Abstract
erm(A) subclass erm(TR), a common macrolide resistance determinant in Streptococcus pyogenes but quite rare in Streptococcus pneumoniae, was found in a clinical S. pneumoniae isolate (AP200) from Italy. In this isolate, erm(TR) was found included in a genetic element approximately 56 kb in size that did not appear to be conjugative but could be transferred by transformation. An erm(TR)-containing DNA fragment of approximately 10 kb was sequenced and 12 open reading frames (ORFs) were identified. Upstream of erm(TR), a regulatory protein of the TetR family and the two components of an efflux pump of the ABC type were found. Downstream of erm(TR), there were ORFs homologous to a spectinomycin phosphotransferase, transposases, and a relaxase. Since the genomic sequence of S. pyogenes MGAS10750 carrying erm(TR) became available, comparison between the erm(TR)-containing genetic elements in AP200 and in MGAS10750 was performed. The region flanking erm(TR) in MGAS10750 showed identity with AP200 for 10 ORFs out of 12. PCR mapping using primers designed on the sequence of MGAS10750 confirmed that AP200 carries a genetic element similar to that of MGAS10750. In AP200 the genetic element was inserted inside an ORF homologous to spr0790 of S. pneumoniae R6, coding for a type I restriction modification system. Homologies between the insertion sites in AP200 and MGAS10750 consisted of eight conserved nucleotides, of which three were duplicated, likely representing target site duplication. The structure of the erm(TR)-carrying genetic element shows characteristics of a transposon/prophage remnant chimera. In AP200 this genetic element was designated Tn1806.
Resistance to macrolide antibiotics is becoming increasingly common in clinical isolates of Streptococcus pneumoniae. The two main resistance mechanisms found in pneumococci are active efflux and modification of the rRNA target. Drug efflux is the most common mechanism in strains of S. pneumoniae in North America (15), while in Europe and the Far East, the prevalent mechanism is rRNA methylation encoded by erm(B) (15, 27). This mechanism confers high-level erythromycin resistance and results in coresistance to lincosamide and streptogramin B antibiotics (macrolide-lincosamide-streptogramin B [MLSB] phenotype). In 2001, another erm determinant, erm(A) subclass erm(TR), was found in pneumococci from young Greek carriers (38). This resistance gene was initially described in Streptococcus pyogenes and was designated erm(TR) (35). Subsequently, it was included in the erm(A) class gene (32) because of its relatedness to erm(A) of Staphylococcus aureus that is located in transposon Tn554 (28).
Macrolide resistance conferred by erm(TR) is usually inducible and regulated in a similar way to that described for erm(C), which is the prototype of inducible erm genes (41). The expression of erm(TR) is controlled by posttranscriptional regulation of the methylase expression (16). This is determined by the presence of an upstream regulatory region, the leader sequence, which is composed of two leader peptides, LP1 and LP2, and which contains a number of inverted repeats that act in translational attenuation (26).
In S. pyogenes erm(TR) represents a common determinant of macrolide resistance (33). Besides S. pyogenes, erm(TR) has been identified in other Streptococcus species such as Streptococcus agalactiae (25) and group G streptococci (42) and in different species of Peptostreptococcus (31). Conjugal transfer of erm(TR) has been demonstrated from S. pyogenes to macrolide-susceptible recipients of the species S. pyogenes, Enterococcus faecalis, and Listeria innocua (18) and from Peptostreptococcus magnus to S. pyogenes (31). Recently, the genetic element carrying erm(TR) has been identified in S. pyogenes MGAS10750 as an integrated conjugative element (ICE) (2).
In S. pneumoniae erm(TR) is quite rare. Studies from Spain (4), Belgium (40), Hungary (12), and the United States (9) reported one erm(TR)-carrying strain in each collection analyzed. A study from a global surveillance network (14) confirmed these data, reporting only two erm(TR)-carrying strains from Australia among 1,043 S. pneumoniae strains collected from 25 countries. In the isolate from Spain, erm(TR) was present together with erm(B) (4).
In the context of a surveillance study to monitor antimicrobial resistance in S. pneumoniae in Italy, out of a collection of more than 1,000 invasive isolates, we found a macrolide-resistant strain (AP200) that carried erm(TR). In this study we report on the identification and characterization of the genetic element carrying erm(TR) in this S. pneumoniae strain.
MATERIALS AND METHODS
Characterization of the bacterial strain.
S. pneumoniae AP200 is a clinical strain isolated in Italy in 2003 from the cerebrospinal fluid of a patient with meningitis. Susceptibility to erythromycin, clindamycin, penicillin, and tetracycline was determined by Etest (AB Biodisk, Solna, Sweden). Inducibility of clindamycin resistance was tested by the erythromycin-clindamycin double-disk test (23) and confirmed by a broth macrodilution method using Mueller-Hinton II broth (BBL Microbiology Systems) supplemented with 3% lysed horse blood with and without the addition of 0.05 μg/ml erythromycin as an inducer (37).
Detection of erythromycin resistance genes commonly found in pneumococci and of mutations in ribosomal genes or ribosomal protein was performed according to published methods (27). PCR amplification of the erm(TR) gene was performed using the primers described by Tait-Kamradt et al. (39).
The isolate was serotyped using the quellung reaction with the antiserum produced by the Staten Serum Institut (Copenhagen, Denmark) and was submitted to multilocus sequence typing (MLST) following the procedure recommended at the MLST website (http://spneumoniae.mlst.net/).
Conjugation and transformation experiments.
Conjugation experiments were carried out using AP200 as the donor strain, and FP10, a streptomycin-and chloramphenicol-resistant derivative of S. pneumoniae Rx1, as the recipient strain. Selection for transconjugants was performed on multilayer plates containing erythromycin (1 μg/ml) and streptomycin (1,000 μg/ml) (22). Transformation assays were carried out as previously described (21) using FP11, a novobiocin-and chloramphenicol-resistant derivative of Rx1, as the recipient strain. Transforming DNA from AP200 and CSP-1 (competence-stimulating peptide 1) were added to the recipient strain during the exponential phase of growth in competence medium. Transformants were selected by plating the transformation mixture onto selective plates containing erythromycin (1 μg/ml), novobiocin (10 μg/ml), and chloramphenicol (5 μg/ml).
