon resonance (SPR) technology. GST-SAG1 purchased from BioDesign International (Saco, ME) was exchanged from storage buffer (50 mM Tris-Cl-1.5 M urea-50% glycerol) into phosphate-buffered saline (PBS) buffer via injection over a semipreparative gel filtration column (SuperSW3000 from Tosoh Biosciences), and the major product was collected at between 7 and 9 min (monitoring A280) with the expected molecular mass (56 kDa) and freeze-dried on a Savant Speed-Vac model 110A for concentration. The purified GST-SAG1 was immobilized via amine coupling onto the Biacore CM5 biosensor chip in the following manner. Sequential injections of N-hydroxysuccinimide-N-(3-dimethylaminopropyl)-N-ethylcarbodiimide (0.05 M/0.2 M; 50 μl), GST-SAG1 (71 μM; 50 μl), 1 M ethanolamine (50 μl; pH 8.8), and 100 mM NaOH (10 μl) covalently linked the proteins to the CM5 sensor chip with the net increase in resonance signal of 594 resonance units (RU). Binding experiments were conducted via sequential injections of 2.7 to 150 nM of cupredoxins or GST-azurin (GST-Az) proteins in HBS-EP running buffer (0.01 M HEPES, pH 7.4, 0.15 M NaCl, 3 mM EDTA, 0.005% [vol/vol] surfactant P20) over the sensor surfaces at flow rates of 30 μl/min for 2.3 min (70-μl injection) with intermediate injections of 100 mM NaOH (10-μl pulse) to regenerate the GST-SAG1-CM5 surface. All binding experiments were run against a bare Au CM5 sensor surface (negative channel) to correct for nonspecific binding. The data were analyzed via subtraction of the negative channel (containing no GST-SAG1) from the positive channel, and the equilibrium resonances were fit to the Langmuir (1:1) binding model.
Computational analysis.
Structural similarities between azurin (1JZG) and anti-SAG1 4F11E12 monoclonal antibody (1YNT) were determined by using the VAST and DALI algorithms (10, 14). Structure-based pairwise sequence alignments were calculated using the VAST algorithm. The assessments of the structures were performed by using the DeepViewer program (12).
Parasite culture.
Toxoplasma tachyzoites (RH strain, from NIH AIDS Research and Reference Reagent Program, catalog no. 2859) were cultivated in confluent monolayers of human foreskin fibroblasts (HFF) or African green monkey kidney (Vero) cells. Host cells were grown in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (Invitrogen) with no antibiotics. Infected host cells were grown in DMEM supplemented with 1% fetal bovine serum, free of antibiotics, in a humidified 37°C incubator with 5% CO2. Parasites were tested to confirm negativity for mycoplasma using the Cambrex MycoAlert Mycoplasma kit (Turner Biosystems) prior to initiation of assays.
Toxoplasma growth assays.
Plaque assays were performed as outlined by Roos et al. (23). Briefly, T 25-cm2 tissue culture flasks containing confluent monolayers of host cells were inoculated with 104 freshly lysed and filtered tachyzoites and allowed to incubate undisturbed at 37°C for 5 days. Infected monolayers were then washed in PBS, fixed in methanol, and stained with crystal violet solution (Sigma). Toxoplasma growth assays based on detection of the parasite-specific B1 gene (2) were carried out as previously described (6). Briefly, genomic DNA from infected wells was harvested using the DNeasy kit (Qiagen) and used in SYBR green-based quantitative PCR with the 7500 real-time PCR system (Applied Biosystems). The parasite count for a given sample was calculated by interpolation from a standard curve (6).
Toxoplasma adhesion/invasion assay.
Filter-purified tachyzoites were pretreated with 1.0 μM Laz for 1 h at 37°C. Parasites were collected by centrifugation (6,000 rpm, 4°C, 5 min) and washed in DMEM. Following washing, 3 × 105 tachyzoites were inoculated into 96-well plates and incubated at 37°C for 30 min. The adhesion/invasion assay was carried out as previously described (21). Parasite quantification was performed using the B1 gene detection assay by real-time PCR as described above. Differences in the number of parasites adhering to host cells can be directly compared between samples. The invasion rate for a given sample is represented as the percentage of parasites that were intracellular relative to the total number of parasites that adhered to host cells.
