Abstract
GS-9131 is a phosphonoamidate prodrug of the novel ribose-modified phosphonate nucleotide analog GS-9148 that demonstrates potent anti-human immunodeficiency virus type 1 (HIV-1) activity and an excellent resistance profile in vitro. Prodrug moieties were optimized for the efficient delivery of GS-9148 and its active diphosphate (DP) metabolite to lymphoid cells following oral administration. To understand the intracellular pharmacology of GS-9131, incubations were performed with various types of lymphoid cells in vitro. The intracellular accumulation and antiviral activity levels of GS-9148 were limited by its lack of cellular permeation, and GS-9131 increased the delivery of GS-9148-DP by 76- to 290-fold relative to that of GS-9148. GS-9131 activation was saturable at high extracellular concentrations, potentially due to a high-affinity promoiety cleavage step. Once inside the cells, GS-9148 was efficiently phosphorylated, forming similar amounts of anabolites in primary lymphoid cells. The levels of GS-9148-DP formed in peripheral blood mononuclear cells infected with HIV-1 were similar to that in uninfected PBMCs, and approximately equivalent intracellular concentrations of GS-9148-DP and tenofovir (TVF)-DP were required to inhibit viral replication by 90%. Once it was formed, GS-9148-DP was efficiently retained in activated CD4+ cells, with a half-life of 19 h. In addition, GS-9131 showed a low potential for drug interactions with other adenine nucleoside/nucleotide reverse transcriptase inhibitors, based on the lack of competition for anabolism between suprapharmacologic concentrations of GS-9148 and TVF and the lack of activity of GS-9131 metabolites against purine nucleoside phosphorylase, an enzyme involved in the clearance of 2′,3′-dideoxyinosine. Together, these observations elucidate the cellular pharmacology of GS-9131 and illustrate its efficient loading of lymphoid cells, resulting in a prolonged intracellular exposure to the active metabolite GS-9148-DP.
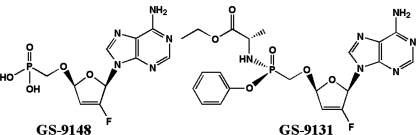
The 2′-fluorine ribose-modified nucleotide phosphonate analog [5-(6-amino-purin-9-yl)-4-fluoro-2,5-dihydro-furan-2- yloxymethyl]-phosphonic acid (GS-9148 or Fd4AP) (Fig. 1) is a selective inhibitor of wild-type and drug-resistant forms of human immunodeficiency virus type 1 (HIV-1) in vitro (5). Strains carrying mutations that cause resistance to nucleoside/nucleotide reverse transcriptase inhibitor (NRTI) therapy showed either a lack of resistance to GS-9148 (K65R, L74V, and M184V) or a less marked effect on the susceptibility to GS-9148 relative to that of FDA-approved NRTIs in vitro (multiple thymidine analog mutations) (5). Fluorine at the 2′ position was rationally designed into the molecule in order to increase the selectivity for HIV-1 reverse transcriptase (RT) over that of mitochondrial DNA polymerase γ (23), and enzymatic studies have illustrated that the diphosphate metabolite of GS-9148 (GS-9148-DP) is a potent inhibitor of wild-type and resistant RT while showing minimal inhibition of host polymerases (5).
FIG. 1.
Structures of the ribose-modified adenosine nucleotide analog GS-9148 (Fd4AP) and its ethanolalaninyl monoamidate, monophenol prodrug GS-9131.
Despite the promising in vitro profile of GS-9148, phosphonate nucleotide analogs have poor cellular permeation and oral bioavailability due to the charges on the phosphonic acid at physiologic pH levels (6, 31). In order to overcome these limitations, GS-9148 was derivatized with ethanolalaninyl monoamidate and monophenol promoieties to create the phosphonoamidate prodrug 9-(R)-4′-(R)-[[[(S)-1-[(ethoxycarbonyl)ethyl]amino]phenoxy-phosphonyl]methoxy]-2′-fluoro-1′-furanyladenine (GS-9131). In activated peripheral blood mononuclear cells (PBMCs) and CD4+ lymphocytes, GS-9131 shows higher anti-HIV-1 activity in vitro than the most potent FDA-approved NRTI 3′-deoxy-3′-azidothymidine (AZT), and following its oral administration to beagle dogs, GS-9131 had approximately 26% oral bioavailability, resulting in high and persistent levels of GS-9148-DP in circulating lymphocytes (5). In this report, we describe studies exploring the cellular pharmacology of GS-9148 and its prodrug, GS-9131, in various lymphoid cells in vitro.
(This work was presented in part at the 13th Conference on Retroviruses and Opportunistic Infections, 5 to 8 February 2006, Denver, CO [25].)
MATERIALS AND METHODS
Materials.
GS-9131, GS-9148, and GS-9148 monophosphate (MP) were prepared by the Medicinal Chemistry Department, Gilead Sciences, Inc. (Foster City, CA). The synthesis of GS-9148 and GS-9131 has been presented elsewhere (C. G. Boojamra, R. L. Mackman, D. Y. Markevitch, V. Prasad, A. S. Ray, J. Douglas, D. Grant, C. U. Kim, and T. Cihlar, submitted for publication; 20). Tenofovir (TFV) and its prodrug, TVF disoproxil fumarate (TDF), were provided by Gilead Sciences, Inc. TFV-DP and GS-9148-DP were synthesized from TFV and GS-9148, respectively, by Trilink Biotechonologies (San Diego, CA). TFV-MP was generously provided by Ivan Rosenberg, Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, Prague, Czech Republic. Tissue culture reagents were obtained from Mediatech Inc. (Herndon, VA). Nyosil M25 oil, used to separate lymphocytes from media, was obtained from TAI Lubricants, Inc. (Hockessin, DE). All other reagents were the highest quality available from Sigma-Aldrich (St. Louis, MO).
Cell culture.
