Abstract
Human polymorphonuclear neutrophils (HPMNs) displayed attenuated hyphal damage associated with impaired O2− release following exposure to Rhizopus oryzae versus that with Aspergillus fumigatus. Exposure of HPMNs to R. oryzae hyphae resulted in upregulation in Toll-like receptor 2 mRNA and a robust proinflammatory gene expression with rapid (1-h) induction of NF-κB pathway-related genes.
Zygomycetes are opportunistic molds that can cause life-threatening infections in a wide range of immunocompromised patients (11, 12). We hypothesized that quantitative and/or qualitative differences in human polymorphonuclear neutrophil (HPMN) responses against zygomycetes compared to those against other, more common opportunistic molds, such as Aspergillus, may partially account for their increased pathogenicity in a particular patient setting (11, 12). As the antifungal activity of immune effector cells is modulated by pattern recognition receptor signaling (1, 5, 6, 10), we additionally assessed differences in gene expression of Toll-like receptors (TLRs), related proinflammatory response pathways, and the β-glucan receptor dectin 1 in HPMNs exposed to Rhizopus oryzae versus Aspergillus fumigatus hyphae.
(Presented in part at the 46th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, 27 to 30 September 2006, abstr. B1332.)
HPMNs from the heparinized whole blood of four healthy adult volunteers were isolated by Ficoll-Hypaque centrifugation (4, 9). Hyphae of a clinical isolate of Rhizopus oryzae 557969 and Aspergillus fumigatus (Af293) were generated by incubating 105 conidia of each isolate in RPMI liquid medium for 18 h at 37°C.
HPMN-induced hyphal damage was assessed by a (2,3)-bis-(2-methoxy-4-nitro-5-sulfenyl)-(2H)-tetrazolium-5-carboxanilide (XTT)-based colorimetric assay (4, 9). Briefly, hyphae of each isolate were incubated for 1 h at 37°C with 106 HPMNs (hyphal/PMN ratio, 1:10) suspended in Hanks' balanced salt solution without Ca2+ and Mg2+ (Gibco). Accordingly, the HPMNs were lysed hypotonically and HPMN-induced hyphal damage was calculated by the following formula: percentage of hyphal damage = [(1 − X)/C] × 100, where X represents the optical density of test wells and C represents the optical density of control wells with hyphae only. Optical densities at 492 nm and 690 nm were measured by using a microplate spectrophotometer (Power Wave X; Biotech Instruments, Winooski, VT).
HPMN-induced hyphal damage was also examined following hypotonic lysis of HPMNs by staining hyphae of each isolate with the fluorescent dye DiBAC (3). Photomicrographs of the hyphae were taken under a fluorescence microscope (Olympus BX-51; Olympus, Melville, NY). All experiments were performed in triplicate.
For assessment of the generation and extracellular release of superoxide anion (O2−), 106 HPMNs were stimulated with hyphae of each isolate (hypha/HPMN ratio, 1:10) and with phorbol myristate acetate (PMA) (0.5 μg/ml) for 1 h. Measurement of the extracellular release of O2− was performed by an assay based on the inhibition of cytochrome c reduction by superoxide dismutase (4). Cytochrome c reduction at 550 nm was calculated using the extinction coefficient: ΔE = 29.5 × 104 liters/mol·cm. Measurement of O2− generation (intracellular and extracellular O2−) was assessed by a luminol-dependent chemiluminescence assay (13).
For quantitative real-time PCR and oligoarray gene expression profiling, 5 × 106 HPMNs collected from three healthy volunteers were exposed to hyphae of each isolate (hypha/HPMN ratio, 1:10) for 1 h. Total RNA was extracted using a commercial kit (RNAeasy; Qiagen). Gene expression profiling of 113 genes encoding TLRs and related pathways (NF-κB and JNK/p38; nuclear factor [NF]/interleukin-6 [IL-6] and interferon regulatory factor [IRF]) was performed by using DNA microarrays (GEArray; Bioscience). All the data sets were corrected using a minimum value for background subtraction and the interquartile for normalization before being compared to each other. A >1.5-fold increase or decrease in signal intensity compared to unexposed control HPMNs was considered significant induction or reduction of gene expression, respectively. Three independent array experiments were performed.
TNFA, IL-1B, and Dectin-1 mRNA expression was confirmed by reverse transcription (RT)-PCR. Forward and reverse PCR primers for TNFA, IL-1B, Dectin-1, and beta-actin were used to simultaneously amplify cDNA using a multiplex chemistry (Qiagen multiplex PCR kit). cDNA samples were analyzed in duplicate by use of an ABI Prism 7000 sequence detection system (Applied Biosystems). The Mann-Whitney U test and Kruskal-Wallis one-way analysis of variance with Dunn's test (for multiple comparisons) were used to determine statistically significant differences where appropriate. P values of less than 0.05 were considered statistically significant.
