Abstract
During intraperiplasmic growth of Bdellovibrio bacteriovorus on Escherichia coli, the substrate cell peptidoglycan is extensively modified as it is converted to bdelloplast peptidoglycan. The initially lysozyme-sensitive peptidoglycan of E. coli was rapidly converted to a lysozyme-resistant form. The conversion was due to the N-deacetylation of a large portion of the peptidoglycan amino sugars. Chemically acetylating the isolated peptidoglycan restored its sensitivity to lysozyme digestion. However, approximately half of the products of lysozyme digestion exhibited hydrophobic interactions that were shown not to be due to the presence of protein. This suggests that a molecule capable of hydrophobic interactions, other than protein, becomes linked to the bdelloplast peptidoglycan. The data also suggest that much of the Braun lipoprotein is removed from the E. coli peptidoglycan early during bdellovibrio development.
Full text
PDF

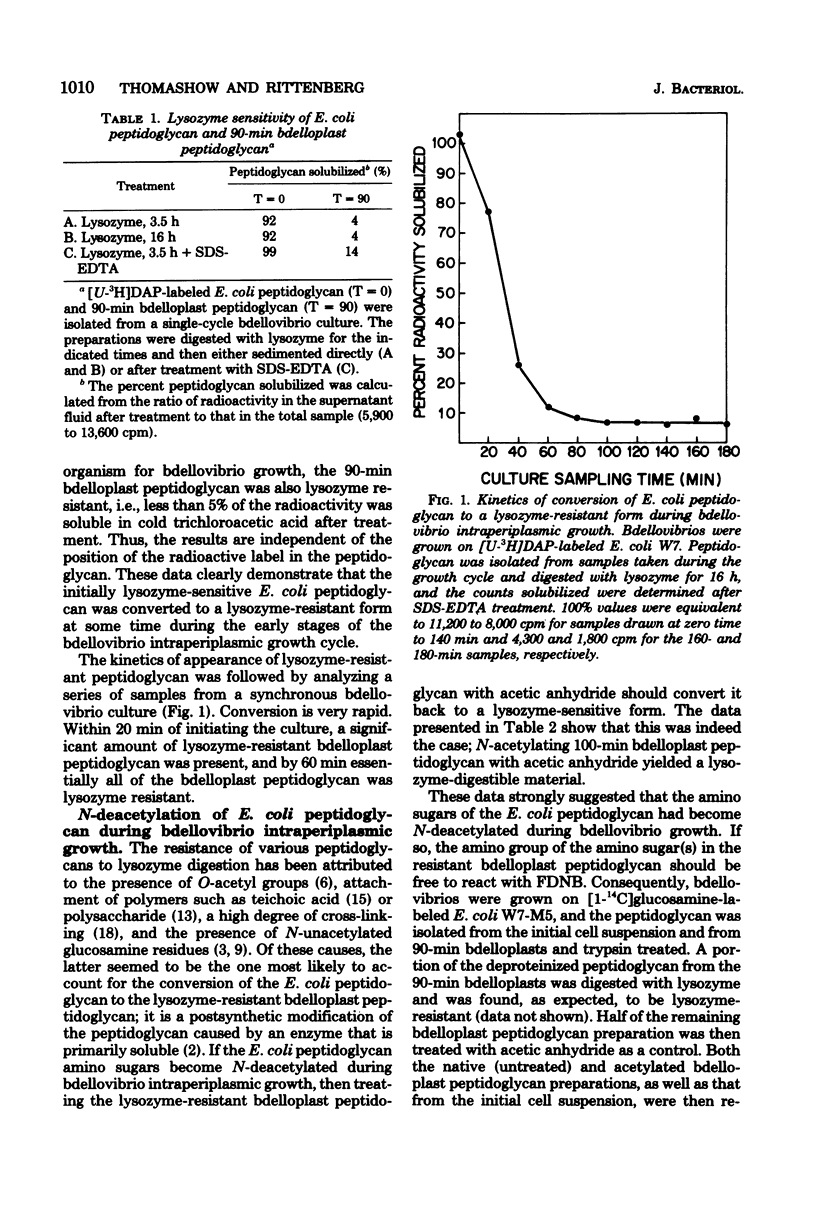
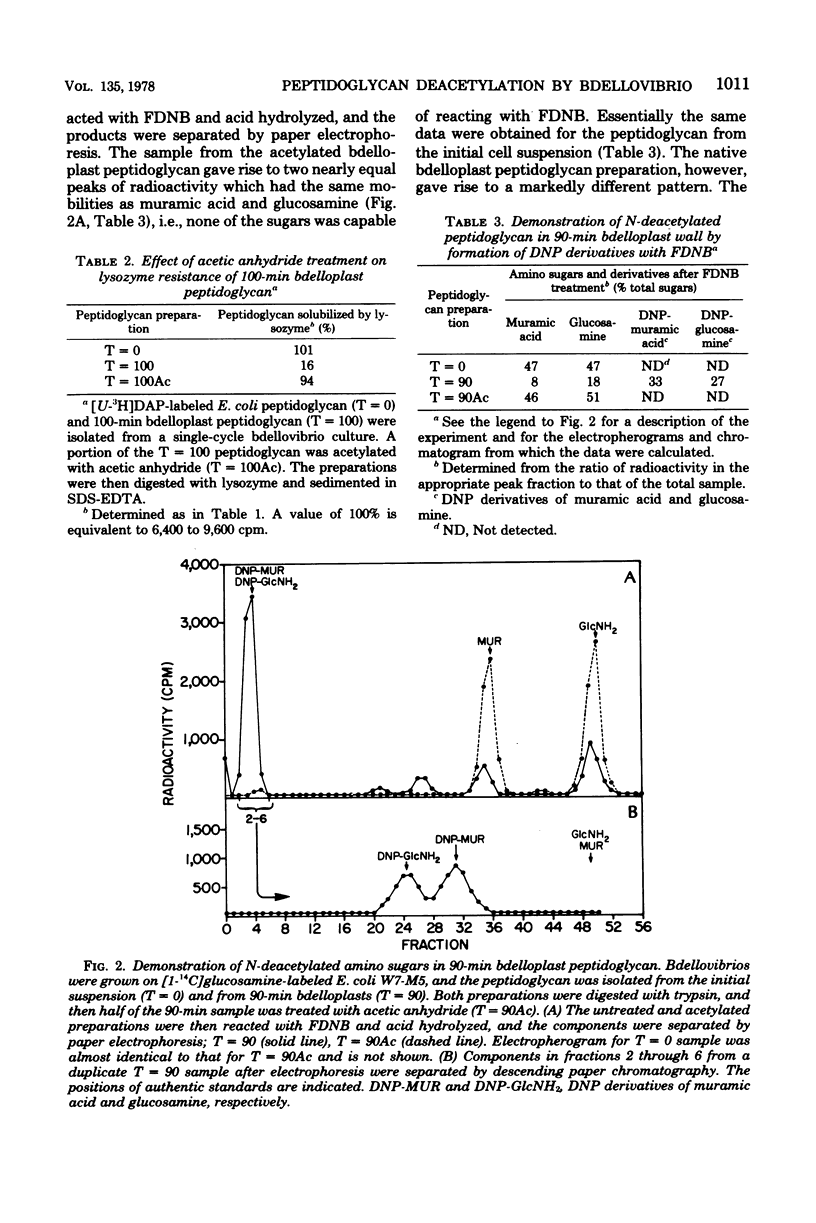

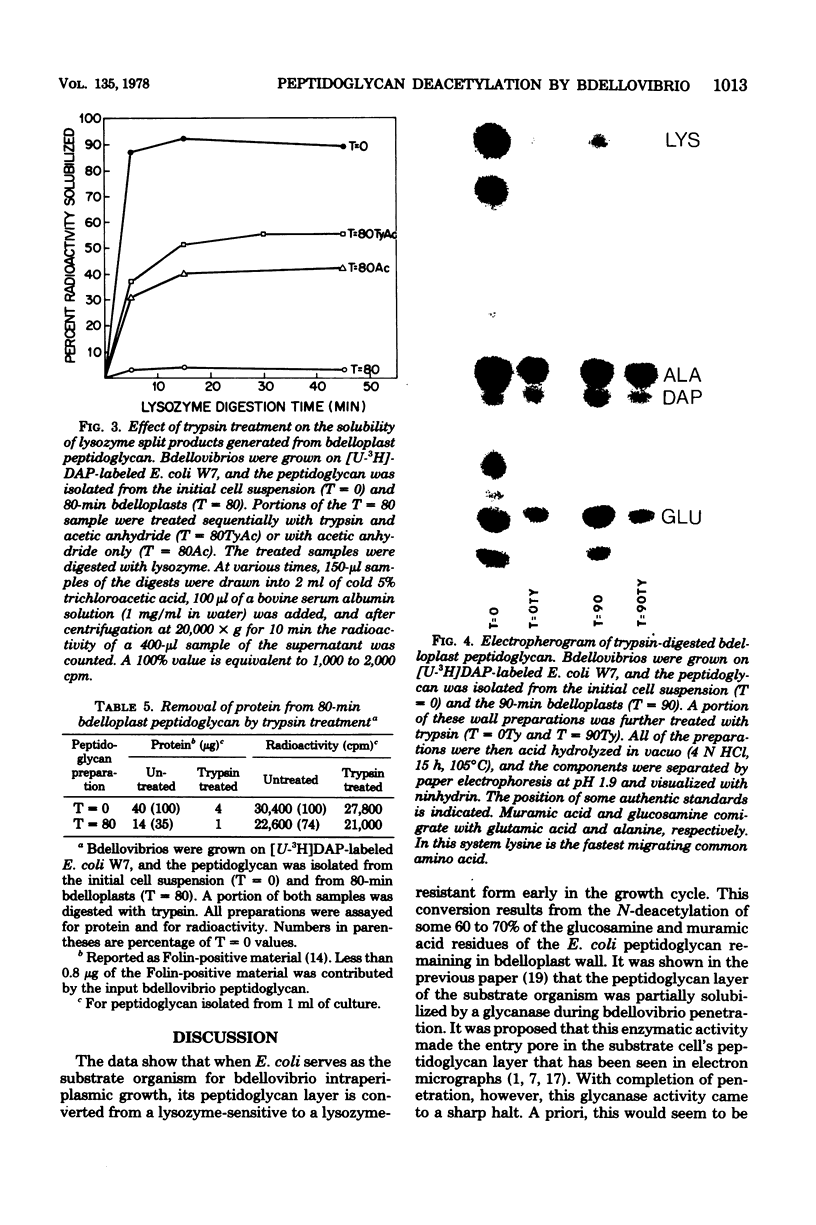

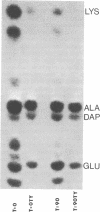
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abram D., Castro e Melo J., Chou D. Penetration of Bdellovibrio bacteriovorus into host cells. J Bacteriol. 1974 May;118(2):663–680. doi: 10.1128/jb.118.2.663-680.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki Y., Fukuoka S., Oba S., Ito E. Enzymatic deacetylation of N-acetylglucosamine residues in peptidoglycan from Bacillus cereus cell walls. Biochem Biophys Res Commun. 1971 Nov 5;45(3):751–758. doi: 10.1016/0006-291x(71)90481-5. [DOI] [PubMed] [Google Scholar]