Immunofluorescence assay.
Freshly lysed, filter-purified parasites (1 × 107) were incubated at 37°C with or without 1 μM Laz for 30 min. Parasites were collected by centrifugation in a microcentrifuge for 5 min at 6,000 rpm and resuspended in 3% paraformaldehyde in PBS. Samples were allowed to settle on poly-l-lysine-coated coverslips for 30 min. After a blocking step (3% bovine serum albumin in PBS for 1 h at room temperature), coverslips were probed with a 1:20 dilution of anti-SAG1 monoclonal antibody conjugated to FITC (C65203M; Biodesign) in 0.3% bovine serum albumin and PBS. For anti-Toxoplasma applications, antiserum was used at 1:1,000, followed by antirabbit-FITC secondary antibody (Molecular Probes).
RESULTS
Effects of azurin and Laz on Toxoplasma in vitro.
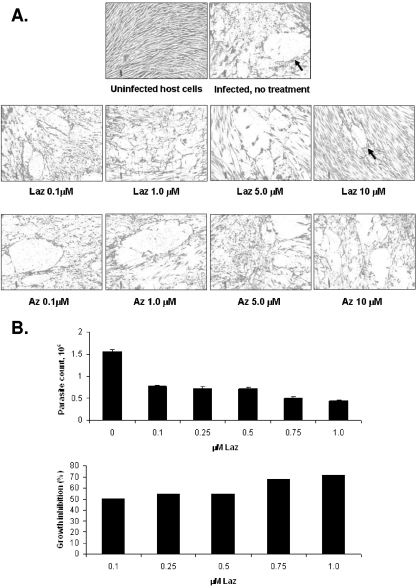
Azurin proteins have been shown to be effective against viruses and Plasmodium falciparum. We tested if purified azurin proteins impact Toxoplasma tachyzoites in vitro. Visual inspection of parasite plaque assays shows that Laz and to a lesser extent azurin clearly reduce the number of plaques in a dose-dependent manner (Fig. 1A). Not only did parasites incubated in the presence of azurin proteins, particularly Laz, exhibit fewer plaques than mock-treated samples (Table 1), but the plaque size was also substantially reduced (Fig. 1A). Uninfected HFF host cells treated with the same concentrations of azurin or Laz showed no overt signs of toxicity or stress, as gauged by microscopic analysis. The lack of toxicity is consistent with results in previous studies on human fibroblasts treated with azurin or Laz (4, 28). Since the effects of Laz were more pronounced than those of azurin, subsequent studies were performed only with the former. PCR-based B1 gene detection was performed as an independent means to verify Laz as a deterrent for Toxoplasma growth. As shown in Fig. 1B, as little as 0.1 μM Laz is sufficient to curtail parasite growth up to ∼50% and 1.0 μM Laz reduces parasite growth by 70%. The results of the B1 assay are consistent with those of the plaque assays, validating Laz as a potent inhibitor of Toxoplasma in culture. The reduction in the number of plaques suggests an effect on adhesion of the parasite to its host cell.
FIG. 1.
Growth-inhibitory properties of azurin proteins for Toxoplasma in vitro. (A) Plaque assay of Toxoplasma cultured with increasing concentrations of azurin proteins. Toxoplasma tachyzoites (2 × 104) were inoculated into culture flasks containing confluent monolayers of HFF host cells. Concurrent with parasite inoculation, the designated concentration of purified azurin protein was added to the media. Infected monolayers were incubated undisturbed at 37°C until day 5, at which point they were fixed and stained. The number of plaques per flask (see Table 1) was determined by visual inspection. Arrows indicate representative examples of a typical plaque size in untreated, infected host cells compared to the smaller plaques observed with Laz treatment. (B) The B1 gene detection assay to determine effects of Laz on Toxoplasma growth was performed in triplicate. Toxoplasma tachyzoites (1,000) were inoculated into 24-well plates containing confluent monolayers of HFF host cells. The indicated amount of Laz was added to culture medium during infection. After 3 days of incubation, the infected monolayer was harvested and DNA purified. Parasite DNA was quantified using real-time PCR for the Toxoplasma B1 gene. Data are represented as the parasite count extrapolated from the B1 PCR assay (upper panel) or percent growth inhibition relative to growth of parasites grown in parallel without Laz (lower panel). The results shown are representative of two independent experiments.