PFGE and hybridization analysis.
The genomic profiles of the donor strain (AP200), the recipient strain (FP11), and one transformant (TP1) were obtained by pulsed-field gel electrophoresis (PFGE) of SmaI-digested chromosomal DNAs following a previously described procedure (10). DNA fragments generated by PFGE were transferred to a nylon membrane and hybridized with an erm(TR) probe obtained by PCR amplification and labeled using an ECL System (GE Healthcare, Milan, Italy). The molecular sizes of the PFGE fragments were determined by the Diversity Database software (Bio-Rad Laboratories, Hercules, CA).
PCR experiments and sequencing.
To detect the flanking region of erm(TR), inverse PCR was carried out with primers ETR3 and ETR4 (Table 1) following digestion of AP200 genomic DNA with SpeI or NdeI and ligation under conditions favoring the production of monomeric circles. The amplicons obtained were sequenced, and direct amplification and sequencing of the AP200 genomic DNA were performed to verify the results.
TABLE 1.
Oligonucleotide primers used for the characterization of the erm(TR) genetic element in AP200
| Primer use and name | Oligonucleotide sequence (5′-3′) | Nucleotides | GenBank accession no. |
|---|---|---|---|
| Inverse PCR | |||
| ETR3 | CGTATTTTTCGGGTTTTTCTGT | 237-216 | AF002716 |
| ETR4 | GCGAATGTTACTGATCTTGATA | 865-886 | AF002716 |
| PCR mapping | |||
| ETR36 | GAGAGCATTTCTATTGAAGCTAAAG | 1621815-1621839 | NC_008024 |
| ETR49 | TCTTAGCTGGTATATACACTTACC | 1626155-1626132 | NC_008024 |
| ETR55 | GGCATACAAACATTGATAACACCA | 1624454-1624477 | NC_008024 |
| ETR57 | AGTTCAATGTGGACACTTGGATC | 1629156-1629134 | NC_008024 |
| ETR63 | AGTTGGCATTGGAAGAAGATAGAG | 1629069-1629092 | NC_008024 |
| ETR89 | GTGTTATACCTGTTGGAATACC | 1630965-1630944 | NC_008024 |
| ETR99 | GGTATTCCAACAGGTATAACAC | 1630944-1630965 | NC_008024 |
| ETR92 | GATCCACATAAGTTTTTACATCC | 1636069-1636047 | NC_008024 |
| ETR95 | GGATGTAAAAACTTATGTGGATC | 1636047-1636069 | NC_008024 |
| ETR94 | CTGTTCTTGGATATGTGATTAGC | 1638270-1638248 | NC_008024 |
| ETR96 | GCTAATCACATATCCAAGAACAG | 1638248-1638270 | NC_008024 |
| ETR97 | GGCATTCACTCTACTTGTACCATA | 1644388-1644365 | NC_008024 |
| ETR50 | TATGGTACAAGTAGAGTGAATGCC | 1644365-1644388 | NC_008024 |
| ETR98 | CCAAATTTACCTCATATTGAATCG | 1647254-1647231 | NC_008024 |
| ETR40 | CGATTCAATATGAGGTAAATTTGG | 1647231-1647254 | NC_008024 |
| ETR27 | CCTTTCTTTATAAACCGATGACC | 1427-1405 | EF469826 |
| ETR26 | CAGTTGTATTAGTAGCCTTGG | 10809-10829 | EF469826 |
| ETR42 | TGAAAGATACCCAAGAGATGTCG | 1663121-1663099 | NC_008024 |
| ETR45 | TCAAAAGCAGCATCTTTATCTTCG | 1662903-1662926 | NC_008024 |
| ETR46 | ATGCATATTCTTTACAAGACAGG | 1667257-1667235 | NC_008024 |
| ETR38 | TGTAATAAACGACTCTCATAAGCC | 1666258-1666281 | NC_008024 |
| ETR39 | ATCCTTGATTGGATCTTCTAGACC | 1669963-1669940 | NC_008024 |
| Chromosomal insertions | |||
| ETR47 | GGTCTAGAAGATCCAATCAAGGAT | 1669940-1669963 | NC_008024 |
| ETR79 | GCGTAAGAAAGATGAATGGGAAAC | 787399-787422 | NC_003098 |
| ETR84 | GATAAACCTGAATACCGTTGG | 786950-786970 | NC_003098 |
| ETR37 | TGGTGTTATCAATGTTTGTATGCC | 1624477-1624454 | NC_008024 |
| ETR44 | CTTCTCATTTTAACGATGTGACG | 1672289-1672267 | NC_008024 |
| Gene SOEinga | |||
| ETR8 | CGATTCTTTTGGCGACCATTAG | 4812-4833 | EF469826 |
| ETR9 | GATAGAATAACAAAGCATCCG | 6742-6722 | EF469826 |
| IF149 | CAAGCTGGGGATCCGTTTGAT | 5-25 | AY334018 |
| IF210 | CTAAAACAATTCATCCAGTAAAATAT | 880-855 | AY334018 |
| ETR56b | ATCAAACGGATCCCCAGCTTGTATAACCTTCTCCTTGCC | AY334018EF469826 | |
| ETR64c | ATATTTTACTGGATGAATTGTTTTAGGCGTTCTCTAACTTTAAGAG | AY334018EF469826 |
SOE, splicing overlap extension.
The underlined nucleotides are complementary to IF149 and the others are complementary to a sequence of AP200.
The underlined nucleotides are complementary to IF210 and the others correspond to a sequence of AP200.
As we were analyzing the region containing erm(TR) in S. pneumoniae, the complete genome sequence of S. pyogenes MGAS10750 was deposited in the GenBank database under the accession number NC_008024 (3). This strain carries erm(TR) enclosed in an ICE designated 10750-RD.2 (2). Comparison between this element of MGAS10750 and the erm(TR)-containing region of AP200 was performed by PCR mapping using 11 primer pairs designed on the basis of sequences of MGAS10750 and AP200 (Table 1).
