Abstract
Human immunodeficiency virus type 1 (HIV-1) can be transmitted through breast-feeding and through contaminated blood donations. Copper has potent biocidal properties and has been found to inactivate HIV-1 infectivity. The objective of this study was to determine the capacity of copper-based filters to inactivate HIV-1 in culture media. Medium spiked with high titers of HIV-1 was exposed to copper oxide powder or copper oxide-impregnated fibers or passed through copper-based filters, and the infectious viral titers before and after treatment were determined. Cell-free and cell-associated HIV-1 infectivity was inhibited when exposed to copper oxide in a dose-dependent manner, without cytotoxicity at the active antiviral copper concentrations. Similar dose-dependent inhibition occurred when HIV-1 was exposed to copper-impregnated fibers. Filtration of HIV-1 through filters containing the copper powder or copper-impregnated fibers resulted in viral deactivation of all 12 wild-type or drug-resistant laboratory or clinical, macrophage-tropic and T-cell-tropic, clade A, B, or C, HIV-1 isolates tested. Viral inactivation was not strain specific. Thus, a novel means to inactivate HIV-1 in medium has been developed. This inexpensive methodology may significantly reduce HIV-1 transmission from “mother to child” and/or through blood donations if proven to be effective in breast milk or plasma and safe for use. The successful application of this technology may impact HIV-1 transmission, especially in developing countries where HIV-1 is rampant.
Transmission of human immunodeficiency virus type 1 (HIV-1) through contaminated milk is a merciless quandary (15, 17, 39). In sub-Saharan Africa, HIV-1 mother-to-child transmission (MTCT) is considered a “disaster” (50), while the growing incidence of breast-feeding-acquired pediatric HIV infection in other developing countries, such as India (47), amplifies the looming threat worldwide. In 2001, breast-feeding was estimated to have contributed 33 to 50% of the >700,000 MTCT cases worldwide (43). Paradoxically, breast-feeding promises the best chance of adequate nutrition and immunological protection for infants born in developing nations; not breast-feeding is estimated to result in 1.5 million child deaths per year from malnutrition and infection (9, 18, 21, 52).
The transmission of HIV-1 in whole blood and its components is also a continuing global problem (2, 24, 46). Currently, blood centers in industrialized countries rigorously screen blood donations for known pathogens and have now implemented nucleic acid testing that further reduces HIV transmission before serological conversion (3, 38). Unfortunately, these highly sensitive detection tests do not eliminate the period of potential infectivity. Furthermore, in many developing countries where the prevalence of HIV infection among blood donors is orders of magnitude greater than that in industrialized countries, the blood supply is either incompletely screened or not screened at all for antibodies against HIV (12-14, 30, 35, 36). The WHO estimates that 80,000 to 160,000 HIV infections occur through blood transfusion each year worldwide (31). The CDC estimates that 5 to 10% of HIV infections in developing countries are due to blood transfusion (30).
Copper has potent bactericidal properties (e.g., references 20 and 37) and virucidal properties (reviewed in reference 7). Copper also inactivates HIV-1 (44). Recently we developed a durable platform technology that introduces copper into cotton fibers, latex, and other polymeric materials (6, 23). These copper-impregnated materials demonstrate broad-spectrum antibacterial, antiviral, and antifungal activity. This technology enabled the production of antibacterial, self-sterilizing fabrics (that kill antibiotic-resistant bacteria, including methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci) and antifungal socks (that alleviate symptoms of athlete's foot) (6, 23). Recently we reported the capacity of copper oxide-containing filters to reduce infectious titers of a panel of viruses spiked into culture media, including enveloped and nonenveloped, RNA and DNA viruses, suggesting the possibility of using copper oxide-containing devices to inactivate a wide spectrum of infectious viruses found in filterable suspensions (8). In the present study, we describe the results and development of inexpensive, copper-based filters that inactivate HIV-1 in media. Preliminary data suggest that these filters are efficacious when HIV-1 is present in breast milk or plasma. Such filters may thus have an important impact on the reduction of mother-to-child and/or blood-borne HIV-1 transmission, especially in developing countries.
MATERIALS AND METHODS
Cell culture.
Peripheral blood mononuclear cells (PBMC), MT2, U937, cMAGI, and H9+ cell lines were cultured in RPMI 1640 medium (GibcoBRL, Life Technologies, Paisley, United Kingdom) containing 10% fetal calf serum (GibcoBRL, United Kingdom). PBMC, isolated from heparinized venous blood by standard centrifugation over Lymphoprep (Nycomed, Oslo, Norway), were washed, counted, and cultured (105 cells/ml) in RPMI 1640 medium supplemented with 10% fetal calf serum, 1 mM glutamine, and 2.5 μg/ml phytohemagglutinin (PHA) (Difco, Detroit, MI). MT2, U937, cMAGI, and H9+ cells (T cells chronically infected with HIV-1IIIB) were obtained from Mark Wainberg, McGill University, Canada. The viability of the cells was assessed by either (i) measuring the reduction of 3-(4,5-dimethythiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) dye reduction to formazan by measuring the absorbance of the culture medium at 550 nm or (ii) using the trypan blue exclusion assay, where cells without an intact membrane take up the coloring agent.
Viruses.
The following HIV-1 isolates were tested: HIV-1IIIB, HIV-1 M461/L63P/V82T/184, HIV-1 L10R/M461/L63P/V82T/184, HIV-1RTMC/MT2, HIV-1 96USNG20, HIV-1IIIB A17 variant, saquinavir-resistant HIV-1, HIV-1Ba-L, HIV-1JR-FL, macrophage-tropic (M-tropic) and T-cell-tropic (T-tropic) HIV-1 clade C clinical isolates, and a HIV-1 clade B clinical isolate resistant to protease inhibitors (PI)/zidovudine (AZT). The first nine HIV-1 isolates listed were obtained from the NIH AIDS Research and Reference Reagent Program, while the clade B and C clinical isolates were isolated at Kaplan Medical Center. HIV-1 T-tropic (X4) isolates were propagated by subculture in MT2 or cMAGI cells. HIV-1 M-tropic (R5) strains were propagated in cMAGI cells or U937 cells. Clarified cell-free supernatant fluids containing 105 to 108 50% cell culture infectious doses per ml served as viral inocula.