TABLE 1.
Effect of azurin proteins on Toxoplasma growth as measured by plaque assaya
| Sample | No. of plaques |
|---|---|
| Control, untreated | >30 |
| Laz, 0.1 μM | 30 |
| Laz, 1.0 μM | 10 |
| Laz, 5.0 μM | 3 |
| Laz, 10 μM | 3 |
| Az, 0.1 μM | >30 |
| Az, 1.0 μM | 15 |
| Az, 5.0 μM | 15 |
| Az, 10 μM | 10 |
Toxoplasma-infected HFF monolayers were treated with increasing concentrations of azurin (Az) or azurin-like (Laz) proteins for 5 days and then processed for plaque assay (23). The number of parasite plaques in each flask is shown. The data shown are the results from one of three independent experiments that produced similar results.
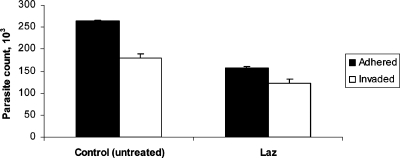
To assess if Laz perturbed the ability of Toxoplasma tachyzoites to adhere and invade HFF host cells, we performed an adhesion/invasion assay following pretreatment of the parasites with 1.0 μM Laz for 1.0 h. Tachyzoites pretreated with Laz exhibited a 41% decrease in their ability to adhere to host cells (Fig. 2). The invasion efficiency, i.e., the ability of Laz-treated tachyzoites to invade once attached to host cells, was not significantly reduced relative to results with the control sample.
FIG. 2.
Effects of Laz on Toxoplasma adhesion to host cells. Toxoplasma tachyzoites were pretreated with 1.0 μM Laz or mock treated (control) for 1 h, washed, and allowed to infect HFF monolayers for 30 min. The adhesion/invasion assay was carried out as described previously (21), with black bars representing the number of parasites adhering to host cells and white bars representing the number of parasites that invaded host cells. Assays were performed in triplicate, with error bars denoting the standard deviation from the mean for the three replicates.
Structural analysis of azurin and surface antigen SAG1.
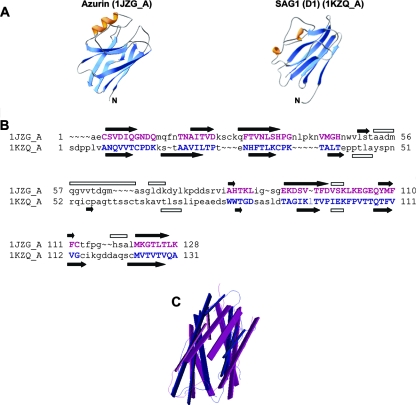
It has previously been noted that azurin exhibits structural similarity to the individual domains D1 and D2 of the surface antigen SAG1 from Toxoplasma (13). Specifically, azurin shows considerable topological similarity (in having a common type of Greek key β-barrel) to SAG1 (Fig. 3A). To expand on this analysis, we used the VAST and DALI programs (10, 14) to perform a structural comparison of these two proteins. The results yielded alignments with significant scores of 11.4 for the VAST score (out of a maximum possible score of 15.7) and 3.0 for the DALI score (scores of <2.0 are structurally dissimilar). Figure 3B and C illustrate that the secondary elements that form the core of the Greek-key β-barrel arranged in two sheets superimpose very well, with a root mean square deviation of 2.6 Å (calculated over 69 structurally equivalent Cα atoms). In contrast, the number and arrangement of α-helices are much less conserved (Fig. 3A and B). This level of tertiary structure is striking considering that azurin and SAG1 exhibit less than 10% primary amino acid sequence identity. These data suggest that azurin may be capable of mimicking SAG1.