To define the insertion site of the genetic element in AP200, amplification of the right junction was attempted using primers ETR47 and ETR44 designed on the right end of the genetic element and the flanking chromosome sequence of MGAS10750, respectively (Table 1). From the sequence of the amplicon obtained, primers specific for the flanking chromosome regions of AP 200 were designed, and the right and left junctions of the element were amplified using the primer pairs ETR47-ETR79 and ETR84-ETR37, respectively (Table 1). The amplicons obtained were sequenced.
Construction of an erm(TR) deletion mutant.
To obtain a deletion mutant, the erm(TR) gene was replaced with a kanamycin resistance cassette. A 2,060-bp fragment containing the kanamycin cassette, flanked by the upstream and downstream regions of erm(TR), was generated by the gene splicing overlap extension technique (19, 20) and used to transform TP1, a transformant carrying the erm(TR) genetic element from AP200. The transforming DNA fragment was the result of the combined amplification of three overlapping amplicons. The kanamycin cassette was amplified from a synthetic construct containing the aphIII gene of plasmid pJH1 (36) using primers IF149 and IF210. The regions upstream and downstream of erm(TR) were obtained with primers ETR8-ETR56 and ETR64-ETR9, respectively (Table 1). Primers ETR8 and ETR9 were designed on sequences flanking erm(TR) in AP200. In primer ETR56, 21 nucleotides at the 5′ end were complementary to IF49, and the last 18 nucleotides were complementary to a sequence upstream of erm(TR). Similarly, in primer ETR64 26 nucleotides at the 5′ end were complementary to IF210, and the last 20 nucleotides corresponded to a sequence downstream of erm(TR) (Table 1). Recombinants were selected by a multilayer plating procedure, using kanamycin (1,000 μg/ml) as the selective antibiotic. Deletion of the erm(TR) gene and replacement with the kanamycin cassette were confirmed by PCR and sequencing.
Nucleotide sequence accession number.
The nucleotide sequences of a 10,582-bp fragment of Tn1806, the erm(TR)-genetic element of AP200, and of its left and right junctions have been assigned GenBank accession number EF469826.
RESULTS
Characterization of strain AP200.
S. pneumoniae strain AP200 belongs to serotype 11A and to sequence type (ST) 2003, which is a single-locus variant of ST62, the most common ST among Italian serotype 11A pneumococci (6). AP200 was resistant to erythromycin, with a MIC of 1 μg/ml, and susceptible to penicillin and tetracycline. Resistance to clindamycin was inducible (Table 2). Double-disk testing showed blunting of the clindamycin inhibition zone in the proximity of the erythromycin disk. By broth dilution, exposure to a subinhibitory concentration of erythromycin (0.05 μg/ml) caused a 1,032-fold increase in the clindamycin MIC (from 0.06 μg/ml to 64 μg/ml). Preinduction with erythromycin did not affect the erythromycin MIC, which is in line with the results of other investigators (37). The presence of erm(TR) was demonstrated by PCR. No other known pneumococcal erythromycin resistance genes or mutations of ribosomal genes or ribosomal proteins were detected.
TABLE 2.
Strains used in this study and their MLSB phenotypes
| Strain | Characteristic | MIC (μg/ml)a
|
CLI D-testb | ||
|---|---|---|---|---|---|
| ERY | CLI | iCLI | |||
| AP200 | Clinical isolate, donor | 1 | 0.06 | 64 | iR |
| FP11 | Recipient | 0.023 | 0.015 | ND | S |
| TP1 | Transformant | 1 | 0.015 | 32 | iR |
| RM5 | TP1 Δerm(TR) | 0.023 | 0.015 | ND | S |
ERY, erythromycin; CLI, clindamycin; iCLI, clindamycin after induction with 0.05 μg/ml erythromycin; ND, not done.
CLI D-test, clindamycin double-disk test; iR, inducible resistance; S, susceptible.
Transferability of erm(TR).
Erythromycin resistance was not transferable by conjugation from AP200 to a pneumococcal recipient (conjugation frequency of <1 × 10−8). However, it could be transferred by transformation to the erythromycin-susceptible recipient FP11 (MIC of 0.023 μg/ml). One transformant (TP1) was selected and further characterized. TP1 showed resistance to erythromycin (MIC of 1 μg/ml) and inducible clindamycin resistance, as shown by the double-disk test and the broth macrodilution method (Table 2). The presence of erm(TR) was confirmed by PCR. In order to establish the size of the transferred DNA fragment, PFGE was performed with transformant TP1, recipient FP11, and donor AP200. Compared with the PFGE profile of the recipient, the transformant TP1 showed the disappearance of a band of approximately 350 kb and the appearance of a new band of ca. 400 kb. The molecular size of the transferred fragment was estimated to be approximately 56 kb. A hybridization assay, carried out using an erm(TR) probe, demonstrated that the fragment acquired by the transformant TP1 contained erm(TR).
Analysis of the flanking regions of erm(TR) in AP200.
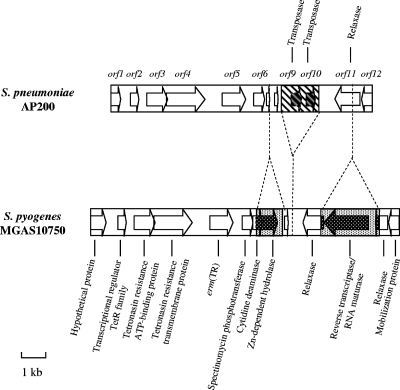
A 10,582-bp DNA fragment including erm(TR) was obtained by a series of inverse PCRs. Sequence analysis of this region revealed the presence of 12 open reading frames (ORFs) (Fig. 1). Homologies with other microbial proteins are described in Table 3. No significant nucleotide identity with known genes was found, with the exception of 95% nucleotide identity between orf10 and the sequence of a transposase of Trichomonas vaginalis. If comparison was carried out with the corresponding erm(TR)-containing region of MGAS10750, an almost total correspondence from orf1 to orf6 was present, with nucleotide and amino acids homologies varying from 93 to 100% and from 97 to 100%, respectively. The erm(TR) gene of AP200, corresponding to orf5, showed 100% identity to erm(TR) of S. pyogenes and 81% homology to erm(A) of S. aureus. A single base change, C145T, was detected in the leader sequence with respect to the deposited erm(TR) sequence of S. pyogenes. This point mutation introduces a stop codon in the coding region of LP2, which results in a truncated peptide of 17 amino acids instead of 19 amino acids.