Detection of HIV-1 infection.
Viral growth was assessed by (i) microscopic assessment of syncytium formation by two independent observers of T-tropic HIV-1 infection of CD4+ MT2 cells or (ii) determining the number of infected cMAGI cells (11). The cMAGI cell line is a lymphocyte cell line stably transfected with a plasmid containing the HIV-1 long terminal repeat fused to β-galactosidase (29). These cells stain blue only when infected with HIV-1.
Inhibition of cell-free HIV-1 infectivity by copper oxide mix.
The capacity of a copper oxide powder mix (70% Cu2O and 30% CuO, >99% purity [SCM Corporation, Durham, NC; hereafter referred to as copper powder mix) to inhibit cell-free HIV-1 infectivity was determined as follows: RPMI 1640 medium only or containing various concentrations of copper powder mix was spiked with 105 50% tissue culture infective doses (TCID50) of HIV-1IIIB and added to 2 × 105 MT2 or cMAGI cells. After 2 hours of incubation at 37°C, the cells were washed thoroughly and incubated in a CO2 humidified incubator at 37°C. Viral infectivity was determined after 3 to 5 days of incubation at 37°C in a 5% CO2 moist incubator as described above.
Inhibition of cell-associated HIV-1 infectivity by copper powder mix.
The capacity of copper powder mix to inhibit cell-associated HIV-1 infectivity was assessed as follows: 106 washed H9+ cells (T cells that constantly produce and secrete HIV-1 virions) were resuspended in media containing different concentrations of cuprous oxide powder mix (percentage [weight/volume]). After 2 h of incubation in a CO2 humidified incubator at 37°C, the cells were centrifuged, and the supernatants were collected. The H9+ cells were centrifuged and washed again before being cocultured with attached cMAGI target cells (1:30 cell-cell ratio) in order to allow cell-associated HIV-1 transmission to occur. After coculture for 3 h, the H9+ cells were thoroughly removed from the attached cMAGI cells and discarded. The cMAGI cells were cultured for 3 days, and the number of cells infected with HIV-1 (stained blue) was then determined.
Determination of HIV-1 infectivity following filtration through filters containing copper powder mix.
We designed filters with a radius of 2.5 cm and a height of 1 cm containing in the bottom a 0.2-μm porous membrane (Pall Corporation) and 0 or 50 mg of copper oxide powder mix. In addition, to some filters we added a lower layer of resin containing activated carbon (Bio Chem Zorb; Aquarium Pharmaceutical Inc., Charlotte, PA). The copper and carbon layers were separated by a 0.2-μm porous membrane (Pall Corporation). Portions (10 ml) of RPMI 1640 culture medium without serum spiked with 105 to 108 50% cell culture infectious doses of HIV-1 per ml were put in a syringe attached to a filter and passed through the dry filter by using a peristaltic pump. The flowthrough was varied between 0.025 and 0.25 ml/cm2/min. The eluates were collected in sterile tubes, and either different aliquots were added to the target cells or the eluates were first subjected to multiple sequential dilutions in appropriate media containing serum. Each dilution was then added to MT2 or cMAGI target cells in 96-well microplates using three replicate wells per dilution. Following 3 or 4 days of culture, the infectious viral titers were determined by using a cytopathic effect assay (MT2) or counting cells stained blue (cMAGI). The titer was calculated by using the Reed-Muench endpoint dilution method (41). The titer of an unfiltered sample from each original clarified viral stock was also determined in parallel. Ten milliliters of culture medium without virus, passed through the filter, was used as a negative control for antiviral activity and for determination of cytotoxicity.
Determination of HIV-1 infectivity following filtration through filters containing copper oxide-impregnated fibers.
We designed filters with a radius of 2.5 cm and a height of 10 cm that were packed only with copper-impregnated polypropylene fibers. The copper oxide used to impregnate these polypropylene fibers had a purity of 99% as determined by the manufacturer (SCM Corporation, Durham, NC). For these experiments, we prepared very large viral stocks by infecting ∼109 MT2 cells with HIV-1IIIB, HIV-1 clade B clinical isolate resistant to AZT and PI, or a HIV-1 clade C clinical isolate resistant to AZT and collecting the supernatants after several days of culture. One hundred twenty-five milliliters of the culture supernatants were passed by gravity filtration through each filter, packed with 10, 20, or 30 g of the copper-impregnated fibers or 30 g of polypropylene fibers containing no copper (control filters). The first milliliter and the 100 ml that eluted from the filters were collected. Replicate 25-μl aliquots of these eluates were then fourfold sequentially diluted with culture medium in four separate wells in a 96-well plate. Each dilution of the virus was then transferred to MT2 target cells prepositioned in four separate wells in a 96-well plate. After 5 days of culture at 37°C, viral infectivity was determined by examining syncytium formation. One hundred twenty-five milliliters of culture medium without virus, passed through the filter, was used as a negative control for antiviral activity and for determination of cytotoxicity.
The capacity of these filters to inhibit HIV-1 infectivity of primary CD4+ cells was assessed as described above by adding the filtered viruses to PHA-activated PBMC in 96-well plates. After overnight incubation at 37°C in a 5% CO2 moist incubator, the PBMC were thoroughly washed with fresh medium and further incubated in the humidified incubator. After 8 days of culture, MT2 cells, at a ratio of 1:5 (PBMC/MT2) were added to the wells, and viral infectivity was determined after 3 to 5 days of incubation by microscopic assessment of syncytium formation in the MT2 cells.
