Abstract
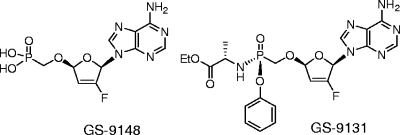
GS-9148 [(5-(6-amino-purin-9-yl)-4-fluoro-2,5-dihydro-furan-2-yloxymethyl)phosphonic acid] is a novel ribose-modified human immunodeficiency virus type 1 (HIV-1) nucleotide reverse transcriptase (RT) inhibitor (NRTI) selected from a series of nucleoside phosphonate analogs for its favorable in vitro biological properties including (i) a low potential for mitochondrial toxicity, (ii) a minimal cytotoxicity in renal proximal tubule cells and other cell types, (iii) synergy in combination with other antiretrovirals, and (iv) a unique resistance profile against multiple NRTI-resistant HIV-1 strains. Notably, antiviral resistance analysis indicated that neither the K65R, L74V, or M184V RT mutation nor their combinations had any effect on the antiretroviral activity of GS-9148. Viruses carrying four or more thymidine analog mutations showed a substantially smaller change in GS-9148 activity relative to that observed with most marketed NRTIs. GS-9131, an ethylalaninyl phosphonoamidate prodrug designed to maximize the intracellular delivery of GS-9148, is a potent inhibitor of multiple subtypes of HIV-1 clinical isolates, with a mean 50% effective concentration of 37 nM. Inside cells, GS-9131 is readily hydrolyzed to GS-9148, which is further phosphorylated to its active diphosphate metabolite (A. S. Ray, J. E. Vela, C. G. Boojamra, L. Zhang, H. Hui, C. Callebaut, K. Stray, K.-Y. Lin, Y. Gao, R. L. Mackman, and T. Cihlar, Antimicrob. Agents Chemother. 52:648-654, 2008). GS-9148 diphosphate acts as a competitive inhibitor of RT with respect to dATP (Ki = 0.8 μM) and exhibits low inhibitory potency against host polymerases including DNA polymerase γ. Oral administration of GS-9131 to beagle dogs at a dose of 3 mg/kg of body weight resulted in high and persistent levels of GS-9148 diphosphate in peripheral blood mononuclear cells (with a maximum intracellular concentration of >9 μM and a half-life of >24 h). This favorable preclinical profile makes GS-9131 an attractive clinical development candidate for the treatment of patients infected with NRTI-resistant HIV.
Therapeutic regimens containing nucleoside and nucleotide reverse transcriptase (RT) inhibitors (NRTIs) in combination with other antiretrovirals represent the current standard of care for the treatment of the majority of both therapy-naïve and therapy-experienced human immunodeficiency virus (HIV)-infected patients (10). However, the extremely effective evolution of HIV may frequently lead to the development of resistance to antiretrovirals, including NRTIs, thereby diminishing their long-term clinical benefit. In addition, cross-resistance among multiple NRTIs due to specific mutations in RT limits the treatment options available for individuals for whom current therapies have failed (11, 46). The prevalence of NRTI resistance mutations among HIV-infected individuals with persistent viremia is estimated to be as high as 70% (35, 40, 43), and an increasing rate of the transmission of drug-resistant HIV variants has been observed among newly infected patients (13). The success of chronic therapy with some of the current NRTIs may also be affected by various adverse symptoms including hepatotoxicity, pancreatitis, neuropathy, peripheral lipoatrophy, and lactic acidosis (4, 5, 31, 32). Some of these toxicities are likely to be related to the effects that NRTIs have on mitochondria due to the ability of the active metabolites to interfere with the replication of mitochondrial DNA (mtDNA) (18, 47). Therefore, a significant need remains for novel NRTIs with favorable resistance profiles, improved safety, long-term tolerability, and the potential for once-daily dosing to facilitate patient compliance.
Multiple novel antiretroviral nucleoside analogs have recently been evaluated, both preclinically and clinically, for the treatment of patients with HIV NRTI resistance (1, 12, 21, 22, 33, 34, 42), but only a few have progressed toward late-stage clinical development. We elected to explore nucleoside phosphonates (nucleotide analogs) as they offer a unique pharmacological properties including long intracellular half-lives (15), efficient activation in both dividing and nondividing lymphoid cells (41), and the opportunity to apply prodrug strategies to optimize in vivo pharmacokinetics and tissue distribution (26). Previously, the design of a wide range of acyclic nucleoside phosphonates yielded a number of promising antivirals (9, 16), culminating in the clinical approval of adefovir dipivoxil and tenofovir disoproxil for the treatment of hepatitis B and HIV infections, respectively. In contrast, comparatively fewer cyclic ribose-modified nucleoside phosphonates with antiviral activities have been identified. Among these, recent examples are nucleotides containing a 2′-deoxythreose sugar moiety (50). Independently, 2′,3′-didehydro-2′,3′-dideoxyribose phosphonates have been explored in the past, with the prototype adenine derivative 9-[(2R,5R-2,5-dihydro-5-phosphonomethoxy)-2-furanyl]adenine (d4AP) identified as a potent antiretroviral inhibitor (20). We subsequently observed that d4AP maintains its potent activity against viruses with major NRTI resistance mutations. To minimize the undesirable mitochondrial toxicity associated with d4AP, its 2′-fluorine analog GS-9148 {[5-(6-amino-purin-9-yl)-4-fluoro-2,5-dihydro-furan-2-yloxymethyl] phosphonic acid} (Fig. 1) was prepared. Here we report on the favorable biological profile of GS-9148 and its orally bioavailable phosphonoamidate prodrug GS-9131 {9-(R)-4′-(R)-[[[(S)-1-[(ethoxycarbonyl)ethyl]amino]phenoxyphosphonyl]methoxy]-2′-fluoro-1′-furanylad-enine} (Fig. 1).
FIG. 1.
Structures of GS-9148 and GS-9131.
MATERIALS AND METHODS
Compounds and reagents.
GS-9148 and GS-9131 and all other tested nucleoside phosphonates and phosphonoamidate prodrugs were synthesized by Gilead Sciences (3a, 19, 28, 29). GS-7340 was prepared as described previously (26). Tenofovir and cidofovir were provided by Process Chemistry (Gilead Sciences). Zidovudine (AZT), stavudine (d4T), didanosine (ddI), and a set of deoxynucleoside triphosphates (dNTPs) were purchased from Sigma (St. Louis, MO). Lamivudine (3TC) was purchased from Moravek Biochemicals (Brea, CA), and abacavir (ABC) was provided by GlaxoSmithKline (Research Triangle Park, NC). Lopinavir was isolated from its therapeutic formulation (Kaletra) by reverse-phase high-performance liquid chromatography. GS-9148-diphosphate (DP) was prepared from GS-9148 by Trilink BioTechnologies (San Diego, CA). Human DNA polymerases α and β were purchased from Replizyme, Inc. (Heslington, United Kingdom). Human DNA polymerase γ expressed in baculovirus was kindly provided by William Copeland (NIEHS, Research Triangle Park, NC) (27). A wild-type HIV-1 RT p66/p51 heterodimer with N-terminal six-His tags was cloned from the HIV-1 strain HXB2, expressed, and isolated as described previously (49). [3H]dATP and activated calf thymus DNA were purchased from GE Healthcare (Piscataway, NJ).
Cells and viruses.
MT-2 cells were provided by Stanford University (Palo Alto, CA) and maintained in RPMI 1640 medium supplemented with antibiotics and 10% fetal bovine serum (FBS). Human peripheral blood mononuclear cells (PBMCs) were isolated from donor buffy coat samples (Stanford Blood Bank, Palo Alto, CA), using centrifugation in Ficoll Paque Plus (Amersham Biosciences, Piscataway, NJ) and activated for 4 to 5 days in RPMI 1640 medium with 20% FBS, antibiotics, interleukin-2 (20 units/ml), and phytohemagglutinin (1 μg/ml). Human CD4+ T lymphocytes were purified from isolated and activated PBMCs, by negative magnetic bead sorting with an AutoMACS using a CD4+ T-cell isolation kit II (Miltenyi Biotec, Auburn, CA). For infection with HIV-1, PBMCs or CD4+ cells from three to four separate donors were pooled. The HepG2 human hepatoma cell line (ATCC, Manassas, VA) was maintained in minimal essential medium supplemented with 10% FBS, 1 mM pyruvate, and antibiotics. Human primary renal proximal tubule epithelial cells (RPTECs) from two independent donors were purchased from Cambrex Bio Science (Walkersville, MD) and cultured according to the vendor's protocol. Chinese hamster ovary (CHO) cells stably transfected with human renal organic anion transporter type 1 (hOAT1) were generated and maintained as described previously (7).
The HIV-1 strain IIIB (Advanced Biotechnologies, Columbia, MD) and strain BaL (Advanced Biotechnologies) were used for the infection of MT-2 cells and primary cells, respectively. HIV-1 and HIV-2 clinical isolates were obtained from the AIDS Research and Reference Reagent Program (NIAID, NIH, Bethesda, MD). HIV-1 recombinant strains carrying RT mutations were prepared by transfecting infectious proviral HXB2-based cDNA clones into MT-2 cells and harvesting the cell supernatants. Clones with the K65R or M184V mutation were prepared by site-directed mutagenesis, and the clone containing the six thymidine analog mutations (6TAMs) (consisting of M41L, D67N, K70R, L210W, T215F, and K219Q) was constructed by cloning a PCR-amplified RT fragment from patient plasma into the HXB2-based proviral clone.
Antiviral activity assays. (i) MT-2 cells.
