Abstract
Among 10,872 isolates of Enterobacteriaceae from a nationwide study of 88 French hospitals in 2005, 169 (1.7%) expressed an extended-spectrum β-lactamase. The most prevalent species were Escherichia coli (48.5%), Enterobacter aerogenes (23.7%), and Klebsiella pneumoniae (14.8%). Molecular analysis underlined the polyclonal spread of CTX-M-expressing E. coli, primarily isolates of the CTX-M-1 subgroup.
Resistance to extended-spectrum cephalosporins in Enterobacteriaceae can be associated with the production of extended-spectrum β-lactamases (ESBL) (11). Since the end of the 1990s, a new family of ESBL, the CTX-M-type β-lactamases, has spread worldwide. To date, over 60 CTX-M-type β-lactamases have been described and divided into five different clusters: CTX-M-1, CTX-M-2, CTX-M-8, CTX-M-9, and CTX-M-25 (2, 17, 20). Several local or regional studies indicate the emergence of CTX-M β-lactamases in France, but concurring data for the nation as a whole are lacking (1, 9, 12-16). We have conducted a nationwide prospective study in 88 nonteaching hospitals affiliated with the Collège de Bactériologie-Virologie-Hygiène des Hôpitaux de France network to evaluate both (i) the prevalence of ESBL-producing Enterobacteriaceae by using phenotypic confirmatory tests and (ii) the prevalence and molecular epidemiology of CTX-M-harboring Enterobacteriaceae (5, 6).
Bacterial isolates.
All ESBL-producing Enterobacteriaceae isolates were collected from clinical samples during a 1-month period (October 2005). For patients with recurrent infections, only isolates from the first episodes were included. Bacterial identification and ESBL screening were performed by the participating institutions according to the recommendations of the Antibiogram Committee of the French Society of Microbiology (10). The data concerning the total number of Enterobacteriaceae isolates and all the ESBL-producing Enterobacteriaceae isolates were centralized at the Service d'Hygiène Hospitalière, Centre Hospitalier de Versailles.
Quality controls.
The following four E. coli isolates were submitted anonymously: a wild type, two isolates harboring an extended-spectrum TEM or CTX-M β-lactamase, and an isolate overexpressing a cephalosporinase. Analyses of the capacity of each participating institution to detect the ESBL phenotype were sent to the central laboratory.
Identification and antimicrobial susceptibility testing.
Analysis at the central laboratory confirmed the identification and ESBL production determined by the API 20E and VITEK 2 systems (bioMérieux, Marcy l'Etoile, France), the double-disc synergy test, the MicroScan ESBL plus ESBL confirmation panel (Dade Behring, Sacramento, CA), and the Etest ESBL (AB Biodisk, Piscataway, NJ). Non-β-lactam antimicrobial susceptibility testing was performed by using disk diffusion according to the French guidelines (for nalidixic acid, ciprofloxacin, gentamicin, amikacin, and cotrimoxazole) (10).
PCR amplification.
blaCTX-M genes were amplified by using PCR, as described previously (9). The determination of subgroups was performed using specific primers (Table 1).
TABLE 1.
List of primers used for PCR amplification
| Primer target | Primer name | Sequence | Reference |
|---|---|---|---|
| CTX-M consensus | MA1 | 5′-SCSATGTGCAGYACCAGTAA-3′ | 21 |
| MA2 | 5′-CCGCRATATGRTTGGTGGTG-3′ | ||
| CTX-M-13 subgroup | M13U | 5′-GGTTAAAAAATCACTGCCTC-3′ | 21 |
| M13L | 5′-TTGGTGACGATTTTAGCCGC-3′ | ||
| CTX-M-25 subgroup | M25U | 5′-ATGATGACTCAGAGCATTCG-3′ | 21 |
| M25L | 5′-TGGGTTACGATTTTAGCCGC-3′ | ||
| CTX-M-9 subgroup | M9U | 5′-ACGGTGACAAAGAGAGTGCA-3′ | 21 |
| M9L | 5′-CCCTTCGGCGATGATTCTC-3′ |
Clonality of the isolates.