FIG. 3.
Structural analysis of azurin and SAG1. (A) Ribbon drawings of azurin (1jzg_A) from P. aeruginosa and the surface antigen (SAG1; D1 monomer) from Toxoplasma showed at a similar orientation and with the same coloring pattern (orange for helices, blues for strands, and gray for loops). Pictures were drawn using the DeepViewer program (12). (B) Structural alignment of azurin (1jzg_A) from P. aeruginosa with surface antigen (SAG1; D1 monomer) from Toxoplasma, as computed by the VAST algorithm (10). Superimposed secondary structure elements are denoted by capital purple and blue letters (for azurin and SAG1, respectively). Dashes and lowercase lettering indicate where there are no alignments. Secondary structure elements according to the structures are illustrated as black arrows for β-sheets and open rectangles for helices. (C) Backbone representation of the superimposition of the aligned residues of azurin (1jzg; shown in purple) and surface antigen (SAG1_D1 monomer; shown in blue).
Binding studies of interaction between SAG1 and azurin proteins.
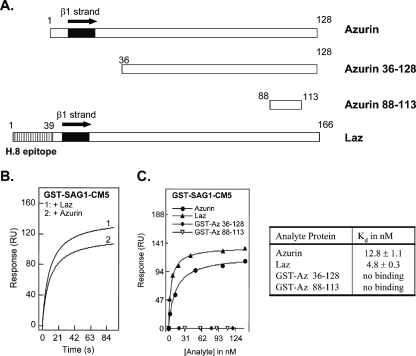
Based on the structural data, in vitro protein-protein interaction studies were performed to assess the potential for binding of SAG1 to azurin proteins. For these assays, purified GST-SAG1 was tested against full-length Laz or azurin. Additionally, two truncated versions of azurin (with GST fusion tags) were available and were used to facilitate mapping of the potential interaction site (Fig. 4A). It should be noted that we previously ruled out any interaction of azurin or Laz with GST alone (4), and this was again confirmed in these experiments (data not shown). Surface plasmon resonance studies yielded positive binding interactions of azurin proteins with GST-SAG1 in the nanomolar concentration range, as depicted in Fig. 4B and C. Each azurin protein, when injected at a fixed concentration (100 nM) over the GST-SAG1 modified sensor surface, led to an increase in the net resonance signals (after subtraction of the negative channel to account for nonspecific binding to the chip), and these changes were recorded over a short time period. The equilibrium resonance values varied from 122 RU (Laz) to 95 RU (azurin), and this trend was indicative of the binding affinities determined after concentration-dependent titrations were conducted for each protein over the GST-SAG1-CM5 sensor surface. Figure 4C depicts the binding curves after injection of the analyte sequentially within the nanomolar concentration regime (2.78 to 142 nM). A Langmuir (1:1) binding model {Req = Rmax/[1 + (Kd/C)], where Req is the equilibrium resonance signal, Rmax is the saturating resonance signal, and C is the analyte concentration} was applied to fit the resonance data for each titration in order to determine equilibrium binding dissociation constants (Kd). Binding constants are 4.8 ± 0.3 nM and 12.8 ± 1.1 nM for Laz and azurin, respectively. Neither of the truncated azurin proteins (GST-Az 36-128 or GST-Az 88-113) could bind to GST-SAG1 (Fig. 4C).
FIG. 4.
Surface plasmon resonance binding titrations depicting the interactions of azurin and Laz with GST-SAG1. (A) Schematic diagram displaying the azurin proteins used in the SPR assay. Note that Laz has an additional 39 amino acids, termed the H.8 epitope, in the N-terminal region of the Neisserial azurin protein. (B) SPR time traces for the injection of 100 nM of the azurin proteins (50 μl) onto a GST-SAG1-CM5 sensor chip at a flow rate of 30 μl/min after subtraction of nonspecific binding to the negative channel (i.e., bare Au CM6 chip). (C) Concentration-dependent binding of the azurin proteins to GST-SAG1 was determined via injection of various amounts (2.7 to 150 nM) over the sensor surface, and the extent of binding was evaluated as a function of the equilibrium resonance response value measured in resonance units. The data table lists the Kd value (nM) for binding of the analyte sample to GST-SAG1.