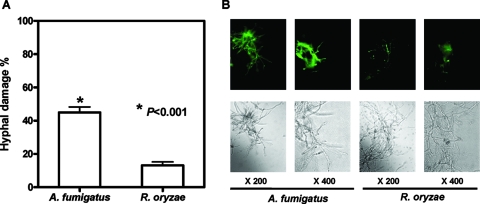
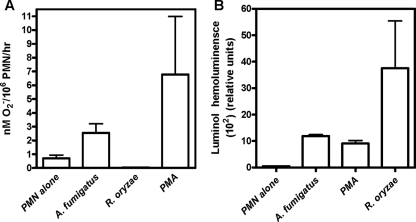
HPMNs were less effective at damaging R. oryzae hyphae than A. fumigatus (Af293) hyphae by the XTT (Fig. 1A) and DiBAC (Fig. 1B) staining assays. The reduction in hyphal damage was consistent with impaired extracellular O2− release by HPMNs compared to O2− release following stimulation with A. fumigatus hyphae or PMA (Fig. 2A). In contrast, total HPMN O2− (intracellular O2− generation and extracellular O2− release) was highly induced upon exposure to Rhizopus hyphae (Fig. 2B).
FIG. 1.
Induction of hyphal damage by HPMNs. (A) Percentage of damage induced by freshly isolated HPMNs on hyphae of R. oryzae and A. fumigatus. The error bars indicate standard deviations. (B) HPMN efficacy for hyphal damage against R. oryzae and A. fumigatus hyphae assessed by the viability dye DiBAC. Hyphae were examined with the use of bright-field (top row) and epifluorescence (bottom row) microscopy at ×400 and ×200 with Nomarski optics and a fluorescein isothiocyanate filter. The fluorescence in the dark boxes is indicative of early hyphal damage by HPMNs.
FIG. 2.
O2− release by HPMNs challenged with no stimulus, PMA, or hyphae of R. oryzae and A. fumigatus. (A) Extracellular O2− release by HPMNs (106 cells) as measured by cytochrome c reduction assay; P = 0.06 for R. oryzae- versus A. fumigatus-induced extracellular O2− release (hypha/HPMN ratio, 1:10). (B) Total (intracellular generation and extracellular release) O2− production by HPMNs (106 cells) assessed by luminol-dependent chemiluminescence. This chemiluminescence assay is based on the ability of luminol to enter cellular membranes and measures the total amount of O2− production (intracellular generation and extracellular release) (13). P = 0.1 for R. oryzae- versus A. fumigatus-induced extracellular O2− release. The error bars indicate standard deviations.
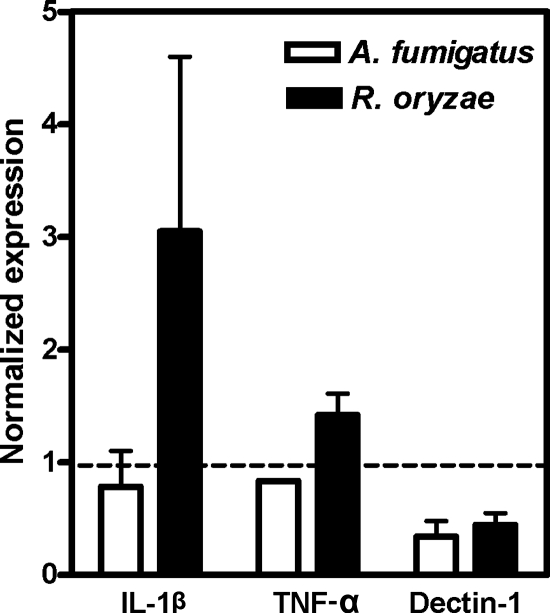
Exposure of HPMNs to hyphae of R. oryzae and Af293 resulted in selective up regulation of TLR2 mRNA (Table 1). Hyphae of both R. oryzae and Af293 increased the HPMN expression of 18 other genes (Table 1). In addition, R. oryzae hyphae selectively induced seven additional genes with proinflammatory functions (TNFA, NFB2, NFKBIE, HMGB1, IL-1B, IRAK1, and IL-8) (Table 1), which was verified by RT-PCR (Fig. 3). Importantly, Af293 hyphae selectively induced IRF3 (Table 1).
TABLE 1.