RESULTS
Inhibition of cell-free HIV-1 infectivity by copper oxide mix.
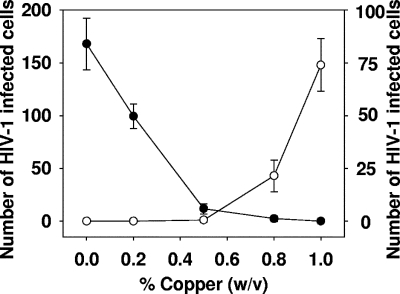
As depicted in Fig. 1 for one representative experiment with HIV-1BaL, there was a dose-dependent inhibition of HIV-1 infectivity with an 50% inhibitory concentration of ∼0.3% (wt/vol) copper oxide powder. Similar results were obtained when three other HIV-1 isolates were tested (HIV-1IIIB and M-tropic and T-tropic HIV-1 clade C clinical isolates). The cytotoxic concentration of drug that reduced the viable cell number by 50% was ∼0.9% (wt/vol) (Fig. 1). At the effective antiviral concentrations of the copper powder mix, there was no discernible cytotoxic effect on the cells (Fig. 1).
FIG. 1.
Inhibition of cell-free HIV-1 infectivity by cupric and cuprous oxide powder mix. cMAGI cells were exposed to HIV-1BaL in the presence of 0 to 1% of copper powder mix. After 2 h of incubation, the cells were washed thoroughly. After 3 days of culture, viral growth (•) was assessed by counting the number of HIV-1-infected cMAGI cells. The results shown are the means ± standard deviations (error bars) of triplicate samples. In parallel, cMAGI cells were exposed for 2 h to 0 to 1% copper powder mix in the absence of HIV-1, and the cytotoxicity was determined (○).
Inhibition of cell-associated HIV-1 infectivity by copper oxide powder mix.
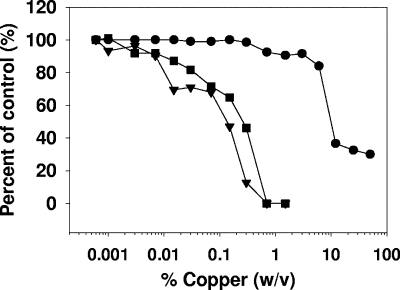
H9+ cells were exposed to copper oxide powder mix for 2 h. Then the cells were washed thoroughly and exposed to cMAGI cells. The infectivity of the virions that budded out of the H9+ cells after removal of the copper mix and which infected cMAGI cells in the process referred to as cell-associated HIV-1 transmission, was attenuated in a dose-dependent manner (Fig. 2).
FIG. 2.
Inhibition of cell-associated HIV-1 infectivity by cupric and cuprous oxide powder mix. H9+ cells, which were exposed for 2 h to different concentrations of copper powder mix, were cocultured with attached cMAGI target cells for 3 h, before being thoroughly removed. The cMAGI cells were then cultured for 3 days, and the number of cells infected with HIV-1 was then determined (▪). In addition, the supernatants containing HIV virions that budded out from the H9+ cells during the period when these cells were exposed to the copper were added to uninfected cMAGI cells. After 3 days of incubation, the number of infected cMAGI cells was determined (▾). The viabilities of the H9+ cells exposed to the various copper concentrations are also shown (•). Cell viability is expressed as a percentage of a control using untreated cells. The data shown are the average of duplicate samples. The differences between the duplicate samples were not more than 5%.
This experiment indicates that copper ions affected not only virions present in media but also virions being formed within the cytoplasm of cells during their exposure to copper and prior to their budding from cells. As expected, the infectivity of the virions that budded out from the H9+ cells during the incubation for 2 h of these cells with the copper oxide powder mix was also attenuated in a dose-dependent manner (Fig. 2).
At the effective antiviral concentrations of the copper powder mix, there was no discernible cytotoxic effect on the cells (Fig. 2), e.g., at 0.8 to 2% of copper powder. At these concentrations, viral infectivity was reduced to an extent that it could not be detected. The cytotoxic concentration found to cause 50% cell death of H9+ cells was ∼9% (wt/vol) of copper powder.
Inhibition of HIV-1 infectivity by filters containing copper powder mix.
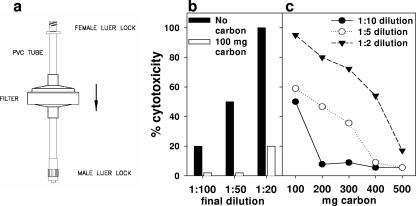
We tested the capacity of filters (Fig. 3a) not containing copper or containing 50 mg copper oxide powder mix (designated as negative-control filters and Cu filters, respectively) to inactivate HIV-1 in media by free-flow filtration. As shown in Fig. 3b, the filter eluate was toxic to MT2 target cells.
FIG. 3.
Cytotoxicity and inhibition of HIV-1 infectivity by filters containing copper powder mix. (a) Sketch of copper oxide-containing filter. (b) The cytotoxicity when applied on MT2 cells of 1:20, 1:50, or 1:100 final dilution of medium that eluted from filters containing 50 mg copper powder mix and no carbon layer or a 100 mg carbon layer is presented. The cytotoxicity after overnight incubation of the cells in a moist 5% CO2 incubator at 37°C was determined by the trypan blue exclusion assay. (C) The cytotoxicity of 1:2, 1: 5, and 1:10 final dilutions of medium that eluted from filters containing 50 mg copper powder mix and 100, 200, 300, 400, or 500 mg carbon, is presented. cMAGI cells were then exposed to these HIV-1-spiked and filtered samples. After 3 days of culture, the number of HIV-1-infected cMAGI cells (stained blue) was determined (see Table 1). The data shown are averages of duplicate samples. The differences between the duplicate samples were not more than 5%.