Laz interferes with SAG1-mediated invasion of host cells.
Based on the structural data obtained by computational approaches and SPR binding assays with azurin proteins and SAG1, we hypothesized that Laz may operate, at least in part, by interfering with SAG1-mediated invasion. To test this, we examined if Laz had the ability to block anti-SAG1 antibody from binding to the parasite surface. Figure 5 shows that incubating tachyzoites with 1.0 μM Laz completely abolishes the ability of anti-SAG1 to bind to SAG1. The ability of Toxoplasma antiserum to bind parasites was generally not affected in the Laz-treated parasites, although a decreased intensity of staining can be observed at the membrane of most tachyzoites (Fig. 5). The observation that only the membrane staining was perturbed by Laz treatment is consistent with Laz acting on a membrane-bound protein.
FIG. 5.
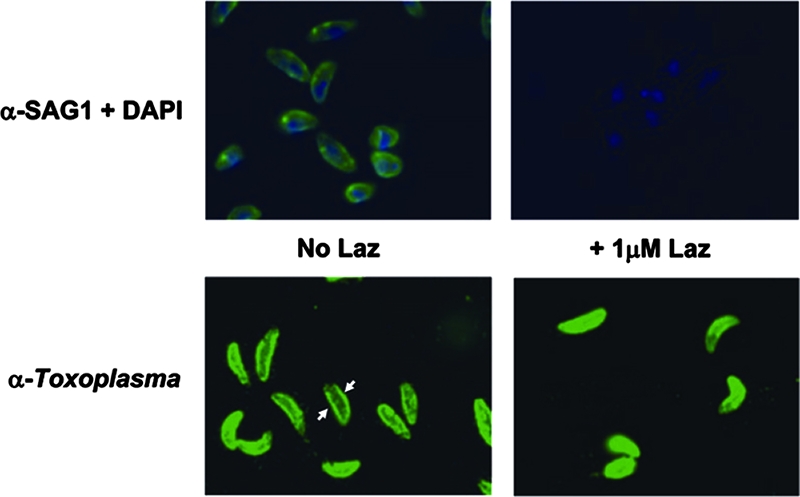
Treatment with Laz blocks anti-SAG1 binding. Tachyzoites were used in an immunofluorescence assay to detect SAG1, which normally stains the outer membrane of the parasite (green, top left panel). When tachyzoites were treated with 1.0 μM Laz, anti-SAG1 is no longer able to bind the parasite surface (top right panel). 4′,6-Diamidino-2-phenylindole (DAPI), which binds DNA, was used as a costain for reference (blue). The lower panels show staining with antibody generated against Toxoplasma (anti-Toxoplasma) with (right) or without (left) the 1.0 μM Laz treatment. Arrows highlight the more-intense staining of the parasite plasma membrane (seen as a ring encircling the parasite), to be contrasted with the less-intense membrane staining (lack of ring) in the Laz-treated parasites.
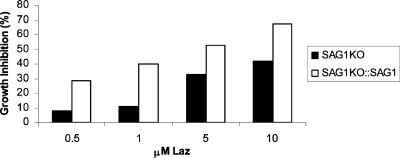
Additionally, we reasoned that if Laz interferes with SAG1, then parasites deficient in SAG1 may be partially immune to the effects of Laz. To test this hypothesis, we monitored the effects of Laz on a mutant Toxoplasma clone lacking SAG1 (SAG1KO) relative to a complemented version of SAG1KO, engineered to stably express ectopic SAG1 (1, 22). Figure 6 shows that the growth inhibition caused by Laz is diminished in parasites lacking the SAG1 protein. The presence of SAG1 thus makes parasites more susceptible to Laz. This argues that the inhibitory effects of Laz can be attributed, at least in part, to interference of SAG1-mediated attachment to host cells.
FIG. 6.
