Abstract
The primary objective of this study was to measure atazanavir-ritonavir and tenofovir pharmacokinetics when the drugs were used in combination in young adults with human immunodeficiency virus (HIV). HIV-infected subjects ≥18 to <25 years old receiving (≥28 days) 300/100 mg atazanavir-ritonavir plus 300 mg tenofovir disoproxil fumarate (TDF) plus one or more other nucleoside analogs underwent intensive 24-h pharmacokinetic studies following a light meal. Peripheral blood mononuclear cells were obtained at 1, 4, and 24 h postdose for quantification of intracellular tenofovir diphosphate (TFV-DP) concentrations. Twenty-two subjects were eligible for analyses. The geometric mean (95% confidence interval [CI]) atazanavir area under the concentration-time curve from 0 to 24 h (AUC0-24), maximum concentration of drug in serum (Cmax), concentration at 24 h postdose (C24), and total apparent oral clearance (CL/F) values were 35,971 ng·hr/ml (30,853 to 41,898), 3,504 ng/ml (2,978 to 4,105), 578 ng/ml (474 to 704), and 8.3 liter/hr (7.2 to 9.7), respectively. The geometric mean (95% CI) tenofovir AUC0-24, Cmax, C24, and CL/F values were 2,762 ng·hr/ml (2,392 to 3,041), 254 ng/ml (221 to 292), 60 ng/ml (52 to 68), and 49.2 liter/hr (43.8 to 55.3), respectively. Body weight was significantly predictive of CL/F for all three drugs. For every 10-kg increase in weight, there was a 10%, 14.8%, and 6.8% increase in the atazanavir, ritonavir, and tenofovir CL/F, respectively (P ≤ 0.01). Renal function was predictive of tenofovir CL/F. For every 10 ml/min increase in creatinine clearance, there was a 4.6% increase in tenofovir CL/F (P < 0.0001). The geometric mean (95% CI) TFV-DP concentrations at 1, 4, and 24 h postdose were 96.4 (71.5 to 130), 93.3 (68 to 130), and 92.7 (70 to 123) fmol/million cells. There was an association between renal function, tenofovir AUC, and tenofovir Cmax and intracellular TFV-DP concentrations, although none of these associations reached statistical significance. In these HIV-infected young adults treated with atazanavir-ritonavir plus TDF, the atazanavir AUC was similar to those of older adults treated with the combination. Based on data for healthy volunteers, a higher tenofovir AUC may have been expected, but was not seen in these subjects. This might be due to faster tenofovir CL/F because of higher creatinine clearance in this age group. Additional studies of the exposure-response relationships of this regimen in children, adolescents, and adults would advance our knowledge of its pharmacodynamic properties.
An increasing number of adolescents and young adults are being infected with human immunodeficiency virus (HIV) (27). However, when new antiretroviral drugs are developed, pharmacokinetic studies are performed in adults and then in children, leaving the adolescent age group often underrepresented. Growth and development are not linear processes (12); thus, antiretroviral pharmacokinetics in adolescents and young adults may differ from those in young children and older adults (4, 15, 26).
Once-daily antiretroviral drugs may be preferred in the treatment of HIV-infected young adults to improve adherence to treatment regimens (24). Tenofovir disoproxil fumarate (TDF [Viread]; Gilead Sciences, Foster City, CA), a nucleotide reverse transcriptase inhibitor, and atazanavir (Reyataz; Bristol Myers Squibb, Princeton, NJ), a protease inhibitor, represent highly efficacious once-daily agents for the treatment of HIV (2, 7, 11, 20, 22, 23). Antiretroviral regimens, including TDF and ritonavir-boosted atazanavir, have proven efficacious in HIV-infected adults (18). Unfortunately, there are no intensive pharmacokinetic data on these agents in combination in HIV-infected adolescents or young adults. Additionally, a high rate of virologic failure was recently observed in a study of adolescents switched to a once-daily regimen that included atazanavir-ritonavir (16). In this study, three of four previously virologically suppressed adolescents who experienced virologic failure upon switching to atazanavir-ritonavir were on TDF. This report highlights the need to fully characterize the pharmacokinetics and potential interactions of antiretroviral drugs in adolescents before prescribing them in this patient population.
Children and adolescents have faster apparent oral clearances of atazanavir and tenofovir than adults. Thus, they require higher doses on a mg/m2 basis to achieve similar exposures (9, 15). The age or size when clearance slows to adult values is unknown and likely differs among antiretroviral drugs. Additionally, there is a bidirectional drug-drug interaction between TDF and atazanavir. In HIV-infected adults, TDF causes an approximate 25% decrease in the atazanavir area under the concentration-time curve (AUC) when the drug is given as either unboosted or ritonavir-boosted atazanavir, and the unboosted atazanavir minimum concentration of drug in serum (Cmin) is reduced 40% when the drug is given with TDF (25). Conversely, the tenofovir AUC and maximum concentration of drug in serum (Cmax) are increased 37% and 34%, respectively, in the presence of atazanavir in healthy volunteers (1). The magnitude of this interaction is not well characterized in HIV-infected adults, and there are no intensive pharmacokinetic data on this interaction in children and adolescents.