FIG. 1.
Schematic representation of a 10,582-bp DNA fragment enclosing erm(TR) in AP200 compared to the corresponding region of MGAS10750 with its annotations (NC_008024). Arrows indicate ORFs and the direction of their transcription. The regions unique to AP200 are indicated by striped areas, and the regions unique to MGAS10750 are indicated by dotted areas.
TABLE 3.
Homologies of the ORFs present in a ∼10-kb region including erm(TR) in S. pneumoniae AP200
| ORF | Homologous protein (microorganism; GenBank accession no.)a | Amino acid identity (%)b | Amino acid similarity (%)b |
|---|---|---|---|
| orf1 | Hypothetical protein (S. pyogenes MGAS10750; ABF38649) | 96/101 (95%) | 98/101 (97%) |
| Putative conjugative transposon protein (C. difficile 630; CAJ68737) | 67/102 (65%) | 84/102 (82%) | |
| orf2 | Transcriptional regulator TetR family (S. pyogenes MGAS10750; ABF38650) | 209/210 (99%) | 210/210 (100%) |
| Transcriptional regulator TetR family (D. hafniense; EAT51867) | 91/209 (43%) | 142/209 (67%) | |
| orf3 | Tetronasin resistance ATP-binding protein (S. pyogenes MGAS10750; ABF38651) | 293/293 (100%) | |
| ABC transporter ATP-binding protein (O. iheyensis; BAC12676) | 251/292 (85%) | 274/292 (93%) | |
| ABC transporter ATP-binding protein (E. faecium; CAL36556) | 250/292 (85%) | 268/292 (91%) | |
| orf4 | Tetronasin resistance transmembrane protein (S. pyogenes MGAS10750; ABF38652) | 567/568 (99%) | 568/568 (100%) |
| Putative ABC-2 type transport system permease protein (E. faecium; EAN08886) | 305/536 (56%) | 424/536 (79%) | |
| orf5 | erm(TR) (S. pyogenes; AAB60941) | 243/243 (100%) | |
| erm(TR) (S. pyogenes MGAS10750; AAB60941) | 243/243 (100%) | ||
| erm(A) (S. aureus; BAB42746) | 197/242 (81%) | 218/242 (90%) | |
| orf6 | Spectinomycin phosphotransferase (S. pyogenes MGAS10750; ABF38655) | 173/174 (99%) | 174/174 (100%) |
| Spectinomycin phosphotransferase (L. pneumophila Lens; CAH15775) | 45/143 (31%) | 73/143 (51%) | |
| orf7 | Cytidine deaminase (S. pyogenes MGAS10750; ABF38656) | 28/28 (100%) | |
| orf8 | DNA recombinase (B.cereus ATCC 10987; AAS39355) | 36/48 (75%) | 40/48 (83%) |
| orf9 | Hypothetical protein CbeiDRAFT_2337 (C. beijerincki; EAP59839) | 62/141 (43%) | 86/141 (60%) |
| Putative transposase (E. faecalis; AAF70552) | 44/141 (31%) | 73/141 (51%) | |
| orf10 | Transposase IS116/IS110/IS902 family protein (T. vaginalis; EAX95005) | 105/110 (95%) | 108/110 (98%) |
| Putative transposase (E. faecalis, AAF70552) | 42/108 (38%) | 63/108 (58%) | |
| Transposase IS116/IS110/IS902 (S. suis; EAP39924) | 37/91 (40%) | 56/91 (61%) | |
| orf11 | Relaxase (S. pyogenes MGAS10750; ABF38658) | 329/332 (99%) | 331/332 (99%) |
| Putative conjugative transposon mobilization protein (C. difficile 630; CAJ68743) | 296/443 (66%) | 353/443 (79%) | |
| Relaxase (S. pyogenes MGAS10750; ABF38660) | 109/112 (97%) | 111/112 (99%) | |
| orf12 | Hypothetical protein (S. pyogenes MGAS10750; ABF38661) | 106/118 (89%) | 113/118 (95%) |
| Putative conjugative transposon mobilization protein (C. difficile 630; CAJ68744) | 85/118 (72%) | 100/118 (84%) |
D. hafniense, Desulphitobacterim hafniense; O. iheyensis, Oceanobacillus iheyensis; E. faecium, Enterococcus faecium; C. beijerincki, Clostridium beijerincki; S. suis, Streptococcus suis.
Values are the ratios of the number of identical or similar amino acids, as indicated, to the total number of amino acids in the region of similarity.
Upstream of erm(TR), orf1 showed homology to a putative conjugative transposon protein of Clostridium difficile 630, and orf2 showed homology to a transcriptional regulator belonging to the TetR family, while orf3 and orf4, two overlapping genes transcribed from two different frames, showed homology to ABC transporter proteins, namely to an ATP-binding protein and to a permease, respectively. In MGAS10750 this putative efflux system is annotated as an exporter of tetronasin, an ionophoric antibiotic belonging to the polyketide group. orf6 corresponded to a spectinomycin phosphotransferase, showing homology with a spectinomycin resistance gene of Legionella pneumophila (2).