- Araki Y., Nakatani T., Nakayama K., Ito E. Occurrence of N-nonsubstituted glucosamine residues in peptidoglycan of lysozyme-resistant cell walls from Bacillus cereus. J Biol Chem. 1972 Oct 10;247(19):6312–6322. [PubMed] [Google Scholar]
- BRUMFITT W., WARDLAW A. C., PARK J. T. Development of lysozyme-resistance in Micrococcus lysodiekticus and its association with an increased O-acetyl content of the cell wall. Nature. 1958 Jun 28;181(4626):1783–1784. doi: 10.1038/1811783a0. [DOI] [PubMed] [Google Scholar]
- Braun V., Bosch V., Hantke K., Schaller K. Structure and biosynthesis of functionally defined areas of the Escherichia coli outer membrane. Ann N Y Acad Sci. 1974 May 10;235(0):66–82. doi: 10.1111/j.1749-6632.1974.tb43257.x. [DOI] [PubMed] [Google Scholar]
- Braun V., Rehn K., Wolff H. Supramolecular structure of the rigid layer of the cell wall of Salmonella, Serratia, Proteus, and Pseudomonas fluorescens. Number of lipoprotein molecules in a membrane layer. Biochemistry. 1970 Dec 22;9(26):5041–5049. doi: 10.1021/bi00828a001. [DOI] [PubMed] [Google Scholar]
