Abstract
The increasing resistance of human pathogens to conventional antibiotics presents a growing threat to the chemotherapeutic management of infectious diseases. The lanthionine antibiotics, still unused as therapeutic agents, have recently attracted significant scientific interest as models for targeting and management of bacterial infections. We investigated the action of one member of this class, subtilin, which permeabilizes lipid membranes in a lipid II-dependent manner and binds bactoprenyl pyrophosphate, akin to nisin. The role the C and N termini play in target recognition was investigated in vivo and in vitro by using the natural N-terminally succinylated subtilin as well as enzymatically truncated subtilin variants. Fluorescence dequenching experiments show that subtilin induces leakage in membranes in a lipid II-dependent manner and that N-succinylated subtilin is roughly 75-fold less active. Solid-state nuclear magnetic resonance was used to show that subtilin forms complexes with membrane isoprenyl pyrophosphates. Activity assays in vivo show that the N terminus of subtilin plays a critical role in its activity. Succinylation of the N terminus resulted in a 20-fold decrease in its activity, whereas deletion of N-terminal Trp abolished activity altogether.
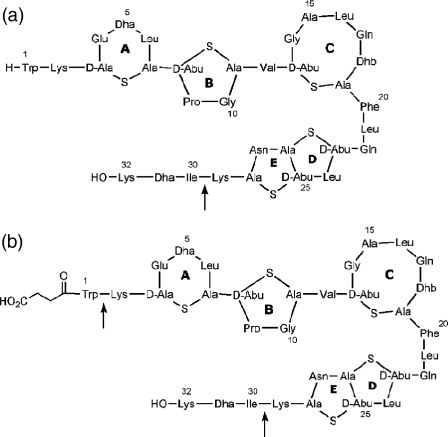
The cationic antimicrobial peptide subtilin (21, 31), produced by the gram-positive Bacillus subtilis strain ATCC 6633, belongs to the family of lanthionine antibiotics (22, 38), which also includes the food preservative nisin (E234) (17, 36). Subtilin is produced by B. subtilis ATCC 6633 and includes two naturally occurring types of the lantibiotic, subtilin and N-succinylated subtilin (Fig. 1), which is less active (14). Biosynthesis of lanthionine antibiotics involves posttranslational modifications of ribosomally synthesized precursor peptides, which result in the formation of unusual amino acids (e.g., lanthionine, dehydroalanine, and dehydrobutyrine) (22, 38).
FIG. 1.
Structures of subtilin (a) and N-succinylated subtilin (b). Arrows indicate points of cleavage that generate fragments.
Subtilin shows antimicrobial activity in the nanomolar range against a broad spectrum of gram-positive bacteria. Its antibacterial activity results from permeabilization of the cytoplasmic membrane of sensitive bacteria (33, 40). It is structurally related to nisin (>60%) (29), which targets in its antimicrobial action the cell wall precursors lipid II (6, 9) and undecaprenyl pyrophosphate (4) on the outer leaflet of the bacterial membranes. The process of molecular recognition by nisin is mediated by binding to pyrophosphates of these precursors (4, 28). Pyrophosphates are ubiquitous and highly conserved across eubacteria, which makes them suitable targets for antimicrobial action.
Nisin-mediated membrane pore formation takes place after target recognition of lipid II (9, 12). The process is rapid, with onset times ranging from milliseconds to seconds, and the lifetime of the pores can extend to over 6 s. Pore formation leads to rapid dissipation of transmembrane electrostatic potential and vital solute gradients (1, 15, 37). Nisin, which has been shown to induce lateral phase separation in membranes (5, 6), recruits lipid II into lateral structures (25). Targeting bactoprenyl pyrophosphate and withdrawing it from the peptidoglycan synthesis into stable membrane complexes is another mechanism by which nisin inhibits bacterial cell wall biosynthesis (4). A complex sequence of events follows the interaction between nisin and cell wall intermediates, which includes accelerated cell division, aberrant morphogenesis, and minicell formation (30).
The implication of targeting the bactoprenyl pyrophosphate moiety in lipid II is obvious, since such biomolecules are crucial in peptidoglycan assembly. The bacterial peptidoglycan encapsulates the plasma membranes, providing structural stability and protecting bacteria from the osmotic challenges of their environment. The peptidoglycan is a dynamic system, in which biosynthetic incorporation of new peptidoglycan and degradation by autolysins are tightly coupled and controlled. The late stages of bacterial cell wall biosynthesis involve the integration of disaccharide pentapeptide monomeric units from lipid II into the linear polysaccharide chains of the peptidoglycan, transglycosylation, which is followed by cross-linking of the linear polymers, transpeptidation (13, 39, 44). These processes take place on the outer leaflet of the bacterial membrane and are targeted by a number of cell wall-inhibiting antibiotics, which interfere with various stages of the pathway (e.g., bacitracin [41], mersacidin [10-12], vancomycin [2], and penicillin [43]).