The region downstream of orf6 was more divergent between AP200 and MGAS10750 (Fig. 1). In AP200, orf7 appears as a truncated gene, corresponding to the first 39 amino acids of Spy1706 of MGAS10750, a cytidine deaminase. In comparison with the sequence of MGAS10750, 1,103 bp were missing in the pneumococcal strain, comprising the 3′ sequence of Spy1706 and the entire Spy1707, a zinc-dependent hydrolase. orf8 appears as a partial sequence, truncated at both the 5′ and 3′ ends, a homologue to a putative DNA recombinase of Bacillus cereus. This recombinase remnant is also present in MGAS10750. Downstream of orf8, AP200 carries a region of 1,589 bp that is not present in MGAS10750 and contains two transposases, designated orf9 and orf10. The amino acid sequence of the two transposases shows conserved domains belonging to the transposase 9 (IS111A/IS1328/IS1533 family) and transposase 20 (IS116/IS110/IS902 family) families, respectively. orf11 codes for a relaxase, which is homologous to Spy1710 of MGAS10750 from amino acids 1 to 112 and to Spy1708 from amino acids 112 to 443. In MGAS10750, Spy1710 and Spy1708 are two distinct relaxases that are interspersed with another ORF that codes for a reverse transcriptase/RNA maturase that is lacking in AP200. orf12, similarly to orf1, showed a high percentage of homology (84%) with a putative conjugative transposon mobilization protein of C. difficile 630.
PCR mapping of the erm(TR)-containing region.
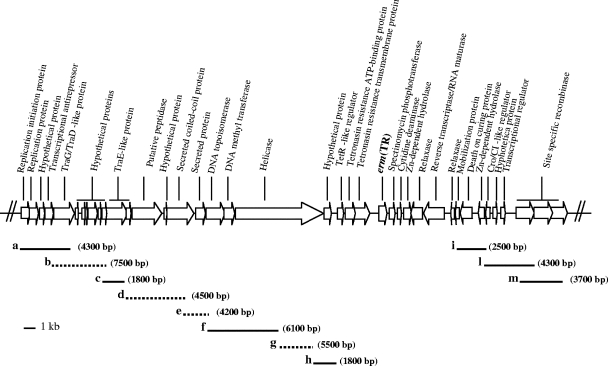
In MGAS10750, ICE 10750-RD.2 containing erm(TR) is approximately 49 kb in size (2). To verify the presence of a similar genetic element in AP200, outside the sequenced region, PCR mapping was performed using primers designed on the basis of MGAS10750 sequence and on the available sequence of AP200 (Table 1). In all cases, the PCR assays yielded amplicons from AP200, indicating that the overall structure of the erm(TR)-containing region in S. pneumoniae is similar to that of MGAS10750 (Fig. 2). Seven amplicons were of the expected size, according to the sequence of MGAS10750, while in four instances the dimensions of the amplicons were different. In particular, three fragments, b, e, and g, were approximately 2.7 kb, 2 kb, and 2.6 kb larger than expected, respectively (Fig. 2). Fragment d appeared approximately 600 bp smaller than expected. On the basis of the amplicon sizes, the erm(TR) genetic element contained in AP200 appears to be approximately 56 kb in size, 7 kb larger than the erm(TR) genetic element in MGAS10750.
FIG. 2.
Schematic representation of ICE 10750-RD.2 of S. pyogenes MGAS10750 (NC_008024). The ORFs are indicated by arrows, and their annotations are reported. The positions and the approximate sizes of the 11 overlapping amplicons (a to m) obtained from AP200 are shown. The amplicons indicated by dotted lines are of different sizes from those predicted from the MGAS10750 sequence (see text).
Chromosomal insertion site.
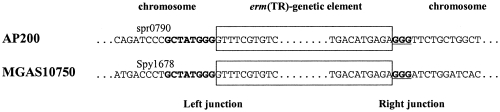
In an attempt to identify the chromosomal insertion sites of the erm(TR) genetic element in AP200, the junctions were explored by PCR using primers designed on the MGAS10750 sequence. An amplicon was obtained for the region spanning the right junction. Sequencing of the amplicon revealed that the primer ETR44, designed on Spy1722 of S. pyogenes, annealed to a conserved pneumococcal gene corresponding to spr0790 in S. pneumoniae R6 (NC_003098) due to a 14-bp sequence identity with the primer. Amplification with primers designed on spr0790 and sequence analysis confirmed that the insertion of the erm(TR) genetic element in AP200 is inside spr0790, at base 787357 of the R6 genome. spr0790 of R6 corresponds to an hsdM gene, coding for a type I restriction modification system. Interestingly, in MGAS10750, the erm(TR) genetic element is inserted in Spy1678, also annotated as an hsdM gene and predicted to encode a type I restriction modification system. However, spr0790 of S. pneumoniae and Spy1678 of S. pyogenes do not share significant nucleotide or amino acids homologies. Comparative analysis of the insertion sites of the erm(TR) genetic elements in AP200 and MGAS10750 showed two conserved features: an eight-nucleotide sequence (GCTATGGG) at the left junction and the duplication of the last three nucleotides of this sequence at the right junction (Fig. 3). In both AP200 and MGAS10750, the insertion of the erm(TR)-carrying genetic element did not interrupt the hsdM coding sequence, which was reconstituted by the first 105 nucleotides of the inserted genetic element.
FIG. 3.
Junctions of the erm(TR) genetic elements with the chromosomes in AP200 and in MGAS10750. The designation of the gene interrupted by the insertion is reported above the corresponding sequence. The sequences belonging to the erm(TR) genetic elements are boxed. At the left junction, the eight conserved nucleotides are in boldface. At the right junction, the three-nucleotide duplication is in boldface and underlined.
Analysis of an erm(TR) deletion mutant.
RM5, one deletion mutant of the transformant TP1 in which erm(TR) was replaced with a kanamycin resistance cassette, was obtained and further investigated. RM5 lost erythromycin resistance, since its erythromycin MIC decreased from 1 to 0.023 μg/ml (Table 2), indicating that the presence of an intact erm(TR) was essential to confer erythromycin resistance.
DISCUSSION
Although rare, the erm(TR) gene has been recently found in S. pneumoniae isolates both from carriers and of invasive diseases (4, 9, 12, 38, 40). This macrolide resistance determinant is far more common in S. pyogenes, where it was originally discovered (35).