The primary objective of this study was to determine the pharmacokinetics of atazanavir-ritonavir and tenofovir when used in combination to treat HIV-infected adolescents and young adults. Secondary objectives included evaluating predictors of atazanavir and tenofovir plasma pharmacokinetics and describing and evaluating predictors of the intracellular concentrations of tenofovir diphosphate (TFV-DP).
(This study was presented at the Eighth International Workshop on Clinical Pharmacology of HIV Therapy, April 16-18, 2007, Budapest, Hungary, as oral abstract 12.)
MATERIALS AND METHODS
Subjects.
HIV-infected persons ≥18 to <25 years old on a stable antiretroviral regimen containing 300 mg TDF once daily plus 300/100 mg atazanavir-ritonavir once daily plus at least one other nucleoside reverse transcriptase inhibitor for at least 28 days were eligible to participate. There were no CD4 count or viral load restrictions. Subjects who were pregnant, required active therapy for a malignancy, had a gastrointestinal condition which could interfere with drug administration or absorption, alanine or aspartate aminotransferase values >5 times the upper limit of normal, serum creatinine ≥2.5 times the upper limit of normal, concurrent treatment with a protease inhibitor other than atazanavir-ritonavir or a nonnucleoside reverse transcriptase inhibitor, hemoglobin of ≤7 g/dl, or a severe clinical toxicity(ies) were excluded. Subjects could not be on the following agents within 1 week prior to the intensive pharmacokinetic visit: antiarrhythmics; warfarin; antiepileptics; itraconazole; voriconazole; astemizole; terfenadine; rifampin; irinotecan; cidofovir; valganciclovir; midazolam; triazolam; bepridil; diltiazem; nifedipine; verapamil; ergot derivatives; cisapride; herbal products, including but not limited to St. John's wort, garlic supplements, and echinacea; lovastatin; simvastatin; cyclosporine; tacrolimus; fluticasone; investigational drugs; pimozide; clarithromycin; erythromycin; or proton pump inhibitors.
This study was conducted through the Adolescent Trials Network for HIV/AIDS Interventions. The study was approved by the institutional review boards at each site recruiting subjects, and all subjects provided written informed consent.
Design.
This was a multisite, open-label, 24-h, intensive pharmacokinetic study. Subjects were contacted via telephone for the 2 days preceding the intensive pharmacokinetic visit to ensure adherence to and appropriate timing of medication administration. For the intensive pharmacokinetic visit, subjects were admitted fasting, a predose concentration for quantification of atazanavir, ritonavir, and tenofovir was obtained, a light-fat meal (400 to 500 kcal and 13 g fat) was consumed, and an observed dose of study medication was administered (time 0). Blood was obtained at the following intervals postdose for quantification of atazanavir, ritonavir, and tenofovir plasma pharmacokinetics: 1, 2, 4, 6, 8, 12, and 24 h. Peripheral blood mononuclear cell samples (PBMCs) were obtained at 1, 4, and 24 h postdose for quantification of intracellular TFV-DP concentrations.
Bioanalyses. (i) Atazanavir-ritonavir in plasma.
Plasma was separated and frozen at −70°C within 30 min of blood collection. Atazanavir and ritonavir plasma concentrations were measured using a simultaneous, validated, reversed-phase high-performance liquid chromatographic (HPLC) UV detection method (University of Colorado antiviral pharmacology laboratory, Denver, CO). Briefly, after the addition of internal standard, a liquid-liquid extraction procedure with t-butylmethylether at basic pH was used to prepare the samples. The chromatographic separation of the compounds and the internal standard was accomplished on a Waters YMC HPLC 100- by 4.6-mm reversed-phase octyl column with a 3-micron particle size (Waters Corp., Milford, MA). The mobile phase consisted of 54.7% 20 mM acetate buffer-45.3% acetonitrile, pH 4.9, with an isocratic flow rate of 1 ml/min. Detection and quantification of the drugs was at 212 nm. For both atazanavir and ritonavir, the assay was linear over the range of 20 to 20,000 ng/ml, with a minimum limit of quantification of 20 ng/ml using 0.2 ml of human plasma. The standard curves generated had coefficients of determination greater than 0.9988. Precision and accuracy were measured in quality controls at 75, 750, and 7,500 ng/ml, and all accuracies were within 15% of the nominal concentration, with percent relative standard deviations of less than 10%.
(ii) Tenofovir in plasma.
Plasma concentrations of tenofovir were determined by a validated LC-tandem mass spectrometry (LC-MS-MS) assay (University of Colorado antiviral pharmacology laboratory, Denver, CO) (6). Briefly, after the addition of adefovir as the internal standard, trifluoroacetic acid was used to produce a protein-free extract. Ten-microliter aliquots of the samples were injected into the HPLC column with the mobile phase (3% acetonitrile-1% acetic acid, aqueous) flowing isocratically at 0.2 ml/min with 7-min sample run times. Chromatographic separation was achieved with a Polar-PR Synergi 2-mm by 150-mm reversed-phase analytical column. Detection of tenofovir was achieved by electrospray ionization MS-MS (TSQ quantum; Thermo Fisher, San Jose, CA) in the positive ion mode using 288/176 and 274/162 transitions, respectively. The method was linear from 10 to 750 ng/ml, with a minimum quantifiable limit of 10 ng/ml when 0.25-ml aliquots were analyzed. Accuracy and precision were within ±15%.
