Abstract
Multiple in vivo studies have characterized the pharmacodynamics of drugs from the triazole and polyene antifungal drug classes. Fewer studies have investigated these pharmacodynamic relationships for the echinocandin drug class. We used a neutropenic murine model of disseminated Candida albicans, Candida tropicalis, and Candida glabrata infection to characterize the time course of activity of the new echinocandin anidulafungin. The pharmacokinetic-pharmacodynamic (PK-PD) indices (the percentage of time that the drug concentration was above the MIC, the ratio of the area under the concentration-time curve from 0 to 24 h [AUC0-24] to the MIC, and the ratio of the maximum serum drug concentration [Cmax] to the MIC) were correlated with in vivo efficacy, as measured by organism numbers in kidney cultures after 96 h of therapy. The kinetics following intraperitoneal anidulafungin dosing in neutropenic infected mice were monitored. Peak levels and AUCs were linear over the 16-fold dose range studied. The drug elimination half-life in serum ranged from 14 to 24 h. Single-dose postantifungal-effect studies demonstrated prolonged suppression of organism regrowth after serum anidulafungin levels had fallen below the MIC. Of the four dosing intervals studied, treatment with the more widely spaced dosing regimens was most efficacious, suggesting the Cmax/MIC ratio as the PK-PD index most predictive of efficacy. Nonlinear regression analysis suggested that both the Cmax/MIC and AUC/MIC ratios were strongly predictive of treatment success. Studies were then conducted with 13 additional C. albicans, C. tropicalis, and C. glabrata isolates with various anidulafungin susceptibilities (MICs of anidulafungin for these strains, 0.015 to 2.0 μg/ml) to determine if similar Cmax/MIC and AUC0-24/MIC ratios for these isolates were associated with efficacy. The anidulafungin exposures associated with efficacy were similar among Candida species.
The incidence of invasive fungal infections continues to rise (36, 42). Despite advances in antifungal therapy, morbidity and mortality associated with these infections remain high. The echinocandins constitute the most recently Food and Drug Administration-approved antifungal drug class (10, 14, 50). The three available drugs from this class include anidulafungin, caspofungin, and micafungin. These compounds exhibit broad-spectrum activity against Candida and Aspergillus species, including emerging non-albicans Candida species (10, 14, 20, 40, 41, 54). The present studies were designed to determine the in vivo pharmacodynamic (PD) characteristics of a new compound against Candida albicans, C. tropicalis, and C. glabrata. We first examined the impact of the anidulafungin concentration on in vivo antimicrobial killing activity over time. Studies were then performed to determine (i) which pharmacokinetic (PK) index (the maximum concentration of the drug in serum [Cmax], the area under the concentration-time curve [AUC], or the duration of time that levels in serum exceed the MIC [T>MIC]) best predicts the efficacy of anidulafungin and (ii) whether the magnitudes of this PK-PD index required for efficacy against multiple C. albicans and C. glabrata strains, including several previously characterized caspofungin-resistant organisms, are similar. The results from these studies provide a PD rationale in support of the current clinical dosing regimens. Furthermore, the data provide information useful in the examination of susceptibility breakpoints for this new compound.
MATERIALS AND METHODS
Organisms.
Fifteen clinical Candida isolates were used, including 4 C. albicans (K-1, 580, 98-17, and 98-210), 1 C. tropicalis (98-234), and 10 C. glabrata (570, 513, 5592, 5376, 33609, 32930, 33616, 34341, 35315, and 37661) isolates. Six of the isolates have been described previously and were kindly provided by J. Knudsen (28). The organisms were maintained, grown, and quantified on Sabouraud's dextrose agar (SDA) plates. Twenty-four hours prior to the study, organisms were subcultured at 35°C. The isolates were chosen to include strains with various echinocandin susceptibilities. We also attempted to choose isolates based upon relatively similar degrees of virulence in this animal model, as determined by the amount of growth in the kidneys of untreated animals over 48 h (Table 1).
TABLE 1.
In vitro susceptibility of select strains of C. albicans, C. tropicalis, and C. glabrata to anidulafungin and relative fitness of these organisms in the disseminated candidiasis modela
| Organism | MIC1 (μg/ml) | MIC2 (μg/ml) | In vivo growth in control animals over 48 h (log10 CFU/kidney) |
|---|---|---|---|
| C. albicans strains | |||
| K-1 | 0.015 | 0.12 | 4.00 |
| 98-17 | 0.03 | 0.12 | 4.30 |
| 98-210 | 0.015 | 0.06 | 3.50 |
| 580 | 0.015 | 0.03 | 3.40 |
| C. tropicalis 98-234 | 0.015 | 0.12 | 3.84 |
| Mean ± SD for C. albicans and C. tropicalis strains | 3.81 ± 0.33 | ||
| C. glabrata strains | |||
| 570 | 0.03 | 0.12 | 2.45 |
| 513 | 0.06 | 0.12 | 2.43 |
| 33609 | 0.03 | 0.12 | 2.37 |
| 32930 | 0.06 | 0.12 | 1.29 |
| 33616 | 1.0 | 2.0 | 1.81 |
| 34341 | 0.12 | 1.0 | 1.82 |
| 35315 | 0.25 | 1.0 | 0.97 |
| 5592 | 0.06 | 0.12 | 2.1 |
| 5376 | 0.03 | 0.06 | 2.1 |
| 37661 | 2.0 | 2.0 | 0.96 |
| Mean ± SD for C. glabrata strains | 1.83 ± 0.54 | ||
| Mean ± SD for all organisms (range) | 2.42 ± 1.06 (0.96-4.30) |
MIC1, partial-inhibition end point; MIC2, complete-inhibition end point.
Antifungal agent.
Anidulafungin was obtained from Pfizer. Stock solutions of anidulafungin were prepared in dimethyl sulfoxide. The stock solution was diluted in 0.9% NaCl to the desired concentrations.
In vitro susceptibility testing.
MICs were determined using the Clinical and Laboratory Standards Institute (CLSI; formerly NCCLS) M27-A2 method (33, 40, 41). The MIC end points for anidulafungin included both partial growth inhibition (the CLSI-recommended end point) and complete growth inhibition relative to the growth in the drug-free control well. Determinations were performed in duplicate on four separate occasions. Final results are expressed as the means of these results.
Animals.
Six-week-old ICR/Swiss specific-pathogen-free female mice (Harlan Sprague-Dawley, Indianapolis, IN) weighing 23 to 27 g were used for all studies. Animals were housed in groups of five and allowed access to food and water ad libitum. Animals were maintained in accordance with the American Association for Assessment and Accreditation of Laboratory Animal Care criteria. Animal studies were approved by the Animal Research Committee of the William S. Middleton Memorial Veterans Affairs Hospital and the University of Wisconsin.
Infection model.
A neutropenic murine disseminated candidiasis model was used for all studies (2, 4, 5, 6). Mice were rendered neutropenic (polymorphonuclear cell count, <100/mm3) by injecting cyclophosphamide (Mead Johnson Pharmaceuticals, Evansville, IN) subcutaneously 4 days before infection (150 mg/kg of body weight), 1 day before infection (100 mg/kg), and 2 days after infection (100 mg/kg). Prior studies have demonstrated that this regimen produces neutropenia (absolute neutrophil counts remained at or below 100/mm3 throughout the 96-h study). Organisms were subcultured on SDA 24 h prior to infection. The inoculum was prepared by placing three to five colonies into 5 ml of sterile pyrogen-free 0.9% saline warmed to 35°C. The final inoculum was adjusted to a 0.6 transmittance at 530 nm. The fungal count of the inoculum determined by counting viable colonies on SDA was 6.1 ± 0.51 log10 CFU/ml (mean ± standard deviation [SD]).
Disseminated infection with the Candida organisms was achieved by the injection of 0.1 ml of the inoculum via the lateral tail vein 2 h prior to the start of drug therapy. At the end of the study period, animals were sacrificed by CO2 asphyxiation. After sacrifice, the kidneys of each mouse were immediately removed and placed into sterile 0.9% saline at 4°C. The homogenate was then serially diluted 1:10, and aliquots were plated onto SDA for counts of viable fungal colonies after incubation for 24 h at 35°C. The lower limit of detection was 100 CFU/ml. Results were expressed as the mean number of CFU per kidney for three mice.