The clonal relationship of the isolates was studied by using pulsed-field gel electrophoresis with the GenePath system and Fingerprinting II software (Bio-Rad, Hercules, CA) in accordance with the manufacturer's recommendations. Clustering was defined when the percent similarity exceeded 80%.
Statistical analysis was performed using a chi-square test.
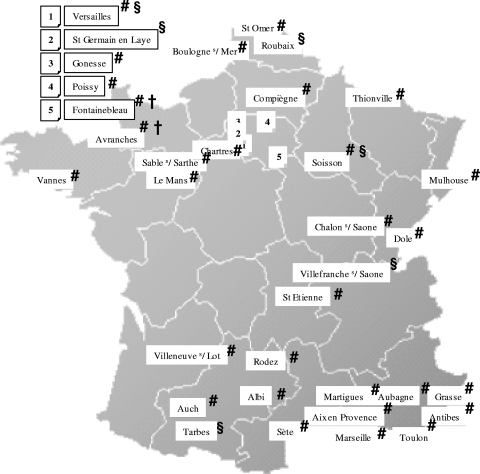
A total of 10,872 Enterobacteriaceae isolates were included in this study, and 169 Enterobacteriaceae isolates (1.6%) were confirmed by the central laboratory to produce ESBL. As with the quality control isolates, the concordance between the results obtained by the participating centers and those by the referent laboratory was above 90%. The percentage of ESBL-harboring isolates, their distribution, and their clinical origins are reported in Tables 2 and 3. blaCTX-M genes were identified in 62/82 E. coli (76%), 6/25 K. pneumoniae (24%), 2/10 Enterobacter cloacae (20%), and 0/40 E. aerogenes isolates. In E. coli, blaCTX-M genes belonged primarily to the CTX-M-1 (53/62) and CTX-M-9 (7/62) groups. No CTX-M-2-related gene was identified, and only two blaCTX-M genes could not be characterized by subtype. Resistance to ceftazidime was identified in 49% of the E. coli isolates harboring CTX-M group 1. The geographical locations of centers that have isolated CTX-M-harboring E. coli in France are reported in Fig. 1.
TABLE 2.
Distribution of each species, main clinical origins, and percentage of ESBL-harboring isolates among 10,872 clinically relevant isolates of Enterobacteriaceaea
| Frequency rankb | Species | Total isolates
|
UTI isolates
|
RTI isolates
|
BI isolates
|
||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % ESBL | No. | % ESBL | No. | % ESBL | No. | % ESBL | ||
| 1 | Escherichia coli | 7,989 | 1.99 | 6,295 | 1.0 | 119 | 2.5 | 666 | 1.5 |
| 2 | Proteus mirabilis | 689 | 1.31 | 454 | 1.8 | 28 | 3.6 | 42 | 0.0 |
| 3 | Salmonella spp. | 70 | 0 | 1 | 0.0 | 0 | 0.0 | 20 | 0.0 |
| 4 | Klebsiella pneumoniae | 647 | 3.71 | 413 | 2.7 | 45 | 8.9 | 65 | 4.6 |
| 5 | Klebsiella oxytoca | 204 | 2.45 | 96 | 4.2 | 15 | 0.0 | 37 | 2.7 |
| 6 | Klebsiella spp. | 10 | 0 | 4 | 0.0 | 1 | 0.0 | 5 | 0.0 |
| 7 | Citrobacter koseri | 112 | 1.79 | 71 | 2.8 | 5 | 0.0 | 10 | 0.0 |
| 8 | Enterobacter cloacae | 392 | 2.55 | 152 | 3.3 | 49 | 6.1 | 70 | 1.4 |
| 9 | Enterobacter aerogenes | 196 | 21.4 | 89 | 25.8 | 43 | 18.6 | 16 | 37.5 |
| 10 | Enterobacter spp. | 16 | 0 | 1 | 0.0 | 3 | 0.0 | 6 | 0.0 |
| 11 | Citrobacter freundii | 120 | 1.66 | 79 | 0.0 | 7 | 0.0 | 8 | 12.5 |
| 12 | Citrobacter spp. | 13 | 0 | 4 | 0.0 | 1 | 0.0 | 2 | 0.0 |
| 13 | Proteus vulgaris | 33 | 0 | 19 | 0.0 | 4 | 0.0 | 3 | 0.0 |
| 14 | Morganella morganii | 185 | 0 | 87 | 0.0 | 24 | 0.0 | 18 | 0.0 |
| 15 | Providencia stuartii | 22 | 0 | 13 | 0.0 | 3 | 0.0 | 2 | 0.0 |
| 16 | Serratia marcescens | 102 | 1.96 | 21 | 4.8 | 28 | 3.6 | 17 | 0.0 |
| 17 | Other Enterobacteriaceae | 63 | 0 | 19 | 0.0 | 6 | 0.0 | 14 | 0.0 |
| Total | 10,872 | 1.7 | 7,818 | 1.5 | 381 | 5.2 | 1,001 | 1.6 | |
UTI, urinary tract infection; RTI, respiratory tract infection; BI, bloodstream infection.