- Burnham J. C., Hashimoto T., Conti S. F. Electron microscopic observations on the penetration of Bdellovibrio bacteriovorus into gram-negative bacterial hosts. J Bacteriol. 1968 Oct;96(4):1366–1381. doi: 10.1128/jb.96.4.1366-1381.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEYMANN H., MANNIELLO J. M., BARKULIS S. S. STRUCTURE OF STREPTOCOCCAL CELL WALLS. 3. CHARACTERIZATION OF AN ALANINE-CONTAINING GLUCOSAMINYLMURAMIC ACID DERIVATIVE LIBERATED BY LYSOZYME FROM STREPTOCOCCAL GLYCOPEPTIDE. J Biol Chem. 1964 Sep;239:2981–2985. [PubMed] [Google Scholar]
- Hayashi H., Araki Y., Ito E. Occurrence of glucosamine residues with free amino groups in cell wall peptidoglycan from bacilli as a factor responsible for resistance to lysozyme. J Bacteriol. 1973 Feb;113(2):592–598. doi: 10.1128/jb.113.2.592-598.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M., Arnheim N., Sternglanz R. Bacteriophage T7 lysozyme is an N-acetylmuramyl-L-alanine amidase. J Biol Chem. 1973 Oct 25;248(20):7247–7252. [PubMed] [Google Scholar]
- KENT P. W., LAWSON G., SENIOR A. Chromatographic separation of amino sugars and amino acids, using the N-(2:4-dinitrophenyl) derivatives. Science. 1951 Mar 30;113(2935):354–355. doi: 10.1126/science.113.2935.354. [DOI] [PubMed] [Google Scholar]
- KRAUSE R. M., MCCARTY M. Studies on the chemical structure of the streptococcal cell wall. I. The identification of a mucopeptide in the cell walls of groups A and A-variant streptococci. J Exp Med. 1961 Jul 1;114:127–140. doi: 10.1084/jem.114.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- PRIMOSIGH J., PELZER H., MAASS D., WEIDEL W. Chemical characterization of mucopeptides released from the E. coli B cell wall by enzymic action. Biochim Biophys Acta. 1961 Jan 1;46:68–80. doi: 10.1016/0006-3002(61)90647-3. [DOI] [PubMed] [Google Scholar]