Pharmacokinetics.
The single-dose pharmacokinetics of anidulafungin in infected neutropenic ICR/Swiss mice were monitored following the intraperitoneal administration of 80, 20, and 5 mg/kg in 0.2-ml volumes. Blood from groups of three isoflurane-anesthetized mice was collected at each of six time points (1, 4, 8, 12, 24, and 48 h after drug administration). Serum was collected by centrifugation, and samples were stored at −80°C until the drug assay. Samples were analyzed by using reverse-phase high-pressure liquid chromatography with fluorescence end point detection. Loratadine was used as the internal standard. The calibration curve was linear over a range of 10 to 50,000 ng/ml (lower limit of detection, 10 ng/ml) in plasma (R2 = 0.999). The mean intraday and interday variations were less than 7 and 10%, respectively.
A noncompartmental model was used in the kinetic analysis. PK parameters including the elimination half-life and the concentration at time zero were calculated via nonlinear least-squares techniques. The AUC was calculated by the trapezoidal rule. For treatment doses for which no kinetics were determined, PK indices were estimated by linear extrapolation from the highest and lowest dose levels used in the kinetic studies described above. An accumulation factor was considered for several of the dosing intervals (every 12 h [q12h], q24h, and q48h).
Protein binding.
Serum protein binding was analyzed using two different methods. The first study was performed at Pfizer by using equilibrium dialysis. The second method examined the impact of 95% mouse serum on the activity of anidulafungin in vitro by using arithmetic dilutions as previously described (3). Specifically, the anidulafungin MICs for C. albicans K-1 in broth, 95% mouse serum treated with heat at 65°C for 30 min, and 95% serum ultrafiltrate were determined by using arithmetic dilutions of 0.02 mg/liter/tube. Reduced potency (higher MICs) in serum was presumed to be due to drug binding to serum protein. The difference in potency was used to estimate the percentage of protein binding by the following equation: (MIC in 95% serum − MIC in serum ultrafiltrate)/MIC in 95% serum.
In vivo time-kill and PAFE studies.
Infection in neutropenic mice was produced as described above. Two hours after infection with C. albicans K-1 or C. glabrata 5376, mice were treated with one of three single intraperitoneal doses of anidulafungin (20, 5, or 1.25 mg/kg). Groups of three treated and three control mice were sacrificed at sampling intervals ranging from 1 to 24 h over a total study period of 96 h. Control growth was determined by sampling at five time points. Samples from anidulafungin-treated animals were collected nine times. Kidneys were removed at each time point and processed immediately for the determination of numbers of CFU as outlined above. The T>MIC for the organism following each of the three doses was calculated from the PK data. Free-drug concentrations of 1% were utilized for kinetic calculations (B. Damle, Pfizer Inc., personal communication). Total drug concentrations remained above the MIC for the entire period of study. The postantifungal effect (PAFE) was calculated by determining the amount of time it took for the organism burden in controls to increase by 1 log10 CFU/kidney (c) and subtracting this value from the amount of time it took organism burdens in the treated animals to increase by 1 log10 CFU/kidney (t) after serum drug levels fell below the MIC for the organism (PAFE = t − c) (13).
PD index determinations.
Neutropenic mice were infected with C. albicans K-1 or C. glabrata 5376 2 h prior to the start of therapy. Twenty dosing regimens were chosen to determine the impact of the dose level and the dosing interval on anidulafungin efficacy. These 20 regimens comprised five total dose levels (1.25, 5, 20, 80, and 320 mg/kg/96 h) divided into one, two, four, or six doses (i.e., doses were given every 96, 48, 24, or 16 h). The drug doses were administered intraperitoneally in 0.2-ml volumes. This wide variety of regimens was used to minimize the interdependence among the three PD indices studied and also to demonstrate the complete dose-response relationship. For each dosing regimen, groups of three mice were treated over a 4-day study period. Mice were sacrificed at the end of therapy, and kidneys were removed for CFU determinations as described above. Untreated control mice were sacrificed just before treatment and at the end of the 4-day experiment. Efficacy was defined by the change in the log10 number of CFU per kidney over the study period and was calculated by subtracting the mean log10 number of CFU per kidney for treated mice from the mean log10 number of CFU per kidney for three untreated mice at the end of therapy.
PD index magnitude determinations.
Studies similar to those described above were performed with 13 additional Candida strains, for a total of 15 strains, including 4 C. albicans (K-1, 98-17, 580, and 98-210), 1 C. tropicalis (98-234), and 10 C. glabrata (5376, 570, 513, 5592, 33609, 32930, 33616, 34341, 35315, and 37661) strains. Dosing studies were designed to vary the magnitudes of the PD indices. The five total dose levels varied from 0.078 to 20 mg/kg/24 h. Doses were administered every 24 h for the 4-day study period. Groups of three mice were used for each dosing regimen. At the end of the study, mice were euthanized and kidneys were immediately processed for CFU determinations.
Data analysis.
A sigmoid dose-effect model was used to measure the in vivo potency of anidulafungin. The model was derived from the following Hill equation: E = (Emax × DN)/(ED50N + DN), where E is the observed effect (the change in the log10 number of CFU per kidney compared with the number for untreated controls at the end of the treatment period), D is the total dose, Emax is the maximum effect, ED50 is the 50% effective dose, or the dose required to achieve 50% of the Emax, and N is the slope of the line depicting the dose-effect relationship. The indices Emax, ED50, and N were calculated by using nonlinear least-squares regression. The correlation between efficacy and each of the three indices studied was determined by nonlinear least-squares regression analysis using Sigma Stat (Jandell Scientific Software, San Rafael, CA). The coefficient of determination (R2) was used to estimate the variance that could be due to regression with each of the PK-PD indices. Calculations were performed using both total and free-drug concentrations.
We also calculated the doses required to produce a net static effect and to reduce the organism burden by 1 log10 CFU/kidney over the treatment period for each of the dosing intervals. The calculated values for each dosing interval were compared by analysis of variance using Sigma Stat (Jandell Scientific Software). If the AUC/MIC ratio was the most predictive of anidulafungin in vivo activity, then these effective total doses would be similar for each dosing interval. If the T>MIC was the most predictive index, the effective total doses would be lower with shorter dosing intervals. And lastly, if the ratio of the peak serum drug level to the MIC was the most pharmacodynamically important index, the effective total doses would be lower with longer dosing intervals.
To allow a comparison of the degrees of potency of anidulafungin against a variety of organisms, we utilized the 24-h static dose and the doses required to achieve a 1 log reduction in colony counts. The magnitude of the PK-PD index associated with each end point dose was calculated from the following equation: log10 D = log10 [E/(Emax − E)]/(N + log10 ED50), where the effect E is the amount of growth relative to the control for static dose D, E is the amount of growth relative to the control minus 1 log for a D killing 1 log10 CFU, and N is the slope of the dose-response curve.
RESULTS
In vitro susceptibility testing and in vivo organism fitness.
The study organisms and the MICs of anidulafungin are listed in Table 1. The 24-h MICs for the 15 Candida organisms studied varied more than 100-fold (range, 0.015 to 2.0 μg/ml). The MICs determined using the partial (50%)-inhibition end point were one- to eightfold lower (mean difference, 3.8-fold) than those determined using a complete-inhibition end point.
Each of the Candida strains grew in the kidneys of untreated control mice over the study period. In general, the C. glabrata isolates were somewhat less fit based on growth in the kidneys over 48 h (mean ± SD of 1.83 ± 0.54 log10 CFU/kidney for C. glabrata versus 3.81 ± 0.33 log10 CFU/kidney for both C. albicans and C. tropicalis). Furthermore, two of the four isolates for which anidulafungin exhibited the highest MICs in vitro were the least fit in vivo (Table 1).
Pharmacokinetics.