MT-2 cells were infected with HIV-1 strain IIIB at a multiplicity of infection (MOI) of 0.01 and added to 96-well plates with serial dilutions of the tested compounds at a density of 20,000 cells/well. After a 5-day incubation, the virus-induced cytopathic effect was determined, using a CellTiter-Glo cell viability assay (Promega, Madison, WI), and expressed as a percentage of the signal from samples with fully suppressed virus replication after subtracting the signal from the untreated control. The concentration of each drug that inhibited the virus-induced cytopathic effect by 50% (EC50) was determined by nonlinear regression, using Prizm software (GraphPad Software, San Diego, CA). Activity against NRTI-resistant mutants was determined in parallel with that of the wild-type control virus, and the change in EC50 was calculated. Activity against HIV-2 isolates was determined with MT-2 cells under identical conditions, except that an XTT formazan-based cell viability assay was used.
(ii) PBMCs and CD4+ T lymphocytes.
Activated PBMCs were infected with the HIV-1 strain BaL for 3 h, washed, seeded into 96-well plates (250,000 cells/well), and incubated with serial dilutions of tested compounds for 5 days, at which point cell supernatants were collected, and virus production was determined by using a commercial HIV-1 p24 antigen enzyme-linked immunosorbent assay (ELISA; Beckman Coulter, Miami, FL). The EC50 of each drug that inhibited the p24 antigen production was determined by regression analysis. Conditions for the antiviral assay with PBMCs infected with clinical isolates were similar, with the exception of a shorter incubation (4-day) period and a 3-fold higher MOI. These conditions are the likely reasons for some differences observed for the activity of GS-9131 against the HIV-1 BaL strain and the clinical isolates. For the single-cycle infection assay, CD4+ lymphocytes were infected with HIV-1 BaL at an MOI of 0.5 to 1.0, washed with cell culture medium containing 50% FBS, and seeded into 96-well plates. Serial dilutions of tested compounds were added, and 36 h later, cell supernatants were collected, and the virus production in each sample was determined by p24 antigen ELISA. Results were processed by regression analysis to determine the EC50 values for the tested compounds.
(iii) Macrophages.
Isolated human PBMCs were resuspended in Dulbecco's modified Eagle's medium supplemented with 10% human pooled serum and antibiotics and seeded in 96-well plates. Monocytes/macrophages were allowed to adhere for 18 h. Cultures were washed to remove nonadherent cells, supplemented with RPMI 1640 medium with 15% FBS and antibiotics, and incubated for an additional 6 to 10 days, at which point the cells were infected with HIV-1 BaL in the presence of serial dilutions of tested compounds for 24 h. After virus was removed, cells were treated with freshly added compounds for an additional 6 days. Virus production was quantified by p24 antigen ELISA, and EC50 values were calculated.
Antiviral drug combination studies.
Two-drug combinations were tested in 96-well plates, in which 1.7-fold serial dilutions of drug 1 (horizontal direction) were combined with 2-fold serial dilutions of drug 2 (vertical direction) and added to MT-2 cells infected with HIV-1 (IIIB). Each combination was tested in three identical plates set up in parallel. The virus-induced cytopathic effect was determined by using a CellTiter-Glo assay as described above. Combination effects were determined by using MacSynergy II software (University of Michigan, Ann Arbor, MI) based on an algorithm described previously (36, 37) and defined according to a specific value of combination volume (concentration2 × percentage of inhibition), as follows: >100, highly synergistic; >50 to 100, moderately synergistic; >25 to 50, slightly synergistic; > −25 to 25, additive; > −50 to −25, slightly antagonistic; > −100 to −50, moderately antagonistic; ≤ −100, highly antagonistic. Combination volume values were calculated at their 95% confidence level. Tenofovir combined with efavirenz and d4T combined with ribavirin (30) served as the controls for synergy and antagonism, respectively.
Determination of mtDNA levels.
The mtDNA assay was based on a sequential hybridization with a mtDNA probe (the cytochrome b gene) and a chromosomal DNA probe (the 18S rRNA gene). The probes were PCR amplified from human DNA, purified by agarose gel electrophoresis, verified by sequencing, and labeled with [α-32P]dATP, using a MegaPrime random priming kit (GE Healthcare, Piscataway, NJ). HepG2 cells were incubated with serial dilutions of test compounds in 96-well plates for 14 days (with medium contents replaced after 3 to 4 days), washed with phosphate-buffered saline, and lysed with 0.5 M NaOH/12.5 mM EDTA for 15 min at room temperature. Lysates were heated for 10 min at 100°C, cooled on ice, and vacuum blotted onto a ZetaProbe membrane (Bio-Rad, Hercules, CA). Each dot blot was rinsed with 0.5 M NaOH, and subsequently, the membrane was washed with an excess of 2× SSC buffer (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). After the membrane was exposed to UV for cross-linking, it was prehybridized in 0.5 M Na2HPO4, (pH 7.2) and 7% sodium dodecyl sulfate (SDS) at 65°C, hybridized with 25 ng of labeled mtDNA probe in the same solution, and washed twice with 40 mM Na2HPO4 (pH 7.2) and 5% SDS and twice more with 40 mM Na2HPO4 (pH 7.2) and 1% SDS. All washing steps were performed at 65°C. The probe signal from each sample was quantified using a PhosphorImager (Molecular Devices/GE Healthcare, Piscataway, NJ). Subsequently, the membrane was stripped in 0.1× SSC solution with 0.5% SDS at 95°C, rehybridized with an rRNA gene fragment, washed, and analyzed as described above for the mtDNA hybridization. The ratio of the mtDNA signal to the chromosomal DNA signal was calculated for each treated sample and compared to the signal from the untreated control.
Cytotoxicity assays.
HepG2 cells and RPTECs were plated in 96-well plates at densities of 10,000 and 4,000 cells/well, respectively. The next day, fresh medium containing serial dilutions of tested compounds was added. The viability of HepG2 cells and RPTECs was determined 4 and 5 days later, respectively, using a CellTiter-Glo cell viability kit. Luminescence signal was quantified using a Victor V3 reader (Perkin-Elmer, Wellesley, MA) and expressed as a percentage of the signal from untreated samples (0% cytotoxicity) after the subtraction of the signal from samples treated with 500 μM arabinosylcytosine (100% cytotoxicity). The concentration of each drug that reduced the cell viability by 50% (CC50) was determined by nonlinear regression analysis using Prizm software. Cytotoxicity assays in CHO-hOAT1 cells were performed as described previously (7).
Enzyme inhibition assays.
The steady-state inhibition of RT by active DP/triphosphate (TP) metabolites was determined as described in prior studies (49). Inhibition assays with DNA polymerase α were conducted in a reaction mixture containing 1 μM [3H]dATP, 10 μM amounts of other dNTPs, 40 mM HEPES (pH 7.5), 200 μg/ml bovine serum albumin, 7.5 mM MgCl2, 10 mM dithiothreitol, 10% glycerol, 400 nM heteropolymeric template (78-mer)/primer (18-mer), 3 units/ml enzyme, and serial dilutions of tested inhibitors. Reaction mixture conditions for polymerase β were identical, except the pH value was 8.6. Reaction mixtures for DNA polymerase γ contained 0.3 μM [3H]dATP, 50 μM amounts of other dNTPs, 30 mM Tris (pH 8.0), 100 mM KCl, 5 mM MgCl2, 3 mM dithiothreitol, 5% glycerol, 100 μg/ml activated calf thymus DNA, 1.0 mg/ml bovine serum albumin, 250 ng/ml human recombinant DNA polymerase γ, and inhibitor. All host polymerase reactions were carried out at 37°C in 96-well reaction plates. Aliquots of reaction mixtures were removed, mixed in 1:10 dilutions with 60 mM EDTA, and loaded onto Unifilter 96-well CE81 DEAE plates (VWR, Brisbane, CA). Plates were washed three times with 125 mM Na2HPO4, once with water, and once with ethanol. After plates were air dried, Microscint 20 (Perkin-Elmer, Wellesley, MA) was added and plates were analyzed in a TopCount counter (Perkin-Elmer, Wellesley, MA). All reactions were carried out in triplicate, and 50% inhibitory concentrations (IC50) were calculated from plotting the inhibitor concentration versus the percentage of inhibition, using nonlinear regression.
Resistance profiling.
A PhenoSense assay (Monogram Biosciences, South San Francisco, CA) was used to compare the in vitro resistance profile of GS-9148 with that of clinically approved NRTIs (AZT, ABC, ddI, emtricitabine [FTC], d4T, and tenofovir; 3TC was not included because its resistance profile closely resembles that of FTC). The susceptibility of 18 recombinant HIV-1 constructs containing protease and RT sequences from NRTI-experienced patients were tested, and susceptibility data were expressed as the change in the EC50 of each patient-derived virus isolate relative to the EC50 of wild-type reference strain NL4-3.
Pharmacokinetic studies in dogs.