To overcome the cytotoxicity problem, we added a layer of resin containing activated carbon capable of reabsorbing any copper released from the upper copper layer during filtration. Addition of the carbon (100 mg) layer to the filter reduced significantly the cytotoxicity of the filter eluate (Fig. 3b). Increase in the quantity of the carbon layer further reduced the cytotoxicity of the eluate to insignificant levels (Fig. 3c).
After overcoming the cytotoxicity problem, our aim was to determine whether the Cu filters (containing 200 mg of carbon) are effective against a wide spectrum of HIV-1 isolates. For this purpose, 10 ml of RPMI 1640 medium, to which 2 × 105 TCID50 units of different HIV-1 were added, were passed through the filters at a flow rate of 5 ml/min. Fifty-microliter aliquots of the filtered medium containing HIV-1 were added to cMAGI target cells (final dilution of the eluate, 1:20). After 3 days of incubation, the number of HIV-infected cells was determined. Filtration of the HIV-1 spiked media at a flow rate of 5 ml/min, resulted in >3-log-unit reduction of all 12 HIV-1 isolates tested (Table 1), which represent a wide variety of strains and include viruses that are resistant to PI, nucleoside inhibitors, nonnucleoside inhibitors, syncytium-inducing and non-syncytium-inducing viruses, clade A, clade B, and clade C viruses, and T-tropic and M-tropic viral isolates. Each virus isolate was tested at least twice. Even when 1 ml of stock virus containing 2 × 106 TCID50 HIV-1 was filtered, no viral infectivity of cMAGI cells was noted. These experiments show that the virus isolates tested do not differ in their high susceptibility to copper.
TABLE 1.
HIV-1 isolates tested in this study
| HIV-1 isolate | Subtype | Phenotypea | Coreceptorb | Resistancec | Isolated |
|---|---|---|---|---|---|
| Ba-L | B | NSI | R5 | wt | Lab |
| IIIB | B | SI | X4 | wt | Lab |
| M461/L63P/V82T/184V | NDe | SI | R5X4 | PI | Lab |
| L10R/M461/L63P/V82T | ND | SI | X4 | PI | Lab |
| RTMC/MT2 | ND | SI | R5X4 | AZT | Lab |
| 96USNG20 | A | SI | R5X4 | wt | Clinical |
| IIIB A17 variant | B | SI | X4 | NNRTI | Lab |
| Saquinavir-resistant | ND | SI | X4 | PI | Lab |
| JR-FL | B | NSI | R5 | wt | Lab |
| ETH33 | C | NSI | R5 | wt | Clinical |
| ETH134 | C | SI | X4 | AZT | Clinical |
| ELI | B | SI | X4 | AZT/PI | Clinical |
Phenotype based according to the env gene.
Coreceptor usage: R5, CCR5; X4, CXCR4; R5X4, both CCR5 and CXCR4.
Abbreviations: wt, wild type; PI, protease inhibitors; NNRTI, nonnucleoside reverse transcriptase inhibitors.
Lab, laboratory-adapted isolate; clinical, clinical isolate.
ND, not determined.
Inhibition of HIV-1 infectivity with copper-impregnated fibers.
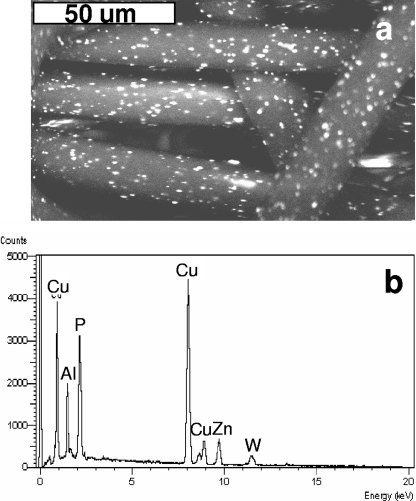
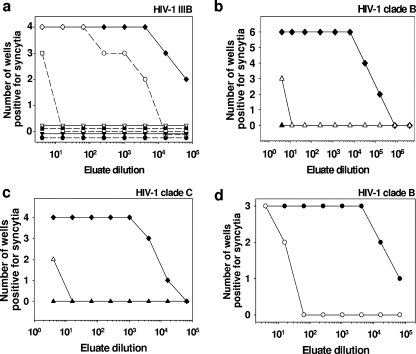
As described elsewhere (6, 23), we developed a platform technology that impregnates synthetic fibers with copper oxide (Fig. 4a). We examined the possibility that these copper-impregnated fibers, which do not allow release of copper particles, may eliminate the need for having 0.2-μm membranes and a carbon layer in the filters. We thus tested the capacity of filters packed only with copper-impregnated polypropylene fibers to inactivate HIV-1. Approximately 10% of the surfaces of the fibers used in these filters was copper, as determined by scanning electron microscopy followed by X-ray photoelectron spectra analysis (Fig. 4b). As possible future applications of these filters would be to inactivate HIV-1 in breast milk or in blood donations, which occur in greater volumes, we determined their capacity to inactivate HIV-1 when 125 ml of HIV-1-spiked culture supernatants were passed by gravity filtration through each filter. We first tested the efficacy of the filters against HIV-1IIIB when passing through filters containing 10, 20, or 30 g of the copper-impregnated fibers. All the wells exposed to the fourfold sequential dilutions of the eluates from the control filter (Fig. 5a) were positive for HIV-1 infection, with the exception of the last dilution (48), in which two out of the four wells were positive for HIV-1. Hence, the TCID50 of the virus that eluted from the control filter was 65,536 per 25 μl. In contrast, all the dilutions of the first milliliter collected from the virus filtered through each of the three filter configurations containing copper-impregnated fibers were negative for HIV-1 infection (Fig. 5a). HIV-1 infectivity in the 100 ml of the supernatant passed through filters containing 10, 20, and 30 g of the copper-impregnated fibers was partially decreased (Fig. 5, TCID50 of 2,560 per 25 μl), almost completely eliminated (Fig. 5a, TCID50 of 5 per 25 μl), or abolished (Fig. 5a), respectively. No cytotoxicity was observed in all wells. On the basis of these results, we then examined the efficacy of the filters containing 30 g of the copper-impregnated fibers against HIV-1 clinical isolates of clade B with resistance to AZT and PI (Fig. 5b) and the HIV-1 clinical isolate of clade C with resistance to AZT (Fig. 5c). Similar results were obtained when the filtered viruses were added to activated PBMC (Fig. 5d). No cytotoxicity was observed in all dilutions tested.