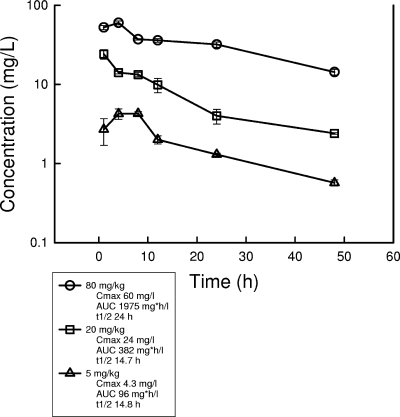
The time courses for anidulafungin in the sera of infected neutropenic mice following intraperitoneal doses of 80, 20, and 5 mg/kg are depicted in Fig. 1. Peak serum drug levels and the AUC increased in a linear fashion with dose escalation. Peak levels were achieved within 4 h for each of the doses and ranged from 4.3 ± 0.24 to 60 ± 3.5 μg/ml. The elimination half-life of the drug in serum was prolonged and ranged from 14 to 24 h. The AUC from 0 h to infinity (AUC0-∞), as determined by the trapezoidal rule, ranged from 96 to 1,975 mg·h/liter with the lowest and highest doses, respectively. Free-drug calculations were based both on protein binding levels in mice and humans determined previously by using equilibrium dialysis (99% bound) (B. Damle, Pfizer, personal communication) and on binding levels determined in the present study by using the arithmetic MICs in serum and serum ultrafiltrate (80% bound).
FIG. 1.
Pharmacokinetics of anidulafungin in sera of neutropenic infected mice following three single intraperitoneal doses of 5, 20, and 80 mg/kg. Each symbol represents the mean concentration for three mice and triplicate assays. The error bars represent the standard deviations. t1/2, half-life of the drug in serum.
In vivo time-kill and PAFE studies.
The effects of single doses of anidulafungin in the in vivo killing of strains of C. albicans and C. glabrata over time are shown in Fig. 2. Following tail vein inoculation with 105.6 CFU/ml, the C. albicans and C. glabrata burdens in the kidneys of untreated mice increased by 4.3 ± 0.24 and 3.2 ± 0.02 log10 CFU/kidney, respectively, over 96 h. Control growth of 1 log10 CFU/kidney in untreated mice was achieved in 10 and 9 h for C. albicans and C. glabrata, respectively. Each of the dose levels of anidulafungin produced extensive and rapid killing, reducing organism burdens in kidneys to levels below the limit of assay detection for both Candida species. Based upon the pharmacokinetics described above, the three doses of anidulafungin studied (1.25, 5, and 20 mg/kg) would produce serum drug levels (free drug, 1%) above the MICs for the C. albicans (0.12 μg/ml) and C. glabrata (0.06 μg/ml) strains for 0, 0, and 12 h and 0, 0, and 32 h, respectively. All the dose levels produced a net reduction in organisms to levels below the limit of detection within 1 to 4 h of drug administration. Anidulafungin at each of the doses studied suppressed the regrowth of organisms. A prolonged period of growth suppression was observed, ranging from 56 to >96 h for C. albicans and from 19 to 67 h for C. glabrata with the different anidulafungin doses.
FIG. 2.
Results of single-dose time-kill and PAFE studies of anidulafungin against strains of C. albicans and C. glabrata following three intraperitoneal doses of 1.25, 5, and 20 mg/kg. Each symbol represents the mean organism burden in the kidneys of three mice (six kidneys, plated in duplicate). The error bars represent the standard deviations. The solid symbols represent organism burdens in saline-treated control mice. The hollow horizontal bars represent the duration of time that total anidulafungin concentrations remained above the MIC for the organism. The solid horizontal bars represent the duration of time that free-anidulafungin concentrations remained above the MIC for the organism.
PD index determinations.
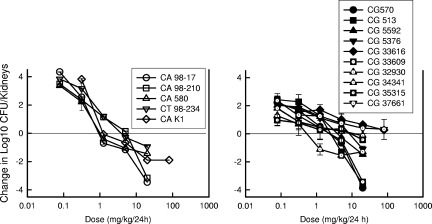
Figure 3 illustrates the anidulafungin dose-response curves for the different dosing intervals for the strains of C. albicans and C. glabrata. At the start of therapy, kidneys had 3.90 ± 0.15 log10 CFU of C. albicans K-1/kidney and 3.71 ± 0.21 log10 CFU of C. glabrata 5376/kidney. As the dosing interval was shortened from a single dose over the 96-h study period to q16h administration, the dose-response curves shifted to the right, indicating greater efficacy of the regimens in which large doses were administered infrequently. Each of the dose-response curves was also mathematically characterized by using a maximum-effect model. The static dose and the dose associated with 1-log killing for each of the drug-organism combinations and the various dosing regimens are shown in Table 2. The anidulafungin doses needed to produce either a net static effect or a 1-log10 reduction in organism burden in this model were three- to fivefold lower for the least fractionated regimens than for the other regimens. A statistical comparison revealed that the differences among all of the regimens, except that between the q24h and q48h regimens, were significant (P < 0.05). Both visual inspection of the dose-response curves in Fig. 3 and these statistical comparisons suggest that infrequent dosing with large amounts of anidulafungin was most effective in this model against both Candida species. This pattern of activity would suggest that either the AUC0-24/MIC ratio or the Cmax/MIC ratio would be the most important PD index for describing the activity of this echinocandin.
FIG. 3.
Impact of dose fractionation on the in vivo efficacy of anidulafungin against a strain of C. albicans (left panel) and a strain of C. glabrata (right panel). Mice were treated with one of a series of five fourfold-increasing total doses of anidulafungin. The total doses were fractionated into one, two, four, or six doses over a 96-h treatment period. Each symbol represents the mean organism burden in kidneys from three mice (six kidneys, plated in duplicate). The dashed horizontal lines represent the burdens of organisms in kidneys at the start of therapy.
TABLE 2.
Impact of anidulafungin dosing intervals on efficacy against C. albicans and C. glabrataa
| Organism | q16h
|
q24h
|
q48h
|
q96h
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ED50 (95% CI) | Static dose (95% CI) | Dose killing 1 log CFU (95% CI) | ED50 (95% CI) | Static dose (95% CI) | Dose killing 1 log CFU (95% CI) | ED50 (95% CI) | Static dose (95% CI) | Dose killing 1 log CFU (95% CI) | ED50 (95% CI) | Static dose (95% CI) | Dose killing 1 log CFU (95% CI) | |
| C. albicans K-1 | 8.74 (7.96-9.52) | 16.5 (14.9-18.1) | 38.3 (34.7-41.9) | 3.50 (3.3-3.7) | 5.00 (4.73-5.27) | 8.10 (7.67-8.53) | 3.14 (2.74-3.54) | 5.01 (4.41-5.61) | 9.19 (8.1-10.3) | 2.13 (2.08-2.18) | 3.07 (3.03-3.11) | 4.97 (4.9-4.93) |
| C. glabrata 5376 | 26.3 (23.4-29.2) | 30.0 (28.5-31.5) | 56.7 (53.9-59.5) | 15.9 (13.6-18.2) | 17.6 (16.5-18.7) | 30.8 (28.7-32.9) | 13.4 (10.9-15.9) | 15.3 (14-16.6) | 27.4 (25-29.8) | 8.12 (6.12-10.1) | 9.06 (6.06-12.1) | 16.6 (10.6-22.6) |
Values are doses in milligrams per kilogram per 96 h. The static dose is the dose needed to maintain the organism burden at the same level as that at the start of therapy. 95% CI, 95% confidence interval.
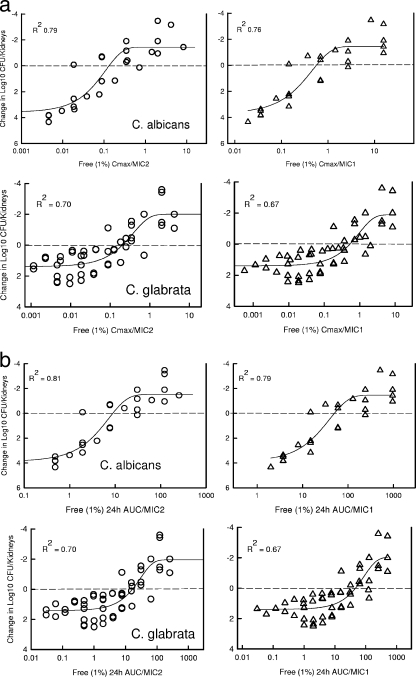
The relationships between the antimicrobial effect and each of the PD indices, the percentage of time that the drug level was above the MIC, the AUC/MIC ratio, and the ratio of the peak drug level to the MIC, are shown in Fig. 4. The data regressed with the AUC and the peak level in relation to the MIC had the strongest relationships, with R2 values of 89 and 93%, respectively, for C. albicans. The T>MIC had the weakest correlation with treatment efficacy, whether total or free-drug levels (1%) were considered, with R2 values of 61 and 52%, respectively. These PD relationships were the same for C. glabrata (R2 values for free drug: AUC/MIC ratio, 94%; Cmax/MIC ratio, 97%; percentage of time above the MIC, 84%).