(iii) Intracellular TFV-DP.
PBMCs were isolated using 8-ml citrate cell preparation tubes, and a hemocytometer was used to obtain the cell count. An indirect method was developed that first isolated TFV-DP from tenofovir-monophosphate and tenofovir. TFV-DP was then dephosphorylated with acid phosphatase to form tenofovir. Tenofovir was desalted and concentrated, making its tandem mass spectral detection possible. Intracellular TFV-DP concentrations in PBMCs were determined with a validated LC-MS-MS assay using a TSQ quantum (ThermoElectron, San Jose, CA) (University of Colorado antiviral pharmacology laboratory, Denver, CO) (13). The assay was linear in the range of 50 fmol to 10,000 fmol per sample. The minimal quantifiable limit is 10 fmol/million cells when 5 million cells are analyzed. Accuracy and precision are within ±15%.
Pharmacokinetic analyses.
The atazanavir, ritonavir, and tenofovir AUCs for the 0- to 24-h dosing intervals (AUC0-24) were determined by using the linear-log trapezoidal rule and noncompartmental methods (WinNonLin version 5.0.1; Pharsight Corporation, Mountain View, CA). The Cmax, time to Cmax (Tmax), and concentration at 24 h postdose (C24) values were determined visually. The total apparent oral clearance (CL/F) was determined as dose/AUC0-24.
Statistical analyses.
This study was designed to enroll 30 individuals, with the goal of accruing 20 evaluable subjects. The following criteria were applied a priori to consider a subject's concentrations evaluable: (i) subject had to have pharmacokinetic samples obtained at the following times during the 24-h study period: predose and 1, 4, 8, 12, and 24-h postdose, and (ii) subject must have been adherent to medication administration as evidenced by detectable predose atazanavir (>20 ng/ml) and tenofovir plasma (>10 ng/ml) concentrations.
Creatinine clearance was estimated by using the Cockcroft-Gault equation (5). Adjusted body weight was used in the Cockcroft-Gault formula if a subject was ≥130% of ideal body weight.
Linear regression analyses were used to evaluate predictors of atazanavir, ritonavir, and tenofovir pharmacokinetics. Data were log transformed to reduce skewness when necessary. All statistical tests were performed in SAS version 9.1 (SAS, Cary, NC). There were no adjustments made for multiple comparisons.
RESULTS
Twenty-five (11 female and 14 male) subjects enrolled and completed the study. The data for three subjects were excluded from the plasma analyses due to tenofovir and/or atazanavir predose concentrations below the limits of assay detection (i.e., nonadherence). Thus, 22 subjects were eligible for the pharmacokinetic analyses. Of the 22 subjects eligible for the data analyses, all subjects were Tanner stage 5. The racial distribution for the 22 subjects was 14 black, 2 white, 5 other or mixed, and 1 unknown. Six of these subjects were of Hispanic or Latino ethnicity. In addition to atazanavir-ritonavir and TDF, concomitant antiretroviral drugs included emtricitabine (n = 17), delayed-release didanosine (n = 2), stavudine (n = 1), and abacavir and lamivudine (n = 2). Seventy-three percent of subjects had viral loads of <400 copies/ml. Among those with detectable HIV-1 RNA values, the values ranged from 431 to 27,914 copies/ml. The characteristics of the study subjects are shown in Table 1.
TABLE 1.
Characteristics of study subjectsa
| Subject characteristic | Value (median [range]) |
|---|---|
| Age (yr) | 23 (18.6-24.9) |
| Weight (kg) | 70.3 (46.9-131.6) |
| Body surface area (m2) | 1.86 (1.45-2.62) |
| Serum creatinine (mg/dl) | 0.8 (0.6-1.3) |
| Total bilirubin (mg/dl) | 1.6 (0.4-4.7) |
| Creatinine clearance (ml/min)b | 129 (78.9-274.8) |
| CD4 count (cells/mm3) | 430 (12-959) |
Twenty-two subjects were evaluated.
Estimated using the Cockcroft-Gault equation.