Genes of the TLR signaling and related pathways significantly (>1.5-fold) induced after 1 h of exposure of HPMNs to hyphae of Af293 or R. oryzae compared to control unstimulated HPMNs
| Gene | Fold increase
|
Function/related pathway | |
|---|---|---|---|
| Af293 | Rhizopus | ||
| TLR2 | 2.5 | 4.4 | TLRs |
| CD14 | 2.1 | 3.4 | Adaptors/TLR signaling |
| MYD88 | 2.7 | 3.7 | Adaptors/TLR signaling |
| HSPA1A | 3.1 | 3.9 | Adaptors/TLR signaling |
| HSPA6 | 1.8 | 2.3 | Adaptors/TLR signaling |
| HSPD1 | 1.7 | 2.2 | Adaptors/TLR signaling |
| RIPK2 | 1.8 | 2.6 | Adaptors/TLR signaling |
| HMGB1 | 3.7 | Adaptors/TLR signaling | |
| IRAK1 | 2.3 | Effectors | |
| IKBKG | 2.7 | 1.7 | NF-κB pathway |
| NFKB1 | 1.9 | 2.8 | NF-κB pathway |
| NFKBIA | 1.6 | 2.5 | NF-κB pathway |
| NFKBIB | 1.7 | 1.8 | NF-κB pathway |
| PTGS2 (Cox-2) | 4.2 | 2.9 | NF/IL-6 pathway |
| RELA | 1.5 | 3.3 | NF-κB pathway |
| RELB | 2.0 | 2.5 | NF-κB pathway |
| TNFRSF1A | 2.4 | 6.0 | NF-κB pathway |
| TRADD | 2.1 | 1.6 | NF-κB pathway |
| TNFA | 2.5 | NF-κB pathway | |
| NFKB2 | 2.6 | NF-κB pathway | |
| NFKBIE | 2.4 | NF-κB pathway | |
| IL-1B | 1.6 | NF-κB pathway | |
| IL-8 | 3.4 | NF-κB pathway | |
| IRF1 | 2.6 | 3.7 | IRF pathway |
| IRF7 | 1.6 | 1.6 | IRF pathway |
| IRF3 | 1.6 | IRF pathway | |
| FOS | 2.7 | 3.2 | JNK/p38 pathway |
FIG. 3.
Relative expression levels of TNFA, IL-1B, and DECTIN-1 mRNAs after 1 h of exposure of HPMNs to hyphae of Af293 or R. oryzae, as assessed by RT-PCR. Forward and reverse PCR primers for human TNF-α (5′-AGG CCA AGC CCT GGT ATG AGC-3′ and 5′-CAC AGG GCA ATG ATC CCA AAG TAG-3′), IL-1β (5′-CAG GGA CAG GAT ATG GAG CAA CAA-3′ and 5′-CAT CTT TCA ACA CGC AGG ACA GGT-3′), Dectin-1 (5′-TGG CAA CTG GGC TCT AAT CTC CT-3′ and 5′-TTT CTT GGG TAG CTG TGG TTC TGA-3′) and beta-actin (5) were used to simultaneously amplify cDNA and the housekeeping gene using multiplex chemistry. The products were subjected to 32 cycles of PCR amplification at 94°C for 30 s, 58°C for 30 s, and 72°C for 2 min. P was nonsignificant for all comparisons. The error bars indicate standard deviations.
In this study, we found that HPMNs exhibit a reduced capacity to induce oxidative damage against unopsonized hyphae of clinical Zygomycetes isolates in comparison with unopsonized hyphae of A. fumigatus. These findings are in line with previous studies using germinating conidia (7) and hyphae of Zygomycetes (4). The attenuated efficacy of HPMN against Zygomycetes hyphae may partially explain the relatively high pathogenicity of these fungi (12, 13).
Similar to previous studies (4, 7), we found that there is reduced superoxide anion (O2−) release by human HPMNs after exposure to Zygomycetes compared to A. fumigatus hyphae. In contrast, by using a chemiluminescence assay, we found that the total production (intracellular generation plus extracellular release) of O2− by human HPMNs was highly induced upon exposure to Rhizopus hyphae. This discordance has also been reported following exposure to Neisseria gonorrhea (8, 14).
We also found that, similar to A. fumigatus hyphae (1, 10), R. oryzae hyphae selectively induced activation of TLR2 mRNA in HPMNs, as well as other key proinflammatory response genes (Table 1). Nonetheless, induction of TLR4 in HPMNs after exposure to A. fumigatus hyphae has been reported in other studies (2, 4). Notably, Rhizopus hyphae specifically induced proinflammatory genes (e.g., TNFA and IL-1B).