FIG. 4.
Copper oxide-impregnated polypropylene fibers. (a) Scanning electron microscopy (JEOL JMS 5410 LV scanning electron microscope, Japan) of a copper oxide-impregnated polypropylene fibers. Bar, 50 μm. (b) X-ray photoelectron spectrum analysis (Link IV, ISIS, Oxford Instruments, England) of the surfaces of the copper-impregnated fibers used to fill filters for deactivation of HIV-1.
FIG. 5.
Inactivation of HIV-1 by filters containing copper oxide-impregnated polypropylene fibers. (a) One hundred twenty-five milliliters of medium containing HIV-1IIIB was passed through filters containing 10 (circles), 20 (squares), or 30 (triangles) grams of copper-impregnated fibers. The first milliliter (black symbols) and the 100 ml (open symbols) that eluted from each filter were collected and subjected to sequential fourfold dilutions before they were added to MT2 target cells. For each viral dilution, four replicate wells were used. The eluate from the control filter (black diamonds) served as a positive control. (b) One hundred twenty-five milliliters of medium containing HIV-1 clinical isolate clade B, resistant to AZT and PI, were passed through filters containing 30 g of copper-impregnated fibers. The first milliliter (black triangles) and the 100 ml (white triangles) that eluted from each filter were collected and subjected to sequential fourfold dilutions before they were added to MT2 target cells. For each viral dilution, six replicate wells were used. The eluate from the control filter (black diamonds) served as a positive control. (c) One hundred twenty-five milliliters of medium containing HIV-1 clinical isolate clade C, resistant to AZT, was passed through filters containing 30 g of copper-impregnated fibers. The first milliliter (black triangles) and the 100 ml (white triangle) that eluted from each filter were collected and subjected to sequential fourfold dilutions before they were added to MT2 target cells. For each viral dilution, four replicate wells were used. The eluate from the control filter (black diamonds) served as a positive control. With all three isolates tested, viral infectivity was determined after 5 days of culture at 37°C by examining syncytium formation. Each well in which even one syncytium was seen was considered a positive well, i.e., infected with HIV-1. No cytotoxicity was observed in all examined wells. (d) Ten milliliters of medium containing HIV-1 clinical isolate clade B, resistant to AZT and PI, were passed through filters containing 10 g of copper-impregnated fibers (○) or control fibers without copper (•). The eluate was fourfold sequentially diluted and then added to PHA-activated PBMC in 96-well plates (triplicate wells). After overnight incubation at 37°C, the cells were thoroughly washed with RPMI 1640 medium and then cultured for additional 8 days at 37°C. MT2 cells were then cocultured with the PBMC (at a MT2/PBMC ratio of 5:1). After an additional 5 days of culture, the presence of syncytia was determined. Each well in which even one syncytium was seen was considered a positive well, i.e., infected with HIV-1. The experiments shown are representative of two similar experiments performed for each virus.
DISCUSSION
As a first step in evaluating the use of copper-based filters in deactivating HIV-1 in filterable solutions, in the current study, we examined the capacity of filters containing copper oxide powder or copper oxide-impregnated fibers to inactivate HIV-1 in medium. We found that the filters containing copper oxide powder efficiently and quickly inactivated HIV-1. Minutes of exposure of the virions to the copper oxide powder rendered them noninfectious. Not only free viruses but also virions being formed within the cytoplasm of cells during cell exposure to copper and before virion budding from the cells were affected. Furthermore, cell-associated HIV-1 transmission was also attenuated in a dose-dependent manner by the copper oxide powder mix.
Depending on the amount of copper used, very large volumes could be “sterilized” by filtration. However, the eluate of the copper oxide-containing filters had a cytotoxic effect on the target cells used for evaluating HIV-1 infection. To overcome this problem, we designed filters with two layers. The upper layer included the copper oxide powder mix, and the second layer included activated carbon, capable of reabsorbing any copper particles released from the upper copper layer during filtration. While we eliminated the problem of cytotoxicity by using the carbon layer, the flow rate of the liquids through the filters was significantly slowed down, apparently due to the presence of a 0.2-μm porous membrane separating the copper and carbon layers. Subsequently, we were able to eliminate the need of the carbon layer and the porous membrane by impregnating the copper oxide particles in polymeric fibers (6, 23). The eluates from filters containing the copper oxide-impregnated fibers showed no cytotoxicity at the dilution tested. Importantly, these filters very efficiently reduced the infectious viral titers (>99.9% reduction) of all viral isolates tested, including wild-type and drug-resistant isolates, laboratory and clinical isolates, and isolates from different clades.
In contrast to the low sensitivity of human tissue (skin or other) to copper (25), microorganisms are highly susceptible to copper (7). Copper ions, either alone or in complexes, have been used for centuries to disinfect fluids, solids, and tissues (5, 19). Bacteria and other microorganisms have different mechanisms to deal with excess copper, such as active transport membrane efflux pumps (e.g., references 22 and 42). Viruses, however, do not possess mechanisms to tolerate excess metal ions, making them extremely susceptible to copper ions. The deactivation by copper of infectious bronchitis virus, poliovirus, herpes simplex virus, and other enveloped or nonenveloped, single- or double-stranded DNA or RNA viruses, has been reported (reviewed in reference 7). Sagripanti and Lightfoote (44) reported in 1996 that HIV-1 was inactivated by cupric ions when the virus was free in solution and also 3 h after cell infection. Subsequently, it was showed that stoichiometric concentrations of copper ions inactivate the HIV-1 protease (27, 28), which is an essential protein for replication of the virus. In addition, copper ions may cause nonspecific damage to HIV virions by damaging their envelope phospholipids and denaturing the virus nucleic acids by binding to and/or disordering helical structures and/or by cross-linking between and within the nucleic acid strands (7). The multisite antiviral mechanism of copper oxide explains the high susceptibilities of all HIV-1 viral isolates tested with no clade or other specificity.