PBMCs were isolated from human buffy coat samples (Stanford Blood Bank, Palo Alto, CA), using centrifugation in Ficoll Paque Plus (GE Healthcare, Piscataway, NJ) according to the manufacturer's procedure. PBMCs isolated from three to four independent donors were maintained in RPMI 1640 medium with 20% fetal bovine serum and antibiotics (the quiescent state) or were activated in the presence of interleukin 2 (20 units/ml; Roche Biochemicals, Indianapolis, IN) and phytohemagglutinin P (PHA-P; 1 μg/ml; Sigma) for 3 to 4 days before experiments were initiated. Human CD4+ T lymphocytes were purified from isolated and activated PBMCs by negative magnetic bead sorting on AutoMACS using a CD4+ T-cell Isolation Kit II (Miltenyi Biotec, Auburn, CA). Human transformed CCRF-CEM T cells were obtained from American Type Culture Collection (Manassas, VA) and cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum and antibiotics.
Uptake and egress of GS-9148 and its anabolites.
To characterize the intracellular metabolism rates of the prodrug and the parent phosphonate nucleotide analog, activated CD4+ lymphocytes were treated with either 1 μM GS-9131 or 100 μM GS-9148 for 2, 6, and 24 h. The retention and intracellular half-life of GS-9148 metabolites in cells were determined following treatment with GS-9131. Cells were preincubated for 24 h with 0.3 μM GS-9131, washed three times in fresh culture medium, and incubated in the absence of compound for 2, 4, 24, and 48 h. In all experiments, an aliquot of cells (2 × 106 to 3 × 106 cells) was collected at each time point, counted, pelleted by centrifugation, resuspended in 0.5 ml of the original treatment medium, and layered onto 0.5 ml of Nyosil M25 oil. Oil separation of cells from extracellular medium was done essentially as described previously (24). The samples were spun in a microcentrifuge for 20 s at the maximum speed (approximately 800 × g). The top layer of medium was removed, and the oil layer was washed twice with 0.8 ml of phosphate-buffered saline. The washing buffer and the oil layer were carefully removed, the cell pellet was resuspended in 0.5 ml of 70% methanol, and incubated overnight at −70°C to facilitate cell lysis.
Correlation of HIV-1 activity and intracellular metabolism for adenosine analogs in activated PBMCs.
The nucleoside analog 2′,3′-dideoxyinosine (ddI) and the amidate prodrugs of TFV and GS-9148 were incubated at concentrations anticipated to cause approximately 90% inhibition of HIV-1 (between 20 and 200 nM for amidate prodrugs and from 10 to 50 μM for ddI). Activated PBMCs were infected in bulk with the HIV-1 BaL strain (Advanced Biotechnologies, Inc., Columbia, MD) for 3 h at 37°C and washed three times in fresh medium. Infected cells were seeded into 24-well plates (3 × 106 cells/well), and dilutions of tested compounds were added to make a final assay volume of 1.5 ml. Viral inhibition was measured by p24 enzyme-linked immunosorbent assay (Beckman Coulter, Miami, FL) with samples taken at 96 and 120 h following the addition of compounds. Cell samples were taken at 24 and 60 h after the initiation of drug treatment in order to measure intracellular nucleotide analog levels and to correlate the levels with the percentage of viral inhibition observed. Samples were collected as described above, and intracellular metabolites were measured as specified in the nucleotide quantitation section below.
Metabolite quantitation.
Cell lysates stored in 70% methanol were centrifuged, and supernatants were collected and dried by vacuum and resuspended in 10 μl of tetrabutyl ammonium acetate containing the nonfluorinated analog of GS-9148-DP. Transient ion-pairing high-performance liquid chromatography coupled to positive ion electrospray tandem mass spectrometry (LC-MS-MS) was used to quantitate intracellular nucleotides. Methods were adapted from those described for the quantitation of acyclic phosphonate nucleotide analog adefovir, its phosphorylated metabolites, and natural nucleotides (30). Standard curves and quality control samples were generated for all analytes using extracts from untreated cells. Seven-point standard curves generally ranged from 0.03 to 20 pmol/million cells and had linearity in excess of an r2 equal to 0.99 for all analytes. The lower limits of quantitations for all analytes ranged from 0.05 to 0.1 pmol/million cells. Low- and high-concentration quality control samples (typically 0.2 and 10 pmol/million cells, respectively) were run with each analyte at the beginning and end of each analytical run to assure accuracy and precision within 20%.
Competition for anabolism between GS-9148 and TFV and their prodrugs.
CEM-CCRF cells and both quiescent and activated PBMCs were incubated with GS-9148 and TFV or with GS-9131 and TDF (the orally bioavailable prodrug of TFV), either alone or in two-drug combinations. Incubations were performed at concentrations expected to generate suprapharmacologic intracellular concentrations, to maximize the possibility of detecting antagonism. Therefore, 100 to 250 μM of the phosphonate nucleotide analogs or 1 μM of their prodrugs were incubated for 24 h in the presence of specified cells. Samples were collected at 2, 6, and 24 h as described above, and intracellular metabolites were measured as described above in Metabolite quantitation.
Inhibition of PNP.
A colorimetric 96-well assay was performed to assess the inhibition of human purine nucleoside phosphorylase (PNP)-mediated phosphorolysis of ddI, essentially as described previously (9, 24). Briefly, inhibition constants (Ki) were generated by obtaining binding curves for ddI (measuring the rate of phosphorolysis between 100 μM and 4 mM ddI) in the presence of different concentrations of GS-9148 or its phosphorylated metabolites or the positive control TFV-MP. Incubations included 20 nM human PNP, 35 mU of xanthine oxidase, 0.075% Triton X-100, 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyltetrazolium chloride, and 1 mM HEPES (pH 7.6). Nonlinear kinetic curve fitting was performed using Kaleidagraph version 3.5 (Synergy Software, Reading, PA).
RESULTS
Relative formation of GS-9148-DP following incubations with either GS-9148 or GS-9131.
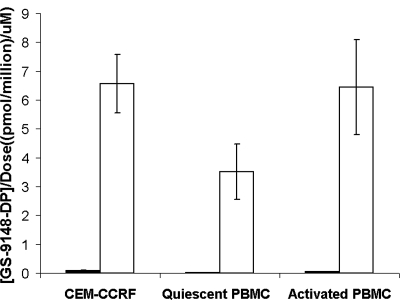
To test the effect of the addition of promoieties on the intracellular accumulation of the active metabolite, incubations were performed with various lymphoid cells in the presence of GS-9131 or GS-9148, and the levels of GS-9148-DP were compared upon quantitation of intracellular extracts. GS-9148 was incubated at a 100-fold higher concentration (100 μM) than GS-9131 (1 μM) to facilitate the accurate analysis of the much lower intracellular accumulation of metabolites observed following GS-9148 incubation. As shown in Fig. 2, GS-9131 induced 76-, 290-, and 140-fold increased levels of GS-9148-DP relative to that of GS-9148 following incubation with CEM-CCRF cells and quiescent and activated PBMCs, respectively.