FIG. 4.
(a) Relationship between the anidulafungin PD indices (the Cmax/MIC ratio, the AUC0-24/MIC ratio [24 h AUC/MIC], the percentage of time that the total drug concentration exceeds the MIC [% T>MIC], and the percentage of time that the free-drug concentration exceeds the MIC [% fT>MIC]], where the level of free drug is estimated at 1%) and in vivo efficacy against a strain of C. albicans. Mice were treated with one of five total doses of anidulafungin. The total doses were fractionated into one, two, four, or six doses over a 96-h treatment period. Each symbol represents the mean organism burden in kidneys from three mice (six kidneys, plated in duplicate). The lines through the data points represent the best-fit curves. R2 is the coefficient of determination. (b) Relationship between the anidulafungin PD indices (the Cmax/MIC ratio, the AUC0-24/MIC ratio, the percentage of time that the total drug concentration exceeds the MIC, and the percentage of time that the free-drug concentration exceeds the MIC [free % T>MIC]) and in vivo efficacy against a strain of C. glabrata. Mice were treated with one of five total doses of anidulafungin. The total doses were fractionated into one, two, four, or six doses over a 96-h treatment period. Each symbol represents the mean organism burden in kidneys from three mice (six kidneys, plated in duplicate). The lines plotted through the data represent the best-fit curves. R2 is the coefficient of determination.
Magnitude of the PD index associated with efficacy.
Both of the concentration-dependent indices were important predictors of the in vivo efficacy of anidulafungin. Thus, for comparisons of the index magnitudes among strains for which the MICs varied, we examined both the AUC0-24/MIC and Cmax/MIC ratios. To determine if the index magnitudes among Candida strains were similar, we studied the activities of anidulafungin in the q24h dosing regimen against 4 strains of C. albicans, 1 strain of C. tropicalis, and 10 strains of C. glabrata. The dose-response curves for anidulafungin against these various strains are shown in Fig. 5. At the start of therapy, the mice infected with C. albicans had 3.63 ± 0.21 log10 CFU of Candida/kidney (range, 3.16 to 3.90 log10 CFU/kidney). The four C. albicans strains and the C. tropicalis strain grew similarly in the animals. However, the growth of the C. glabrata strains was less than that of the other Candida strains studied. The mean level of organism growth in control animals was 3.81 ± 0.33 log10 CFU/kidney for C. albicans and C. tropicalis and 1.83 ± 0.54 log10 CFU/kidney for C. glabrata. The group of 10 C. glabrata isolates demonstrated relatively equivalent levels of growth. In general, the shapes of the dose-response curves were similar for all strains. The location of each dose-response curve was related to the MIC for the organism. The static doses, the doses associated with 1-log killing, and the associated free-drug Cmax/MIC and AUC0-24/MIC ratios are shown in Tables 3 and 4. The extent of organism killing varied among the strains and was related to the anidulafungin exposure-MIC relationship. The majority of strains exhibited an extensive drop in the numbers of CFU following anidulafungin therapy over the 4-day study compared to the numbers of CFU in the untreated controls (mean reduction ± SD, 4.13 ± 1.9 log10 CFU/kidney). For the anidulafungin regimens with q24h dosing, the free-drug Cmax/MIC ratio associated with a static effect against the 15 Candida strains was calculated to be 0.26 ± 0.22 by using 1% free-drug concentrations and a complete-inhibition end point for the MIC. The corresponding PD index value (Cmax/MIC ratio) determined by using a partial-inhibition end point was 0.91 ± 0.76. The free-drug AUC0-24/MIC ratios associated with the same stasis end point for these organisms were 5.23 ± 5.01 for the complete-inhibition MIC and 18 ± 15 for the partial-inhibition MIC end point. The PD index values determined by using the protein binding value from the arithmetic MIC studies (80%) are also provided in Tables 3 and 4. The relationships between the anidulafungin free-drug Cmax/MIC and AUC0-24/MIC ratios and efficacy for the 15 strains are displayed in Fig. 6. The relationship among the treatment groups was strong (R2 values for C. albicans: Cmax/MIC ratio, 76%, and AUC/MIC ratio, 82%; R2 values for C. glabrata: Cmax/MIC ratio, 70%, and AUC/MIC ratio, 82%). The in vivo anidulafungin drug exposures associated with a 1-log reduction in organism burden are shown in Table 4. The mean anidulafungin drug exposures, relative to the MIC, that were necessary to produce a 1-log reduction in the yeast burden were 2.5-fold greater than those associated with fungal stasis in this model.
FIG. 5.
In vivo anidulafungin dose-response curves for multiple strains of C. albicans (5 strains; left panel) and C. glabrata (10 strains; right panel). Mice received one of a series of five fourfold-increasing doses of anidulafungin every 24 h over a 96-h treatment period. Each symbol represents the mean organism burden in the kidneys of three mice (six kidneys, plated in duplicate). The error bars represent the standard deviations. The solid horizontal lines at 0 on the y axis represent the organism burdens at the start of therapy. Symbols below the line represent organism reduction or killing over the treatment period compared to the burden at the start of therapy. Symbols above the line represent organism growth.
TABLE 3.
Doses and PD indices associated with a net static effect of anidulafungin against multiple Candida organisms in a murine neutropenic disseminated candidiasis modela
| Strain | Static dose (mg/kg/24 h) | Cmax/MIC | F1 Cmax/MIC | F2 Cmax/MIC | AUC0-24/MIC | F1 AUC0-24/MIC | F2 AUC0-24/MIC |
|---|---|---|---|---|---|---|---|
| C. albicans K-1 | 1.25 | 9 (72) | 0.09 (0.72) | 1.89 (14.4) | 200 (1,600) | 2.0 (16) | 42 (320) |
| C. albicans 98-17 | 1.15 | 42 (165) | 0.42 (1.65) | 8.66 (33) | 184 (736) | 1.84 (7.37) | 38.6 (147) |
| C. albicans 98-210 | 3.37 | 48 (193) | 0.48 (1.93) | 10.2 (39) | 1,078 (4,313) | 10.8 (43.1) | 226 (863) |
| C. albicans 580 | 0.94 | 27 (53) | 0.27 (0.53) | 5.6 (10.7) | 600 (1,200) | 6.0 (12) | 126 (240) |
| C. tropicals 98-234 | 3.81 | 27 (216) | 0.27 (2.16) | 5.4 (43.2) | 610 (4,880) | 6.1 (48.8) | 122 (976) |
| C. glabrata 570 | 1.97 | 14 (57) | 0.14 (0.57) | 2.97 (11.3) | 315 (1,260) | 3.15 (12.6) | 66.2 (252) |
| C. glabrata 513 | 2.45 | 18 (35) | 0.18 (0.35) | 3.69 (7.0) | 392 (783) | 3.92 (7.83) | 82.3 (157) |
| C. glabrata 33609 | 2.00 | 14 (57) | 0.14 (0.57) | 2.86 (11.5) | 317 (1,267) | 3.17 (12.7) | 63.4 (253) |
| C. glabrata 32930 | 1.58 | 11.3 (23) | 0.11 (0.23) | 2.26 (4.53) | 250 (500) | 2.5 (5.0) | 50 (100) |
| C. glabrata 33616 | NA | NA | NA | NA | NA | NA | |
| C. glabrata 34341 | 6.9 | 6.9 (58) | 0.07 (0.58) | 1.38 (11.5) | 132 (1,100) | 1.32 (11) | 26.4 (220) |
| C. glabrata 37661 | NA | NA | NA | NA | NA | NA | |
| C. glabrata 35315 | 6.4 | 6.2 (25) | 0.06 (0.25) | 1.24 (4.96) | 123 (492) | 1.23 (4.92) | 24.6 (98.4) |
| C. glabrata 5592 | 3.57 | 25 (50) | 0.25 (0.50) | 5.0 (10) | 570 (1,141) | 5.7 (11.4) | 114 (228) |
| C. glabrata 5376 | 6.8 | 92 (183) | 0.92 (1.83) | 18.3 (36.7) | 2,033 (4,047) | 20.3 (40.6) | 407 (813) |
| Mean ± SD | 26.1 ± 22 (91 ± 70) | 0.26 ± 0.22 (0.91 ± 0.70) | 5.47 ± 4.94 (18 ± 14) | 523 ± 521 (1,795 ± 1,538) | 5.23 ± 5.01 (18 ± 15) | 108 ± 110 (359 ± 307) |
NA indicates that the end point was not achieved. The static dose is the dose needed to maintain the organism burden at the same level as that at the start of therapy. F1 and F2 indicate that values were determined using free-drug concentrations of 1 and 20%, respectively. Values in parentheses are those determined using the partial-inhibition end point; other values were calculated using the complete-inhibition end point.