The geometric mean (95% confidence interval [CI]) atazanavir, ritonavir, and tenofovir plasma pharmacokinetic parameters are shown in Table 2. In regression modeling, gender and race were not predictive of atazanavir or tenofovir pharmacokinetics. No significant association between atazanavir concentrations and total bilirubin levels was observed in this study. Ritonavir concentrations were associated with atazanavir concentrations. For every 1,000 ng/hr/ml increase in the ritonavir AUC, there was, on average, a 6.4% increase in the atazanavir AUC (P = 0.02). Weight was associated with both the atazanavir (Fig. 1) and tenofovir CL/F. For every 10-kg increase in weight, there was, on average a 10% increase in the atazanavir CL/F (P = 0.0005) and a 6.8% increase in the tenofovir CL/F (P = 0.003). The relationships were similar for body surface area and the atazanavir (P = 0.004) and tenofovir (P = 0.013) CL/F values. When data for the four patients weighing >120 kg were removed, the slopes for both drugs remained similar, though the P values were no longer significant (P value of 0.1 for atazanavir clearance and P value of 0.2 for tenofovir). Renal function was predictive of tenofovir CL/F. For every 10 ml/min increase in creatinine clearance, there was, on average, a 4.6% increase in the tenofovir CL/F (P < 0.0001) (Fig. 2). This association remained significant even after the data for the subject with an estimated creatinine clearance of 274 ml/min was removed (P = 0.003). There was no association between HIV-1 RNA level and atazanavir or tenofovir pharmacokinetics.
TABLE 2.
Geometric mean atazanavir, ritonavir, and tenofovir plasma pharmacokinetic parametersa
| Drug | Value (geometric mean [95% CI])
|
|||
|---|---|---|---|---|
| AUC0-24 (ng·hr/ml) | Cmax (ng/ml) | C24 (ng/ml) | CL/F (liter/hr) | |
| Atazanavir | 35,971 (30,853-41,898) | 3,504 (2,978-4,105) | 578 (474-704) | 8.3 (7.2-9.7) |
| Ritonavir | 7,840 (6,292-9,769) | 842 (664-1,064) | 71 (53-102) | 12.8 (10.3-16) |
| Tenofovir | 2,762 (2,392-3,041) | 254 (221-292) | 60 (52-68) | 49.2 (43.8-55.3) |
Pharmacokinetics for 22 subjects were measured.
FIG. 1.
Weight (in kilograms) is shown on the x axis. Atazanavir CL/F (in liters/h) is shown on the y axis. For every 10-kg increase in weight, there was, on average, a 10% increase in atazanavir CL/F (P = 0.0005).
FIG. 2.
Creatinine clearance (in milliliters/minute, estimated using the Cockcroft-Gault equation) is shown on the x axis. Tenofovir CL/F (in milliliters/minute) is shown on the y axis. For every 10 ml/min increase in creatinine clearance, there was, on average, a 4.6% increase in tenofovir CL/F (P < 0.0001).
Multiple regression confirmed the contribution of weight to the atazanavir CL/F and renal function to the tenofovir CL/F. When weight, gender, and race were included in a multivariate model, weight remained the only significant predictor of atazanavir CL/F (P = 0.0015). When renal function, weight, race, and gender were included in a multivariate model, only estimated creatinine clearance remained significantly predictive of the tenofovir CL/F (P = 0.008).
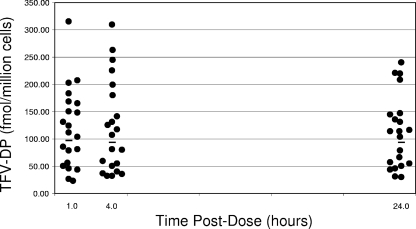
The intracellular TFV-DP concentrations for 21 subjects are shown in Fig. 3. One subject had suspect samples (hemolyzed, very-low cell counts, and concentrations above the limits of assay detection), and that subject's data were therefore excluded from these analyses. The geometric mean (95% CI) TFV-DP concentrations at 1, 4, and 24 h postdose were 96.4 (71.5 to 130), 93.3 (68 to 130), and 92.7 (70 to 123) fmol/million cells. There was an association between renal function, tenofovir AUC, and tenofovir Cmax and intracellular TFV-DP concentrations, although none of these associations reached statistical significance. For every 10 ml/min increase in creatinine clearance, there was, on average, a 4% decrease in intracellular TFV-DP concentrations (P = 0.12). For every 1,000 ng·hr/ml increase in the tenofovir AUC, there was, on average, a 25% increase in TFV-DP (P = 0.16). For every 100 ng/ml increase in the tenofovir Cmax, there was, on average, a 21% increase in TFV-DP concentrations (P = 0.15).
FIG. 3.
Time postdose (in hours) is shown on the x axis. Intracellular TFV-DP concentrations (in femtomoles/million cells) are shown on the y axis. The horizontal lines indicate the geometric mean TFV-DP concentrations at each of the three time points. The geometric mean (95% CI) TFV-DP concentrations at 1, 4, and 24 h postdose were 96.4 (71.5 to 130), 93.3 (68 to 130), and 92.7 (70 to 123) fmol/million cells.