The accumulation of GS-9148 and its metabolites in PBMCs in vivo was assessed in male beagle dogs. Prodrugs formulated in citric acid solutions (containing propylene glycol or polyethylene glycol when necessary to enhance solubility) were administered either by a 30-min intravenous (i.v.) infusion (0.5 mg/kg of body weight) or by oral gavage (3 to 5 mg/kg). PBMCs were isolated from blood samples using Vacutainer cell preparation tubes containing sodium citrate anticoagulant (BD Biosciences, San Jose, CA). Isolated PBMCs were resuspended in phosphate-buffered saline, counted, pelleted, and extracted with 70% methanol. Following methanol evaporation, GS-9148 metabolites were converted to GS-9148 by calf intestinal phosphatase (Sigma-Aldrich). Samples were precipitated in 60% acetonitrile and analyzed by liquid chromatography-tandem mass spectrometry (LC-MS-MS). Samples from dogs dosed orally with GS-9131 were processed and analyzed identically except that the levels of GS-9148 and GS-9148 DP in PBMCs were determined individually. All LC-MS-MS analyses used an HTS PAL autosampler (Leap Technologies, Carrboro, NC) with cooled stacks, a Shimadzu LC-20AD ternary pump system (Shimadzu Scientific Instruments, Columbia, MD), and a Sciex API-4000 mass spectrometer (Applied Biosystems, Foster City, CA) operating in a multiple-reaction-monitoring mode. LC separation for the analysis of plasma levels of GS-9131 and GS-9148 was performed by using a Fusion RP 80, 4-μm 50- by 4.6-mm column (Phenomenex, Torrance, CA) and a linear gradient of 0 to 95% acetonitrile in 0.2% formic acid at a flow rate of 1 ml/min. Samples were quantified based on a standard curve for each metabolite analyzed. The parent/daughter mass transition of 507.1/220.3 and 332.0/220.3 were used for GS-9131 and GS-9148, respectively. Intracellular levels of GS-9148 and its phosphorylated metabolites were analyzed by using capillary ion pairing LC-MS-MS essentially as described previously (45). The parent/daughter mass transition of 492.2/219.9 was used for GS-9148-DP. Noncompartmental pharmacokinetic analysis was performed to obtain parameters for plasma and PBMC metabolite levels, using WinNonLin version 5.0.1. software (Pharsight Corporation, Mountain View, CA).
RESULTS
Rational design of GS-9148.
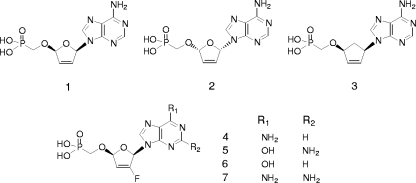
As a part of our search for novel NRTIs with favorable biological profiles, a structurally diverse set of both acyclic and ribose-modified nucleoside phosphonate analogs were evaluated with various in vitro assays that characterize anti-HIV-1 activity, cytotoxicity, and resistance profiles against viruses carrying major NRTI resistance mutations. The previously described nucleoside phosphonate analog d4AP (Fig. 2, compound 1) (20) was identified as a potent inhibitor of both wild-type and NRTI-resistant HIV-1 strains (Table 1). However, d4AP interfered strongly with the replication of mtDNA (Table 1), and so it was precluded from further consideration for clinical development. Related 2′,3′-dideoxyribose and 2′3′-didehydro-2′,3′-dideoxyribose analogs with different nucleobases were evaluated in parallel, but despite somewhat reduced effects on mtDNA replication, these analogs had inferior resistance profiles (29). Since isomeric l-nucleosides (e.g., 3TC) and carbocyclic nucleosides (e.g., ABC) are known to exhibit minimal effects on mtDNA replication (2), we sought to utilize similar approaches to reduce the toxicity of d4AP. Although the l-isomer of d4AP (l-d4AP; Fig. 2, compound 2) had good potency and abrogated the mitochondrial toxicity, its resistance profile was inferior to those of both d4AP and several approved NRTIs. The previously reported carbocyclic analog of d4AP (Cd4AP; Fig. 2, compound 3) (19) showed only marginal antiretroviral activity in comparison with that of d4AP. Finally, a third approach to reduce mitochondrial toxicity of d4AP, based on structural differences in the active site domain of RT (17) and mtDNA polymerase γ (14) interacting with the substrate 2′-deoxyribose region, was initiated. In this context, prior studies have demonstrated that 2′-fluoro-2′,3′-dideoxynucleosides exhibit reduced mitochondrial toxicity compared to that of corresponding 2′-unsubstituted compounds (44). The analogous modification of d4AP resulted in 2′-fluoro-d4AP (Fd4AP, GS-9148; Fig. 2, compound 4), which exhibited both sufficient antiretroviral activity and a favorable resistance profile against viral strains containing major NRTI mutations (Table 1). Importantly, the introduction of 2′-fluorine into the ribose ring of d4AP eliminated its effect on mtDNA (Table 1). As demonstrated by further analyses using both DNA hybridization and real-time PCR, the treatment of HepG2 cells for up to 21 days with 50 to 300 μM GS-9148 did not produce any measurable reduction of mtDNA, which contrasted with some of the approved NRTIs such as ddI, d4T, and ddC that reduced the content of mtDNA by 70 to 90% under the same conditions (data not shown). Based on these results, additional 2′-fluoro-2′,3′-didehydro-2′,3′-dideoxy nucleoside phosphonates with guanine (Fd4GP), hypoxanthine (Fd4IP), and 2,6-diaminopurine (Fd4DAPP) bases (Fig. 2, compounds 5 to 7) were synthesized and characterized. Among these analogs, only Fd4GP was active against HIV-1 but was overall less attractive because of its reduced selectivity (i.e., the ratio of CC50 to EC50) in MT-2 cells compared to GS-9148.
FIG. 2.
Structures of the nucleotide analogs tested. Compounds: 1, nucleoside phosphonate analog d4AP; 2, l-d4AP; 3, Cd4AP; 4, Fd4AP; 5, Fd4GP; 6, Fd4IP; and 7, Fd4DAPP.
TABLE 1.
In vitro anti-HIV-1 activity, toxicity, and resistance profile of ribose-modified nucleoside phosphonates and approved NRTIsa
| Compound no. | Name | EC50 ± SD (μM)b | CC50 ± SD (μM)b | Fold change ± SD in HIV resistance mutations relative to wild typec
|
MTC50 ± SD required to induce mtDNA reduction (μM)e | ||
|---|---|---|---|---|---|---|---|
| 6TAMsd | K65R | M184V | |||||
| 1 | d4AP | 2.1 ± 1.0d | >1,000 | 2.9 ± 1.0 | 2.9 ± 1.1 | 0.9 ± 0.6 | 3.6 ± 1.5 |
| 2 | l-d4AP | 5.9 ± 0.3 | >1,000 | 13.7 ± 2.3 | 3.6 ± 2.0 | 10.6 ± 4.8 | >300 |
| 3 | Cd4AP | 52 ± 13.5 | |||||
| 4 | Fd4AP (GS-9148) | 10.6 ± 2.4 | >1,000 | 4.3 ± 0.4 | 1.1 ± 0.2 | 0.8 ± 0.1 | >300 |
| 5 | Fd4GP | 17.4 ± 5.3 | 700 | ||||
| 6 | Fd4IP | >300 | |||||
| 7 | Fd4DAPP | >300 | |||||
| Tenofovir | 3.6 ± 1.5 | >1,000 | 8.8 ± 3.7 | 4.3 ± 1.5 | 0.7 ± 0.2 | >300 | |
| AZT | 0.14 ± 0.1 | >70 | 1.0 ± 0.5 | 0.7 ± 0.2 | |||
| d4T | 4.8 ± 2.3 | 286 ± 122 | 5.5 ± 2.5 | 2.3 ± 1.1 | 1.1 ± 0.2 | 125 ± 53 | |
| ddI | 3.1 ± 1.7 | 2.5 ± 0.9 | 3.4 ± 1.5 | 2.5 ± 1.7 | 28 ± 11 | ||
| FTC | 0.39 ± 0.17 | >1,000 | 29.6 | 14.6 ± 5.1 | >500 | >300 | |
| Abacavir | 0.32 ± 0.15 | 55 ± 7.1 | 6.7 ± 2.9 | 3.7 ± 1.9 | 7.1 ± 4.4 | >300 | |
All assay results are presented as means ± standard deviations (SD) from at least two independent experiments performed in triplicate.
EC50 and CC50 were determined with uninfected and HIV-1-infected MT-2 cells following a 5-day incubation.
Recombinant site-directed mutant viruses were used for side-by-side comparison with wild-type HIV-1.
6TAMs, the M41L, D67N, K70R, L210W, T215F, and K219Q mutations.
MTC50, concentration of the tested compound inducing a 50% reduction in mtDNA content relative to that of chromosomal DNA in HepG2 cells following a 14-day treatment.
Selection of GS-9131 as an optimal prodrug of GS-9148.
