Abstract
We amplified, cloned, and sequenced the β-tubulin gene of Vittaforma corneae, a microsporidium causing human infections. The β-tubulin gene sequence has a substitution at Glu198 (with glutamine), which is one of six amino acids reported to be associated with benzimidazole sensitivity. Benzimidazoles were assayed for antimicrosporidial activity and showed poor parasite inhibition.
Microsporidia are obligate intracellular protozoan parasites that have emerged as major opportunistic human pathogens since the onset of the AIDS pandemic (6, 11). Vittaforma corneae is responsible for keratitis in otherwise healthy persons (2, 9, 10, 18), but disseminated infections have occurred in human immunodeficiency virus-infected patients as well (3). V. corneae was first described as Nosema corneum in a stromal biopsy from a non-human immunodeficiency virus-infected man who suffered from central disciform keratitis for 18 months (2). The parasite was cultivated in cell culture and identified as a new species, N. corneum (19). Infection with this organism was subsequently established in athymic mice (20), and new taxonomically significant features found warranted placing this organism within a new genus, Vittaforma, as V. corneae (21). In the second reported case of V. corneae infection, dual microsporidial infection was detected in a patient with AIDS—Encephalitozoon hellem in the sinonasal aspirate and V. corneae in the urine (3), indicating that V. corneae is capable of dissemination and survival in deep tissues, at least in an immunocompromised host. Three further cases involving V. corneae infection of the corneal stroma of immunocompetent patients were published (9, 10, 18). In addition to these confirmed cases, there is molecular evidence (obtained by PCR) for Vittaforma infections as a cause of keratitis in India (15 cases) (12) and of enteritis in Portugal (25 cases) (23).
Benzimidazoles are widely used as anthelmintic drugs in veterinary and human medicine and have been used as antifungal agents in agriculture. Albendazole is one of the most commonly used drugs for treating microsporidiosis in humans (5). Infections with Encephalitozoon spp. respond especially well to therapy with albendazole, whereas infections with Enterocytozoon bieneusi do not respond to albendazole, and the sequence of the β-tubulin gene of E. bieneusi suggests that this species is resistant to albendazole (1, 5). No clinical data about the response of Vittaforma infections to albendazole are available, and molecular data on the β-tubulin gene are not available for this species either. We therefore tested the susceptibility of V. corneae to different benzimidazoles in vitro and amplified, cloned, and sequenced the β-tubulin gene of V. corneae.
MRC-5 cells were seeded on 24-well culture plates at a concentration of 5 × 105/ml in minimal essential medium and incubated overnight to confluence. Confluent monolayers of cells were inoculated with 2 × 106 microsporidian spores (V. corneae and E. cuniculi as a control). Three hours later, noninternalized spores were washed off and fresh medium with or without drugs was added. Albendazole and fenbendazole were purchased from Sigma (St. Louis, MO), and stock solutions were dissolved in dimethyl sulfoxide at a final concentration of 10 mg/ml and thereafter diluted in culture medium for use in the assay. Culture medium was replaced every 3 days, and on day 10, 100 μl of 10% (wt/vol) sodium dodecyl sulfate was added to each well to release newly developed spores from infected host cells. The spores were counted in a hemocytometer. Each treatment was done in triplicate, and the percent inhibition of microsporidian replication was calculated as 100 − [(number of spores counted in treated culture/number of spores in nontreated cultures) × 100]. Comparison of antimicrosporidial activities was done by Student's t test.
DNA was isolated from V. corneae-infected cell cultures, and the β-tubulin sequences were amplified with the degenerate primers btubf (5′-GCC TGC AGG NCA RTG YGG NAA YCA-3′) and btubr (5′-GGC CTC AGT RAA YTC CAT YTC RTC CAT-3′) (15). PCR fragments were ligated into the vector pCR4-TOPO (Invitrogen, Carlsbad, CA) and cloned into TOP10 competent cells (Invitrogen). Plasmids were isolated from white colonies and sequenced on an automated DNA sequencer (ABI PRISM, Applied Biosystems, Foster City, CA) with vector-directed primers T7 and M13. Sequencing of both strands of each PCR fragment was done twice, and several plasmids were sequenced. The resulting DNA sequences were assembled with GeneTool Lite, version 1.0, and a consensus sequence was generated.