DISCUSSION
These are the first intensive pharmacokinetic data on the combination of atazanavir-ritonavir and TDF in young adults. The atazanavir concentrations observed in our patients in the presence of tenofovir were similar to what was previously observed in older adults receiving this combination (25). The Puzzle 2 pharmacokinetic substudy included 10 HIV-infected males aged 33 to 59 receiving atazanavir-ritonavir plus TDF and at least one other nucleoside reverse transcriptase inhibitor. In that study, the mean (percent coefficient of variation [%CV]) atazanavir AUC0-24, Cmax, C24, and CL/F values were 39,231 ng·hr/ml (59), 3,443 ng/ml (41), 665 ng/ml (84), and 9.8 liter/hr (51), very similar to the values observed in our patients. This compares with mean (%CV) atazanavir AUC0-24, Cmax, Cmin, and CL/F values of 53,761 ng·hr/ml (66), 5,233 ng/ml (58), 862 ng/ml (97), and 5.6 liter/hr (66) when atazanavir is administered without TDF in HIV-infected adults (Reyataz product information; Bristol-Myers Squibb). We also found weight to be significantly predictive of atazanavir CL/F. These data suggest that higher doses of atazanavir may be required in very-large patients to achieve the same exposures. Unlike the results of several previous studies (17, 19, 21), we did not detect a strong and statistically significant correlation between atazanavir concentrations and total bilirubin levels. The atazanavir AUC and Cmax values were very weakly (positively) correlated with total bilirubin concentrations (0.02 and 0.04, respectively) in this study. The correlation between the atazanavir Cmin and total bilirubin was 0.37, and this increased to approximately 0.5 if we removed the data for three subjects with total bilirubin concentrations of >4 mg/dl. We can only assume that the correlation with Cmin and total bilirubin concentrations was not significant in our study due to the small sample size and also, possibly, the racial heterogeneity of our patient population. The majority of our subjects were African American; however, several previous studies identifying a correlation included mainly Caucasian subjects. Also, it could be possible that UGT1A1 activity is greater in this age group. There are no published data correlating bilirubin and atazanavir concentrations in children or adolescents.
The tenofovir prescribing information reports the mean (%CV) tenofovir AUC0-24 and Cmax from seven patients as 3.3 μg·hr/ml (42) and 326 ng/ml (37) (tenofovir [Viread] prescribing information; Gilead, Foster City, CA [accessed January 14, 2007]), very similar to the values observed in our patients. In the aforementioned Puzzle 2 substudy, the tenofovir AUC0-24, Cmax, and C24 values were 2.3 μg·hr/ml, 234 ng/ml, and 45 ng/ml (28), also comparable to the concentrations observed in our patients. A separate study of 28 healthy volunteers aged 19 to 43 found that the tenofovir AUC0-24, Cmax, and C24 values were increased 37%, 34%, and 29%, respectively, when TDF was given with atazanavir-ritonavir (1). Based on those findings, we anticipated that tenofovir concentrations in our patients would be higher than the values reported in the literature for tenofovir without a protease inhibitor, but this was not the case. The lower-than-anticipated tenofovir concentrations found in our study subjects may be due to faster tenofovir clearance as a result of increased creatinine clearance in this young age group. Indeed, we found estimated creatinine clearance to be significantly predictive of tenofovir clearance. Tenofovir concentrations were also lower in obese subjects.
This was the first study to describe TFV-DP concentrations in young adults and in combination with atazanavir-ritonavir. The TFV-DP concentrations observed in this study were similar to those described previously by Hawkins et al. (8) in subjects 31 to 65 years of age (median, 85 to 110 fmol/million cells) and by Kiser et al. (14) in subjects aged 25 to 60 (mean ± standard deviation, 76 ± 40 fmol/million cells). The previous study by Kiser at al. also found tenofovir AUC and renal function to be associated with intracellular TFV-DP concentrations (14).
There are limitations to this study. First, this was an observational trial that did not allow within-person comparisons of changes in tenofovir pharmacokinetics after the addition of atazanavir-ritonavir and/or changes in atazanavir pharmacokinetics after the addition of tenofovir. Therefore, we are only able to describe our subjects' pharmacokinetic parameters and make comparisons to historical data. Also, several PBMC pellet samples arrived hemolyzed or frozen. These were included in the analyses, which may have affected TFV-DP quantification in unpredictable ways.
Although we may have expected higher tenofovir concentrations in our subjects, based on a previous interaction study of healthy volunteers, the atazanavir concentrations observed in this study were similar to historical data. The fact that 16 of 22 subjects had HIV-1 RNA less than or equal to 400 copies/ml suggests that the tenofovir and atazanavir exposures were therapeutic in the majority of these subjects. There are some concentration-effect data for atazanavir with which to compare our data. In the BMS-089 study, in which subjects received stavudine and lamivudine in combination with either atazanavir alone or ritonavir-boosted atazanavir, 85% of treatment-naïve subjects with atazanavir troughs between 327 to 764 ng/ml had an undetectable viral load at week 48 of treatment (3). However, there are very limited data correlating tenofovir levels with response. A previous study of TDF in 18 children aged 8.3 to 16.2 found that tenofovir serum AUCs in virologic responders (median, 3,800 ng·hr/ml) were higher than in nonresponders (median, 2,510 ng·hr/ml) (10). The geometric mean tenofovir exposures in our study subjects (2,762 ng·hr/ml) were closer to the exposures in the “virologic nonresponder” subjects in that study. Considering that atazanavir and atazanavir-ritonavir have been shown to increase tenofovir concentrations in prior studies, our findings provide a basis for concern about tenofovir exposures in young adults not receiving atazanavir-ritonavir or another protease inhibitor. Specifically, could tenofovir exposures in young adults be even lower when used in regimens that do not include atazanavir or ritonavir? If they are lower, does this have implications for virologic response in this age group? Unfortunately, there are very limited concentration-effect data with tenofovir; thus, it is currently unclear what the lower threshold for tenofovir exposures should be. The lack of exposure-response relationships in our study may also be a function of the heterogenous patient population included (i.e., the study included treatment-naïve and experienced subjects, and the other nucleoside reverse transcriptase inhibitor(s) the subjects were taking were not controlled for).