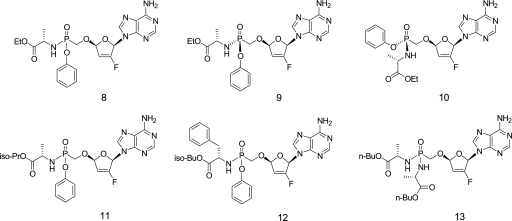
The cellular permeation of nucleotides is limited by their negative charge at a physiological pH level, which requires the use of lipophilic prodrugs for their in vivo oral administration. Recently described phosphonoamidates of tenofovir improve the oral bioavailability of the parent nucleotide and allow for its effective in vivo delivery into target lymphoid cells and tissues (26). Hence, a series of phosphonoamidate prodrugs of GS-9148 with various amino acid residues and ester moieties were prepared and characterized for their antiviral activity, cytotoxicity, and in vivo delivery of GS-9148 metabolites into PBMCs following administration to dogs. Both mono-phenol mono-phosphonoamidates and bis-phosphonoamidates substantially enhanced the antiretroviral potency of GS-9148 while they maintained favorable selectivity in MT-2 cells. Consistent with the increase in the cellular permeation of GS-9148, prodrugs with the highest lipophilicity such as mono(isobutyl-phenylalanine) amidate (Fig. 3, compound 12) and bis(n-butyl-alanine) amidate (Fig. 3, compound 13) were more than 1,000- and 250-fold more active, respectively, than GS-9148 by itself (Table 2). In comparison, mono(ethyl-alanine) amidate (Fig. 3, compound 8) improved the activity of GS-9148 by approximately 50-fold, whereas the mono(isopropyl-alanine) prodrug (Fig. 3, compound 11) was only poorly active relative to that of other prodrugs of GS-9148. Despite its potent in vitro activity, bis-amidate (Fig. 3, compound 13) was quite ineffective in delivering GS-9148 metabolites into PBMCs following i.v. administration to dogs, producing only approximately threefold higher intracellular levels of GS-9148 metabolites compared to that from the same dose of GS-9148 by itself (Table 2). This is likely a consequence of the extensive metabolic clearance of this compound. In contrast, monoamidate prodrugs (Fig. 3, compounds 8 and 12) administered i.v. enhanced the in vivo intracellular delivery of GS-9148 metabolites by 57- and 106-fold, respectively, relative to that of GS-9148 by itself. However, in comparison with what was seen with compound 12, the oral administration of compound 8 to dogs resulted in fourfold higher levels of GS-9148 metabolites in PBMCs. Therefore, the two diastereomers of mono(ethyl-alanine) prodrug were separated and further characterized. Diastereomer A, designated GS-9131 (Fig. 3, compound 9), showed twofold more potent antiretroviral activity in MT-2 cells and produced threefold higher levels of GS-9148 metabolites in PBMCs from orally dosed dogs than the diastereomer B (Fig. 3, compound 10). Based on this profile, GS-9131 was selected for further evaluation.
FIG. 3.
Structures of GS-9148 amidate prodrugs. Compounds: 8, mono(ethyl-alanine) amidate; 9, diastereomer A (designated GS-9131); 10, diastereomer B; 11, mono(isopropyl-alanine) prodrug; 12, mono(isobutyl-phenylalanine) amidate; 13, bis(n-butyl-alanine) amidate.
TABLE 2.
In vitro anti-HIV-1 activity and cytotoxicity in MT-2 cells and in vivo PBMC loading for GS-9148 and its amidate prodrugs
| Compound (designation) | Prodrug moiety | Diastereomeric preparation | In vitro activity and cytotoxicity valuesa
|
In vivo PBMC exposure AUC0-24 ± SD (μM/h/dose)b
|
||
|---|---|---|---|---|---|---|
| EC50 ± SD (μM) | CC50 ± SD (μM) | i.v. | Oral | |||
| 4 (GS-9148) | None | 10.6 ± 2.4 | >1,000 | 14.7 ± 0.2 | ||
| 8 | Mono(Ala-Et) | Mixture | 0.23 ± 0.07 | >50 | 845 ± 201 | 65.3 ± 17.1 |
| 9 (GS-9131) | Mono(Ala-Et) | Diastereomer A | 0.15 ± 0.04 | >50 | 359 ± 23 | 91.6 ± 25.0 |
| 10 | Mono(Ala-Et) | Diastereomer B | 0.32 ± 0.10 | >50 | 324 ± 29 | 31.2 ± 9.0 |
| 11 | Mono(Ala-iPr) | Mixture | 0.81 ± 0.23 | |||
| 12 | Mono(Phe-iBu) | Mixture | 0.006 ± 0.004 | 38 ± 4 | 1,560 ± 430 | 17.2 ± 1.6 |
| 13 | Bis(Ala-Bu) | Mixture | 0.041 ± 0.012 | >50 | 49.1 ± 13.9 | |
EC50 and CC50 were determined with HIV-1 (IIIB)-infected and uninfected MT-2 cells, respectively, following a 5-day incubation. The data represent the means ± standard deviations (SD) from two to eight independent experiments.
In vivo PBMC exposure (0 to 24 h) to all GS-9148-related metabolites following intravenous or oral administration of tested compounds to beagle dogs normalized to a dose of 1 mg of GS-9148 eq/kg. The total intracellular level of all GS-9148-related metabolites was determined in PBMC extracts following phosphatase treatment. Values of the area under the concentration-time curve from 0 to 24 h (AUC0-24) represent the means ± SD of results from three to eight individual animals.
Characterization of antiretroviral activities of GS-9131 and GS-9148.
GS-9131 is substantially more potent in activated PBMCs (EC50 = 3.7 nM; Table 3) than in MT-2 cells (EC50 = 150 nM; Table 2), whereas GS-9148 showed levels of antiviral potency that were similar in both cell types, indicating that the improvement in GS-9131 activity in PBMCs is likely due to its enhanced cellular permeation and/or more effective intracellular hydrolysis. GS-9131 is also a potent HIV-1 inhibitor in a single-cycle infection assay with primary CD4+ T lymphocytes (Table 3). Furthermore, both GS-9148 and GS-9131 inhibit HIV-1 in primary macrophages.
TABLE 3.
Anti-HIV-1 activity in primary cells
| Compound | EC50 ± SD (μM) for anti-HIV-1 activity ina:
|
||
|---|---|---|---|
| PBMCs | CD4+ lymphocytesb | Macrophages | |
| GS-9148 | 8.5 ± 7.7 | 2.5 | |
| GS-9131 | 0.0037 ± 0.0001 | 0.024 ± 0.0 | 0.051 |
| Tenofovir | 1.5 ± 1.3 | 0.41 | |
| AZT | 0.015 ± 0.006 | 0.055 ± 0.028 | 0.005 |
| Lopinavir | 0.0079 ± 0.0023 | 0.0073 ± 0.0087 | 0.14 |
EC50s in PBMCs and CD4+ lymphocytes represent means ± standard deviations (SD) from at least two independent experiments. EC50s in macrophages are the means from two experiments performed in triplicate.
Data are results from a single-cycle replication assay.
GS-9148 and GS-9131 maintain consistent levels of activity across multiple HIV-1 clinical isolates of different subtypes, with 4- to 10-fold higher potency for GS-9131 than for AZT (Table 4). Differences between the activity of GS-9131 against clinical isolates and against the laboratory BaL strain are likely due to different conditions for both assays, as described in Materials and Methods. Both GS-9148 and GS-9131 are also active in MT-2 cells against multiple isolates of HIV-2, with mean EC50s of 14 and 0.36 μM, respectively (Table 5). Importantly, GS-9131 exhibits a minimal approximately 1.7-fold increase in its EC50 against HIV-1 in the presence of 50% human serum. When GS-9131 was tested in parallel with the HIV protease inhibitor nelfinavir, the potency of the protease inhibitor was reduced by more than 28-fold by the addition of 50% human serum (data not shown).
TABLE 4.
Activities of GS-9148 and GS-9131 against different subtypes of HIV-1 clinical isolates in PBMCs
| Compound | EC50 (μM)a
|
Mean EC50 ± SD (μM)b | ||||
|---|---|---|---|---|---|---|
| UG-92-031 subtype A | B940374 subtype B | LJM subtype B | BR-92-025 subtype C | UG-92-024 subtype D | ||
| GS-9148 | 12.5 | 17.6 | 8.2 | 5.1 | 12.6 | 11.2 ± 4.8 |
| GS-9131 | 0.037 | 0.030 | 0.027 | 0.068 | 0.023 | 0.037 ± 0.018 |
| Tenofovir | 1.8 | 2.8 | 2.0 | 1.9 | 3.9 | 2.5 ± 0.9 |
| AZT | 0.16 | 0.31 | 0.33 | 0.26 | 0.13 | 0.24 ± 0.09 |
EC50s for individual HIV-1 isolates represent the means from three to four independent experiments, with standard deviations (SD) (not shown) of <50% of the mean.
Means ± standard deviations of EC50s for the five clinical isolates tested.
TABLE 5.
Activity of GS-9148 and GS-9131 against HIV-2 isolates in MT-2 cells
| Compound | EC50 (μM)a
|
Mean EC50 ± SD (μM)b | ||
|---|---|---|---|---|
| CDD77618 subtype A | CDD310248 subtype A | CDD310319 subtype B | ||
| GS-9148 | 14.2 | 21.0 | 6.8 | 14.0 ± 7.1 |
| GS-9131 | 0.39 | 0.65 | 0.039 | 0.36 ± 0.30 |
| Tenofovir | 5.7 | 3.3 | 2.0 | 3.7 ± 1.9 |
| AZT | 0.32 | 0.35 | 0.26 | 0.31 ± 0.05 |
EC50s for individual HIV-2 strains represent the means from three to four independent experiments, with standard deviations (SD) (not shown) of <50% of the mean.
Means ± SD of EC50s for the three HIV-2 isolates tested.
Activity in combination with other antiretrovirals.
The activity of GS-9148 in combination with multiple approved antiretrovirals was assessed by using a cytopathic assay with HIV-1-infected MT-2 cells. Results indicated a high level of synergy in the activity of GS-9148 in combination with AZT, ABC, or efavirenz (Table 6). Moderate synergy was found for combinations of GS-9148 with FTC or lopinavir, and the combination of GS-9148 with tenofovir showed an additive to slightly synergistic antiviral effect. Since GS-9148 and tenofovir are both adenine nucleotide analogs, their combination was further characterized mechanistically by using the RT enzyme inhibition assay. In accordance with the antiviral drug combination studies, an additive inhibitory effect was detected with combinations of the DP metabolites of GS-9148 and tenofovir (data not shown). In addition, no in vitro intracellular metabolic drug-drug interactions between GS-9148 and tenofovir or their prodrugs were detected in PBMCs treated with supratherapeutic concentrations of these compounds (39).