Species were ranked according to their frequency of isolation among clinical samples, independent of the expression or lack of expression of an ESBL.
TABLE 3.
Origins of ESBL-producing Enterobacteriaceae
| No. of isolates from indicated source
| |||||||
|---|---|---|---|---|---|---|---|
| Species | Urine | Bloodstream infections | Respiratory tract infections | Suppurations | Intravascular catheter infections | Other | All sources |
| Escherichia coli | 60 | 9 | 2 | 6 | 2 | 3 | 82 |
| Enterobacter aerogenes | 18 | 6 | 10 | 5 | 1 | 40 | |
| Klebsiella pneumoniae | 11 | 3 | 2 | 3 | 2 | 4 | 25 |
| Enterobacter cloacae | 3 | 1 | 2 | 1 | 1 | 2 | 10 |
| Proteus mirabilis | 6 | 6 | |||||
| Citrobacter koseri | 2 | 2 | |||||
| Citrobacter freundii | 1 | 1 | |||||
| Klebsiella oxytoca | 1 | 1 | |||||
| Salmonella spp. | 1 | 1 | |||||
| Serratia marcescens | 1 | 1 | |||||
| Total | 101 | 20 | 16 | 15 | 5 | 12 | 169 |
FIG. 1.
Geographical locations of the centers that have isolated Escherichia coli harboring the following ESBL: CTX-M group 1 (#), CTX-M group 9 (§), other CTX-M groups (†).
An analysis of pulsed-field gel electrophoresis profiles showed 10 clusters including 35.4% of the E. coli isolates, 6 of which included 63.6% of the K. pneumoniae isolates and 3 of which included 71.4% of the E. aerogenes isolates (data not shown). Statistical analysis showed that the presence of CTX-M was significantly associated with nalidixic acid or ciprofloxacin resistance (P < 0.001) but not with cotrimoxazole, gentamicin, or amikacin resistance (P = 0.49, 0.67, and 0.67, respectively).
The last national study of ESBL prevalence in France was performed in 1998, before the rise of Enterobacteriaceae producing CTX-M ESBL, when levels of CTX-M ESBL reached 3.2% (4). Only 1.6% of the Enterobacteriaceae isolates in our work produced ESBL. This national study does not confirm the increase in the prevalence of ESBL-producing Enterobacteriaceae reported in some local or regional settings (3, 13, 20). Such data could have been influenced by the spread of a particular isolate locally. Compared to results in previous studies, the percentages of ESBL-producing isolates decreased for Proteus mirabilis (1.31% in this study versus 3.7% in 1998), E. aerogenes (21.4% versus 53.5%), and K. pneumoniae (3.71% versus 9.4%) but increased for E. coli (1.99% versus 0.2%) (4). Until the late 1990s, E. aerogenes TEM-3 and TEM-24 clones dominated the species and ESBL group distribution in France (3, 4, 7, 17). CTX-M ESBL appeared in 1989 and then were reported only occasionally (1, 4, 8, 16, 18). Lavigne et al. underline the increase of ESBL prevalence in E. coli isolates in 2004 (0.68% versus 0.2% in 1998) from a sample of isolates in southern and central France, which were caused primarily by the rise of the CTX-M-15 subtype (14). Our work confirms these findings at the national level: half of the E. coli isolates harboring the CTX-M-1 subgroup show decreased susceptibility to ceftazidime, which indicates the putative presence of CTX-M-15.