TABLE 4.
Doses and PD indices associated with killing of 1 log CFU of various Candida organisms by anidulafungin in a murine neutropenic disseminated candidiasis modela
| Strain | Dose (mg/kg/24 h) killing 1 log CFU | Cmax/MIC | F1 Cmax/MIC | F2 Cmax/MIC | AUC0-24/MIC | F1 AUC0-24/MIC | F2 AUC0-24/MIC |
|---|---|---|---|---|---|---|---|
| C. albicans K-1 | 2 | 14.3 (114) | 0.14 (1.2) | 2.86 (22.9) | 320 (2,560) | 3.2 (25.6) | 64 (512) |
| C. albicans 98-17 | 1.95 | 14 (56) | 0.14 (0.56) | 2.80 (11.2) | 312 (1,247) | 3.12 (12.5) | 62.4 (249) |
| C. albicans 98-210 | 13 | 255 (1020) | 2.55 (10.2) | 51 (204) | 4,133 (16,533) | 41.3 (165) | 824 (3307) |
| C. albicans 580 | 2.31 | 66.3 (132) | 0.66 (1.33) | 13.3 (26.5) | 1,480 (2,960) | 14.8 (29.6) | 296 (592) |
| C. tropicals 98-234 | NA | NA | NA | NA | NA | ||
| C. glabrata 570 | 3.72 | 26.7 (107) | 0.27 (1.07) | 5.34 (21.3) | 595 (2,380) | 6.0 (23.8) | 119 (476) |
| C. glabrata 513 | 4.13 | 29.6 (59) | 0.30 (0.59) | 5.92 (11.8) | 658 (1,317) | 6.58 (13.2) | 132 (263) |
| C. glabrata 33609 | 4.21 | 30.2 (121) | 0.30 (1.21) | 6.04 (24.1) | 673 (2,693) | 6.73 (26.9) | 135 (539) |
| C. glabrata 32930 | 3.31 | 23.8 (48) | 0.24 (0.48) | 4.76 (9.50) | 530 (1,060) | 5.3 (10.6) | 106 (212) |
| C. glabrata 33616 | NA | NA | NA | NA | NA | ||
| C. glabrata 34341 | NA | NA | NA | NA | NA | ||
| C. glabrata 37661 | NA | NA | NA | NA | NA | ||
| C. glabrata 35315 | NA | NA | NA | NA | NA | ||
| C. glabrata 5592 | 11.8 | 114 (228) | 1.14 (2.28) | 22.8 (45.7) | 1,875 (3,750) | 18.8 (37.5) | 375 (750) |
| C. glabrata 5376 | 7.5 | 129 (258) | 1.29 (2.58) | 25.8 (51.7) | 2,383 (4,766) | 23.8 (47.7) | 477 (953) |
| Mean ± SD | 70.3 ± 72 | 0.70 ± 0.72 | 14.1 ± 14.5 | 1,296 ± 1,157 | 13 ± 11.7 | 259 ± 231 | |
| (214 ± 292) | (2.14 ± 2.92) | (42.3 ± 58.3) | (3,927 ± 4,577) | (39.3 ± 45.8) | (785 ± 915) |
NA indicates that the end point was not achieved. The static dose is the dose needed to maintain the organism burden at the same level as that at the start of therapy. F1 and F2 indicate that values were determined using free-drug concentrations of 1 and 20%, respectively. Values in parentheses are those determined using the partial-inhibition end point; other values were calculated using the complete-inhibition end point.
FIG. 6.
(a) Relationship between the anidulafungin Cmax/MIC ratio (using free-drug concentrations of 1%) and in vivo efficacy against multiple strains of C. albicans (5 strains; top panels) and C. glabrata (10 strains; bottom panels). Left panels present data obtained using total-inhibition drug MICs, and right panels present data obtained using partial-inhibition end points. Mice were treated with one of five total doses of anidulafungin. The total doses were fractionated into four doses (q24h) over a 96-h treatment period. Each symbol represents the mean organism burden in kidneys from three mice (six kidneys, plated in duplicate). The dashed horizontal lines represent the organism burdens in the kidneys at the start of therapy. The sigmoid lines represent the best-fit curves. R2 is the coefficient of determination. (b) Relationship between the anidulafungin AUC0-24/MIC ratio (using free-drug concentrations of 1%) and in vivo efficacy against multiple strains of C. albicans (5 strains; top panels) and C. glabrata (10 strains; bottom panels). Left panels present data obtained using total-inhibition drug MICs, and right panels present data obtained using partial-inhibition end points. Mice were treated with one of five total doses of anidulafungin. The total doses were fractionated into four doses (q24h) over a 96-h treatment period. Each symbol represents the mean organism burden in kidneys from three mice (six kidneys, plated in duplicate). The dashed horizontal lines represent the organism burdens in the kidneys at the start of therapy. The sigmoid lines represent the best-fit curves. R2 is the coefficient of determination.
DISCUSSION
The study of antimicrobial pharmacodynamics provides insight into the link among drug exposures, in vitro susceptibility, and treatment efficacy (12, 16). These investigations have been useful for optimizing dosing regimens and for defining clinical resistance or guiding the development of susceptibility breakpoints (1, 7, 12, 16, 18, 43, 44, 47). The majority of these studies are undertaken using either in vitro or animal infection models, and the results are used to predict outcomes in humans. These types of studies have been useful in the development of dosing strategies for a variety of antivirals, antibacterials, and more recently, antifungals (1, 3, 7, 9, 11, 12, 16, 44, 46, 48, 49). The predictions from these studies have been shown to be relevant for optimizing treatment efficacy in patients. For example, animal studies with triazole antifungals have shown that the PD index best associated with treatment outcome is an AUC0-24/MIC ratio near 25 (4). The examination of large clinical databases has demonstrated the relevance of this PD target (4, 43, 44, 47). Furthermore, this PD relationship has been useful for supporting the development of susceptibility breakpoints for these compounds and Candida species (43, 44, 47).
Studies have begun to explore these PD relationships for drugs from the most recently Food and Drug Administration-approved antifungal drug class, the echinocandins (2, 11, 17, 18, 19, 21, 22, 23, 27, 37, 38, 39, 51, 52, 53). Both in vitro and in vivo studies have demonstrated that the killing activities of echinocandin antifungals, such as anidulafungin, are enhanced by exposure to drug concentrations far exceeding the MICs. For example, Walsh et al. found that the extent and rate of killing of Candida in vitro with cilofungin were enhanced with each increase in concentration over a 50-fold range (51). Ernst et al. observed similar concentration-dependent activity for other echinocandins in vitro (17, 18, 19). These studies also demonstrated a prolonged period of growth suppression following brief echinocandin exposures (i.e., a PAFE). An in vitro pattern of activity characterized by concentration-dependent killing and a prolonged PAFE would suggest that large, infrequent drug doses may be most efficacious (12). The PD indices that correlate with this pattern of activity include the Cmax/MIC and AUC0-24/MIC ratios. A prior animal model PD study of a new echinocandin, aminocandin, showed that large, infrequent doses were the most efficient strategy to reduce the Candida burden and that the PD index that best described the dosing relationship was the Cmax/MIC ratio (2). However, the AUC/MIC ratio was also a strong predictor of efficacy. Investigators in a similar study of caspofungin in an aspergillosis model also observed this relationship (53). A subsequent study with caspofungin in a candidiasis model confirmed the importance of this dosing strategy (27). However, an analysis of PD relationships provided a stronger fit for the AUC/MIC ratio. Interestingly, the investigators in the latter study examined the treatment outcome over time. The outcome early in therapy (up to day 3) appears to be most closely linked to the Cmax/MIC ratio; however, the outcome later in the treatment period (day 7) is better predicted by the AUC/MIC ratio, presumably due to the prolonged tissue distribution of these compounds. The impact of these subtle differences may not affect dosing strategies. Both PD relationships support the administration of large doses of echinocandin infrequently. However, the possibility that maximizing the Cmax early in therapy may improve the outcome or shorten treatment courses should be explored in future studies.