TABLE 6.
Anti-HIV-1 activity of GS-9148 in combination with selected antiretrovirals
| Drug combination | Synergy/antagonism volumea
|
Combination effect | |
|---|---|---|---|
| Type | Combination value ± SD | ||
| GS-9148 plus AZT | Synergy | 193.5 ± 94.4 | Highly synergistic |
| Antagonism | −0.6 ± 0.8 | ||
| GS-9148 plus FTC | Synergy | 78.6 ± 16.5 | Moderately synergistic |
| Antagonism | −2.1 ± 2.5 | ||
| GS-9148 plus abacavir | Synergy | 112.0 ± 6.3 | Highly synergistic |
| Antagonism | −9.1 ± 12.8 | ||
| GS-9148 plus tenofovir | Synergy | 27.0 ± 27.1 | Additive to slightly synergistic |
| Antagonism | −5.9 ± 8.3 | ||
| GS-9148 plus efavirenz | Synergy | 152.1 ± 62.9 | Highly synergistic |
| Antagonism | 0 ± 0 | ||
| GS-9148 plus lopinavir | Synergy | 52.8 ± 17.8 | Moderately synergistic |
| Antagonism | −3.7 ± 2.1 | ||
| Tenofovir plus efavirenz | Synergy | 184.7 ± 115.6 | Highly synergistic |
| Antagonism | −2.6 ± 3.7 | ||
| d4T plus ribavirin | Synergy | 0 | Highly antagonistic |
| Antagonism | −379.3 ± 35.0 | ||
Data represent means ± standard deviations (SD) from two independent experiments performed in triplicate. Combination values were defined according to a specific value of combination volume (concentration2 × percentage of inhibition).
Cytotoxicity in liver and renal cells.
In addition to MT-2 cells (Tables 1 and 2), the in vitro cytotoxicity levels of GS-9148 and GS-9131 were tested in a human liver cell line (HepG2) and in RPTECs. Both GS-9148 and GS-9131 exhibited low levels of cytotoxicity in HepG2 cells and in RPTECs from two separate donors, with CC50s above 2,000 and 50 μM for both cell types, respectively (Table 7). In comparison, tenofovir and GS-7340, a tenofovir prodrug from the same class as GS-9131, had CC50s of 1,530 and 6.9 μM, respectively, in HepG2 cells. The acyclic nucleotide analog cidofovir, known to cause renal tubular dysfunction (24), was significantly cytotoxic in RPTECs from both donors. GS-9148 was also substantially less cytotoxic than cidofovir in CHO cells stably expressing functional renal transporter hOAT1 (Table 7). We have confirmed that this last difference is due to the weaker affinity of hOAT1 for GS-9148 than for cidofovir (data not shown), suggesting a less effective renal transport and possibly a lower potential for nephrotoxicity than that of acyclic nucleotide analogs. Finally, quantitative studies confirmed that despite increased cellular permeation, GS-9131 at concentrations of up to 50 μM did not cause any selective depletion of mtDNA in HepG2 cells (data not shown), an observation consistent with the low potential of GS-9148 to interfere with the replication of mtDNA.
TABLE 7.
Cytotoxicity of GS-9148 and GS-9131 compared with that of other NRTIs
| Compound | CC50 ± SD (μM)a
|
|||
|---|---|---|---|---|
| HepG2 | RPTECs (donor 1) | RPTECs (donor 2) | CHO (hOAT1)b | |
| GS-9148 | >2,000 | >2,000 | >2,000 | 435 ± 148 |
| GS-9131 | >50 | >50 | >50 | |
| Tenofovir | 1,530 ± 205 | >2,000 | >2,000 | 21.0 ± 7.0 |
| GS-7340 | 6.9 ± 4.0 | 22.0 ± 8.5 | 8.2 ± 0.5 | |
| d4T | 1,042 ± 681 | |||
| Cidofovir | 291 ± 55 | 308 ± 84 | 3.0 ± 1.4 | |
CC50s represent means ± standard deviations (SD) from two independent experiments.
CHO cells stably expressing hOAT1.
Inhibition of HIV-1 RT and host DNA polymerases.
In vitro metabolism experiments demonstrated effective intracellular conversion of GS-9131 to the active metabolite GS-9148-DP in various lymphoid cells (39). In steady-state enzyme kinetic experiments, GS-9148-DP acts as a competitive inhibitor of HIV-1 RT with respect to dATP, exhibiting a level of potency comparable to that of the active metabolites of 3TC and FTC (Table 8).
TABLE 8.
Inhibition of HIV-1 reverse transcriptase by active metabolites of NRTIs
| Compound | Ki value (μM) ± SD for HIV RT inhibitiona |
|---|---|
| GS-9148-DP | 0.80 ± 0.07 |
| Tenofovir-DP | 0.18 ± 0.03 |
| AZT-TP | 0.038 ± 0.006 |
| d4T-TP | 0.06 ± 0.02 |
| 3TC-TP | 2.0 ± 0.4 |
| FTC-TP | 1.2 ± 0.4 |
| ddA-TPb | 0.021 ± 0.004 |
| Carbovir-TPb | 0.11 ± 0.03 |
Ki values represent means ± standard deviations (SD) from three independent experiments.
ddA-TP and carbovir-TP are active intracellular metabolites of ddI and abacavir, respectively.
To establish its selectivity toward RT, GS-9148-DP was also tested for the inhibition of three major human DNA polymerases (α, β, and γ). The steady-state enzyme inhibition kinetics demonstrated that GS-9148-DP is not an inhibitor of DNA polymerases β and γ, with IC50s of >175 and >300 μM, respectively (Table 9). A moderate inhibitory effect of GS-9148-DP was detected against DNA polymerase α (IC50 = 43.5 μM). However, RT is substantially more sensitive to GS-9148-DP under comparable substrate conditions (IC50 = 2.3 μM), indicating a good selectivity toward the viral target enzyme. As shown in Table 9, the inhibitory profile of GS-9148-DP against host DNA polymerases resembles that of tenofovir DP and is more favorable than that of ddATP.
TABLE 9.
Comparison of inhibition values for RT and for host DNA polymerases by active NRTI metabolitesa
| Compound | IC50 for RT (μM) | Inhibition of host DNA polymerases
|
|||||
|---|---|---|---|---|---|---|---|
| α
|
β
|
γ
|
|||||
| IC50 ± SD (μM) | SI | IC50 ± SD (μM) | SI | IC50 ± SD (μM) | SI | ||
| GS-9148-DP | 2.3 ± 1.0 | 43.5 ± 33.0 | 18.7 | >175 | >76 | >300 | >130 |
| Tenofovir-DP | 0.45 ± 0.20 | 60.5 ± 23.3 | 133 | 50.5 ± 3.5 | 111 | >300 | >667 |
| ddA-TP | 0.18 ± 0.10 | >100 | >555 | 2.4 ± 0.6 | 13.3 | 1.0 ± 0.4 | 5.4 |
IC50s represent the means ± standard deviations (SD) from two independent experiment performed in triplicate. The selectivity index (SI) is derived from the IC50 for DNA polymerase/IC50 for RT.
Activity of GS-9148 against NRTI-resistant HIV-1 variants.
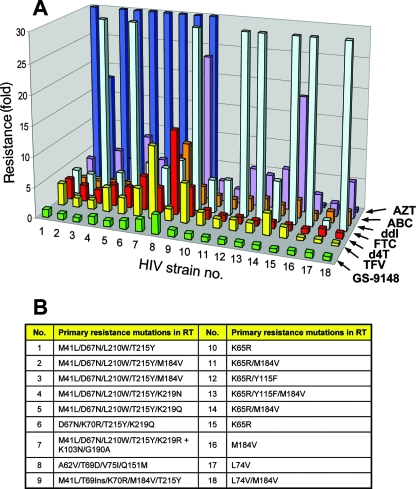
Characterization of the resistance profile of GS-9148 in comparison with the approved NRTIs was carried out using a PhenoSense assay. All inhibitors were tested in parallel against 18 patient-derived HIV-1 recombinant strains containing various combinations of all major known NRTI resistance-associated mutations. Viruses carrying the K65R, K70E, L74V, or M184V mutation, as well as various combinations thereof, were fully susceptible or slightly hypersensitive to GS-9148, responses that were similar to those of AZT and unlike that of most of the other marketed NRTIs (Fig. 4 and see supplemental material). Furthermore, while most viruses with thymidine analog mutations (TAMs) showed substantial cross-resistance to all of the marketed NRTIs that were tested, GS-9148 retained its activity against viruses with four or more TAMs, including combinations containing the M41L and L210W mutations (Fig. 4 and see supplemental material).
FIG. 4.
(A) Comparison of the resistance profile of GS-9148 with those of clinically approved NRTIs. The bars represent the change in the susceptibility of each mutant virus relative to that of the wild-type control strain, using a PhenoSense assay (Monogram Biosciences). GS-9148 (green), TFV (yellow), d4T (red), FTC (light blue), ddI (orange), ABC (purple), AZT (dark blue). To improve the resolution of lower resistance, a greater than 30-fold change is not depicted. The exact values of each virus’ resistance to the NRTIs tested are provided in the supplemental material. (B) List of HIV-1 strains used for the resistance analysis, with primary resistance mutations present in RT.