- Starr M. P., Baigent N. L. Parasitic interaction of Bdellovibrio bacteriovorus with other bacteria. J Bacteriol. 1966 May;91(5):2006–2017. doi: 10.1128/jb.91.5.2006-2017.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strominger J. L., Ghuysen J. M. Mechanisms of enzymatic bacteriaolysis. Cell walls of bacteri are solubilized by action of either specific carbohydrases or specific peptidases. Science. 1967 Apr 14;156(3772):213–221. doi: 10.1126/science.156.3772.213. [DOI] [PubMed] [Google Scholar]
- Thomashow M. F., Rittenberg S. C. Intraperiplasmic growth of Bdellovibrio bacteriovorus 109J: attachment of long-chain fatty acids to escherichia coli peptidoglycan. J Bacteriol. 1978 Sep;135(3):1015–1023. doi: 10.1128/jb.135.3.1015-1023.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow M. F., Rittenberg S. C. Intraperiplasmic growth of Bdellovibrio bacteriovorus 109J: solubilization of Escherichia coli peptidoglycan. J Bacteriol. 1978 Sep;135(3):998–1007. doi: 10.1128/jb.135.3.998-1007.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W., FRANK H., MARTIN H. H. The rigid layer of the cell wall of Escherichia coli strain B. J Gen Microbiol. 1960 Feb;22:158–166. doi: 10.1099/00221287-22-1-158. [DOI] [PubMed] [Google Scholar]