Breast-feeding is an important source of HIV-1 MTCT. While milk substitutes may be given by HIV-1-infected mothers to their infants, breast-feeding remains the recommendation of the WHO, UNICEF, and CDC in developing countries, especially in sub-Saharan Africa, where children suffer from malnutrition and infection, and where over 50% of adults living with HIV/AIDS are women of childbearing age (10, 51, 53). However, developing a means to inactivate the >600,000 HIV-1 virions estimated to be ingested by an infant on a daily basis during the first few months of life through breast milk (32) is critical. To reduce infant illness and mortality, a low-cost approach to infant feeding is needed that prevents HIV MTCT and enteric/respiratory infections from formula use.
At present, several approaches are being developed to inactivate HIV-1 and other pathogens in contaminated fluids. Most inactivation approaches use chemicals that bind nucleic acids either directly (40) or when subjected to UV light (16), preventing viral replication. The toxicity and mutagenicity of these chemicals raise significant concern. In addition, these deactivation modalities, especially those requiring exposure to UV light, are highly expensive and are not practicable in regards to MTCT. Thus, the importance of finding new modes to inactivate pathogens in fluids cannot be overemphasized. Furthermore, if such means were inexpensive, their employment by blood banks and/or HIV-1-infected mothers in developing countries would be facilitated and have a significant and immediate impact on reducing the transmission of HIV-1 and other pathogens.
Copper is considered safe for humans, as demonstrated by the widespread and prolonged use by women of copper intrauterine devices (4, 26). The biochemistry of copper in humans has long been studied and is well understood (45, 49). Excess copper in humans at the level which is expected to be leached out into the milk, blood, or plasma as it passes through the copper filters (less than 2 μg/dl [data not shown]) is not toxic. This represents only a minor perturbation to the normal serum levels of copper in blood, which range from approximately 80 to 160 μg/dl (1, 54). As a relatively benign trace element, humans physiologically adapt to changes in copper intake (33). The National Academy of Sciences Committee recently established the U.S. recommended daily allowance of 0.9 mg of copper for healthy adults. This committee also noted that daily intakes up to 3 mg/day in children and 8 to 10 mg/day for adults are considered tolerable and nontoxic (48). In healthy humans, any excess copper absorbed is readily excreted (34).
The use of copper is a radical departure from technologies currently being developed for antiviral inactivation. Obviously, the effect of copper filters on important components found in breast milk, such as the protecting leukocytes and immunoglobulins, or those components found in blood, such as coagulation factors, should be thoroughly studied before this technology may be implemented. We are indeed in the process of carrying out these studies. Additionally, a filter strategy tailored to the uniqueness of the mother-child feeding relationship within cultural sanctions of resource-poor societies must be developed. We realize that the use of bottled milk acquired with a breast pump with copper filters is not feasible in most African countries, mainly due to the absence of “safe” water and social constraints. We are thus in the process of designing a breast shield that may be used by HIV-1-infected mothers that will allow them to safely breast-feed their children without posing a cultural or social threat to them.
Acknowledgments
This study was funded by Cupron Inc., Greensboro, NC. G.B. is the Chief Medical Scientist, and J.G. is the Chief Executive Officer of Cupron Inc.
Footnotes
Published ahead of print on 10 December 2007.
REFERENCES
- 1.Ahmed, M. J., I. Jahan, and S. Banoo. 2002. A simple spectrophotometric method for the determination of copper in industrial, environmental, biological and soil samples using 2,5-dimercapto-1,3,4-thiadiazole. Anal. Sci. 18:805-810. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez, M., S. Oyonarte, P. M. Rodriguez, and J. M. Hernandez. 2002. Estimated risk of transfusion-transmitted viral infections in Spain. Transfusion 42:994-998. [DOI] [PubMed] [Google Scholar]
- 3.Bamaga, M. S., F. F. Bokhari, A. M. Aboud, M. Al Malki, and F. Q. Alenzi. 2006. Nucleic acid amplification technology screening for hepatitis C virus and human immunodeficiency virus for blood donations. Saudi Med. J. 27:781-787. [PubMed] [Google Scholar]
- 4.Bilian, X. 2002. Intrauterine devices. Best. Pract. Res. Clin. Obstet. Gynaecol. 16:155-168. [DOI] [PubMed] [Google Scholar]
- 5.Block, S. S. 2001. Definition in terms, p. 19-31. In S. S. Block (ed.), Disinfection, sterilization, and preservation, 5th ed. Lippincott Williams & Wilkins, Hagerstown, MD.
- 6.Borkow, G., and J. Gabbay. 2004. Putting copper into action: copper-impregnated products with potent biocidal activities. FASEB J. 18:1728-1730. [DOI] [PubMed] [Google Scholar]
- 7.Borkow, G., and J. Gabbay. 2005. Copper as a biocidal tool. Curr. Med. Chem. 12:2163-2175. [DOI] [PubMed] [Google Scholar]
- 8.Borkow, G., R. W. Sidwell, D. F. Smee, D. L. Barnard, J. D. Morrey, H. H. Lara-Villegas, Y. Shemer-Avni, and J. Gabbay. 2007. Neutralizing viruses in suspensions by copper oxide-based filters. Antimicrob. Agents Chemother. 51:2605-2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brahmbhatt, H., and R. H. Gray. 2003. Child mortality associated with reasons for non-breastfeeding and weaning: is breastfeeding best for HIV-positive mothers? AIDS 17:879-885. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. 2003. Preventing mother-to-child transmission (MTCT). In Global AIDS program technical strategies overview. Centers for Disease Control and Prevention, Atlanta, GA.