A direct comparison between GS-9148 and tenofovir revealed statistically significant differences in the degrees of their resistance among K65R-containing isolates (mean changes of 0.8-fold and 2.1-fold for GS-9148 and tenofovir, respectively; n = 6; P < 0.003) and TAM-containing isolates (mean changes of 1.2-fold and 4.3-fold for GS-9148 and tenofovir, respectively; n = 7; P < 0.02). In addition, unlike any of the marketed NRTIs that were tested, GS-9148 showed a minimal loss of activity against a multidrug-resistant virus containing TAMs in combination with M184V and an insertion at T69. The only virus from the tested set of mutants displaying more than twofold reduced susceptibility to GS-9148 contained the Q151M multi-NRTI resistance complex. However, this virus also showed considerable resistance to all approved NRTIs except tenofovir. Collectively, these data indicate that among the NRTIs tested, GS-9148 is the only inhibitor exhibiting uncompromised activity against the K65R, L74V, and M184V mutation-carrying viruses, combined with minimal resistance due to multiple TAMs (Fig. 4).
Pharmacokinetic profile of GS-9131 administered orally to dogs.
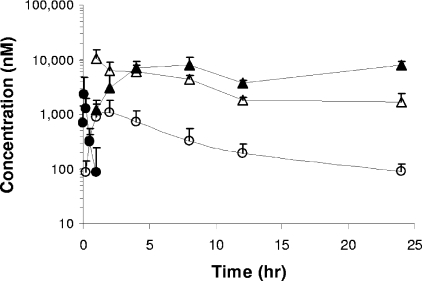
As illustrated in Fig. 5, after GS-9131 was given orally to male beagle dogs at 3 mg/kg, it was rapidly absorbed, generating a maximum serum drug concentration (Cmax) of 2.5 μM, and was subsequently eliminated from plasma with an apparent terminal half-life (t1/2) of less than 20 min. As determined following i.v. administration, the systemic clearance of GS-9131 was approximately 1.4 liters/h/kg. Combined i.v. and oral data for GS-9131 indicate a mean oral bioavailability of 26%. Concomitant with the elimination of GS-9131 from plasma, GS-9148 was observed to reach a Cmax of 1.1 μM within approximately 2 h after dosing. A comparison of the exposure to GS-9148 following i.v. and oral administration suggests that the majority of the oral dose (>60%) is absorbed from the gastrointestinal tract.
FIG. 5.
Plasma and PBMC pharmacokinetic profiles for GS-9131 and its metabolites following the oral administration of 3 mg/kg to male beagle dogs. GS-9131 is rapidly absorbed and has a short t1/2 in plasma (solid circles). Following the disappearance of the prodrug, a concomitant increase in plasma levels of GS-9148 is observed (open circles). Analysis of intracellular PBMC drug levels indicates that GS-9148 reaches a maximal concentration at the first measurement following a slow disappearance (open triangles). GS-9148-DP (solid triangles) forms over time and reaches a persistent maximal level at between 8 and 24 h postdose. All values represent the mean ± standard deviations of data from eight dogs dosed in two independent studies.
The short plasma exposure to GS-9131 following its oral administration to dogs was sufficient to effectively deliver high levels of GS-9148 and its DP into PBMCs. Analysis of cellular extracts from isolated PBMCs demonstrated rapid intracellular accumulation of GS-9148 (Fig. 5), with its intracellular decay mirroring the increase in the levels of GS-9148-DP. These results indicate that GS-9148 undergoes effective intracellular phosphorylation in PBMCs in vivo. Notably, GS-9148-DP reached a peak concentration of more than 9 μM in PBMCs, which persisted for more than 24 h after dosing. Together, these data indicate that GS-9131 administered orally to dogs delivers high and persistent intracellular levels of the active metabolite GS-9148-DP to PBMCs.
DISCUSSION
The design of novel nucleotide analogs and their prodrugs described in the present study was motivated primarily by the need for new agents that exhibit clinical efficacy for HIV-infected patients with NRTI resistance. Because of the extensive existing experimental and clinical experience with the NRTI class, broad in vitro and in vivo profiling of new candidate molecules are required, with a special emphasis on minimizing class-specific toxicity, maintaining activity against a broad spectrum of NRTI-resistant viruses, and achieving high intracellular levels of the active metabolite in target cells in vivo. Based on these and other criteria, a novel nucleotide, GS-9148, and its phosphonoamidate prodrug GS-9131 were selected as candidates for development.
The discovery of the favorable resistance profile of the previously described nucleoside phosphonate d4AP (20) was an important initial step toward the design of GS-9148. However, the mitochondrial toxicity observed with d4AP precluded considering it for further development. The toxicity of d4AP was not completely unexpected given the potent inhibition of DNA polymerase γ by its DP metabolite (6). Multiple rational approaches have been taken to address this limitation. Among the modifications of d4AP that have been explored, only the 2′-fluorine substitution resulted in both the elimination of effects on mtDNA and the retention of potency and favorable resistance profile, leading to the selection of GS-9148. The precedent for this modification was originally established with 2′-β-fluoro-2′,3′-dideoxyadenosine, which showed a markedly reduced effect on mtDNA compared to that of the unsubstituted 2′,3′-dideoxyadenosine. This can be explained by substantial differences in the affinity levels of the corresponding active TPs for DNA polymerase γ (44). Similarly, the 2′-fluorine substitution on the d4AP scaffold leads to a marked reduction in the inhibition of DNA polymerase γ by the corresponding DP metabolite (38). Although 2′-fluoro-2′,3′-didehydro-2′,3′-dideoxyadenosine (the corresponding nucleoside analog of GS-9148) was prepared previously and its potent in vitro antiretroviral activity has been demonstrated (25), its potential for mitochondrial toxicity has not been studied in detail. Hence, at present, it is not clear whether the phosphonate in the context of 2′-fluorine substitution offers additional benefit with respect to a reduction in the mitochondrial toxicity potential.
Results from a PhenoSense analysis indicate that GS-9148 meets the target resistance profile set for the development candidate by maintaining its activity against multiple patient-derived HIV strains with the major known NRTI resistance mutations. Importantly, its profile appears to be distinct from that of the clinically approved NRTIs in that GS-9148 was the only inhibitor tested that showed no reduction in potency due to K65R, L74V, and M184V mutations, together with the minimal resistance associated with multiple TAMs. Recently, we have also shown that GS-9148 selects for the rare K70E primary resistance mutation in RT (8). In vitro exposure to increasing concentrations of GS-9148 for 7 months resulted in the emergence of an HIV-1 variant with the K70E, D123N, and T165I mutations in RT and an approximately threefold reduced susceptibility to GS-9148. A search of the Stanford HIV drug resistance database (http://hivdb.stanford.edu/) indicated that the K70E mutation is present only in NRTI-experienced patients at a frequency of less than 0.5% of database samples, which sharply contrasts with the much higher incidence of other NRTI resistance mutations such as M184V (>45% of database samples) and various TAMs (10 to 35% of database samples).
Effective oral administration and intracellular delivery of GS-9148 require the use of lipophilic prodrug moieties to mask the negative charges present on the phosphonate group at a physiological pH level. Based on the favorable pharmacokinetic profile and tissue distribution pattern demonstrated with phosphonoamidate prodrugs of tenofovir (26), we decided to explore a similar strategy with GS-9148 rather than rely on the diester prodrug concept employed in the prior design of tenofovir disoproxil or adefovir dipivoxil (9). Among multiple characterized phosphonoamidates, GS-9131 was selected as the optimal prodrug of GS-9148. GS-9131 is a potent inhibitor of in vitro HIV-1 replication, both in established T-cell lines and in various primary cells, including macrophages. In activated human PBMCs, GS-9131 inhibits replication in multiple subtypes of HIV-1 clinical isolates with a 4- to 10-fold higher potency than that of AZT. At the same time, GS-9131 has good in vitro selectivity in several human cell types, including renal proximal tubule cells, the target for dose-limiting clinical adverse effects of some nucleoside phosphonates (7, 23, 24).
Inside lymphoid cells, GS-9131 is readily hydrolyzed to the parent nucleotide GS-9148, which undergoes effective phosphorylation to the active DP metabolite (39). In vitro metabolism studies demonstrated that a GS-9131 concentration approximately 100-fold lower than GS-9148 is required to achieve similar intracellular levels of GS-9148-DP, a result consistent with a marked enhancement of cellular permeation of GS-9148 by its conversion to GS-9131 (39). Once formed, GS-9148-DP is effectively retained in cells (39) and acts as a competitive inhibitor of HIV-1 RT with respect to the natural substrate dATP. Additional mechanistic enzymology studies have demonstrated the ability of GS-9148 to function as a chain terminator following its incorporation into DNA by RT (48).
When GS-9131 was administered to dogs, it exhibited an oral bioavailability of more than 20% as an intact prodrug. The comparison of GS-9148 levels in plasma following oral and i.v. administration of GS-9131 demonstrated an oral bioavailability of more than 60% as the parent GS-9148, indicating effective intestinal absorption. This is likely a consequence of the well-balanced properties of GS-9131, including its lipophilicity, solubility, and stability in the gastrointestinal tract. In addition, GS-9131 exhibits good stability in both dog and human plasma, with a t1/2 of 1 h, but undergoes fast hydrolysis in lymphoid cells and cellular extracts (3, 39). This profile is in contrast with that of diester nucleotide prodrugs such as tenofovir disoproxil, which displays limited stability in plasma (t1/2 < 1 min) and no selectivity of intracellular activation over systemic hydrolysis (26). Thus, GS-9131 acts in vivo as an intracellular prodrug that allows for the effective delivery of GS-9148 and its metabolites into PBMCs. In this context and somewhat similar to other NRTIs, the intracellular level of GS-9148-DP in target cells rather than the plasma level of parent GS-9148 should be taken into account as a predictor for the antiretroviral activity of GS-9131 in a clinical setting. Following the oral administration of GS-9131 to dogs at 3 mg/kg, the concentration of GS-9148-DP in PBMCs approached 10 μM, a level approximately 20-fold higher than that of the tenofovir DP detected in PBMCs of patients treated with tenofovir disoproxil fumarate at the standard clinical dose of 300 mg (15). As discussed in further detail in the report by Ray et al., these intracellular levels of GS-9148-DP are expected to result in an antiretroviral effect in patients (39). In addition, prolonged in vivo intracellular retention of GS-9148-DP suggests the possibility for once-daily dosing, an important aspect for facilitating the compliance of patients. Finally, both the antiviral combination experiments described here and the in vitro metabolic drug-drug interaction studies described by Ray et al. (39) suggest that GS-9131 should be suitable for use in combination with multiple antiretrovirals including other NRTIs.