FIG. 2.
Enhanced intracellular levels of GS-9148-DP following the incubation of lymphoid cells with GS-9131 (open bars) relative to that of GS-9148 (filled bars). Intracellular GS-9148-DP levels are normalized based on extracellular concentration following incubation with 1 μM GS-9131 or with 100 μM GS-9148. Values represent the means ± standard deviations of three independent incubations performed in duplicate.
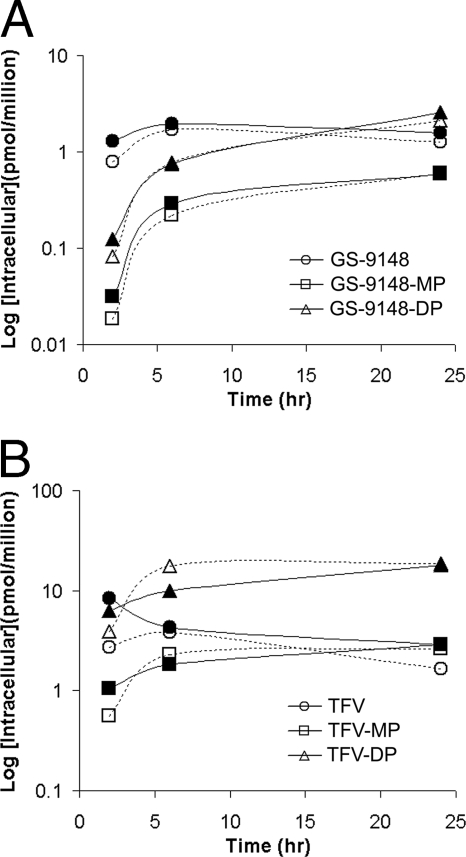
Intracellular anabolism of GS-9148 following incubations of lymphoid cells with GS-9131.
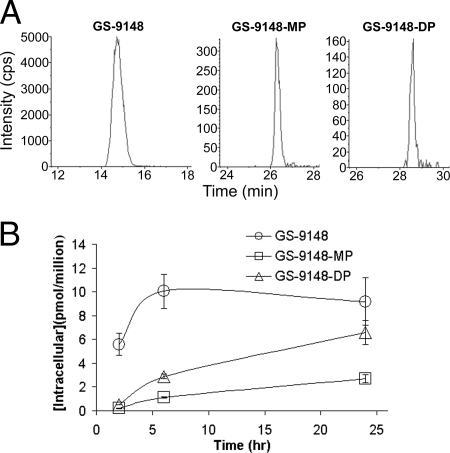
In the next set of studies, a more detailed investigation of the metabolism of GS-9131 in various lymphoid cells was performed. Intracellular GS-9148, GS-9148-MP, and GS-9148-DP were readily detected in cell extracts by LC-MS-MS (Fig. 3A). When CEM-CCRF T cells (a human lymphoblast cell line) were incubated with 1 μM GS-9131, GS-9148 was rapidly released inside the cells, and the drug levels appeared to plateau following approximately 6 h. GS-9148-MP accumulated slowly, followed by the formation of GS-9148-DP that increased linearly over 24 h (Fig. 3B). A similar concentration-versus-time profile was observed for GS-9148 and its phosphorylated metabolites with both quiescent and activated PBMCs, as well as with activated CD4+ cells isolated as the most relevant target subpopulation of PBMCs (data not shown). Taking into consideration the relative intracellular volume of the cell populations tested (4), no marked differences in the intracellular anabolism of GS-9131 were observed across primary lymphoid cells, while the CEM-CCRF T-cell line, with approximately fivefold larger intracellular volume, showed two- to fivefold less efficient accumulation (Table 1).
FIG. 3.
Intracellular metabolism of GS-9131 following incubation of CEM-CCRF cells with 1 μM GS-9131. (A) Detection of intracellular metabolites following a 24-h incubation by LC-MS-MS. GS-9148, GS-9148-MP, and GS-9148-DP were monitored in multiple-reaction-monitoring mode following the molecular ion transitions of 331.9 to 220.1, 412.1 to 220.1, and 492.0 to 220.1 m/z, respectively. Calculation of levels by comparing peak areas to authentic standard curves led to the determination of 7.15, 3.10, and 5.56 pmol/million cells of GS-9148, GS-9148-MP, and GS-9148-DP, respectively. (B) Intracellular accumulation of GS-9148 and its phosphorylated metabolites. Values represent the means ± standard deviations of three independent experiments done in duplicate.
TABLE 1.
Comparison of GS-9131 metabolism in different lymphoid cell types
| Cell type | Extracellular concn of GS-9131 (μM)a | Intracellular concn ± SD (pmol/million cells)b
|
||
|---|---|---|---|---|
| GS-9148 | GS-9148-MP | GS-9148-DP | ||
| CEM-CCRF | 1.0 | 9.18 ± 1.99 | 2.70 ± 0.33 | 6.60 ± 1.00 |
| Quiescent PBMCs | 1.0 | 7.92 ± 1.35 | 2.07 ± 0.90 | 3.90 ± 0.55 |
| Activated PBMCs | 1.0 | 11.6 ± 7.7 | 3.95 ± 2.63 | 5.64 ± 2.42 |
| Activated CD4+ | 0.3 | 1.70 ± 0.34 | 0.14 ± 0.01 | 1.50 ± 0.17 |
Cells were incubated for 24 h at the indicated concentrations.
Values represent the means ± standard deviations (SD) from two to three independent experiments done in duplicate. Quiescent and activated PBMC experiments were done with cells isolated from single donors, while activated CD4+ cell experiments were done with cells pooled from four donors.
Concentration dependence of GS-9131 activation in activated PBMCs.