In our collection comprising 300 erythromycin-resistant invasive S. pneumoniae isolates obtained in the years from 1997 to 2003 (27), one strain (AP200) was found to possess erm(TR) and no other additional resistance mechanisms, such as erm(B), mef, or mutations in the ribosomal components. AP200 belongs to serotype 11A, the same serotype of the first reported erm(TR)-positive pneumococci from Greek carriers (38). In an attempt to characterize the genetic element carrying erm(TR) in this strain, we sequenced an approximately 10-kb DNA region surrounding erm(TR).
The erm(TR) leader sequence in AP200 showed a single nucleotide mutation in LP2 (C145T). This mutation, which was previously described in S. pyogenes and found to be associated with a constitutive MSLB phenotype (13), determines a truncated LP2 peptide but does not appear to alter the secondary mRNA structure (16). In AP200, this point mutation does not affect the inducible MSLB phenotype that is characteristic of erm(TR). It is unclear why the C145T mutation is associated with different phenotypes in these two species.
Analysis of an erm(TR) deletion mutant demonstrated that erythromycin resistance was conferred by the presence of an intact erm(TR).
In the region flanking erm(TR), other genes involved in antibiotic resistance were found, such as the transcriptional regulator tetR. In Escherichia coli, TetR is implicated in controlling the expression of tet(A), an efflux pump conferring tetracycline resistance (30). In AP200 downstream of TetR, there are two components of an efflux system, an ATP-binding protein and a permease that is predicted to export tetronasin, a polyketide-polyether antibiotic which acts as an ionophore depolarizing the bacterial membrane (24). Although in AP200 an intact erm(TR) appears to be sufficient to confer erythromycin resistance, Giovanetti et al. found that in S. pyogenes carrying erm(TR), high-level erythromycin resistance was conferred by the contribution of a drug efflux pump (17). Immediately downstream of erm(TR) another antibiotic resistance determinant, a gene encoding a spectinomycin phosphotransferase, was found. In the staphylococcal transposon Tn554, erm(A) is associated with another spectinomycin resistance gene, spc, encoding a spectinomycin adenyltransferase (28).
While we were characterizing the genetic context of erm(TR) in AP200, the genomic sequence of S. pyogenes MGAS10750 harboring erm(TR) in ICE 10750-RD.2 was deposited in the GenBank database. From a comparison of the sequenced region of AP200 with the corresponding region of the MGAS10750 element, an overall similarity was found. Among the 12 ORFs sequenced in AP200, 10 showed a high degree of similarity with the corresponding ORFs of MGAS10750. The absence of two transposases and the presence of two separate relaxases were the only differences between MGAS10750 and AP200 in this region.
The presence in AP200 of a large genetic element similar to that of MGAS10750 was ascertained by PCR mapping. Only a few differences in the size of the individual amplicons were observed, indicating that the AP200 genetic element was larger than that of MGAS10750. The erm(TR) genetic elements in both AP200 and MGAS10750 were found inserted into the hsdM gene, encoding a type I restriction modification system. Although the respective genes code for a similar product, they do not show significant nucleotide or amino acid homologies. Nevertheless, the chromosomal insertion sites of both erm(TR) genetic elements share a sequence of eight nucleotides with duplication of the last three nucleotides, likely representing the target sequence for integration of the erm(TR) genetic element. Both in AP200 and MGAS10750 the coding sequences of the target gene hsdM are not interrupted by insertion of the genetic element, which introduces an alternative 105-bp 3′ end for the gene. This is a mechanism frequently occurring at the chromosomal insertion site of a prophage, which is able to complement the coding sequence of the interrupted gene, either precisely or approximately, leading to an altered but functional protein (7).
Along the erm(TR) genetic element in MGAS10750, genes coding for phage proteins can be identified, such as a phage antirepressor or a transcriptional regulator belonging to the Cro/CI family, although essential structural proteins like phage tail or phage head/capsid proteins are missing. Prophage remnants are characterized by the loss of substantial amounts of the prophage genome, especially genes that do not represent a selective advantage for the host. In contrast, genes with positive selective value, such as those that confer immunity functions, are preserved (8). The phage CI-like repressor can have an immunological role in protecting the bacterial cell against superinfection, with temperate phages sharing identical or related repressor DNA recognition sites (5), and is conserved also in severely deleted prophage remnants such as that of S. pyogenes SF370 (8).
At the 3′ end of the genetic element, three serine recombinases are present. This is reminiscent of the three recombinases present at the ends of Tn1207.3/θ10394.4, a transposon or transposon/prophage chimeric element carrying the macrolide efflux gene mef(A) in S. pyogenes (1, 34).
Although a fragment of 30 to 40 kb including erm(TR) was demonstrated to transfer conjugatively from S. pyogenes to macrolide-susceptible S. pyogenes or other gram-positive species (18), erm(TR) could not be transferred by conjugation from AP200. The lack of conjugal transfer of transposons or other genetic elements from pneumococci is not unusual. For instance, Tn2009 is not conjugative in pneumococcus (11) but appears to be conjugative in Acinetobacter junii (29).
Taken together, these characteristics suggest that the erm(TR)-carrying genetic element is a transposon/prophage remnant chimera. In S. pyogenes this element has been defined as an ICE, a chimeric structure containing antibiotic resistance genes and genes related to lateral transfer (2). The genetic element found in AP200 was designated Tn1806.
The frequent presence of erm(TR) in S. pyogenes (33) and in P. magnus (31), another constituent of the oropharyngeal flora, indicates that this resistance determinant circulates among gram-positive aerobic and anaerobic species inhabiting the oral cavity. However, S. pneumoniae appears only as an occasional host for this resistance determinant. It is possible that such a large element is not easily acquired or maintained in this species.
Acknowledgments
This study was supported in part by grants from the European Commission (6th Framework, DRESP2 project) and from the Italian Ministry of University and Research (FIRB 2005).
Footnotes
Published ahead of print on 10 December 2007.