Results from the present in vitro and in vivo pharmacological profiling and from additional preclinical evaluations indicate that the nucleotide prodrug GS-9131 is an attractive clinical development candidate for the treatment of patients infected with NRTI-resistant HIV.
Supplementary Material
Acknowledgments
We thank William Lee, Choung Kim, Manoj Desai, Michael Miller, Gerald Rhodes, Hans Reiser, Laura Lehman, and Katyna Borroto-Esoda from Gilead for their continual support and useful feedback during the course of this project. Additionally, we thank many other colleagues from Gilead, namely, Kirsten Stray and Christian Callebaut for determining the antiviral activity in activated CD4+ lymphocytes, Manuel Tsiang for help with DNA polymerase alpha assays, John Ly for evaluating the inhibitor combinations in HIV-1 RT assay, Nicolas Margot for kindly sharing several recombinant HIV-1 clones with NRTI resistance mutations, and Xiaohong Liu and Ruth Wang for producing recombinant HIV-1 reverse transcriptase. In addition, we are grateful to Roger Ptak and Deborah Hill from Southern Research Institute for testing the activity in macrophages, William Copeland from the National Institute of Environmental Health Sciences for kindly providing human DNA polymerase γ, and the NIH AIDS Research and Reference Reagent Program for providing various HIV-1 and HIV-2 isolates.
Footnotes
Published ahead of print on 3 December 2007.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Bethell, R. C., Y. S. Lie, and N. T. Parkin. 2005. In vitro activity of SPD754, a new deoxycytidine nucleoside reverse transcriptase inhibitor (NRTI), against 215 HIV-1 isolates resistant to other NRTIs. Antivir. Chem. Chemother. 16:295-302. [DOI] [PubMed] [Google Scholar]
- 2.Birkus, G., M. J. Hitchcock, and T. Cihlar. 2002. Assessment of mitochondrial toxicity in human cells treated with tenofovir: comparison with other nucleoside reverse transcriptase inhibitors. Antimicrob. Agents Chemother. 46:716-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birkus, G., R. Wang, X. Liu, N. Kutty, H. MacArthur, T. Cihlar, C. Gibbs, S. Swaminathan, W. Lee, and M. McDermott. 2007. Cathepsin A is the major hydrolase catalyzing the intracellular hydrolysis of the antiretroviral nucleotide phosphonoamidate prodrugs GS-7340 and GS-9131. Antimicrob. Agents Chemother. 51:543-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.Boojamra, C. G., R. L. Mackman, D. Y. Markevitch, V. Prasad, A. S. Ray, J. Douglas, D. Grant, C. U. Kim, and T. Cihlar. Bioorg. Med. Chem. Lett., in press. [DOI] [PubMed]
- 4.Carr, A. 2003. Toxicity of antiretroviral therapy and implications for drug development. Nat. Rev. Drug Discov. 2:624-634. [DOI] [PubMed] [Google Scholar]
- 5.Carr, A., J. Miller, M. Law, and D. A. Cooper. 2000. A syndrome of lipoatrophy, lactic acidaemia and liver dysfunction associated with HIV nucleoside analogue therapy: contribution to protease inhibitor-related lipodystrophy syndrome. AIDS 14:F25-F32. [DOI] [PubMed] [Google Scholar]
- 6.Cherrington, J. M., S. J. W. Allen, N. Bischofberger, and M. S. Chen. 1995. Kinetic incorporation of the diphosphates of 9-(2-phosphonylmethoxyethyl)adenine and other anti-HIV active purine congeners with HIV reverse transcriptase and human DNA polymerases alpha, beta, and gamma. Antivir. Chem. Chemother. 6:217-221. [Google Scholar]
- 7.Cihlar, T., E. S. Ho, D. C. Lin, and A. S. Mulato. 2001. Human renal organic anion transporter 1 (hOAT1) and its role in the nephrotoxicity of antiviral nucleotide analogs. Nucleosides Nucleotides Nucleic Acids. 20:641-648. [DOI] [PubMed] [Google Scholar]
- 8.Cihlar, T., A. Ray, C. Boojamra, L. Zhang, H. Hui, D. Grant, K. White, M. Desai, N. Parkin, and R. Mackman. 2006. GS9148: a novel nucleotide active against HIV-1 variants with drug resistance mutations in reverse transcriptase, abstr. 45. Abstr. 13th Conference on Retroviruses and Opportunistic Infections., Denver, CO.
- 9.De Clercq, E., and A. Holy. 2005. Acyclic nucleoside phosphonates: a key class of antiviral drugs. Nat. Rev. Drug Discov. 4:928-940. [DOI] [PubMed] [Google Scholar]
- 10.Department of Health and Human Services. 10 October 2006, posting date. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. http://AIDSinfo.nih.gov.
- 11.Gallant, J. E., P. Z. Gerondelis, M. A. Wainberg, N. S. Shulman, R. H. Haubrich, M. St Clair, E. R. Lanier, N. S. Hellmann, and D. D. Richman. 2003. Nucleoside and nucleotide analogue reverse transcriptase inhibitors: a clinical review of antiretroviral resistance. Antivir. Ther. 8:489-506. [PubMed] [Google Scholar]
- 12.Geleziunas, R., K. Gallagher, H. Zhang, L. Bacheler, S. Garber, J. T. Wu, G. Shi, M. J. Otto, R. F. Schinazi, and S. Erickson-Viitanen. 2003. HIV-1 resistance profile of the novel nucleoside reverse transcriptase inhibitor beta-D-2′,3′-dideoxy-2′,3′-didehydro-5-fluorocytidine (Reverset). Antivir. Chem. Chemother. 14:49-59. [DOI] [PubMed] [Google Scholar]
- 13.Geretti, A. M. 2007. Epidemiology of antiretroviral drug resistance in drug-naive persons. Curr. Opin. Infect. Dis. 20:22-32. [DOI] [PubMed] [Google Scholar]
- 14.Graziewicz, M. A., M. J. Longley, R. J. Bienstock, M. Zeviani, and W. C. Copeland. 2004. Structure-function defects of human mitochondrial DNA polymerase in autosomal dominant progressive external ophthalmoplegia. Nat. Struct. Mol. Biol. 11:770-776. [DOI] [PubMed] [Google Scholar]
- 15.Hawkins, T., W. Veikley, R. L. I. St. Claire, B. Guyer, N. Clark, and B. P. Kearney. 2005. Intracellular pharmacokinetics of tenofovir diphosphate, carbovir triphosphate, and lamivudine triphosphate in patients receiving triple-nucleoside regimens. J. Acquir. Immune Defic. Syndr. 39:406-411. [DOI] [PubMed] [Google Scholar]
- 16.Holy, A. 2003. Phosphonomethoxyalkyl analogs of nucleotides. Curr. Pharm. Des. 9:2567-2592. [DOI] [PubMed] [Google Scholar]
- 17.Huang, H., R. Chopra, G. L. Verdine, and S. C. Harrison. 1998. Structure of a covalently trapped catalytic complex of HIV-1 reverse transcriptase: implications for drug resistance. Science 282:1669-1675. [DOI] [PubMed] [Google Scholar]
- 18.Kakuda, T. N. 2000. Pharmacology of nucleoside and nucleotide reverse transcriptase inhibitor-induced mitochrondrial toxicity. Clin. Ther. 22:685-708. [DOI] [PubMed] [Google Scholar]
- 19.Khandazhinskaya, A. L., E. A. Shirokova, Y. S. Skoblov, L. S. Victorova, L. Y. Goryunova, R. S. Beabealashvilli, T. R. Pronyaeva, N. V. Fedyuk, V. V. Zolin, A. G. Pokrovsky, and M. K. Kukhanova. 2002. Carbocyclic dinucleoside polyphosphonates: interaction with HIV reverse transcriptase and antiviral activity. J. Med. Chem. 45:1284-1291. [DOI] [PubMed] [Google Scholar]
- 20.Kim, C., B. Luh, and J. C. Martin. 1991. Regiospecific and highly stereoselective electrophilic addition to furanoid glycals: synthesis of phosphonate nucleotide analogues with potent activity against HIV. J. Org. Chem. 56:2642-2647. [Google Scholar]
- 21.Kim, E. Y., L. Vrang, B. Oberg, and T. C. Merigan. 2001. Anti-HIV type 1 activity of 3′-fluoro-3′-deoxythymidine for several different multidrug-resistant mutants. AIDS Res. Hum. Retroviruses 17:401-407. [DOI] [PubMed] [Google Scholar]
- 22.Kodama, E. I., S. Kohgo, K. Kitano, H. Machida, H. Gatanaga, S. Shigeta, M. Matsuoka, H. Ohrui, and H. Mitsuya. 2001. 4′-Ethynyl nucleoside analogs: potent inhibitors of multidrug-resistant human immunodeficiency virus variants in vitro. Antimicrob. Agents Chemother. 45:1539-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lacy, S. A., M. J. M. Hitchcock, W. A. Lee, P. Tellier, and K. C. Cundy. 1998. Effect of oral probenecid coadministration on the chronic toxicity and pharmacokinetics of intravenous cidofovir in cynomolgus monkeys. Toxicol. Sci. 4:97-106. [DOI] [PubMed] [Google Scholar]
- 24.Lalezari, J. P., R. J. Stagg, B. D. Kuppermann, G. N. Holland, F. Kramer, D. V. Ives, M. Youle, M. R. Robinson, W. L. Drew, and H. S. Jaffe. 1997. Intravenous cidofovir for peripheral cytomegalovirus retinitis in patients with AIDS. A randomized, controlled trial. Ann. Intern. Med. 126:257-263. [DOI] [PubMed] [Google Scholar]
- 25.Lee, K., Y. Choi, G. Gumina, W. Zhou, R. F. Schinazi, and C. K. Chu. 2002. Structure-activity relationships of 2′-fluoro-2′,3′-unsaturated D-nucleosides as anti-HIV-1 agents. J. Med. Chem. 45:1313-1320. [DOI] [PubMed] [Google Scholar]
- 26.Lee, W. A., G. X. He, E. Eisenberg, T. Cihlar, S. Swaminathan, A. Mulato, and K. C. Cundy. 2005. Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrob. Agents Chemother. 49:1898-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Longley, M. J., P. A. Ropp, S. E. Lim, and W. C. Copeland. 1998. Characterization of the native and recombinant catalytic subunit of human DNA polymerase gamma: identification of residues critical for exonuclease activity and dideoxynucleotide sensitivity. Biochemistry 37:10529-10539. [DOI] [PubMed] [Google Scholar]
- 28.Mackman, R., C. Boojamra, J. Chen, J. Douglas, D. Grant, C. Kim, K. M. Lin, D., V. Prasad, A. Ray, and T. Cihlar. 2006. Discovery of GS-9148, a novel nucleotide HIV reverse transcriptase inhibitor. Antivir. Res. 70:A40.