One goal of the present studies was to further examine these relationships for the echinocandin anidulafungin against C. albicans and to determine if the relationships were similar for the treatment of infection with C. glabrata. Similar to the results of studies of other drugs from this class, the degree of in vivo killing in our observations was enhanced with higher drug doses over a 16-fold dose range in single-dose studies and over a 125-fold dose range in multiple-dose investigations. In postantifungal observations, the duration of regrowth suppression was as long as 4 days. These time course characteristics would also suggest that one of the concentration-dependent PK-PD indices would best predict drug efficacy. These observations were similar for both C. albicans and C. glabrata.
The observations from multiple anidulafungin dosing regimens in these studies further demonstrated that the infrequent administration of large drug doses was most effective. Three- to fourfold-lower total drug doses were necessary to produce a net fungistatic effect and 1-log killing in the q96h dosing regimen than in the q16h dosing regimen. As one would expect, when these dosing regimen data were regressed with each of the three PK-PD indices, the strongest relationships were observed when the Cmax/MIC and AUC/MIC ratios were utilized. Similar to the results of our studies of aminocandin in this model, the Cmax/MIC ratio regression was slightly stronger; however, the differences were small. Similar to the findings of time course studies, the PD relationships were similar for both C. albicans and C. glabrata. These dose-response relationships clearly support the use of large, infrequent doses to optimize in vivo treatment efficacy.
Drugs from the echinocandin class, including caspofungin, micafungin, and anidulafungin, exert potent activity against many fungal pathogens, including Candida species (20, 40, 41). The degrees of potency of this class against C. albicans and C. glabrata are similar. The most common Candida species for which echinocandin in vitro potency is reduced is C. parapsilosis. Most C. parapsilosis isolates are roughly 50- to 100-fold less susceptible to echinocandins than are other common Candida species. Clinical trials have demonstrated the effectiveness of these compounds for the management of both mucosal and systemic candidiasis (10, 29, 31, 34, 45, 50). In these large trials, the MICs for the majority of organisms are very low and there has been no discernible relationship between in vitro susceptibility and treatment efficacy. The only isolates for which the MICs are elevated are a few C. parapsilosis isolates from cases of infection in which patients appeared to fare well. However, case reports describing treatment failure and elevated MICs for C. albicans and C. glabrata have begun to accumulate (24, 25, 26, 28, 30, 32). A second goal of the present studies was to determine the amount of the drug relative to the MIC or the magnitude of the predictive PD index required for treatment efficacy. In addition, we wished to discern if the PD targets for C. albicans and C. glabrata were similar. We attempted to utilize strains with various anidulafungin in vitro susceptibilities. The less susceptible strains used in these studies have been clinically characterized previously and were isolated from patients who received prolonged courses of treatment with an echinocandin (caspofungin) and experienced treatment failure or organism persistence. We considered both partial- and complete-inhibition MIC end points. Not surprisingly, the values for the complete-inhibition end points were higher than those for the partial-inhibition end points currently recommended by the CLSI. Similar to the results in previous reports, the response to the echinocandin therapy was related to the MIC for the organism with both end points (11).
One treatment variable that we encountered in the present study and that can be difficult to account for in modeling is the impact of organism fitness. Not all strains and species produce similar degrees of disease in this model or, for that matter, in cases of clinical disease (36, 42). In the past and in the present investigations, we considered the level of organism growth in the kidneys to provide an estimate of fitness. In general, the C. albicans strains were more fit in this model (the C. albicans organism burden increased twofold more than the C. glabrata organism burden). Among the C. glabrata isolates, two of the four strains for which MICs were higher grew less well than the other strains, suggesting a fitness cost associated with resistance development, as has been described previously for C. albicans (8). Despite this fitness cost, the response to anidulafungin in the present studies was related to the MIC for all of the organisms. We had hoped to be able to discern the impact of higher echinocandin MICs for C. parapsilosis. However, this species does not grow well in mice, even with significant immunosuppression (unpublished observations).
Since both the Cmax/MIC and AUC0-24/MIC ratios were important in the dosing interval studies, we considered both indices in the PD target investigations. Previous PD studies with triazole antifungals have demonstrated the importance of considering protein binding; thus, we considered both the total (including protein-bound) and free (unbound)-drug concentrations in these analyses (7). We performed the study using an additional protein binding method, given the variation in the protein binding values determined by other methods (anidulafungin ultrafiltration, 84% binding, and equilibrium dialysis, 99% binding) (15, 35, 54). We report free-drug values using results from both methods, as it is not known which, if either, is relevant in vivo. This information may become important for PD comparisons as similar data emerge for other drugs from the echinocandin class.
Because it is not known which echinocandin treatment end point in this model will correlate with outcomes in patients, we considered both an inhibitory (static-dose) and a killing (1-log-reduction) end point. In studies with 13 of the 15 organisms, we observed an inhibitory effect. For the two organisms for which we did not observe growth inhibition, the MIC was 2.0 μg/ml. We observed a 1-log reduction in vivo for 10 of the 15 strains. For four of the five strains for which we did not observe a killing effect, the MIC was 1 or 2 μg/ml. Of note, for the organisms for which the MIC was 1 μg/ml, the calculations were based on a complete-inhibition end point. These data suggest a strong relationship between exposure and effect and further demonstrate the relevance of the MIC.
The pharmacokinetics of anidulafungin in humans demonstrate that the drug has a long half-life and protein binding values essentially the same as those observed in mice (15; B. Damle, Pfizer Inc., personal communication). A dose of 100 mg/day produces a Cmax of 11 μg/ml (free-drug concentration of 0.11 μg/ml for 1% binding) and a steady-state AUC of 112 mg·h/liter (free-drug value of 1.12 mg·h/liter for 1% of the total). If one considers the inhibitory PD targets identified in the present in vivo models, current anidulafungin dosing regimens would give free-drug AUC0-24/MIC ratios exceeding those for both C. albicans and C. glabrata for more than 99% of the organisms from large surveillance databases (MIC for C. albicans at which 90% of the isolates were inhibited [MIC90], 0.06 μg/ml, and MIC90 for C. glabrata, 0.12 μg/ml) (20, 40). Thus far, there is minimal clinical data to reliably discern the impact of echinocandin MICs on treatment outcome. The present in vivo pharmacodynamic studies would suggest that at least for C. albicans and C. glabrata, the lack of correlation between MICs and outcomes is related to the very low MIC distribution observed in these clinical trials.
In summary, the present studies demonstrate that anidulafungin has concentration-dependent in vivo efficacy against both C. albicans and C. glabrata. The Cmax/MIC and AUC0-24/MIC ratios were very highly associated with in vivo anidulafungin activity. These PD characteristics support the infrequent administration of large doses. These studies also suggest that anidulafungin at a dose of 100 mg/day achieves an inhibitory PD target against C. albicans and C. glabrata organisms for which MICs are up to 0.12 μg/ml, as determined by using a partial-inhibition MIC end point, and 0.5 μg/ml, as determined by using a complete-inhibition MIC end point. This concentration is lower than the MIC90 distribution for C. parapsilosis. We suspect that the successful treatment of infection with these reduced-fitness organisms may require a lower PD target due to the reduced virulence of this Candida species. In the absence of a large series of echinocandin clinical successes against C. albicans and C. glabrata infections with organisms for which MICs are in the same range as those for C. parapsilosis, the concept of species-specific susceptibility breakpoints should be explored.