REFERENCES
- 1.Banks, D. J., S. F. Porcella, K. D. Barbian, J. M. Martin, and J. M. Musser. 2003. Structure and distribution of an unusual chimeric genetic element encoding macrolide resistance in phylogenetically diverse clones of group A Streptococcus. J. Infect. Dis. 188:1898-1908. [DOI] [PubMed] [Google Scholar]
- 2.Beres, S. B., and J. M. Musser. 2007. Contribution of exogenous genetic elements to the Group A Streptococcus metagenome. PLoS ONE 2:e800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beres, S. B., E. W. Richter, M. J. Nagiec, P. Sumby, S. F. Porcella, F. R. DeLeo, and J. M. Musser. 2006. Molecular genetic anatomy of inter- and intraserotype variation in the human bacterial pathogen group A Streptococcus. Proc. Natl. Acad. Sci. USA 103:7059-7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Betriu, C., M. Redondo, M. L. Palau, A. Sanchez, M. Gomez, E. Culebras, A. Boloix, and J. J. Picazo. 2000. Comparative in vitro activities of linezolid, quinupristin-dalfopristin, moxifloxacin, and trovafloxacin against erythromycin-susceptible and-resistant streptococci. Antimicrob. Agents Chemother. 44:1838-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruttin, A., S. Foley, and H. Brussow. 2002. DNA-binding activity of the Streptococcus thermophilus phage Sfi21 repressor. Virology 303:100-109. [DOI] [PubMed] [Google Scholar]
- 6.Camilli, R., E. Pettini, M. Del Grosso, G. Pozzi, A. Pantosti, and M. R. Oggioni. 2006. Zinc metalloproteinase genes in clinical isolates of Streptococcus pneumoniae: association of the full array with a clonal cluster comprising serotypes 8 and 11A. Microbiology 152:313-321. [DOI] [PubMed] [Google Scholar]
- 7.Campbell, A. M. 2003. Prophage insertion sites. Res. Microbiol. 154:277-282. [DOI] [PubMed] [Google Scholar]
- 8.Canchaya, C., F. Desiere, M. McShan, J. J. Ferretti, J. Parkhill, and H. Brussow. 2002. Genome analysis of an inducible prophage and prophage remnants integrated in the Streptococcus pyogenes strain SF370. Virology 302:245-258. [DOI] [PubMed] [Google Scholar]
- 9.Davies, T. A., K. Bush, D. Sahm, and A. Evangelista. 2005. Predominance of 23S rRNA mutants among non-Erm, non-Mef macrolide resistant clinical isolates of Streptococcus pneumoniae collected in the United States in 1999-2000. Antimicrob. Agents Chemother. 49:3031-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Del Grosso, M., F. Iannelli, C. Messina, M. Santagati, N. Petrosillo, S. Stefani, G. Pozzi, and A. Pantosti. 2002. Macrolide efflux genes mef(A) and mef(E) are carried by different genetic elements in Streptococcus pneumoniae. J. Clin. Microbiol. 40:774-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Grosso, M., A. Scotto d'Abusco, F. Iannelli, G. Pozzi, and A. Pantosti. 2004. Tn2009, a Tn916-like element containing mef(E) in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 48:2037-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dobay, O., F. Rozgonyi, and G. B. Amyes. 2005. Molecular characterization of Hungarian macrolide-resistant Streptococcus pneumoniae isolates, including three highly resistant strains with mef gene. Int. J. Antimicrob. Agents 25:488-495. [DOI] [PubMed] [Google Scholar]
- 13.Doktor, S. Z., and V. Shortridge. 2005. Differences in the DNA sequences in the upstream attenuator region of erm(A) in clinical isolates of Streptococcus pyogenes and their correlation with macrolide/lincosamide resistance. Antimicrob. Agents Chemother. 49:3070-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farrell, D. J., I. Morrissey, S. Bakker, and D. Felmingham. 2002. Molecular characterization of macrolide resistance mechanisms among Streptococcus pneumoniae and Streptococcus pyogenes isolated from the PROTEKT 1999-2000 study. J. Antimicrob. Chemother. 50(Suppl. S1):39-47. [DOI] [PubMed] [Google Scholar]
- 15.Farrell, D. J., I. Morrissey, S. Bakker, L. Morris, S. Buckridge, and D. Felmingham. 2004. Molecular epidemiology of multiresistant Streptococcus pneumoniae with both erm(B)-and mef(A)-mediated macrolide resistance. J. Clin. Microbiol. 42:764-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fines, M., M. Gueudin, A. Ramon, and R. Leclercq. 2001. In vitro selection of resistance to clindamycin related to alterations in the attenuator of the erm(TR) gene of Streptococcus pyogenes UCN1 inducibly resistant to erythromycin. J. Antimicrob. Chemother. 48:411-416. [DOI] [PubMed] [Google Scholar]
- 17.Giovanetti, E., A. Brenciani, R. Burioni, and P. E. Varaldo. 2002. A novel efflux system in inducibly erythromycin-resistant strains of Streptococcus pyogenes. Antimicrob Agents Chemother. 46:3750-3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giovanetti, E., G. Magi, A. Brenciani, C. Spinaci, R. Lupidi, B. Facinelli, and P. E. Varaldo. 2002. Conjugative transfer of the erm(A) gene from erythromycin-resistant Streptococcus pyogenes to macrolide-susceptible S. pyogenes, Enterococcus faecalis and Listeria innocua. J. Antimicrob. Chemother. 50:249-252. [DOI] [PubMed] [Google Scholar]
- 19.Horton, R. M. 1995. PCR-mediated recombination and mutagenesis. SOEing together tailor-made genes. Mol. Biotechnol. 3:93-99. [DOI] [PubMed] [Google Scholar]
- 20.Iannelli, F., D. Chiavolini, S. Ricci, M. R. Oggioni, and G. Pozzi. 2004. Pneumococcal surface protein C contributes to sepsis caused by Streptococcus pneumoniae in mice. Infect. Immun. 72:3077-3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iannelli, F., and G. Pozzi. 2004. Method for introducing specific and unmarked mutations into the chromosome of Streptococcus pneumoniae. Mol. Biotechnol. 26:81-86. [DOI] [PubMed] [Google Scholar]