- 11.Chackerian, B., E. M. Long, P. A. Luciw, and J. Overbaugh. 1997. Human immunodeficiency virus type 1 coreceptors participate in postentry stages in the virus replication cycle and function in simian immunodeficiency virus infection. J. Virol. 71:3932-3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatterjee, R., P. Tarwater, D. Neogi, A. Ghosh, P. Roy, S. Sengupta, and P. Gupta. 2004. Estimation of HIV seroprevalence in blood bank camps in Kolkata, India. Transfus. Med. 14:77-78. [DOI] [PubMed] [Google Scholar]
- 13.Chikwem, J. O., I. Mohammed, G. C. Okara, N. C. Ukwandu, and T. O. Ola. 1997. Prevalence of transmissible blood infections among blood donors at the University of Maiducuri Teaching Hospital, Maiduguri, Nigeria. East Afr. Med. J. 74:213-216. [PubMed] [Google Scholar]
- 14.Consten, E. C., J. T. van der Meer, F. de Wolf, H. A. Heij, P. C. Henny, and J. J. van Lanschot. 1997. Risk of iatrogenic human immunodeficiency virus infection through transfusion of blood tested by inappropriately stored or expired rapid antibody assays in a Zambian hospital. Transfusion 37:930-934. [DOI] [PubMed] [Google Scholar]
- 15.Coovadia, H. M., N. C. Rollins, R. M. Bland, K. Little, A. Coutsoudis, M. L. Bennish, and M. L. Newell. 2007. Mother-to-child transmission of HIV-1 infection during exclusive breastfeeding in the first 6 months of life: an intervention cohort study. Lancet 369:1107-1116. [DOI] [PubMed] [Google Scholar]
- 16.Corbin, F. 2002. Pathogen inactivation of blood components: current status and introduction of an approach using riboflavin as a photosensitizer. Int. J. Hematol. 76(Suppl. 2):253-257. [DOI] [PubMed] [Google Scholar]
- 17.Coutsoudis, A., and N. Rollins. 2003. Breast-feeding and HIV transmission: the jury is still out. J. Pediatr. Gastroenterol. Nutr. 36:434-442. [DOI] [PubMed] [Google Scholar]
- 18.De Cock, K. M., M. G. Fowler, E. Mercier, I. de Vincenzi, J. Saba, E. Hoff, D. J. Alnwick, M. Rogers, and N. Shaffer. 2000. Prevention of mother-to-child HIV transmission in resource-poor countries: translating research into policy and practice. JAMA 283:1175-1182. [DOI] [PubMed] [Google Scholar]
- 19.Dollwet, H. H. A., and J. R. J. Sorenson. 2001. Historic uses of copper compounds in medicine. Trace Elements Med. 2:80-87. [Google Scholar]
- 20.Faundez, G., M. Troncoso, P. Navarrete, and G. Figueroa. 2004. Antimicrobial activity of copper surfaces against suspensions of Salmonella enterica and Campylobacter jejuni. BMC Microbiol. 4:19-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fowler, M. G., and M. L. Newell. 2002. Breast-feeding and HIV-1 transmission in resource-limited settings. J. Acquir. Immune Defic. Syndr. 30:230-239. [DOI] [PubMed] [Google Scholar]
- 22.Franke, S., G. Grass, C. Rensing, and D. H. Nies. 2003. Molecular analysis of the copper-transporting efflux system CusCFBA of Escherichia coli. J. Bacteriol. 185:3804-3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gabbay, J., J. Mishal, E. Magen, R. C. Zatcoff, Y. Shemer-Avni, and G. Borkow. 2006. Copper oxide impregnated textiles with potent biocidal activities. J. Ind. Textiles 35:323-335. [Google Scholar]
- 24.Goodnough, L. T., A. Shander, and M. E. Brecher. 2003. Transfusion medicine: looking to the future. Lancet 361:161-169. [DOI] [PubMed] [Google Scholar]
- 25.Hostynek, J. J., and H. I. Maibach. 2003. Copper hypersensitivity: dermatologic aspects—an overview. Rev. Environ. Health. 18:153-183. [DOI] [PubMed] [Google Scholar]
- 26.Hubacher, D., R. Lara-Ricalde, D. J. Taylor, F. Guerra-Infante, and R. Guzman-Rodriguez. 2001. Use of copper intrauterine devices and the risk of tubal infertility among nulligravid women. N. Engl. J. Med. 345:561-567. [DOI] [PubMed] [Google Scholar]
- 27.Karlstrom, A. R., and R. L. Levine. 1991. Copper inhibits the protease from human immunodeficiency virus 1 by both cysteine-dependent and cysteine-independent mechanisms. Proc. Natl. Acad. Sci. USA 88:5552-5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karlstrom, A. R., B. D. Shames, and R. L. Levine. 1993. Reactivity of cysteine residues in the protease from human immunodeficiency virus: identification of a surface-exposed region which affects enzyme function. Arch. Biochem. Biophys. 304:163-169. [DOI] [PubMed] [Google Scholar]
- 29.Kimpton, J., and M. Emerman. 1992. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated β-galactosidase gene. J. Virol. 66:2232-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lackritz, E. M. 1998. Prevention of HIV transmission by blood transfusion in the developing world: achievements and continuing challenges. AIDS 12(Suppl. A):S81-S86. [PubMed] [Google Scholar]
- 31.Larkin, M. 2000. WHO's blood-safety initiative: a vain effort? Lancet 355:1245. [DOI] [PubMed] [Google Scholar]
- 32.Lewis, P., R. Nduati, J. K. Kreiss, G. C. John, B. A. Richardson, D. Mbori-Ngacha, J. Ndinya-Achola, and J. Overbaugh. 1998. Cell-free human immunodeficiency virus type 1 in breast milk. J. Infect. Dis. 177:34-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linder, M. 2002. Biochemistry and molecular biology of copper in mammals, p. 1-32. In E. J. Massaro (ed.), Handbook of copper pharmacology and toxicology. Humana Press, Totowa, NJ.