- 29.Mackman, R. L., L. Zhang, V. Prasad, C. G. Boojamra, J. Douglas, D. Grant, H. Hui, C. U. Kim, G. Laflamme, J. Parrish, A. D. Stoycheva, S. Swaminathan, K. Wang, and T. Cihlar. 2007. Synthesis, anti-HIV activity, and resistance profile of thymidine phosphonomethoxy nucleosides and their bis-isopropyloxymethylcarbonyl (bisPOC) prodrugs. Bioorg. Med. Chem. 15:5519-5528. [DOI] [PubMed] [Google Scholar]
- 30.Margot, N. A., and M. D. Miller. 2005. In vitro combination studies of tenofovir and other nucleoside analogues with ribavirin against HIV-1. Antivir. Ther. 10:343-348. [PubMed] [Google Scholar]
- 31.Moyle, G. 2000. Clinical manifestations and management of antiretroviral nucleoside analog-related mitochondrial toxicity. Clin. Ther. 22:911-936. [DOI] [PubMed] [Google Scholar]
- 32.Nolan, D., and S. Mallal. 2004. Complications associated with NRTI therapy: update on clinical features and possible pathogenic mechanisms. Antivir. Ther. 9:849-863. [PubMed] [Google Scholar]
- 33.Ohrui, H., S. Kohgo, H. Hayakawa, E. Kodama, M. Matsuoka, T. Nakata, and H. Mitsuya. 2006. 2′-Deoxy-4′-C-ethynyl-2-fluoroadenosine: a nucleoside reverse transcriptase inhibitor with highly potent activity against all HIV-1 strains, favorable toxic profiles and stability in plasma. Nucleic Acids Symp. Ser. 50:1-2. [DOI] [PubMed] [Google Scholar]
- 34.Otto, M. J. 2004. New nucleoside reverse transcriptase inhibitors for the treatment of HIV infections. Curr. Opin. Pharmacol. 4:431-436. [DOI] [PubMed] [Google Scholar]
- 35.Pillay, D., H. Green, R. Matthias, D. Dunn, A. Phillips, C. Sabin, and B. Evans. 2005. Estimating HIV-1 drug resistance in antiretroviral-treated individuals in the United Kingdom. J. Infect. Dis. 192:967-973. [DOI] [PubMed] [Google Scholar]
- 36.Prichard, M. N., L. E. Prichard, W. A. Baguley, M. R. Nassiri, and C. Shipman, Jr. 1991. Three-dimensional analysis of the synergistic cytotoxicity of ganciclovir and zidovudine. Antimicrob. Agents Chemother. 35:1060-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prichard, M. N., and C. Shipman, Jr. 1990. A three-dimensional model to analyze drug-drug interactions. Antiviral Res. 14:181-205. [DOI] [PubMed] [Google Scholar]
- 38.Ray, A. S., C. G. Boojamra, N. Parkin, K. L. White, G. R. Rhodes, R. Mackman, and T. Cihlar. 2006. Amidate prodrug of nucleotide analog GS-9148 has favorable in vitro activity against resistant HIV and in vivo pharmacokinetics. Global Antivir. J. 2(Suppl. 2):37. [Google Scholar]
- 39.Ray, A. S., J. E. Vela, C. G. Boojamra, L. Zhang, H. Hui, C. Callebaut, K. Stray, K.-Y. Lin, Y. Gao, R. L. Mackman, and T. Cihlar. 2008. Intracellular metabolism of the nucleotide prodrug GS-9131, a potent anti-human immunodeficiency virus agent. Antimicrob. Agents Chemother. 52:648-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richman, D. D., S. C. Morton, T. Wrin, N. Hellmann, S. Berry, M. F. Shapiro, and S. A. Bozzette. 2004. The prevalence of antiretroviral drug resistance in the United States. AIDS 18:1393-1401. [DOI] [PubMed] [Google Scholar]
- 41.Robbins, B., R. Srinivas, C. Kim, N. Bischofberger, and A. Fridland. 1998. Anti-human immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleosides phosphate 9-R-(2-phosphonomethoxypropyl)adenine (PMPA), bis(isopropyloxymethylcarbonyl)PMPA. Antimicrob. Agents Chemother. 42:612-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schinazi, R. F., J. Mellors, H. Bazmi, S. Diamond, S. Garber, K. Gallagher, R. Geleziunas, R. Klabe, M. Pierce, M. Rayner, J. T. Wu, H. Zhang, J. Hammond, L. Bacheler, D. J. Manion, M. J. Otto, L. Stuyver, G. Trainor, D. C. Liotta, and S. Erickson-Viitanen. 2002. DPC 817: a cytidine nucleoside analog with activity against zidovudine- and lamivudine-resistant viral variants. Antimicrob. Agents Chemother. 46:1394-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tamalet, C., J. Fantini, C. Tourres, and N. Yahi. 2003. Resistance of HIV-1 to multiple antiretroviral drugs in France: a 6-year survey (1997-2002) based on an analysis of over 7000 genotypes. AIDS 17:2383-2388. [DOI] [PubMed] [Google Scholar]
- 44.Tsai, C. H., S. L. Doong, D. G. Johns, J. S. Driscoll, and Y. C. Cheng. 1994. Effect of anti-HIV 2′-beta-fluoro-2′,3′-dideoxynucleoside analogs on the cellular content of mitochondrial DNA and on lactate production. Biochem. Pharmacol. 48:1477-1481. [DOI] [PubMed] [Google Scholar]
- 45.Vela, J. E., L. Y. Olson, A. Huang, A. Fridland, and A. S. Ray. 2007. Simultaneous quantitation of the nucleotide analog adefovir, its phosphorylated anabolites and 2′-deoxyadenosine triphosphate by ion-pairing LC/MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 848:335-343. [DOI] [PubMed] [Google Scholar]
- 46.Wainberg, M. A., and D. Turner. 2004. Resistance issues with new nucleoside/nucleotide backbone options. J. Acquir. Immune Defic. Syndr. 37(Suppl. 1):S36-S43. [DOI] [PubMed] [Google Scholar]
- 47.White, A. J. 2001. Mitochondrial toxicity and HIV therapy. Sex. Transm. Infect. 77:158-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.White, K., J. Feng, A. Ray, G. Laflamme, F. Yu, M. Tsiang, R. Wang, M. McDermott, M. Miller, R. Mackman, and T. Cihlar. 2006. GS-9148 diphosphate, an active metabolite of a novel adenine nucleotide analogue, is an effective inhibitor of HIV-1 reverse transcriptase. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother., abstr. H-251.
- 49.White, K. L., N. A. Margot, J. K. Ly, J. M. Chen, A. S. Ray, M. Pavelko, R. Wang, M. McDermott, S. Swaminathan, and M. D. Miller. 2005. A combination of decreased NRTI incorporation and decreased excision determines the resistance profile of HIV-1 K65R RT. AIDS 19:1751-1760. [DOI] [PubMed] [Google Scholar]
- 50.Wu, T., M. Froeyen, V. Kempeneers, C. Pannecouque, J. Wang, R. Busson, E. De Clercq, and P. Herdewijn. 2005. Deoxythreosyl phosphonate nucleosides as selective anti-HIV agents. J. Am. Chem. Soc. 127:5056-5065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.