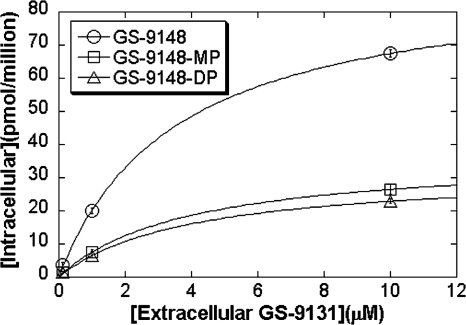
The dependence of the intracellular levels of GS-9148 and its phosphorylated metabolites on extracellular GS-9131 concentration was studied by incubating activated PBMCs with 0.1, 1, and 10 μM GS-9131 and measuring intracellular metabolites at 24 h. The results indicate that over the tested range of concentrations, intracellular metabolites do not increase proportionately with extracellular GS-9131 concentrations (Fig. 4). However, the ratio of all intracellular metabolites remained constant at different GS-9131 concentrations, suggesting that the process of the intracellular release of GS-9148 from its prodrug is the limiting step reaching saturation rather than the phosphorylation of GS-9148.
FIG. 4.
Concentration dependence of GS-9131 metabolism following the incubation of activated PBMCs with 0.1, 1, and 10 μM extracellular GS-9131 for 24 h. Data were fitted to hyperbolic equations showing that GS-9148, GS-9148-MP, and GS-9148-DP had an apparent saturation constant (the concentration required to cause 50% saturation) of approximately 4 μM. Values represent the means ± standard deviations of two independent experiments performed in duplicate in activated PBMCs pooled from three donors.
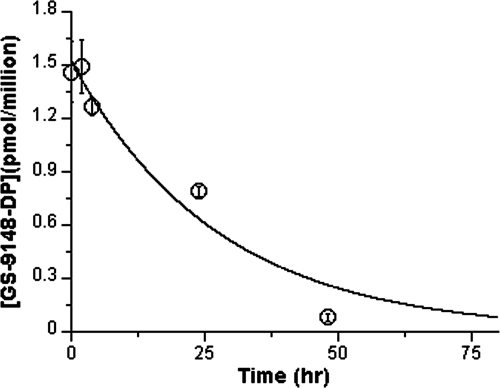
Persistence of GS-9148-DP in activated CD4+ cells.
Phosphonate nucleotide analogs have been shown to exhibit long intracellular half-lives with a variety of cell types in vitro (8, 26, 28) and in vivo (13, 21). To understand the persistence of GS-9148-DP, egress studies were performed with activated CD4+ cells. When cells were pretreated with GS-9131 for 24 h and GS-9131 was subsequently removed from the culture medium, GS-9148-DP was observed to persist, with a half-life of 19 h (Fig. 5). Similarly long intracellular half-lives were observed for GS-9148 and GS-9148-MP (data not shown).
FIG. 5.
Egress of GS-9148-DP following the incubation of activated CD4+ lymphocytes with 0.3 μM GS-9131 for 24 h. Following the removal of GS-9131 from culture medium, GS-9148-DP persisted in activated CD4+ lymphocytes, with a half-life of 19 h. Results represent the means ± standard deviations of two independent experiments done in duplicate, with cells pooled from four donors. Data were fitted to a single exponential decay curve.
Intrinsic intracellular anti-HIV-1 potency of GS-9148-DP relative to that of active metabolites of other adenosine NRTIs.
The intrinsic intracellular potency levels of the active metabolites of GS-9148, TFV, and ddI were compared by determining the intracellular concentrations of GS-9148-DP, TFV-DP, and 2′,3′-ddATP, causing approximately 90% inhibition of viral replication in HIV-1-infected PBMCs in vitro (Table 2). The intracellular concentration of active metabolites generally varied by less than twofold between cell samples taken after 24 and 60 h of incubation, indicating that steady-state concentrations were reached over the time interval during which viral replication was measured. At 60 h postinfection, intracellular concentrations of 1.86, 3.65, and 0.20 pmol/million cells correlated with 99.3, 91.6, and 93% viral inhibition at 120 h for GS-9148-DP, TFV-DP, and ddATP, respectively. These results suggest a ranked order in which the intrinsic intracellular potency of ddATP > GS-9148-DP > TFV-DP. A similar conclusion can be drawn from the intracellular levels generated under different incubation conditions, the metabolite levels at 24 h, and the antiviral activity at 96 h.
TABLE 2.
Comparison of intracellular concentrations of active metabolites of GS-9148, TFV, and ddIa
| NRTI | Monoamidate prodrugb | Incubation concn (nM) | Active metabolite concn (pmol/ million cells)c
|
% Inhibition of HIV-1 activity
|
||
|---|---|---|---|---|---|---|
| 24 h | 60 h | 96 h | 120 h | |||
| GS-9148 | Ethanolalaninyld | 200 | 0.85 | 0.90 | 79.5 | NDe |
| Cyclobutanolalaninyl | 100 | 1.55 | 1.86 | 92.0 | 99.3 | |
| 500 | 4.62 | 4.95 | 94.5 | 100 | ||
| TFV | Isopropanolalaninyl | 20 | 0.63 | 0.81 | 83.8 | ND |
| 25 | 1.55 | 3.65 | 84.4 | 91.6 | ||
| 125 | 7.89 | 12.7 | 96.1 | 100 | ||
| ddI | NAf | 10,000 | 0.12 | 0.20 | 87.2 | 93.0 |
| 50,000 | 0.67 | 0.80 | 96.0 | 100 | ||
Intracellular concentrations of the di- or triphosphorylated active metabolites of GS-9148, TFV or ddI are compared to antiviral effects with activated PBMCs infected with HIV-1.
Incubations were done with a stereoisomeric mixture (around phosphorus) of two GS-9148 monoamidate monophenol prodrugs and a single stereoisomer of TFV isopropanolalaninyl monophenol (GS-7340).
Intracellular concentrations of GS-9148-DP, TFV-DP, and ddATP were measured after the incubation of phosphonoamidate prodrugs GS-9148 and TFV or ddI, respectively. Values represent the means of active metabolite levels and antiviral activities from duplicate wells. A difference of less than 15% was observed between duplicate samples, and consistent results were observed across test concentrations.
GS-9131 is a single stereoisomer of the ethanolalaninyl monophenol mixture.
ND, not determined.