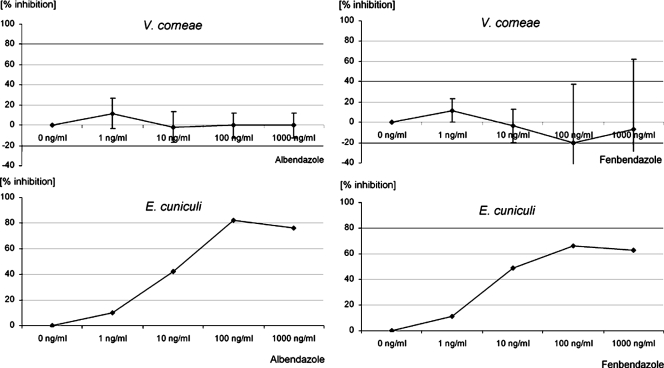
Results in Fig. 1 demonstrated that albendazole displayed significantly lower (P < 0.001) antimicrosporidial in vitro activities against V. corneae compared to E. cuniculi. Significant antimicrosporidial activities were observed in E. cuniculi cultures treated with albendazole or fenbendazole at concentrations of 10 ng/ml and above, whereas in V. corneae cultures no antimicrosporidial activities could be detected up to 1,000 ng/ml. At concentrations of 1,000 ng/ml, clear signs of host cell toxicity were observed after treatment of host cells with albendazole or fenbendazole for 10 days.
FIG. 1.
Dose-response curves for albendazole and fenbendazole. Values are means and standard deviations of three replications (V. corneae) or from one experiment (E. cuniculi).
PCR amplification revealed a DNA fragment that was ∼1,000 bp long. Sequencing of the cloned DNA fragment provided a 1,024-bp gene sequence with a CG content of 46%. The predicted β-tubulin amino acid sequence has only five of the six sites that have been reported to be associated with benzimidazole activity (His6 is not included, as the fragment is truncated at the 5′ end) and has a substitution at Glu198 (with glutamine) (numbering is based on the Saccharomyces cerevisiae sequence in Fig. 2).
FIG. 2.
Clustal W alignment of partial β-tubulin amino acid sequences from V. corneae, E. cuniculi (GenBank accession no. NM_001040955), E. hellem (GenBank accession no. L47271), E. intestinalis (GenBank accession no. AF297876), E. bieneusi (GenBank accession no. DQ242640), Trachipleistophora hominis (GenBank accession no. AF162081), Nosema plutellae (GenBank accession no. DQ083404), Nosema bombycis (GenBank accession no. DQ663475), S. cerevisiae (GenBank accession no. V01296), and Homo sapiens (GenBank accession no. NM_178014). Single-letter amino acid abbreviations are used. Five of the six amino acids of β-tubulin that are reported to be associated with benzimidazole sensitivity (Ala165, Phe1167, Glu1198, Phe200, and Arg241; numbering based on the S. cerevisiae sequence shown) are indicated by plus signs. Glu198, which is associated with benzimidazole sensitivity, is changed to glutamine in V. corneae.
Some microsporidia (especially the Encephalitozoon spp.) and other protists (e.g., Giardia lamblia and Cryptococcus neoformans) are very sensitive to several derivatives of a group of drugs called the benzimidazoles, which are widely used to treat helminth infections in humans and animals and as systemic fungicides in agriculture (13, 17). Benzimidazoles have been shown to disrupt mitosis in sensitive organisms through binding to the β-tubulin subunit of microtubules. The benzimidazole binding site and the basis for the selective toxicity of these compounds remain incompletely defined (17). Six amino acids of β-tubulin are reported to be associated with benzimidazole sensitivity (His6, Ala165, Phe167, Glu198, Phe200, and Arg241; numbering is based on the S. cerevisiae sequence in Fig. 2) (7, 8, 13, 14, 16, 24). The β-tubulin amino acid fragment of V. corneae has a substitution at Glu198 (with glutamine), which suggests resistance to benzimidazoles. The same substitution was found recently in the β-tubulin gene of E. bieneusi, another human-infecting microsporidium with clinically observed resistance to benzimidazoles (1). Several papers have indicated that V. corneae and E. bieneusi evolved simultaneously and are closely related microsporidia. The β-tubulin data presented here support this relationship, and V. corneae may be a useful surrogate organism for studies on Enterocytozoon drug resistance; such studies have so far been hampered as E. bieneusi could not be cultivated in long-term culture.
Organisms resistant to benzimidazoles lack one or more of the six amino acids mentioned above. Besides E. bieneusi, Entamoeba histolytica also has changes at Glu198 and is relatively resistant to albendazole, and Cryptosporidium parvum and Acanthamoeba polyphaga, which have an additional change at Phe200 (in addition to Glu198), are resistant to benzimidazoles as well. V. corneae has Ala165, whereas E. cumiculi, E. hellem, and E. intestinalis have a change from Ala165 to Cys165. Nevertheless, V. corneae is resistant to benzimidazoles, whereas the Encephalitozoon spp. are highly susceptible. Cys165 is also present in G. lamblia and C. neoformans, which are sensitive to albendazole (7, 16, 17). So changes in Glu198 and/or Phe200 seem to be associated with resistance to benzimidazoles, whereas changes in Ala165 seem not to be highly predictive of benzimidazole sensitivity.