Parasites lacking SAG1 are not as susceptible to the growth-inhibitory effects of Laz. Toxoplasma containing a disruption at the SAG1 genomic locus (SAG1KO; black bars) or SAG1KO complemented with recombinant SAG1 (SAG1KO::SAG1; white bars) was incubated in the presence of increasing concentrations of the Laz protein. Percentages of growth inhibition were determined using the B1 parasite growth assay.
DISCUSSION
We report here that the azurin protein Laz is a novel inhibitor effective against Toxoplasma. Results from our in vitro adhesion-and-invasion assays support the idea that Laz impairs parasite growth by interfering with attachment of the tachyzoite to the host cell (Fig. 2). Once the parasite attaches, the capacity to invade is not greatly diminished. Structural analysis of azurin and SAG1 suggests the former may mimic the latter, which could interfere with parasite invasion by (i) blocking dimerization of SAG1 monomers and/or (ii) competing for SAG1-binding sites on host cell surface receptors. Additional analysis identified that azurin also has structural similarity to the immunoglobulin variable domains (light and heavy chains) of the anti-SAG1 4F11E12 monoclonal antibody (data not shown). This analysis is coincident with a previous observation that demonstrates a low but discernible superposition between cupredoxins, such as azurin, and members of the immunoglobulin superfamily (8, 25). It is thus plausible that azurin proteins may mimic the Fab fragment of anti-SAG1 and bind to SAG1, thereby interfering with Toxoplasma invasion of host cells. The areas of similarity to anti-SAG1 and the SAG1 protein itself are located in different regions of the azurin protein.
Azurin proteins are also effective against fellow apicomplexan parasite P. falciparum (4), but our studies described here show that inhibition of Toxoplasma occurs through a different portion of Laz and via a different surface antigen. We previously reported that GST-Az 36-128 showed significant binding with the P. falciparum MSP1 (cleavage product MSP1-19), with Kd values of 24.5 nM, while binding of azurin to MSP1 showed a Kd value of 32.2 nM, suggesting that the extreme N terminus of azurin (amino acids 1 to 35) played no role in MSP1 binding (4). In contrast, this 35-amino-acid N-terminal portion of azurin is important for its binding to Toxoplasma SAG1. Thus, these data illustrate a novel feature of azurin-mediated inhibition of parasite invasion. It is also worth noting that Laz binds with greater affinity to SAG1 than azurin, consistent with our studies showing that Laz is a more potent inhibitor of Toxoplasma growth than azurin (Fig. 1A).
Azurin has been shown to associate with dendritic cell-specific adhesion receptor and intercellular adhesion molecule 3 on human cells (4), as well as receptor tyrosine kinase EphB2 (5). While pretreatment of parasites alone is sufficient to significantly prevent invasion, the possibility that Laz may also disrupt Toxoplasma invasion through actions on the host cell surface is an interesting question subject to future investigation.
Azurin proteins display a remarkable promiscuity in targeting multiple diseases caused by both viral and protozoal infections and thus may represent a type of “progenitor” immune response used by prokaryotes. It is important to note that a single protein, such as Laz or azurin, inhibits not only HIV-1 growth but also that of the malarial parasite and Toxoplasma. It would be valuable to test if azurin proteins could be developed as a novel broad-spectrum drug to treat not only disease caused by apicomplexan parasites or HIV-1 but coinfections as well.
Acknowledgments
This investigation was supported by a Sponsored Research Agreement between CDG Therapeutics and the University of Illinois at Chicago (UIC) and by a contract with the Indiana University School of Medicine (IUSM), Indianapolis.
We thank Michael Grigg, John Boothroyd, and Ira Blader for kindly providing SAG1 mutant parasites and Andrew Hemphill for supplying anti-Toxoplasma antibody. We are grateful to Sherry Queener (IUSM) for critically reading the manuscript.
Footnotes
Published ahead of print on 10 December 2007.