- 34.Massaro, E. J. (ed.). 2002. Handbook of copper pharmacology and toxicology. Humana Press, Totowa, NJ.
- 35.McFarland, W., D. Mvere, and D. Katzenstein. 1997. Risk factors for prevalent and incident HIV infection in a cohort of volunteer blood donors in Harare, Zimbabwe: implications for blood safety. AIDS 11(Suppl. 1):S97-S102. [PubMed] [Google Scholar]
- 36.Moore, A., G. Herrera, J. Nyamongo, E. Lackritz, T. Granade, B. Nahlen, A. Oloo, G. Opondo, R. Muga, and R. Janssen. 2001. Estimated risk of HIV transmission by blood transfusion in Kenya. Lancet 358:657-660. [DOI] [PubMed] [Google Scholar]
- 37.Mulligan, A. M., M. Wilson, and J. C. Knowles. 2003. The effect of increasing copper content in phosphate-based glasses on biofilms of Streptococcus sanguis. Biomaterials 24:1797-1807. [DOI] [PubMed] [Google Scholar]
- 38.Offergeld, R., D. Faensen, S. Ritter, and O. Hamouda. 2005. Human immunodeficiency virus, hepatitis C and hepatitis B infections among blood donors in Germany 2000-2002: risk of virus transmission and the impact of nucleic acid amplification testing. Eurosurveillance 10:8-11. [PubMed] [Google Scholar]
- 39.Ogundele, M. O., and J. B. Coulter. 2003. HIV transmission through breastfeeding: problems and prevention. Ann. Trop. Paediatr. 23:91-106. [DOI] [PubMed] [Google Scholar]
- 40.Ohagen, A., V. Gibaja, S. Aytay, J. Horrigan, D. Lunderville, and A. Lazo. 2002. Inactivation of HIV in blood. Transfusion 42:1308-1317. [DOI] [PubMed] [Google Scholar]
- 41.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 42.Rensing, C., and G. Grass. 2003. Escherichia coli mechanisms of copper homeostasis in a changing environment. FEMS Microbiol. Rev. 27:197-213. [DOI] [PubMed] [Google Scholar]
- 43.Rousseau, C. M., R. W. Nduati, B. A. Richardson, M. S. Steele, G. C. John-Stewart, D. A. Mbori-Ngacha, J. K. Kreiss, and J. Overbaugh. 2003. Longitudinal analysis of human immunodeficiency virus type 1 RNA in breast milk and of its relationship to infant infection and maternal disease. J. Infect. Dis. 187:741-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sagripanti, J. L., and M. M. Lightfoote. 1996. Cupric and ferric ions inactivate HIV. AIDS Res. Hum. Retrovir. 12:333-337. [DOI] [PubMed] [Google Scholar]
- 45.Sarkar, B. 2000. Copper transport and its defect in Wilson disease: characterization of the copper-binding domain of Wilson disease ATPase. J. Inorg. Biochem. 79:187-191. [DOI] [PubMed] [Google Scholar]
- 46.Shang, G., C. R. Seed, F. Wang, D. Nie, and A. Farrugia. 2007. Residual risk of transfusion-transmitted viral infections in Shenzhen, China, 2001 through 2004. Transfusion 47:529-539. [DOI] [PubMed] [Google Scholar]
- 47.Suryavanshi, N., S. Jonnalagadda, A. S. Erande, J. Sastry, H. Pisal, K. E. Bharucha, A. Shrotri, P. M. Bulakh, M. A. Phadke, R. C. Bollinger, and A. V. Shankar. 2003. Infant feeding practices of HIV-positive mothers in India. J. Nutr. 133:1326-1331. [DOI] [PubMed] [Google Scholar]
- 48.Trumbo, P., A. A. Yates, S. Schlicker, and M. Poos. 2001. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J. Am. Diet. Assoc. 101:294-301. [DOI] [PubMed] [Google Scholar]
- 49.Turnlund, J. R. 1998. Human whole-body copper metabolism. Am. J. Clin. Nutr. 67:960S-964S. [DOI] [PubMed] [Google Scholar]
- 50.UNAIDS. 2002. Table of country-specific HIV/AIDS estimates and data report on the global HIV/AIDS epidemic, June 2000. Report on the HIV/AIDS epidemic June 2000. UNAIDS (Joint United Nations Program on HIV/AIDS, Geneva, Switzerland.
- 51.United Nations. 2001. SCN 28th session, p. 25-30. In United Nations system's forum on nutrition. SCN News no. 22. United Nations Administrative Committee on Coordination Sub-Committee on Nutrition (ACC/SCN), United Nations. http://acc.unsystem.org/scn.
- 52.Van de Perre, P. 2003. Transfer of antibody via mother's milk. Vaccine 21:3374-3376. [DOI] [PubMed] [Google Scholar]
- 53.World Health Organization. 2001. New data on the prevention of mother-to-child transmission of HIV and their policy implications. Conclusions and recommendations. WHO Technical Consultation on behalf of the UNFPA/UNICEF/WHO/UNAIDS Inter-Agency Task Team on Mother-to-Child Transmission of HIV. WHO publication no. WHO/RHR/01.28. World Health Organization, Geneva, Switzerland.
- 54.Yilmaz, M. E., M. Kiraz, and I. H. Kara. 2000. The evaluation of serum zinc and copper levels in hemodialysis patients in Southeast Turkey. Dialysis Transplant. 29:718-721. [Google Scholar]