NA, not applicable. The nucleoside ddI was incubated in its parent form.
Interaction studies between GS-9148 and TFV.
In order to determine if the metabolism of the ribose-modified adenosine phosphonate nucleotide analog GS-9148 might potentially be antagonized by the presence of the acyclic adenosine phosphonate nucleotide analog TFV and vice versa, coincubations were performed with the parent molecules and their prodrugs in lymphoid cells. The incubation of CEM-CCRF cells or pooled quiescent PBMCs from five donors with 100 μM TFV and 100 μM GS-9148 resulted in no intracellular drug interaction. Similarly, the coincubation of activated PBMCs with 250 μM TFV and 157 μM GS-9148 resulted in no evidence of an alteration in the intracellular anabolism of either phosphonate nucleotide analog (data not shown). The nucleotide prodrugs TDF and GS-9131 increase cellular loading and require hydrolytic steps to release their parent phosphonate nucleotide analogs for further phosphorylation. The coincubations of 1 μM TDF with 1 μM GS-9131 resulted in no marked change in the levels of their respective intracellular metabolites in CEM-CCRF cells and in both quiescent and activated PBMCs (Fig. 6 and data not shown). The levels of TFV-DP observed for activated PBMCs under these incubation conditions exceeded the levels measured in patients by greater than 100-fold (85 to 110 fmol/million cells observed with patient PBMCs [13, 21] versus approximately 20 pmol/million cells observed for this study). In addition, no evidence for inhibition of the phosphorylation of TFV and GS-9148 was observed following incubation with several other phosphonoamidate prodrugs of either agent in lymphoid cells (data not shown).
FIG. 6.
Lack of an intracellular metabolic interaction between TDF and GS-9131 in activated PBMCs. (A) GS-9131 (1 μM) either alone (closed symbols and solid lines) or in combination with 1 μM TDF (open symbols and dotted lines) was metabolized to GS-9148, GS-9148-MP, and GS-9148-DP to similar extents. (B) TDF (1 μM) either alone (closed symbols and solid lines) or in combination with 1 μM GS-9131 (open symbols and dotted lines) was metabolized to TFV, TFV-MP, and TFV-DP to similar extents. Values represent the averages of duplicate samples from a representative experiment performed in activated PBMCs from a single donor.
Inhibition of PNP by GS-9148 and its phosphorylated metabolites.
PNP is an enzyme involved in the purine nucleoside salvage pathway. Previous studies have shown that the phosphorylated metabolites of acyclic nucleoside and acyclic phosphonate nucleotide analogs can potently inhibit PNP (1, 17, 24, 35). To determine if the ribose-modified phosphonate nucleotide analog GS-9148 and its anabolites have a similar effect, enzymatic studies were performed with purified human PNP. GS-9148, GS-9148-MP, and GS-9148-DP all showed Ki values of >100 μM for the PNP-catalyzed phosphorolysis of ddI (data not shown). In a side-by-side comparison, TFV-MP was found to have a Ki value of 74.7 nM, a result consistent with the Ki value reported previously (24).
DISCUSSION
While the current standard-of-care first-line therapies for HIV-1 have been shown to be safe and efficacious (11), the need for therapy for patients harboring viral strains with high-level resistance to NRTIs has not been met (33). Extensive pharmacological profiling suggests that GS-9131 may be effective against a broad range of NRTI-resistant HIV-1 variants based on (i) the unparalleled resistance profile of GS-9148, including in vitro activity against common NRTI resistance mutations (5); and (ii) the ability of the phosphonoamidate prodrug to deliver sufficiently high levels of active metabolite to infected cells to potentially overcome resistant virus (5, 19). Here, we have explored in detail the intracellular pharmacology of GS-9131 and have characterized its activation in lymphoid cells.
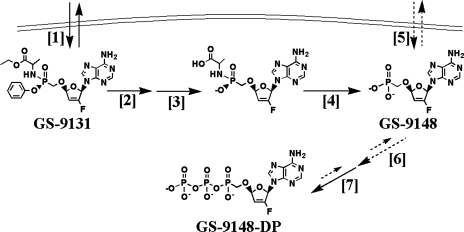
The putative mechanism for the intracellular activation of GS-9131 is summarized in Fig. 7. The marked increase in intracellular levels following incubation with GS-9131, relative to that of GS-9148, reflects both the increased permeation of the more lipophilic prodrug and the efficient intracellular promoiety cleavage. Similar increases in intracellular accumulation and antiviral activity have been observed for GS-7340, a related phosphonoamidate prodrug of TFV (19). A recent report found that lysosomal serine protease cathepsin A is at least in part responsible for the intracellular activation of GS-9131 and GS-7340 in lymphoid cells (3). Increased intracellular accumulation of the substantially more carboxylesterase-reactive substrate TDF has also been observed in vitro; however, these effects are likely less relevant in vivo due to the substantially lower stability of disoproxil promoieties in the systemic circulation (28). Saturable cell loading following incubation at different concentrations of GS-9131 was observed. Based on the constant ratio of GS-9148 to its phosphorylated metabolites, it does not appear that this phenomenon is due to the saturation of GS-9148 phosphorylation. While the observed saturation likely reflects a high affinity step in the cleavage of GS-9131, the saturation is probably not due to limiting GS-9131 activation by cathepsin A based on the reported Km of 363 μM for GS-9131 (3). Saturation may occur during the subsequent step of phosphonoamidate bond cleavage. Importantly, the saturation is likely to have little impact on the in vivo loading of lymphoid cells as GS-9131 is expected to reach a maximal plasma concentration substantially below 10 μM at efficacious clinical doses.
FIG. 7.
Putative mechanism of intercellular activation of GS-9131 and GS-9148. Arrows with solid lines represent efficient metabolic steps, while arrows with dashed lines represent more inefficient metabolic processes. First, the lipophilic prodrug GS-9131 freely permeates the cell [1] and is cleaved by lysosomal protease cathepsin A and possibly by other cellular hydrolases [2]. Phenol is lost by spontaneous nucleophilic release [3], and l-alanine is then released through hydrolysis of the P-N bond yielding GS-9148, either by a previously described phosphoamidase (29) or spontaneously due to the low pH present in the lysosomal compartment [4]. The poor cell membrane permeation of the nucleotide analog GS-9148 [5] is then phosphorylated by the rate-limiting first phosphorylation step, likely catalyzed by an adenylate kinase [6], followed by the more efficient second phosphorylation step, putatively catalyzed by creatine kinase or other kinases [7], generating the active metabolite GS-9148-DP.