The suspected resistance against albendazole was further evaluated in vitro. Previous studies have shown inconsistent results. In one study, albendazole at 2.1 or 4.2 μg/ml in minimal essential medium was tested against V. corneae in MDCK cell monolayers and showed some antimicrosporidial activity (22). The percentage of infected cells was reduced in the presence of the drug, and there were ultrastructural abnormalities at all stages of the life cycle. The drug prevented parasite division (22). However, such high concentrations cannot be reached under therapy. In another study, albendazole was less effective against V. corneae than against E. intestinalis, based on an approximately sevenfold higher minimal inhibitory concentration for 50% of the isolates tested (4). The in vitro data presented here also show that benzimidazoles are not effective against V. corneae whereas E. cuniculi parasite growth was inhibited very effectively. There are limited clinical data about the application of albendazole in Vittaforma infections. One patient with keratitis due to V. corneae was treated with topical steroids and broad-spectrum antibiotics but ultimately required a corneal transplant (2). Another patient was treated initially with topical acyclovir and steroid, but penetrating keratoplasty was performed later and he was given two courses of oral albendazole (400 mg daily), each for 14 days, and no microsporidia were detected in a biopsy of the rejected graft 6 months later (18). A third patient underwent penetrating keratoplasty as well but was not treated with albendazole (10), and another patient was treated with both topical fumagillin bicyclohexylammonium salt and oral albendazole but failed to improve or control the progression of the infection after lamellar keratoplasty (9). Thus, there is no clinical evidence for or against the use of albendazole in Vittaforma infections. Our experimental data show resistance of V. corneae to benzimidazoles. The molecular data obtained suggest that V. corneae should be resistant to benzimidazoles, and this was shown in vitro. Infections with V. corneae should not be treated with benzimidazoles, and other treatment options, such as fumagillin or TNP-470, should be chosen.
Nucleotide sequence accession number.
The consensus sequence generated from several sequencing reactions in this study was submitted to the GenBank database under accession no. EU031749.
Acknowledgments
This work was supported by a grant from the Bundesministerium für Bildung und Forschung (BMBF) Programm zur Förderung von Klinischen Forschergruppen in der Klinischen Infektiologie, Förderkennzeichen 01 KI 9952.
Footnotes
Published ahead of print on 3 December 2007.
REFERENCES
- 1.Akiyoshi, D. E., L. M. Weiss, X. Feng, B. A. P. Williams, P. J. Keeling, Q. Zhang, and S. Tzipori. 2007. Analysis of the β-tubulin genes from Enterocytozoon bieneusi isolates from a human and rhesus macaque. J. Eukaryot. Microbiol. 54:38-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis, R. M., R. L. Font, M. S. Keisler, and J. A. Shadduck. 1990. Corneal microsporidiosis. A case report including ultrastructural observations. Ophthalmology 97:953-957. [PubMed] [Google Scholar]
- 3.Deplazes, P., A. Mathis, M. van Saanen, A. Iten, R. Keller, I. Tanner, M. P. Glauser, R. Weber, and E. U. Canning. 1998. Dual microsporidial infection due to Vittaforma corneae and Encephalitozoon hellem in a patient with AIDS. Clin. Infect. Dis. 27:1521-1524. [DOI] [PubMed] [Google Scholar]
- 4.Didier, E. S. 1997. Effects of albendazole, fumagillin, and TNP-470 on microsporidial replication in vitro. Antimicrob. Agents Chemother. 41:1541-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Didier, E. S., J. A. Maddry, P. J. Brindley, M. E. Stovall, and P. J. Didier. 2005. Therapeutic strategies for human microsporidia infections. Expert Rev. Anti-infect. Ther. 3:419-434. [DOI] [PubMed] [Google Scholar]
- 6.Didier, E. S., and L. M. Weiss. 2006. Microsporidiosis: current status. Curr. Opin. Infect. Dis. 19:485-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edlind, T., G. Visvesvara, J. Li, and S. Katiyar. 1994. Cryptosporidium and microsporidial beta-tubulin sequences: predictions of benzimidazole sensitivity and phylogeny. J. Eukaryot. Microbiol. 41:38S. [PubMed] [Google Scholar]