In conclusion, the pharmacokinetic characteristics of atazanavir, ritonavir, and tenofovir in these young adults are consistent with historical data, though we anticipated higher tenofovir concentrations, based on a tenofovir/atazanavir-ritonavir interaction study of healthy volunteers. Additional studies of exposure-response relationships of this regimen in children, adolescents, and adults would advance our knowledge of its pharmacodynamic properties.
Acknowledgments
This work was supported by the Adolescent Trials Network for HIV/AIDS Interventions (ATN) (grant no. U01-HD040533 from the National Institutes of Health through the National Institute of Child Health and Human Development [B. Kapogiannis and L. Serchuck]), with supplemental funding from the National Institutes on Drug Abuse (N. Borek) and Mental Health (P. Brouwers and S. Allison). Five of the sites utilized their General Clinical Research Center (GCRC)/Pediatric Clinical Research Center (PCRC) for this study. These centers were supported by grants from the general clinical research center program of the National Center for Research Resources, National Institutes of Health, Department of Health and Human Services, as follows: Children's Hospital of Los Angeles, grant no. M01 RR00043; Mt. Sinai Medical Center, grant no. M01 RR00071; University of Maryland, grant no. M01 RR165001; University of Pennsylvania/Children's Hospital of Philadelphia, grant no. M01 RR00240; and University of South Florida/All Children's Hospital Clinical Research Center, grant no. R60 MC00003-01.
The study was scientifically reviewed by the ATN′s Therapeutic Leadership Group. Network, scientific, and logistical support was provided by the ATN coordinating center (C. Wilson and C. Partlow) at the University of Alabama at Birmingham. Network operations and analytic support was provided by the ATN data and operations center at Westat, Inc. (J. Korelitz and B. Driver).
We acknowledge the contribution of the investigators and staff at the following ATN sites that participated in this study: Children's Diagnostic and Treatment Center (Ana Puga, Esmine Leonard, and Zulma Eysallenne); Childrens Hospital of Los Angeles (Marvin Belzer, Cathy Salata, and Diane Tucker); John H. Stroger, Jr., Hospital of Cook County and the CORE Center (Jaime Martinez, Kelly Bojan, and Rachel Jackson); Montefiore Medical Center (Donna Futterman, Elizabeth Enriquez-Bruce, and Maria Campos); Mount Sinai Medical Center (Linda Levin-Carmine, Mary Geiger, and Angela Lee); St. Jude Children's Research Hospital (Nehali Patel, Aditya Gaur, and Mary Dillard); University of Maryland (Ligia Peralta, Leonel Flores, and Esther Collinetti); University of Miami School of Medicine (Lawrence Friedman and Donna Maturo); University of Pennsylvania and the Children's Hospital of Philadelphia (Bret Rudy, Mary Tanney, and Adrienne DiBenedetto); and University of South Florida (Patricia Emmanuel, Silvia Callejas, and Priscilla Julian).
We are grateful to the members of the local youth community advisory boards for their insight and counsel and are particularly indebted to the youths who participated in this study. We also acknowledge Michelle Ray, Thomas Delahunty, and Tracy King with the University of Colorado antiviral pharmacology laboratory (director, Courtney V. Fletcher) for analyzing the atazanavir-ritonavir, tenofovir, and intracellular tenofovir diphosphate concentrations, respectively, and Sushma Ahmad and Rick Mitchell with Westat for their invaluable assistance with this study.
Footnotes
Published ahead of print on 19 November 2007.
This is Adolescent Trials Network for HIV/AIDS Interventions study ATN056.
REFERENCES
- 1.Agarwala, S., T. Eley, C. Villegas, Y. Wang, E. Hughes, J. Xie, and D. Grasela. 2005. Pharmacokinetic interaction between tenofovir and atazanavir coadministered with ritonavir in healthy subjects, abstr. 16. Sixth Int. Workshop Clin. Pharmacol. HIV Ther., Quebec City, Quebec, Canada, 26 to 29 April 2005.
- 2.Barditch-Crovo, P., S. G. Deeks, A. Collier, S. Safrin, D. F. Coakley, M. Miller, B. P. Kearney, R. L. Coleman, P. D. Lamy, J. O. Kahn, I. McGowan, and P. S. Lietman. 2001. Phase I/II trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob. Agents Chemother. 45:2733-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertz, R., Y. Wang, L. Mahnke, A. Persson, E. Chung, M. Mathew, S. Agarwala, D. Filoramo, J. Hammond, and D. Grasela. 2007. Assessment of pharmacokinetic/pharmacodynamic relationships through 48 weeks from a study in HIV+, antiretroviral-naive subjects receiving antiretroviral regimens containing atazanavir 400 mg or atazanavir/ritonavir 300/100 mg once daily, Abstr. 565. Fourteenth Conf. Retrovir. Opportunistic Infect., Los Angeles, CA, 25 to 28 February 2007.