The lack of dependence on cell type or activation state of GS-9148 phosphorylation may suggest that, like acyclic adenosine phosphonate nucleotide analogs (15, 27, 28), GS-9148 is initially phosphorylated by the ubiquitous activity of adenylate kinases. The lower levels of intracellular GS-9148-MP in all cell types tested in the present study (with the exception of activated PBMCs, where GS-9148-MP and -DP levels were not markedly different) may indicate that the initial phosphorylation step is rate limiting and is followed by a more rapid enzymatic conversion to GS-9148-DP. While the identity of the enzyme(s) catalyzing the more efficient second phosphorylation step cannot be ascertained from these studies, various nucleoside DP analogs have been found to be phosphorylated by several enzymes such as nucleoside DP kinase, creatine kinase, and pyruvate kinase (16). The equivalent in vitro activation of GS-9131 to its active metabolite in multiple lymphoid cell types suggests that the prodrug should be able to efficiently deliver GS-9148 to various reservoirs for viral replication.
The intracellular half-life observed for GS-9148-DP in activated CD4+ lymphocytes is not unlike the 15.4-h half-life observed for TFV-DP in activated PBMCs (28). The persistence observed for GS-9148-DP in vitro is consistent with that of the in vivo PBMC profile observed with dogs following the oral administration of GS-9131 (5). In general, intracellular persistence is an advantage for phosphonate nucleotide analogs over their nucleoside analog counterparts. For example, the active metabolites of the nucleoside analogs AZT, d4T, and abacavir have all been reported to have half-lives of between 3 and 5 h in vitro (7, 10, 14, 18). The long half-life observed with lymphocytes for TFV-DP in vitro has been found to translate into a median half-life of 150 h in patient PBMCs relative to the shorter 12 to 19 h half-life measured for carbovir triphosphate (the active metabolite of abacavir) in the same study (13). The long intracellular persistence measured for GS-9148 and its phosphorylated metabolites suggests that once-daily dosing should allow for consistent lymphoid exposure and marked accumulation in target cells upon repeat dosing.
The Ki values for the inhibition of purified RT for GS-9148-DP, TFV-DP, and ddATP have been determined to be 0.80, 0.18, and 0.021 μM, respectively (5). To obtain a more pharmacologically relevant understanding of the in situ inhibition of RT by these compounds, studies were performed that found correlations between the intracellular active metabolite concentrations and the inhibition of virus replication in HIV-1-infected PBMCs. While the finding of a ≥10-fold higher intracellular potency for ddATP compared to that of GS-9148-DP and TFV-DP was consistent with data from enzymatic assays, the fourfold enhanced potency observed for TFV-DP over GS-9148-DP in enzymatic assays was not found in HIV-1-infected cells. The more equivalent in situ activity levels of GS-9148-DP and TFV-DP may reflect the decreased rate of GS-9148 excision from chain-terminated primers relative to that of TFV (34). In HIV-1-infected patients treated with TDF or ddI, the intracellular levels of active metabolites in their circulating PBMCs have been found to be approximately 100 and 8 fmol/million cells, respectively (2, 13). While intracellular levels following GS-9131 administration have not yet been assessed, GS-9131 administration to beagle dogs at a potentially clinically relevant dose (3 mg/kg of body weight/day) was observed to yield intracellular GS-9148-DP levels of approximately 1,800 fmol/million cells (5). The relative intrinsic intracellular potency of GS-9148-DP coupled with its ability to effectively load lymphoid cells in vivo suggests that GS-9131 should generate intracellular concentrations sufficient to have efficacy against both the wild-type and the NRTI-resistant strains of HIV-1.
The coadministration of NRTIs and the dependence of their activation on the metabolic machinery responsible for maintaining natural nucleotide pools for activation create the potential for intracellular drug interactions (22). This is particularly true for analogs that share the same base and can compete for the same intracellular phosphorylation pathways. Drug interactions between the thymidine analogs 3′-deoxy-2′,3′-didehydrothymidine (d4T) and AZT and between the cytidine analogs l-2′-deoxy-3′-thiacytidine (3TC) and 2′-deoxy-3′-oxa-4′-thiocytidine (SPD754) have been found to be clinically relevant and to limit treatment consisting of the coadministration of these agents (12, 32). No antagonism was found between TFV and GS-9148 with respect to their intracellular phosphorylation, even when they were incubated at suprapharmacologic concentrations. While TFV and GS-9148 probably share similar anabolic pathways, this result likely reflects the high capacity of the enzymes involved in adenosine phosphorylation. The lack of an anabolic drug interaction between GS-9148 and TFV is consistent with the additive to slightly synergistic anti-HIV-1 activity observed in other in vitro studies (5). Having established the lack of an anabolic drug interaction with TFV, we wanted to assess the potential for GS-9148 and its metabolites to interfere with the catabolism of another NRTI commonly used in patients harboring highly drug resistant HIV-1, ddI. The putative mechanism for the observed increase in ddI exposure when it is coadministered with TFV is the inhibition of PNP by the phosphorylated metabolites of TFV (24). Our studies show that there is a low potential for GS-9131 to affect ddI pharmacokinetics due to the lack of PNP inhibition observed for GS-9131 metabolites. Antiviral combination studies of GS-9148 and other nucleoside, protease, and nonnucleoside HIV inhibitors have shown additive to synergistic activity in vitro (5).
GS-9131 is a promising new agent for the treatment of drug-resistant HIV-1 based on (i) the ability to load lymphoid cells to high levels; (ii) the efficient phosphorylation of GS-9148; (iii) the long intracellular half-life of GS-9148-DP, supporting once daily dosing; (iv) the low potential for drug interactions with TFV and ddI, two NRTIs often used in treatment-experienced NRTI-resistant patients; and (v) the favorable resistance profile of GS-9148 compared to that of other marketed NRTIs (5). Its favorable biological and pharmacokinetic properties make GS-9131 an attractive candidate for clinical development.