For our study, several local outbreaks with the same CTX-M E. coli isolate were identified, as reported by different hospitals in the Paris area or in southern France (9, 12, 13, 15, 16). Though E. coli CTX-M-15 has spread nationally in the United Kingdom, we failed to identify a single CTX-M clone being so widespread in France (23). Nevertheless, additional studies must be performed to explore the putative spread of the same plasmid in unrelated isolates (19, 20, 22).
A statistical link between CTX-M production and nalidixic acid or fluoroquinolone resistance has been established; this association can be explained at least in part by the high incidence of qnr genes in this ESBL type (14, 20).
The results of our work must be interpreted in light of its limitations. The number of ESBL-producing Enterobacteriaceae isolates is quite low; our findings must be confirmed by a larger study. Isoelectric focusing experiments and sequencing of each PCR product would provide more accurate data about the epidemiology of ESBL-producing Enterobacteriaceae. We lack extensive analyses of clinical data to establish the ratio of hospital-acquired to community-acquired infections for each ESBL type. Nevertheless, to the best of our knowledge, this work is the largest study of ESBL-producing Enterobacteriaceae epidemiology performed in France. This national study supports the previous local and regional observations that CTX-M-harboring E. coli isolates are now the most frequent ESBL-producing Enterobacteriaceae in France.
Acknowledgments
We thank the ColBVH Study Group participants: C. Alba-Sauviat, C. Auvray, A. Bailly, D. Barraud, C. Bartizel-Ferdrin, I. Baudinat, E. Benabid, C. Benoit, Z. Benseddik, F. Bessis, H. Biessy, M. Bietrix, V. Blanc, S. Bland, Y. Boucaud-Maitre, C. Bouquigny-Saison, P. Brisou, S. Brovedani, B. Cancet, J. Carre, B. Cartier, B. Cattier, A. Cecille, G. Cellier-Julienne, G. Chambreuil, P. Chantelat, C. Chaplain, H. Chardon, M. Cheron, A. Clarac, P. Clergeau, E. Collot, F. Crepet, M. F. Danjoux, J. P. Darchis, H. De Montclos, J. M. Delarbre, P. Deligne, F. Desroys du Roure, J. Didion, V. Doat, B. Dubourdieu, D. R. Duffaut, C. Eloy, P. Emerique, F. Evreux, D. Fevre, J. Flipo, N. Fonsale, S. Gabriel, G. Gallou, F. Gandilhon-Crepet, I. Ganivala, M. Gavignet, F. Geffroy, C. Grasmick, B. Gravagna, G. Grise, P. Guiet, D. R. Hautefort, J. Heurte, E. Heusse, M. C. Jaffar-Bandjee, E. Jaouen, G. Julienne, L. Koulmann, K. Krechiem, J. P. Lafargue, S. Laluque, R. Lamarca, B. Lamy, V. Larroque, E. Laurens, F. Laurent, J. Y. Le Berre, A. Le Coustumier, J. Y. Leberre, E. Lecaillon Thibon, P. Lemaitre, M. Leneveu, S. Leotard, M. N. Letouzey, A. Mandjee, A. Marmonier, R. Martin, R. Meley, M. Menouar, A. Michel, A. Morel, D. Neri Schiavini, M. N. Noulard, X. Palette, B. Pangon, J. Paul, C. Payen, M. Perrin, N. Petitboulanger, D. Pierrejean, A. S. Poirier, H. Porcheret, P. Pouedras, G. Rast, Y. Rio, B. Riviere, C. Rolland, S. Samaille, R. Sanchez, A. Sarran, Y. Scat, A. Secher, J. Semon, H. Sep-Hieng, D. P. Simeon, S. Smati, D. R. Sorlin, P. Stolidi, D. R. Terki, J. P. Thellier, M. Thouvenin, B. Tourrand, A. Vachee, E. Vallee, M. Vasseur, J. Vaucel, A. Verhaeghe, M. Villemain, L. Villeneuve, and N. Wilhelm.