- 22.Iannelli, F., and G. Pozzi. 2007. Protocol for conjugal transfer of genetic elements in Streptococcus pneumoniae, p. 525-529. In R. Hakenbeck and S. Chhatwal (ed.), Molecular biology of streptococci, vol. 21. Horizon Bioscience, Norwich, United Kingdom. [Google Scholar]
- 23.Kataja, J., P. Huovinen, M. Skurnik, the Finnish Study Group for Antimicrobial Resistance, and H. Seppala. 1999. Erythromycin resistance genes in group A streptococci in Finland. Antimicrob. Agents Chemother. 43:48-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linton, K. J., H. N. Cooper, I. S. Hunter, and P. F. Leadlay. 1994. An ABC-transporter from Streptomyces longisporoflavus confers resistance to the polyether-ionophore antibiotic tetronasin. Mol. Microbiol. 11:777-785. [DOI] [PubMed] [Google Scholar]
- 25.Marimon, J. M., A. Valiente, M. Ercibengoa, J. M. Garcia-Arenzana, and E. Perez-Trallero. 2005. Erythromycin resistance and genetic elements carrying macrolide efflux genes in Streptococcus agalactiae. Antimicrob. Agents Chemother. 49:5069-5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molhoi, M., and F. Dal Degan. 2004. Leader sequences are not signal peptides. Nat. Biotechnol. 22:1502. [DOI] [PubMed] [Google Scholar]
- 27.Monaco, M., R. Camilli, F. D'Ambrosio, M. Del Grosso, and A. Pantosti. 2005. Evolution of erythromycin resistance in Streptococcus pneumoniae in Italy. J. Antimicrob. Chemother. 55:256-259. [DOI] [PubMed] [Google Scholar]
- 28.Murphy, E., L. Huwyler, and M. do Carmo de Freire Bastos. 1985. Transposon Tn554: complete nucleotide sequence and isolation of transposition-defective and antibiotic-sensitive mutants. EMBO J. 4:3357-3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ojo, K. K., N. L. Ruehlen, N. S. Close, H. Luis, M. Bernardo, J. Leitao, and M. C. Roberts. 2006. The presence of a conjugative gram-positive Tn2009 in gram-negative commensal bacteria. J. Antimicrob. Chemother. 57:1065-1069. [DOI] [PubMed] [Google Scholar]
- 30.Orth, P., D. Schnappinger, W. Hillen, W. Saenger, and W. Hinrichs. 2000. Structural basis of gene regulation by the tetracycline-inducible Tet repressor-operator system. Nat. Struct. Biol. 7:215-219. [DOI] [PubMed] [Google Scholar]
- 31.Reig, M., J. Galan, F. Baquero, and J. C. Perez-Diaz. 2001. Macrolide resistance in Peptostreptococcus spp. mediated by erm(TR): possible source of macrolide-lincosamide-streptogramin B resistance in Streptococcus pyogenes. Antimicrob. Agents Chemother. 45:630-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts, M. C., J. Sutcliffe, P. Courvalin, L. B. Jensen, J. Rood, and H. Seppala. 1999. Nomenclature for macrolide and macrolide-lincosamide-streptogramin B resistance determinants. Antimicrob. Agents Chemother. 43:2823-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson, D. A., J. A. Sutcliffe, W. Tewodros, A. Manoharan, and D. E. Bessen. 2006. Evolution and global dissemination of macrolide-resistant group A streptococci. Antimicrob. Agents Chemother. 50:2903-2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santagati, M., F. Iannelli, C. Cascone, F. Campanile, M. R. Oggioni, S. Stefani, and G. Pozzi. 2003. The novel conjugative transposon Tn1207.3 carries the macrolide efflux gene mef(A) in Streptococcus pyogenes. Microb. Drug Resist. 9:243-247. [DOI] [PubMed] [Google Scholar]
- 35.Seppala, H., M. Skurnik, H. Soini, M. C. Roberts, and P. Houovinen. 1998. A novel erythromycin resistance methylase gene (ermTR) in Streptococcus pyogenes. Antimicrob. Agents Chemother. 42:257-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sung, C. K., H. Li, J. P. Claverys, and D. A. Morrison. 2001. An rpsL cassette, Janus, for gene replacement through negative selection in Streptococcus pneumoniae. Appl. Environ. Microbiol. 67:5190-5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Syrogiannopoulos, G. A., I. N. Grivea, L. M. Ednie, B. Bozdogan, G. D. Katopodis, N. G. Beratis, T. A. Davies, and P. C. Appelbaum. 2003. Antimicrobial susceptibility and macrolide resistance inducibility of Streptococcus pneumoniae carrying erm(A), erm(B), or mef(A). Antimicrob. Agents Chemother. 47:2699-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Syrogiannopoulos, G. A., I. N. Grivea, A. Tait-Kamradt, G. D. Katopodis, N. G. Beratis, J. Sutcliffe, P. C. Appelbaum, and T. A. Davies. 2001. Identification of an erm(A) erythromycin resistance methylase gene in Streptococcus pneumoniae isolated in Greece. Antimicrob. Agents Chemother. 45:342-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tait-Kamradt, A., T. Davies, M. Cronan, M. R. Jacobs, P. C. Appelbaum, and J. Sutcliffe. 2000. Mutations in 23S rRNA and ribosomal protein L4 account for resistance in pneumococcal strains selected in vitro by macrolide passage. Antimicrob. Agents Chemother. 44:2118-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Eldere, J., E. Meekers, K. Lagrou, C. Massonet, A. Canu, I. Devenyns, J. Verhaegen, G. Syrogiannopoulos, and R. Leclercq. 2005. Macrolide-resistance mechanisms in Streptococcus pneumoniae isolates from Belgium. Clin. Microbiol. Infect. 11:332-334. [DOI] [PubMed] [Google Scholar]
- 41.Weisblum, B. 1995. Erythromycin resistance by ribosome modification. Antimicrob. Agents Chemother. 39:577-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woo, P. C., A. P. To, H. Tse, S. K. Lau, and K. Y. Yuen. 2003. Clinical and molecular epidemiology of erythromycin-resistant beta-hemolytic Lancefield group G streptococci causing bacteremia. J. Clin. Microbiol. 41:5188-5191. [DOI] [PMC free article] [PubMed] [Google Scholar]