Further studies are needed to examine if there is a link between preferential induction of these genes with attenuated hyphal damage and impaired extracellular release of O2− by HPMNs following exposure to the hyphae of R. oryzae.
Footnotes
Published ahead of print on 19 November 2007.
REFERENCES
- 1.Bellocchio, S., S. Moretti, K. Perruccio, F. Fallarino, S. Bozza, C. Montagnoli, P. Mosci, G. B. Lipford, L. Pitzurra, and L. Romani. 2004. TLRs govern neutrophil activity in aspergillosis. J. Immunol. 173:7406-7415. [DOI] [PubMed] [Google Scholar]
- 2.Bochud, P. Y., J. Chien, M. Janer, K. A. Marr, A. Aderem, and M. Boeckh. 2006. Donor's Toll like receptor 4 haplotypes increase the incidence of invasive mold infections in hematopoietic stem cell transplant recipients, abstr. B1329. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA, 27 to 30 September 2006.
- 3.Bowman, J. C., P. S. Hicks, M. B. Kurtz, H. Rosen, D. M. Schmatz, P. A. Liberator, and C. M. Douglas. 2002. The antifungal echinocandin caspofungin acetate kills growing cells of Aspergillus fumigatus in vitro. Antimicrob. Agents Chemother. 46:3001-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gil-Lamaignere, C., M. Simitsopoulou, E. Roilides, A. Maloukou, R. M. Winn, and T. J. Walsh. 2005. Interferon-gamma and granulocyte-macrophage colony-stimulating factor augment the activity of polymorphonuclear leukocytes against medically important zygomycetes. J. Infect. Dis. 191:1180-1187. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi, F., T. K. Means, and A. D. Luster. 2003. Toll-like receptors stimulate human neutrophil function. Blood 102:2660-2669. [DOI] [PubMed] [Google Scholar]
- 6.Hohl, T. M., A. Rivera, and E. G. Pamer. 2006. Immunity to fungi. Curr. Opin. Immunol. 18:465-472. [DOI] [PubMed] [Google Scholar]
- 7.Liles, W. C., J. E. Huang, J. A. van Burik, R. A. Bowden, and D. C. Dale. 1997. Granulocyte colony-stimulating factor administered in vivo augments neutrophil-mediated activity against opportunistic fungal pathogens. J. Infect. Dis. 175:1012-1015. [DOI] [PubMed] [Google Scholar]
- 8.Lorenzen, D. R., D. Gunther, J. Pandit, T. Rudel, E. Brandt, and T. F. Meyer. 2000. Neisseria gonorrhoeae porin modifies the oxidative burst of human professional phagocytes. Infect. Immun. 68:6215-6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meshulam, T., S. M. Levitz, L. Christin, and R. D. Diamond. 1995. A simplified new assay for assessment of fungal cell damage with the tetrazolium dye, (2,3)-bis-(2-methoxy-4-nitro-5-sulphenyl)-(2H)-tetrazolium-5-carboxanilide (XTT). J. Infect. Dis. 172:1153-1156. [DOI] [PubMed] [Google Scholar]
- 10.Netea, M. G., A. Warris, J. W. Van der Meer, M. J. Fenton, T. J. Verver-Janssen, L. E. Jacobs, T. Andersen, P. E. Verweij, and B. J. Kullberg. 2003. Aspergillus fumigatus evades immune recognition during germination through loss of toll-like receptor-4-mediated signal transduction. J. Infect. Dis. 188:320-326. [DOI] [PubMed] [Google Scholar]
- 11.Ribes, J. A., C. L. Vanover-Sams, and D. J. Baker. 2000. Zygomycetes in human disease. Clin. Microbiol. Rev. 13:236-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roden, M. M., T. E. Zaoutis, W. L. Buchanan, T. A. Knudsen, R. L. Schaufele, M. Sein, T. Sein, C. C. Chiou, J. H. Chu, D. P. Kontoyiannis, and T. J. Walsh. 2005. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin. Infect. Dis. 41:634-653. [DOI] [PubMed] [Google Scholar]
- 13.Voie, O. A., M. Tysklind, P. L. Andersson, and F. Fonnum. 2000. Activation of respiratory burst in human granulocytes by polychlorinated biphenyls: a structure-activity study. Toxicol. Appl. Pharmacol. 167:118-124. [DOI] [PubMed] [Google Scholar]
- 14.Zheng, H. Y., D. J. Hassett, K. Bean, and M. S. Cohen. 1992. Regulation of catalase in Neisseria gonorrhoeae. Effects of oxidant stress and exposure to human neutrophils. J. Clin. Investig. 90:1000-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]