We thank E. Cambeau, G. Arlet, and P. Nordmann from the Assistance de Paris—Hôpitaux de Paris for additional contributions.
Footnotes
Published ahead of print on 19 November 2007.
REFERENCES
- 1.Arpin, C., V. Dubois, L. Coulange, C. Andre, I. Fischer, P. Noury, F. Grobost, J. P. Brochet, J. Jullin, B. Dutilh, G. Larribet, I. Lagrange, and C. Quentin. 2003. Extended-spectrum β-lactamase-producing Enterobacteriaceae in community and private health care centers. Antimicrob. Agents Chemother. 47:3506-3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonnet, R. 2004. Growing group of extended-spectrum β-lactamases: the CTX-M enzymes. Antimicrob. Agents Chemother. 48:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Champs, C., C. Chanal, D. Sirot, R. Baraduc, J. P. Romaszko, R. Bonnet, A. Plaidy, M. Boyer, E. Carroy, M. C. Gbadamassi, S. Laluque, O. Oules, M. C. Poupart, M. Villemain, and J. Sirot. 2004. Frequency and diversity of class A extended-spectrum β-lactamases in hospitals of the Auvergne, France: a 2 year prospective study. J. Antimicrob. Chemother. 54:634-639. [DOI] [PubMed] [Google Scholar]
- 4.De Champs, C., D. Sirot, C. Chanal, R. Bonnet, J. Sirot, and the French Study Group. 2000. A 1998 survey of extended-spectrum β-lactamases in Enterobacteriaceae in France. Antimicrob. Agents Chemother. 44:3177-3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Decousser, J.-W., P. Pina, F. Picot, C. Delalande, B. Pangon, P. Courvalin, P. Allouch, and the ColBVH Study Group. 2003. Frequency of isolation and antimicrobial susceptibility of bacterial pathogens isolated from patients with bloodstream infections: a French prospective national survey. J. Antimicrob. Chemother. 51:1213-1222. [DOI] [PubMed] [Google Scholar]
- 6.Decousser, J.-W., P. Pina, F. Viguier, F. Picot, P. Courvalin, P. Allouch, and the ColBVH Study Group. 2004. Invasive Streptococcus pneumoniae in France: antimicrobial resistance, serotype, and molecular epidemiology findings from a monthly national study in 2000 to 2002. Antimicrob. Agents Chemother. 48:3636-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dumarche, P., C. De Champs, D. Sirot, C. Chanal, R. Bonnet, and J. Sirot. 2002. TEM derivative-producing Enterobacter aerogenes strains: dissemination of a prevalent clone. Antimicrob. Agents Chemother. 46:1128-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dutour, C., R. Bonnet, H. Machandin, M. Boyer, C. Chanal, D. Sirot, and J. Sirot. 2002. CTX-M-1, CTX-M-3, and CTX-M-14 β-lactamases from Enterobacteriaceae isolated in France. Antimicrob. Agents Chemother. 46:534-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckert, C., V. Gautier, M. Saladin-Allard, N. Hidri, C. Verdet, Z. Ould-Hocine, G. Barnaud, F. Delisle, A. Rossier, T. Lambert, A. Philippon, and G. Arlet. 2004. Dissemination of CTX-M-type β-lactamases among clinical isolates of Enterobacteriaceae in Paris, France. Antimicrob. Agents Chemother. 48:1249-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.French Society of Microbiology. 2007. Guidelines of the Antibiogram Committee of the French Society of Microbiology. http://www.sfm.asso.fr/nouv/general.php?pa=2. Accessed 1 July 2007.