- 8.Edlind, T., S. Katiyar, G. Visvesvara, and J. Li. 1996. Evolutionary origins of microsporidia and basis for benzimidazole sensitivity: an update. J. Eukaryot. Microbiol. 43:109S. [DOI] [PubMed] [Google Scholar]
- 9.Font, R. L., A. N. Samaha, M. J. Keener, P. Chevez-Barrios, and J. D. Goosey. 2000. Corneal microsporidiosis. Report of case, including electron microscopic observations. Ophthalmology 107:1769-1775. [DOI] [PubMed] [Google Scholar]
- 10.Font, R. L., G. W. Su, and A. Y. Matoba. 2003. Microsporidial stromal keratitis. Arch. Ophthalmol. 121:1045-1047. [DOI] [PubMed] [Google Scholar]
- 11.Franzen, C., and A. Müller. 2001. Microsporidiosis: human diseases and diagnosis. Microbes Infect. 3:389-400. [DOI] [PubMed] [Google Scholar]
- 12.Joseph, J., S. Sharma, S. I. Murthy, P. V. Krishna, P. Garg, R. Nutheti, J. Kenneth, and D. Balasubramanian. 2006. Microsporidial keratitis in India: 16S rRNA gene-based PCR assay for diagnosis and species identification of microsporidia in clinical samples. Investig. Ophthalmol. Vis. Sci. 47:4468-4473. [DOI] [PubMed] [Google Scholar]
- 13.Jung, M. K., I. B. Wilder, and B. R. Oakley. 1992. Amino acid alterations in the BenA (beta-tubulin) gene of Aspergillus nidulans that confer benomyl resistance. Cell Motil. Cytoskelet. 22:170-174. [DOI] [PubMed] [Google Scholar]
- 14.Katiyar, S. K., V. R. Gordon, G. L. McLaughlin, and T. D. Edlind. 1994. Antiprotozoal activities of benzimidazoles and correlations with β-tubulin sequence. Antimicrob. Agents Chemother. 38:2086-2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keeling, P. J. 2003. Congruent evidence from α-tubulin and β-tubulin gene phylogenies for a zygomycete origin of microsporidia. Fungal Genet. Biol. 38:298-309. [DOI] [PubMed] [Google Scholar]
- 16.Li, J., S. K. Katiyar, A. Hamelin, G. S. Visvesvara, and T. D. Edlind. 1996. Tubulin genes from AIDS-associated microsporidia and implications for phylogeny and benzimidazole sensitivity. Mol. Biochem. Parasitol. 78:289-295. [DOI] [PubMed] [Google Scholar]
- 17.MacDonald, L. M., A. Armson, R. C. Thompson, and J. A. Reynoldson. 2004. Characterisation of benzimidazole binding with recombinant tubulin from Giardia duodenalis, Encephalitozoon intestinalis, and Cryptosporidium parvum. Mol. Biochem. Parasitol. 138:89-96. [DOI] [PubMed] [Google Scholar]
- 18.Rauz, S., S. Tuft, J. K. Dart, R. Bonshek, P. Luthert, and A. Curry. 2004. Ultrastructural examination of two cases of stromal microsporidial keratitis. J. Med. Microbiol. 53:775-781. [DOI] [PubMed] [Google Scholar]
- 19.Shadduck, J. A., R. A. Meccoli, R. Davis, and R. L. Font. 1990. Isolation of a microsporidian from a human patient. J. Infect. Dis. 162:773-776. [DOI] [PubMed] [Google Scholar]
- 20.Silveira, H., E. U. Canning, and J. A. Shadduck. 1993. Experimental infection of athymic mice with the human microsporidian Nosema corneum. Parasitology 107:489-496. [DOI] [PubMed] [Google Scholar]
- 21.Silveira, H., and E. U. Canning. 1995. Vittaforma corneae n. comb. for the human microsporidium Nosema corneum Shadduck, Meccoli, 1990 Davis & Font, based on its ultrastructure in the liver of experimentally infected athymic mice. J. Eukaryot. Microbiol. 42:158-165. [DOI] [PubMed] [Google Scholar]
- 22.Silveira, H., and E. U. Canning. 1995. In vitro cultivation of the human microsporidium Vittaforma corneae: development and effect of albendazole. Folia Parasitol. 42:241-250. [PubMed] [Google Scholar]
- 23.Sulaiman, I. M., O. Matos, M. L. Lobo, and L. Xiao. 2003. Identification of a new microsporidian parasite related to Vittaforma corneae in HIV-positive and HIV-negative patients from Portugal. J. Eukaryot. Microbiol. 50:S586-S590. [DOI] [PubMed] [Google Scholar]
- 24.Thomas, J. H., N. F. Neff, and D. Botstein. 1985. Isolation and characterization of mutations in the β-tubulin gene of Saccharomyces cerevisiae. Genetics 111:715-734. [DOI] [PMC free article] [PubMed] [Google Scholar]