Acknowledgments
We thank members of the GS-9131 project team, including Anne Carey, Rowchanak Pakdaman, and James Chen from Gilead Sciences, Inc., for their contributions to the drug discovery process.
Footnotes
Published ahead of print on 3 December 2007.
REFERENCES
- 1.Beauchamp, L. M., J. V. Tuttle, M. E. Rodriguez, and M. L. Sznaidman. 1996. Guanine, pyrazolo[3,4-d]pyrimidine, and triazolo[4,5-d]pyrimidine (8-azaguanine) phosphonate acyclic derivatives as inhibitors of purine nucleoside phosphorylase. J. Med. Chem. 39:949-956. [DOI] [PubMed] [Google Scholar]
- 2.Becher, F., R. Landman, S. Mboup, C. N. Kane, A. Canestri, F. Liegeois, M. Vray, M. H. Prevot, G. Leleu, and H. Benech. 2004. Monitoring of didanosine and stavudine intracellular trisphosphorylated anabolite concentrations in HIV-infected patients. AIDS 18:181-187. [DOI] [PubMed] [Google Scholar]
- 3.Birkus, G., R. Wang, X. Liu, N. Kutty, H. MacArthur, T. Cihlar, C. Gibbs, S. Swaminathan, W. Lee, and M. McDermott. 2007. Cathepsin A is the major hydrolase catalyzing the intracellular hydrolysis of the antiretroviral nucleotide phosphonoamidate prodrugs GS-7340 and GS-9131. Antimicrob. Agents Chemother. 51:543-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapman, E. H., A. S. Kurec, and F. R. Davey. 1981. Cell volumes of normal and malignant mononuclear cells. J. Clin. Pathol. 34:1083-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cihlar, T., A. S. Ray, C. G. Boojamra, L. Zhang, H. Hui, G. Laflamme, J. E. Vela, D. Grant, J. Chen, F. Myrick, K. L. White, Y. Gao, K.-Y. Lin, J. L. Douglas, N. T. Parkin, A. Carey, R. Pakdaman, and R. L. Mackman. 2008. Design and profiling of GS-9148, a novel nucleotide analog active against nucleoside-resistant variants of human immunodeficiency virus type 1, and its orally bioavailable phosphonoamidate prodrug, GS-9131. Antimicrob. Agents Chemother. 52:655-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cundy, K. C., P. Barditch-Crovo, R. E. Walker, A. C. Collier, D. Ebeling, J. Toole, and H. S. Jaffe. 1995. Clinical pharmacokinetics of adefovir in human immunodeficiency virus type 1-infected patients. Antimicrob. Agents Chemother. 39:2401-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daluge, S. M., S. S. Good, M. B. Faletto, W. H. Miller, M. H. St Clair, L. R. Boone, M. Tisdale, N. R. Parry, J. E. Reardon, R. E. Dornsife, D. R. Averett, and T. A. Krenitsky. 1997. 1592U89, a novel carbocyclic nucleoside analog with potent, selective anti-human immunodeficiency virus activity. Antimicrob. Agents Chemother. 41:1082-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delaney, W. E. t., A. S. Ray, H. Yang, X. Qi, S. Xiong, Y. Zhu, and M. D. Miller. 2006. Intracellular metabolism and in vitro activity of tenofovir against hepatitis B virus. Antimicrob. Agents Chemother. 50:2471-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erion, M. D., K. Takabayashi, H. B. Smith, J. Kessi, S. Wagner, S. Honger, S. L. Shames, and S. E. Ealick. 1997. Purine nucleoside phosphorylase. 1. Structure-function studies. Biochemistry 36:11725-11734. [DOI] [PubMed] [Google Scholar]
- 10.Furman, P. A., J. A. Fyfe, M. H. St Clair, K. Weinhold, J. L. Rideout, G. A. Freeman, S. N. Lehrman, D. P. Bolognesi, S. Broder, H. Mitsuya, et al. 1986. Phosphorylation of 3′-azido-3′-deoxythymidine and selective interaction of the 5′-triphosphate with human immunodeficiency virus reverse transcriptase. Proc. Natl. Acad. Sci. USA 83:8333-8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallant, J. E., E. DeJesus, J. R. Arribas, A. L. Pozniak, B. Gazzard, R. E. Campo, B. Lu, D. McColl, S. Chuck, J. Enejosa, J. J. Toole, and A. K. Cheng. 2006. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N. Engl. J. Med. 354:251-260. [DOI] [PubMed] [Google Scholar]
- 12.Havlir, D. V., C. Tierney, G. H. Friedland, R. B. Pollard, L. Smeaton, J. P. Sommadossi, L. Fox, H. Kessler, K. H. Fife, and D. D. Richman. 2000. In vivo antagonism with zidovudine plus stavudine combination therapy. J. Infect. Dis. 182:321-325. [DOI] [PubMed] [Google Scholar]
- 13.Hawkins, T., W. Veikley, R. L. St Claire, 3rd, B. Guyer, N. Clark, and B. P. Kearney. 2005. Intracellular pharmacokinetics of tenofovir diphosphate, carbovir triphosphate, and lamivudine triphosphate in patients receiving triple-nucleoside regimens. J. Acquir. Immune Defic. Syndr. 39:406-411. [DOI] [PubMed] [Google Scholar]
- 14.Ho, H. T., and M. J. Hitchcock. 1989. Cellular pharmacology of 2′,3′-dideoxy-2′,3′-didehydrothymidine, a nucleoside analog active against human immunodeficiency virus. Antimicrob. Agents Chemother. 33:844-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krejcova, R., K. Horska, I. Votruba, and A. Holy. 2000. Phosphorylation of purine (phosphonmethoxy)alkyl derivatives by mitochondrial AMP kinase (AK2 type) from L1210 cells. Collect. Czech. Chem. Commun. 65:1653-1668. [Google Scholar]
- 16.Krishnan, P., Q. Fu, W. Lam, J. Y. Liou, G. Dutschman, and Y. C. Cheng. 2002. Phosphorylation of pyrimidine deoxynucleoside analog diphosphates: selective phosphorylation of l-nucleoside analog diphosphates by 3-phosphoglycerate kinase. J. Biol. Chem. 277:5453-5459. [DOI] [PubMed] [Google Scholar]
- 17.Kulikowska, E., A. Bzowska, A. Holy, L. Magnowska, and D. Shugar. 1998. Antiviral acyclic nucleoside phosphonate analogues as inhibitors of purine nucleoside phosphorylase. Adv. Exp. Med. Biol. 431:747-752. [DOI] [PubMed] [Google Scholar]
- 18.Ledford, R. M., J. E. Vela, A. S. Ray, C. Callebaut, M. D. Miller, and D. J. McColl. 2006. The longer intracellular half-life of tenofovir diphosphate correlates with persistent inhibition of HIV-1 replication in vitro. Antivir. Res. 70:A45.