REFERENCES
- 1.Brenier-Pinchart, M. P., I. Villena, C. Mercier, F. Durand, J. Simon, M. F. Cesbron-Delauw, and H. Pelloux. 2006. The Toxoplasma surface protein SAG1 triggers efficient in vitro secretion of chemokine ligand 2 (CCL2) from human fibroblasts. Microbes Infect. 8:254-261. [DOI] [PubMed] [Google Scholar]
- 2.Burg, J. L., C. M. Grover, P. Pouletty, and J. C. Boothroyd. 1989. Direct and sensitive detection of a pathogenic protozoan, Toxoplasma gondii, by polymerase chain reaction. J. Clin. Microbiol. 27:1787-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carruthers, V., and J. C. Boothroyd. 2007. Pulling together: an integrated model of Toxoplasma cell invasion. Curr. Opin. Microbiol. 10:83-89. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhari, A., A. M. Fialho, D. Ratner, P. Gupta, C. S. Hong, S. Kahali, T. Yamada, K. Haldar, S. Murphy, W. Cho, V. S. Chauhan, T. K. Das Gupta, and A. M. Chakrabarty. 2006. Azurin, Plasmodium falciparum malaria and HIV/AIDS: inhibition of parasitic and viral growth by Azurin. Cell Cycle 5:1642-1648. [DOI] [PubMed] [Google Scholar]
- 5.Chaudhari, A., M. Mahfouz, A. M. Fialho, T. Yamada, A. T. Granja, Y. Zhu, W. Hashimoto, B. Schlarb-Ridley, W. Cho, T. K. Das Gupta, and A. M. Chakrabarty. 2007. Cupredoxin-cancer interrelationship: azurin binding with EphB2, interference in EphB2 tyrosine phosphorylation, and inhibition of cancer growth. Biochemistry 46:1799-1810. [DOI] [PubMed] [Google Scholar]
- 6.Costa, J. M., C. Pautas, P. Ernault, F. Foulet, C. Cordonnier, and S. Bretagne. 2000. Real-time PCR for diagnosis and follow-up of Toxoplasma reactivation after allogeneic stem cell transplantation using fluorescence resonance energy transfer hybridization probes. J. Clin. Microbiol. 38:2929-2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferreira, M. S., and A. S. Borges. 2002. Some aspects of protozoan infections in immunocompromised patients—a review. Mem. Inst. Oswaldo Cruz 97:443-457. [DOI] [PubMed] [Google Scholar]
- 8.Fialho, A. M., F. J. Stevens, T. K. Das Gupta, and A. M. Chakrabarty. 2007. Beyond host-pathogen interactions: microbial defense strategy in the host environment. Curr. Opin. Biotechnol. 18:279-286. [DOI] [PubMed] [Google Scholar]
- 9.Gagne, S. S. 2001. Toxoplasmosis. Prim. Care Update Ob/Gyns 8:122-126. [DOI] [PubMed] [Google Scholar]
- 10.Gibrat, J. F., T. Madej, and S. H. Bryant. 1996. Surprising similarities in structure comparison. Curr. Opin. Struct. Biol. 6:377-385. [DOI] [PubMed] [Google Scholar]
- 11.Goto, M., T. Yamada, K. Kimbara, J. Horner, M. Newcomb, T. K. Gupta, and A. M. Chakrabarty. 2003. Induction of apoptosis in macrophages by Pseudomonas aeruginosa azurin: tumour-suppressor protein p53 and reactive oxygen species, but not redox activity, as critical elements in cytotoxicity. Mol. Microbiol. 47:549-559. [DOI] [PubMed] [Google Scholar]
- 12.Guex, N., and M. C. Peitsch. 1997. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18:2714-2723. [DOI] [PubMed] [Google Scholar]
- 13.He, X. L., M. E. Grigg, J. C. Boothroyd, and K. C. Garcia. 2002. Structure of the immunodominant surface antigen from the Toxoplasma gondii SRS superfamily. Nat. Struct. Biol. 9:606-611. [DOI] [PubMed] [Google Scholar]
- 14.Holm, L., and C. Sander. 1993. Protein structure comparison by alignment of distance matrices. J. Mol. Biol. 233:123-138. [DOI] [PubMed] [Google Scholar]