- 4.Chiba, K., T. Ishizaki, H. Miura, and K. Minagawa. 1980. Michaelis-Menten pharmacokinetics of diphenylhydantoin and application in the pediatric age patient. J. Pediatr. 96:479-484. [DOI] [PubMed] [Google Scholar]
- 5.Cockcroft, D. W., and M. H. Gault. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31-41. [DOI] [PubMed] [Google Scholar]
- 6.Delahunty, T., L. Bushman, and C. V. Fletcher. 2006. Sensitive assay for determining plasma tenofovir concentrations by LC/MS/MS. J. Chromatogr. B 830:6-12. [DOI] [PubMed] [Google Scholar]
- 7.Gallant, J. E., E. DeJesus, J. R. Arribas, A. L. Pozniak, B. Gazzard, R. E. Campo, B. Lu, D. McColl, S. Chuck, J. Enejosa, J. J. Toole, and A. K. Cheng. 2006. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N. Engl. J. Med. 354:251-260. [DOI] [PubMed] [Google Scholar]
- 8.Hawkins, T., W. Veikley, R. L. St. Claire III, B. Guyer, N. Clark, and B. P. Kearney. 2005. Intracellular pharmacokinetics of tenofovir diphosphate, carbovir triphosphate, and lamivudine triphosphate in patients receiving triple-nucleoside regimens. J. Acquir. Immune Defic. Syndr. 39:406-411. [DOI] [PubMed] [Google Scholar]
- 9.Hazra, R., F. M. Balis, A. N. Tullio, E. DeCarlo, C. J. Worrell, S. M. Steinberg, J. F. Flaherty, K. Yale, M. Poblenz, B. P. Kearney, L. Zhong, D. F. Coakley, S. Blanche, J. L. Bresson, J. A. Zuckerman, and S. L. Zeichner. 2004. Single-dose and steady-state pharmacokinetics of tenofovir disoproxil fumarate in human immunodeficiency virus-infected children. Antimicrob. Agents Chemother. 48:124-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hazra, R., R. I. Gafni, F. Maldarelli, F. M. Balis, A. N. Tullio, E. DeCarlo, C. J. Worrell, S. M. Steinberg, J. Flaherty, K. Yale, B. P. Kearney, and S. L. Zeichner. 2005. Tenofovir disoproxil fumarate and an optimized background regimen of antiretroviral agents as salvage therapy for pediatric HIV infection. Pediatrics 116:e846-e854. [DOI] [PubMed] [Google Scholar]
- 11.Johnson, M., B. Grinsztejn, C. Rodriguez, J. Coco, E. DeJesus, A. Lazzarin, K. Lichtenstein, V. Wirtz, A. Rightmire, L. Odeshoo, and C. McLaren. 2006. 96-week comparison of once-daily atazanavir/ritonavir and twice-daily lopinavir/ritonavir in patients with multiple virologic failures. AIDS 20:711-718. [DOI] [PubMed] [Google Scholar]
- 12.Kearns, G. L., S. M. Abdel-Rahman, S. W. Alander, D. L. Blowey, J. S. Leeder, and R. E. Kauffman. 2003. Developmental pharmacology: drug disposition, action, and therapy in infants and children. N. Engl. J. Med. 349:1157-1167. [DOI] [PubMed] [Google Scholar]
- 13.King, T., L. Bushman, J. Kiser, P. L. Anderson, M. Ray, T. Delahunty, and C. V. Fletcher. 2006. Liquid chromatography-tandem mass spectrometric determination of tenofovir-diphosphate in human peripheral blood mononuclear cells. J. Chromatogr. B 843:147-156. [DOI] [PubMed] [Google Scholar]
- 14.Kiser, J. J., M. L. Carten, C. L. Aquilante, P. L. Anderson, P. Wolfe, T. M. King, T. Delahunty, L. R. Bushman, and C. V. Fletcher. 27 June 2007, posting date. The effect of lopinavir/ritonavir on the renal clearance of tenofovir in HIV-infected patients. Clin. Pharmacol. Ther. doi: 10.1038/sj.clpt.6100269. [DOI] [PubMed]
- 15.Kiser, J. J., R. Rutstein, G. Aldrovandi, P. Samson, B. Graham, S. Schnittman, M. E. Smith, L. Mofenson, and C. V. Fletcher. 2005. Pharmacokinetics of atazanavir/ritonavir in HIV-infected infants, children, and adolescents: PACTG 1020, abstr. 767. Twelfth Conf. Retrovir. Opportunistic Infect., Boston, MA, 22 to 25 February 2005.