Acknowledgments
Research was funded with a grant from Pfizer.
Footnotes
Published ahead of print on 10 December 2007.
REFERENCES
- 1.Ambrose, P. G., S. M. Bhavnani, C. M. Rubino, A. Louie, T. Gumbo, A. Forrest, and G. L. Drusano. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin. Infect. Dis. 44:79-86. [DOI] [PubMed] [Google Scholar]
- 2.Andes, D., K. Marchillo, J. Lowther, A. Bryskier, T. Stamstad, and R. Conklin. 2003. In vivo pharmacodynamics of HMR 3270, a glucan synthase inhibitor, in a murine candidiasis model. Antimicrob. Agents Chemother. 47:1187-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andes, D., and W. A. Craig. 2007. In vivo pharmacodynamic activity of the glycopeptide dalbavancin. Antimicrob. Agents Chemother. 51:1633-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andes, D., and M. I. van Ogtrop. 1999. Characterization and quantitation of the pharmacodynamics of fluconazole in a neutropenic murine disseminated candidiasis infection model. Antimicrob. Agents Chemother. 43:2116-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andes, D., and M. I. van Ogtrop. 2000. In vivo characterization of the pharmacodynamics of flucytosine in a neutropenic murine candidiasis model. Antimicrob. Agents Chemother. 44:938-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andes, D. 2001. In vivo pharmacodynamics of amphotericin B against selected Candida species. Antimicrob. Agents Chemother. 45:922-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andes, D. 2003. In vivo pharmacodynamics of antifungal drugs in treatment of candidiasis. Antimicrob. Agents Chemother. 47:1179-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andes, D., K. Marchillo, J. Nett, A. Pitula, and J. Smith. 2006. In vivo fluconazole pharmacodynamics and resistance development in a previously susceptible Candida albicans population examined by microbiologic and transcriptional profiling. Antimicrob. Agents Chemother. 50:2384-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blaser, J., B. B. Stone, M. C. Groner, and S. H. Zinner. 1987. Comparative study with enoxacin and netilmicin in a pharmacodynamic model to determine importance of ratio of antibiotic peak concentration to MIC for bactericidal activity and emergence of resistance. Antimicrob. Agents Chemother. 31:1054-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandrasekar, P. H., and J. D. Sobel. 2006. Micafungin: a new echinocandin. Clin. Infect. Dis. 42:1171-1178. [DOI] [PubMed] [Google Scholar]
- 11.Cota, J., M. Carden, J. R. Graybill, L. K. Najvar, D. S. Burgess, and N. P. Wiederhold. 2006. In vitro pharmacodynamics of anidulafungin and caspofungin against Candida glabrata isolates, including strains with decreased caspofungin susceptibility. Antimicrob. Agents Chemother. 50:3926-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-12. [DOI] [PubMed] [Google Scholar]
- 13.Craig, W. A., and S. Gudmundsson. 1996. Postantibiotic effect, p. 296-329. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. Williams and Wilkins, Baltimore, MD.
- 14.Denning, D. W. 2003. Echinocandin antifungal drugs. Lancet 362:1142-1151. [DOI] [PubMed] [Google Scholar]
- 15.Dowell, J. A., W. Knebel, T. Ludden, M. Stogniew, D. Krause, and T. Henkel. 2004. Population pharmacokinetic analysis of anidulafungin, an echinocandin antifungal. J. Clin. Pharmacol. 44:590-598. [DOI] [PubMed] [Google Scholar]
- 16.Drusano, G. L. 2004. Antimicrobial pharmacodynamics: critical interactions of bug and drug. Nat. Rev. Microbiol. 2:289-300. [DOI] [PubMed] [Google Scholar]
- 17.Ernst, E. J., M. E. Klepser, and M. A. Pfaller. 2000. Postantifungal effects of echinocandin, azole, and polyene antifungal agents against Candida albicans and Cryptococcus neoformans. Antimicrob. Agents Chemother. 44:1108-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ernst, E. J., M. E. Klepser, M. E. Ernst, S. A. Messer, and M. A. Pfaller. 1999. In vitro pharmacodynamic properties of MK-0991 determined by timekill methods. Diagn. Microbiol. Infect. Dis. 33:75-80. [DOI] [PubMed] [Google Scholar]
- 19.Ernst, M. E., M. E. Klepser, E. J. Wolfe, and M. A. Pfaller. 1996. Antifungal dynamics of LY 303366, an investigational echinocandin B analog, against Candida ssp. Diagn. Microbiol. Infect. Dis. 26:125-131. [DOI] [PubMed] [Google Scholar]
- 20.Espinel-Ingroff, A. 2003. In vitro antifungal activities of anidulafungin and micafungin, licensed agents and the investigational triazole posaconazole as determined by NCCLS methods for 12,052 fungal isolates: review of the literature. Rev. Iberoam. Micol. 20:121-136. [PubMed] [Google Scholar]
- 21.Groll, A. H., D. Mickiene, R. Petrainiene, V. Pettraitis, C. A. Lyman, J. S. Bacher, S. C. Piscitelli, and T. J. Walsh. 2001. Pharmacokinetic and pharmacodynamic modeling of anidulafungin (LY30336): reappraisal of its efficacy in neutropenic animal models of opportunistic mycoses using optimal plasma sampling. Antimicrob. Agents Chemother. 45:2845-2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gumbo, T., G. L. Drusano, W. Liu, R. W. Kulawy, C. Fregeau, V. Hsu, and A. Louie. 2007. Once-weekly micafungin therapy is as effective as daily therapy for disseminated candidiasis in mice with persistent neutropenia. Antimicrob. Agents Chemother. 51:968-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gumbo, T., G. L. Drusano, W. Liu, L. Ma, M. R. Deziel, M. F. Drusano, and A. Louie. 2006. Anidulafungin pharmacokinetics and microbial response in neutropenic mice with disseminated candidiasis. Antimicrob. Agents Chemother. 50:3695-3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hakki, M., J. F. Staab, and K. A. Marr. 2006. Emergence of a Candida krusei isolate with reduced susceptibility to caspofungin during therapy. Antimicrob. Agents Chemother. 50:2522-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernandez, S., J. L. Lopez-Ribot, L. K. Najvar, D. I. McCarthy, R. Bocanegra, and J. R. Graybill. 2004. Caspofungin resistance in Candida albicans: correlating clinical outcome with laboratory susceptibility testing of three isogenic isolates serially obtained from a patient with progressive Candida esophagitis. Antimicrob. Agents Chemother. 48:1382-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laverdière, M., R. G. Lalonde, J. G. Baril, D. C. Sheppard, S. Park, and D. S. Perlin. 2006. Progressive loss of echinocandin activity following prolonged use for treatment of Candida albicans oesophagitis. J. Antimicrob. Chemother. 57:705-708. [DOI] [PubMed] [Google Scholar]
- 27.Louie, A., M. Deziel, W. Liu, M. F. Drusano, T. Gumbo, and G. L. Drusano. 2005. Pharmacodynamics of caspofungin in a murine model of systemic candidiasis: importance of persistence of caspofungin in tissues to understanding drug activity. Antimicrob. Agents Chemother. 49:5058-5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krogh-Madsen, M., M. C. Arendrup, L. Heslet, and J. D. Knudsen. 2006. Amphotericin B and caspofungin resistance in Candida glabrata isolates recovered from a critically ill patient. Clin. Infect. Dis. 42:938-944. [DOI] [PubMed] [Google Scholar]
- 29.Kuse, E. R., P. Chetchotisakd, C. A. da Cunha, M. Ruhnke, C. Barrios, D. Raghunadharao, J. S. Sekhon, A. Freire, V. Ramasubramanian, I. Demeyer, M. Nucci, A. Leelarasamee, F. Jacobs, J. Decruyenaere, D. Pittet, A. J. Ullmann, L. Ostrosky-Zeichner, O. Lortholary, S. Koblinger, H. Diekmann-Berndt, and O. A. Cornely. 2007. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomized double-blind trial. Lancet 369:1519-1527. [DOI] [PubMed] [Google Scholar]
- 30.Miller, C. D., B. W. Lomaestro, S. Park, and D. S. Perlin. 2006. Progressive esophagitis caused by Candida albicans with reduced susceptibility to caspofungin. Pharmacotherapy 26:877-880. [DOI] [PubMed] [Google Scholar]
- 31.Mora-Durate, J. M., R. Betts, C. Rotstein, A. Pescolombo, L. Thompson-Moya, J. S. Meitana, R. Lupinacci, C. Sable, N. Kartsonis, and J. Perfect. 2002. Comparison of caspofungin and amphotericin B for invasive candidiasis. N. Engl. J. Med. 347:2020-2029. [DOI] [PubMed] [Google Scholar]
- 32.Moudgal, V., T. Little, D. Boikov, and J. A. Vazquez. 2005. Multiechinocandin- and multiazole-resistant Candida parapsilosis isolates serially obtained during therapy for prosthetic valve endocarditis. Antimicrob. Agents Chemother. 49:767-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.NCCLS. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard, 2nd ed. Document M27-A2. NCCLS, Wayne, PA.