- 19.Lee, W. A., G. X. He, E. Eisenberg, T. Cihlar, S. Swaminathan, A. Mulato, and K. C. Cundy. 2005. Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrob. Agents Chemother. 49:1898-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackman, R. L., C. G. Boojamra, J. Chen, J. Douglas, D. Grant, C. U. Kim, K. Lin, D. Y. Markevitch, V. Prasad, A. S. Ray, and T. Cihlar. 2006. Discovery of GS9148, a novel nucleotide HIV reverse transcriptase (RT) inhibitor. Antivir. Res. 70:A40.
- 21.Pruvost, A., E. Negredo, H. Benech, F. Theodoro, J. Puig, E. Grau, E. Garcia, J. Molto, J. Grassi, and B. Clotet. 2005. Measurement of intracellular didanosine and tenofovir phosphorylated metabolites and possible interaction of the two drugs in human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 49:1907-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ray, A. S. 2005. Intracellular interactions between nucleos(t)ide inhibitors of HIV reverse transcriptase. AIDS Rev. 7:113-125. [PubMed] [Google Scholar]
- 23.Ray, A. S., C. G. Boojamra, N. Parkin, K. L. White, G. R. Rhodes, R. Mackman, and T. Cihlar. 2006. Amidate prodrug of nucleotide analog GS-9148 has favorable in vitro activity against resistant HIV and in vivo pharmacokinetics. Global Antivir. J. 2(Suppl. 2):37. [Google Scholar]
- 24.Ray, A. S., L. Olson, and A. Fridland. 2004. Role of purine nucleoside phosphorylase in interactions between 2′,3′-dideoxyinosine and allopurinol, ganciclovir, or tenofovir. Antimicrob. Agents Chemother. 48:1089-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ray, A. S., J. E. Vela, R. Mackman, L. Zhang, H. Hui, R. Packdaman, A. Carey, M. Wright, G. R. Rhodes, and T. Cihlar. 2006. Amidate prodrug of a nucleotide analog GS9148 enhances the in vivo intracellular delivery of the active diphosphate metabolite: potential for clinical efficacy, abstr. 498. Abstr. 13th Conference on Retroviruses and Opportunistic Infections., Denver, CO.
- 26.Ray, A. S., J. E. Vela, L. Olson, and A. Fridland. 2004. Effective metabolism and long intracellular half life of the anti-hepatitis B agent adefovir in hepatic cells. Biochem. Pharmacol. 68:1825-1831. [DOI] [PubMed] [Google Scholar]
- 27.Robbins, B. L., J. Greenhaw, M. C. Connelly, and A. Fridland. 1995. Metabolic pathways for activation of the antiviral agent 9-(2-phosphonylmethoxyethyl)adenine in human lymphoid cells. Antimicrob. Agents Chemother. 39:2304-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robbins, B. L., R. V. Srinivas, C. Kim, N. Bischofberger, and A. Fridland. 1998. Anti-human immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleoside phosphonate 9-R-(2-phosphonomethoxypropyl)adenine (PMPA), Bis(isopropyloxymethylcarbonyl)PMPA. Antimicrob. Agents Chemother. 42:612-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saboulard, D., L. Naesens, D. Cahard, A. Salgado, R. Pathirana, S. Velazquez, C. McGuigan, E. De Clercq, and J. Balzarini. 1999. Characterization of the activation pathway of phosphoramidate triester prodrugs of stavudine and zidovudine. Mol. Pharmacol. 56:693-704. [PubMed] [Google Scholar]
- 30.Vela, J. E., L. Y. Olson, A. Huang, A. Fridland, and A. S. Ray. 2007. Simultaneous quantitation of the nucleotide analog adefovir, its phosphorylated anabolites and 2′-deoxyadenosine triphosphate by ion-pairing LC/MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 848:335-343. [DOI] [PubMed] [Google Scholar]
- 31.Wachsman, M., B. G. Petty, K. C. Cundy, H. S. Jaffe, P. E. Fisher, A. Pastelak, and P. S. Lietman. 1996. Pharmacokinetics, safety and bioavailability of HPMPC (cidofovir) in human immunodeficiency virus-infected subjects. Antiviral Res. 29:153-161. [DOI] [PubMed] [Google Scholar]
- 32.Wainberg, M. A., P. Cahn, R. C. Bethell, J. Sawyer, and S. Cox. 2007. Apricitabine: a novel deoxycytidine analogue nucleoside reverse transcriptase inhibitor for the treatment of nucleoside-resistant HIV infection. Antivir. Chem. Chemother. 18:61-70. [DOI] [PubMed] [Google Scholar]
- 33.Wainberg, M. A., and D. Turner. 2004. Resistance issues with new nucleoside/nucleotide backbone options. J. Acquir. Immune Defic. Syndr. 37:S36-43. [DOI] [PubMed] [Google Scholar]
- 34.White, K. L., J. Y. Feng, A. S. Ray, G. Laflamme, F. Yu, M. Tsiang, R. Wang, M. McDermott, M. D. Miller, R. L. Mackman, and T. Cihlar. 2006. GS-9148-diphosphate (GS-9148-DP), an active metabolite of a novel adenine nucleotide analogue, is an effective inhibitor of HIV-1 reverse transcriptase. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother., abstr. H-0251.
- 35.Wierzchowski, J., E. Kulikowska, A. Bzowska, A. Holy, L. Magnowska, and D. Shugar. 1999. Interactions of calf spleen purine nucleoside phosphorylase with antiviral acyclic nucleoside phosphonate inhibitors: kinetics and emission studies. Nucleosides Nucleotides 18:875-876. [DOI] [PubMed] [Google Scholar]