- 16.Macassa, E., C. Delaugerre, J. P. Teglas, V. Jullien, J. M. Treluyer, F. Veber, C. Rouzioux, and S. Blanche. 2006. Change to a once-daily combination including boosted atazanavir in HIV-1-infected children. Pediatr. Infect. Dis. J. 25:809-814. [DOI] [PubMed] [Google Scholar]
- 17.Molto, J., J. R. Santos, M. Valle, C. Miranda, J. Miranda, A. Blanco, E. Negredo, and B. Clotet. 2007. Monitoring atazanavir concentrations with boosted or unboosted regimens in HIV-infected patients in routine clinical practice. Ther. Drug Monit. 29:648-651. [DOI] [PubMed] [Google Scholar]
- 18.Piketty, C., L. Gerard, C. Chazallon, A. G. Marcelin, F. Clavel, A. M. Taburet, V. Calvez, I. Madelaine-Chambrin, J. M. Molina, J. P. Aboulker, and P. M. Girard. 2006. Salvage therapy with atazanavir/ritonavir combined to tenofovir in HIV-infected patients with multiple treatment failures: randomized ANRS 107 trial. Antivir. Ther. 11:213-221. [PubMed] [Google Scholar]
- 19.Rodriguez-Novoa, S., L. Martin-Carbonero, P. Barreiro, G. Gonzalez-Pardo, I. Jimenez-Nacher, J. Gonzalez-Lahoz, and V. Soriano. 2007. Genetic factors influencing atazanavir plasma concentrations and the risk of severe hyperbilirubinemia. AIDS 21:41-46. [DOI] [PubMed] [Google Scholar]
- 20.Sanne, I., P. Piliero, K. Squires, A. Thiry, and S. Schnittman. 2003. Results of a phase 2 clinical trial at 48 weeks (AI424-007): a dose-ranging, safety, and efficacy comparative trial of atazanavir at three doses in combination with didanosine and stavudine in antiretroviral-naive subjects. J. Acquir. Immune Defic. Syndr. 32:18-29. [DOI] [PubMed] [Google Scholar]
- 21.Smith, D. E., S. Jeganathan, and J. Ray. 2006. Atazanavir plasma concentrations vary significantly between patients and correlate with increased serum bilirubin concentrations. HIV Clin. Trials 7:34-38. [DOI] [PubMed] [Google Scholar]
- 22.Squires, K., A. Lazzarin, J. M. Gatell, W. G. Powderly, V. Pokrovskiy, J. F. Delfraissy, J. Jemsek, A. Rivero, W. Rozenbaum, S. Schrader, M. Sension, A. Vibhagool, A. Thiry, and M. Giordano. 2004. Comparison of once-daily atazanavir with efavirenz, each in combination with fixed-dose zidovudine and lamivudine, as initial therapy for patients infected with HIV. J. Acquir. Immune Defic. Syndr. 36:1011-1019. [DOI] [PubMed] [Google Scholar]
- 23.Squires, K., A. L. Pozniak, G. Pierone, Jr., C. R. Steinhart, D. Berger, N. C. Bellos, S. L. Becker, M. Wulfsohn, M. D. Miller, J. J. Toole, D. F. Coakley, and A. Cheng. 2003. Tenofovir disoproxil fumarate in nucleoside-resistant HIV-1 infection: a randomized trial. Ann. Intern. Med. 139:313-320. [DOI] [PubMed] [Google Scholar]
- 24.Stone, V. E., J. Jordan, J. Tolson, R. Miller, and T. Pilon. 2004. Perspectives on adherence and simplicity for HIV-infected patients on antiretroviral therapy: self-report of the relative importance of multiple attributes of highly active antiretroviral therapy (HAART) regimens in predicting adherence. J. Acquir. Immune Defic. Syndr. 36:808-816. [DOI] [PubMed] [Google Scholar]
- 25.Taburet, A. M., C. Piketty, C. Chazallon, I. Vincent, L. Gerard, V. Calvez, F. Clavel, J. P. Aboulker, and P. M. Girard. 2004. Interactions between atazanavir-ritonavir and tenofovir in heavily pretreated human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 48:2091-2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi, H., S. Ishikawa, S. Nomoto, Y. Nishigaki, F. Ando, T. Kashima, S. Kimura, M. Kanamori, and H. Echizen. 2000. Developmental changes in pharmacokinetics and pharmacodynamics of warfarin enantiomers in Japanese children. Clin. Pharmacol. Ther. 68:541-555. [DOI] [PubMed] [Google Scholar]
- 27.UNAIDS. 2006. 2006 report on the global AIDS epidemic: a UNAIDS 10th anniversary special edition. Joint United Nations Program on HIV/AIDS. http://www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/default.asp.
- 28.Vincent, I., A. Barrail, C. Piketty, L. Gerard, C. Chazallon, J. P. Aboulker, P. M. Girard, B. P. Kearney, and A. M. Taburet. 2005. Pharmacokinetic parameters of tenofovir when combined with atazanavir/ritonavir in HIV-infected patients with multiple treatment failures: a substudy of Puzzle 2-ANRS 107 trial, abstr. 55. Sixth Int. Workshop Clin. Pharmacol. HIV Ther., Quebec City, Quebec, Canada, 26 to 29 April 2005.