- 34.Ostrosky-Zeichner, L., D. Kontoyiannis, R. Raffalli, K. M. Mullane, J. Vazquez, E. J. Anaissie, J. Lipton, P. Jacobs, J. H. van Rensburg, J. H. Rex, W. Lau, D. Facklam, and D. N. Buell. 2005. International, open-label, noncomparative, clinical trial of micafungin alone and in combination for treatment of newly diagnosed and refractory candidemia. Eur. J. Clin. Microbiol. Infect. Dis. 24:654-661. [DOI] [PubMed] [Google Scholar]
- 35.Paderu, P., G. Garcia-Effron, S. Balashov, G. Delmas, S. Park, and D. S. Perlin. 2007. Serum differentially alters the antifungal properties of echinocandin drugs. Antimicrob. Agents Chemother. 51:2253-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pappas, P. G., J. H. Rex, J. Lee, R. J. Hamill, R. A. Larsen, W. Powderly, C. A. Kauffman, N. Hyslop, J. E. Mangino, S. Chapman, H. W. Horowitz, J. E. Edwards, W. E. Dismukes, and the NIAID Mycoses Study Group. 2003. A prospective observational study of candidemia: epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients. Clin. Infect. Dis. 37:634-643. [DOI] [PubMed] [Google Scholar]
- 37.Petraitiene, R., V. Petraitis, A. H. Groll, T. Sein, R. L. Schaufele, A. Francesconi, J. Bacher, N. A. Avila, and T. J. Walsh. 2002. Antifungal efficacy of caspofungin (MK-0991) in experimental pulmonary aspergillosis in persistently neutropenic rabbits: pharmacokinetics, drug disposition, and relationship to galactomannan antigenemia. Antimicrob. Agents Chemother. 46:12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petraitiene, R., V. Petraitis, A. H. Groll, M. Candelario, T. Sein, A. Bell, C. A. Lyman, C. L. McMillian, J. Bacher, and T. J. Walsh. 1999. Antifungal activity of LY303366, a novel echinocandin B, in experimental disseminated candidiasis in rabbits. Antimicrob. Agents Chemother. 43:2148-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petraitis, V., R. Petraitiene, A. H. Groll, K. Roussillon, M. Hemmings, C. A. Lyman, T. Sein, J. Bacher, I. Bekersky, and T. J. Walsh. 2002. Comparative antifungal activities and plasma pharmacokinetics of micafungin (FK463) against disseminated candidiasis and invasive pulmonary aspergillosis in persistently neutropenic rabbits. Antimicrob. Agents Chemother. 46:1857-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pfaller, M. A., L. Boyken, R. J. Hollis, S. A. Messer, S. Tendolkar, and D. J. Diekema. 2005. In vitro activities of anidulafungin against more than 2,500 clinical isolates of Candida spp., including 315 isolates resistant to fluconazole. J. Clin. Microbiol. 43:5425-5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfaller, M. A., L. Boyken, R. J. Hollis, S. A. Messer, S. Tendolkar, and D. J. Diekema. 2006. Global surveillance of in vitro activity of micafungin against Candida: a comparison with caspofungin by CLSI-recommended methods. J. Clin. Microbiol. 44:3533-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pfaller, M. A., and D. J. Diekema. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20:133-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfaller, M. A., D. J. Diekema, J. H. Rex, A. Espinel-Ingroff, E. M. Johnson, D. Andes, V. Chaturvedi, M. A. Ghannoum, F. C. Odds, M. G. Rinaldi, D. J. Sheehan, P. Troke, T. J. Walsh, and D. W. Warnock. 2006. Correlation of MIC with outcome for Candida species tested against voriconazole: analysis and proposal for interpretive breakpoints. J. Clin. Microbiol. 44:819-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pfaller, M. A., D. J. Diekema, and D. J. Sheehan. 2006. Interpretive breakpoints for fluconazole and Candida revisited: a blueprint for the future of antifungal susceptibility testing. Clin. Microbiol. Rev. 19:435-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pfaller, M. A., D. J. Diekema, L. Boyken, S. A. Messer, S. Tendolkar, R. J. Hollis, and B. P. Goldstein. 2005. Effectiveness of anidulafungin in eradicating Candida species in invasive candidiasis. Antimicrob. Agents Chemother. 49:4795-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Preston, S. L., P. J. Piliero, and G. L. Drusano. 2003. Pharmacodynamics and clinical use of anti-HIV drugs. Infect. Dis. Clin. N. Am. 17:651-674. [DOI] [PubMed] [Google Scholar]
- 47.Rex, J. H., M. A. Pfaller, J. N. Galgiani, M. S. Bartlett, A. Espinel-Ingroff, M. A. Ghannoum, M. Lancaster, F. C. Odds, M. G. Rinadli, T. J. Walsh, and A. L. Barry for the NCCLS Subcommittee on Antifungal Susceptibility Testing. 1997. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro and in vivo correlation data for fluconazole, itraconazole, and candida infections. Clin. Infect. Dis. 24:235-247. [DOI] [PubMed] [Google Scholar]
- 48.te Dorsthorst, D. T. A., P. E. Verweij, J. F. G. M. Meis, and J. W. Mouton. 2005. Efficacy and pharmacodynamics of flucytosine monotherapy in a nonneutropenic murine model of invasive aspergillosis. Antimicrob. Agents Chemother. 49:4220-4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turnidge, J. D., S. Gudmundsson, B. Vogelman, and W. A. Craig. 1994. The postantibiotic effect of antifungal agents against common pathogenic yeasts. J. Antimicrob. Chemother. 34:83-92. [DOI] [PubMed] [Google Scholar]
- 50.Vazquez, J. A., and J. D. Sobel. 2006. Anidulafungin: a novel echinocandin. Clin. Infect. Dis. 43:215-222. [DOI] [PubMed] [Google Scholar]
- 51.Walsh, T. J., J. W. Lee, P. Kelly, J. Bacher, J. Lecciones, V. Thomas, C. Lyman, D. Coleman, R. Gordee, and P. A. Pizzo. 1991. Antifungal effects of the nonlinear pharmacokinetics of cilofungin, a 1,3-3-glucan synthetase inhibitor, during continuous and intermittent intravenous infusions in treatment of experimental disseminated candidiasis. Antimicrob. Agents Chemother. 35:1321-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiederhold, N. P., L. K. Najvar, R. Bocanegra, D. Molina, M. Olivo, and J. R. Graybill. 2007. In vivo efficacy of anidulafungin and caspofungin against Candida glabrata and association with in vitro potency in the presence of sera. Antimicrob. Agents Chemother. 51:1616-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wiederhold, N. P., D. P. Kontoyiannis, J. Chi, R. A. Prince, V. H. Tam, and R. E. Lewis. 2004. Pharmacodynamics of caspofungin in a murine model of invasive pulmonary aspergillosis: evidence of concentration-dependent activity. J. Infect. Dis. 190:1464-1471. [DOI] [PubMed] [Google Scholar]
- 54.Wiederhold, N. P., and R. E. Lewis. 2003. The echinocandin antifungals: an overview of the pharmacology, spectrum and clinical efficacy. Expert Opin. Investig. Drugs 12:1313-1333. [DOI] [PubMed] [Google Scholar]