- 11.Jacoby, G. A., and L. S. Munoz-Price. 2005. The new β-lactamases. N. Engl. J. Med. 352:380-391. [DOI] [PubMed] [Google Scholar]
- 12.Kassis-Chikhani, N., S. Vimont, K. Asselat, C. Trivalle, B. Minassian, C. Sengelin, V. Gautier, D. Mathieu, E. Dussaix, and G. Arlet. 2004. CTX-M β-lactamase-producing Escherichia coli in long-term care facilities, France. Emerg. Infect. Dis. 10:1697-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavigne, J.-P., N. Bouziges, C. Chanal, A. Mahamat, S. Michaux-Charachon, and A. Sotto. 2004. Molecular epidemiology of Enterobacteriaceae isolates producing extended-spectrum β-lactamases in a French hospital. J. Clin. Microbiol. 42:3805-3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lavigne, J.-P., H. Marchandin, J. Delmas, J. Moreau, N. Bouziges, E. Lecaillon, L. Cavalie, H. Jean-Pierre, R. Bonnet, and A. Sotto. 2007. CTX-M β-lactamase-producing Escherichia coli in French hospitals: prevalence, molecular epidemiology, and risk factors. J. Clin. Microbiol. 45:620-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lavollay, M., K. Mamlouk, T. Frank, A. Akpabie, B. Burghoffer, S. Ben Redjeb, R. Bercion, V. Gautier, and G. Arlet. 2006. Clonal dissemination of a CTX-M-15 β-lactamase-producing Escherichia coli strain in the Paris area, Tunis, and Bangui. Antimicrob. Agents Chemother. 50:2433-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leflon-Guibout, V., C. Jurand, S. Bonacorsi, F. Espinasse, M. C. Guelfi, F. Duportail, B. Heym, E. Bingen, and M. H. Nicolas-Chanoine. 2004. Emergence and spread of three clonally related virulent isolates of CTX-M-15-producing Escherichia coli with variable resistance to aminoglycosides and tetracycline in a French geriatric hospital. Antimicrob. Agents Chemother. 48:3736-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livermore, D. M., R. Canton, M. Gniadkowski, P. Nordmann, G. M. Rossolini, G. Arlet, J. Ayala, T. M. Coque, I. Kern-Zdanowicz, F. Luzzaro, L. Poirel, and N. Woodford. 2006. CTX-M: changing the face of ESBLs in Europe. J. Antmicrob. Chemother. 59:165-174. [DOI] [PubMed] [Google Scholar]
- 18.Neuwirth, C., E. Siebor, M. Pruneaux, M. Zarnayova, C. Simonin, J. P. Kisterman, and R. Labia. 2003. First isolation of CTX-M15-producing Escherichia coli from two French patients. J. Antmicrob. Chemother. 51:471-473. [DOI] [PubMed] [Google Scholar]
- 19.Paterson, D. L., and R. A. Bonomo. 2005. Extended-spectrum β-lactamases: a clinical update. Clin. Microbiol. Rev. 18:657-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pitou, J. D. D., P. Nordmann, K. V. Laupland, and L. Poirel. 2005. Emergence of Enterobacteriaceae producing extend-spectrum β-lactamases (ESBL) in the community. J. Antimicrob. Chemother. 56:52-59. [DOI] [PubMed] [Google Scholar]
- 21.Saladin, M., V. T. B. Cao, T. Lambert, J. L. Donay, J. L. Herrmann, Z. Ould-Hocine, C. Verdet, F. Delisle, A. Philippon, and G. Arlet. 2002. Diversity of CTX-M β-lactamases and their promoter regions from Enterobacteriaceae isolated in three Parisian hospitals. FEMS Microbiol. Lett. 209:161-168. [DOI] [PubMed] [Google Scholar]
- 22.Velasco, C., L. Romero, J. M. Martinez, J. Rodriguez-Bano, and A. Pascual. 2007. Analysis of plasmids encoding extended-spectrum β-lactamases (ESBLs) from Escherichia coli isolated from non-hospitalised patients in Seville. Int. J. Antimicrob. Agents 29:89-92. [DOI] [PubMed] [Google Scholar]
- 23.Woodford, N., M. E. Ward, M. E. Kaufmann, J. E. Turton, E. J. Fagan, D. James, A. P. Johnson, R. Pike, M. Warmer, T. Cheasty, A. Pearson, S. Harry, J. B. Leach, A. Loughrey, J. A. Lowes, R. E. Warren, and D. M. Livermore. 2004. Community and hospital spread of Escherichia coli producing CTX-M extended-spectrum beta-lactamases in the UK. J. Antimicrob. Chemother. 54:735-743. [DOI] [PubMed] [Google Scholar]