- 15.Hong, C. S., T. Yamada, W. Hashimoto, A. M. Fialho, T. K. Das Gupta, and A. M. Chakrabarty. 2006. Disrupting the entry barrier and attacking brain tumors: the role of the Neisseria H.8 epitope and the Laz protein. Cell Cycle 5:1633-1641. [DOI] [PubMed] [Google Scholar]
- 16.Jacquet, A., L. Coulon, J. De Neve, V. Daminet, M. Haumont, L. Garcia, A. Bollen, M. Jurado, and R. Biemans. 2001. The surface antigen SAG3 mediates the attachment of Toxoplasma gondii to cell-surface proteoglycans. Mol. Biochem. Parasitol. 116:35-44. [DOI] [PubMed] [Google Scholar]
- 17.Johnson, A. M., P. J. McDonald, and S. H. Neoh. 1983. Monoclonal antibodies to Toxoplasma cell membrane surface antigens protect mice from toxoplasmosis. J. Protozool. 30:351-356. [DOI] [PubMed] [Google Scholar]
- 18.Jung, C., C. Y. Lee, and M. E. Grigg. 2004. The SRS superfamily of Toxoplasma surface proteins. Int. J. Parasitol. 34:285-296. [DOI] [PubMed] [Google Scholar]
- 19.Mineo, J. R., and L. H. Kasper. 1994. Attachment of Toxoplasma gondii to host cells involves major surface protein, SAG-1 (P30). Exp. Parasitol. 79:11-20. [DOI] [PubMed] [Google Scholar]
- 20.Mineo, J. R., R. McLeod, D. Mack, J. Smith, I. A. Khan, K. H. Ely, and L. H. Kasper. 1993. Antibodies to Toxoplasma gondii major surface protein (SAG-1, P30) inhibit infection of host cells and are produced in murine intestine after peroral infection. J. Immunol. 150:3951-3964. [PubMed] [Google Scholar]
- 21.Naguleswaran, A., N. Muller, and A. Hemphill. 2003. Neospora caninum and Toxoplasma gondii: a novel adhesion/invasion assay reveals distinct differences in tachyzoite-host cell interactions. Exp. Parasitol. 104:149-158. [DOI] [PubMed] [Google Scholar]
- 22.Rachinel, N., D. Buzoni-Gatel, C. Dutta, F. J. Mennechet, S. Luangsay, L. A. Minns, M. E. Grigg, S. Tomavo, J. C. Boothroyd, and L. H. Kasper. 2004. The induction of acute ileitis by a single microbial antigen of Toxoplasma gondii. J. Immunol. 173:2725-2735. [DOI] [PubMed] [Google Scholar]
- 23.Roos, D. S., R. G. Donald, N. S. Morrissette, and A. L. Moulton. 1994. Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 45:27-63. [DOI] [PubMed] [Google Scholar]
- 24.Slifko, T. R., H. V. Smith, and J. B. Rose. 2000. Emerging parasite zoonoses associated with water and food. Int. J. Parasitol. 30:1379-1393. [DOI] [PubMed] [Google Scholar]
- 25.Stevens, F. J., C. Kuemmel, G. Babnigg, and F. R. Collart. 2005. Efficient recognition of protein fold at low sequence identity by conservative application of Psi-BLAST: application. J. Mol. Recognit. 18:150-157. [DOI] [PubMed] [Google Scholar]
- 26.Vijgenboom, E., J. E. Busch, and G. W. Canters. 1997. In vivo studies disprove an obligatory role of azurin in denitrification in Pseudomonas aeruginosa and show that azu expression is under control of rpoS and ANR. Microbiology 143:2853-2863. [DOI] [PubMed] [Google Scholar]
- 27.Wong, S. Y., and J. S. Remington. 1993. Biology of Toxoplasma gondii. AIDS 7:299-316. [DOI] [PubMed] [Google Scholar]
- 28.Yamada, T., M. Goto, V. Punj, O. Zaborina, M. L. Chen, K. Kimbara, D. Majumdar, E. Cunningham, T. K. Das Gupta, and A. M. Chakrabarty. 2002. Bacterial redox protein azurin, tumor suppressor protein p53, and regression of cancer. Proc. Natl. Acad. Sci. USA 99:14098-14103. [DOI] [PMC free article] [PubMed] [Google Scholar]