Here we report the results from a study of the molecular mechanism of target recognition by subtilin. We used a carboxyfluorescein (CF) leakage assay to investigate the role of cell wall precursor lipid II as target for subtilin, and we used solid-state nuclear magnetic resonance (NMR) to assess the role of membrane pyrophosphates in target recognition. We carried out enzymatic digestion of the peptide to investigate the involvement of N-terminal tryptophan in the recognition site within subtilin, which is responsible for its interaction with the target.
MATERIALS AND METHODS
All chemicals were of analytical grade or better. CF and chymotrypsin were purchased from Sigma. The phospholipids, 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) and 1,2-dioleoyl-sn-glycero-3-phosphoglycerol (DOPG), were purchased from Avanti-Polar Lipids, Inc., and stored at −20°C in a 1:1 chloroform-methanol solution.
Bacterial strains, media, and culture conditions.
The plasmid-free subtilin-producing strain B. subtilis ATCC 6633 was grown at 30°C in modified M9 medium with pyruvate as the sole carbon source (47.9 mM sodium phosphate dibasic, 17.2 mM potassium phosphate monobasic, 8.55 mM sodium chloride, 18.7 mM ammonium chloride, 2 mM magnesium sulfate, 0.1 mM calcium chloride, 0.02 M pyruvate, 0.15 mM zinc chloride, 0.2 mM iron chloride, and 0.27 mM manganese chloride).
Isolation of the two variants of subtilin.
Subtilin was isolated from a 24-h B. subtilis ATCC 6633 culture as follows. Cultures were acidified to pH 2.5 by using 80% phosphoric acid and then centrifuged (8,000 × g, 20 min, 12°C). The sodium chloride concentration of the supernatant was adjusted to 1 M, and the pH was adjusted to 4.0 by using 2.5 M acetate buffer solution.
A chromatographic column 15 cm long and 1.5 cm in diameter was packed with 20 ml of Tosohaas Toyopearl Butyl-650 resin. The column was equilibrated with 7 volumes of equilibrating-washing buffer (0.05 M sodium acetate [pH 4.0] and 1 M sodium chloride). The supernatant was placed on ice and loaded onto the column.
The column was then washed with 7 volumes of washing buffer and 2 volumes of 0.05 M sodium acetate (pH 4.0). Finally, subtilin was eluted with 2 volumes of 50% acetonitrile-0.05% trifluoroacetic acid buffer.
The different fractions were tested by an agar diffusion assay against nisin-sensisitive strain Lactococcus lactis MG1614 (20). The fractions showing antimicrobial activity were collected and the subtilin and its naturally occurring variant (14) were purified by reversed-phase high-pressure liquid chromatography (HPLC) using a C18 column (250 by 10 mm). The solvents used were 0.1% aqueous trifluoroacetic acid (solvent A) and 0.1% trifluoroacetic acid in 70% aqueous acetonitrile (solvent B). Elution was performed with a linear gradient from 5 to 100% of solvent B over 30 min, with monitoring of the effluent at 220 nm. Fractions containing subtilin and N-succinylated subtilin were characterized by matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry (MS) analysis and subsequently lyophilized.
Production and purification of subtilin fragments.
The sites of cleavage of subtilin by the proteases used are indicated in Fig. 1. Subtilin or N-succinylated subtilin (5 mg) was dissolved in 15 ml of buffer (25 mM sodium acetate, 5 mM Tris acetate, 5 mM calcium chloride [pH 7.0]), 2 mg of chymotrypsin was added, and the reaction mixture was incubated at 30°C; a further 1 mg of chymotrypsin was added after 24 and 48 h. After 72 h, the reaction was stopped by acidification. The progress of the cleavage reaction was monitored by MS of aliquots obtained at intervals. The desired fragments were purified from the acidified reaction mixtures by reversed-phase HPLC. A C18 column (250 by 10 mm) was used with a gradient from solvent A to solvent B. Elution was performed with a linear gradient from 5 to 100% of solvent B over 30 min, with monitoring of the effluent at 220 nm. Fractions containing the subtilin fragments were collected, characterized by MALDI-TOF MS (Fig. 2), and lyophilized.
FIG. 2.
Mass spectra of subtilin, N-succinylated subtilin, and subtilin fragments. (A) Subtilin, m/z 3,321 Da; (B) N-succinylated subtilin, m/z 3,421 Da; (C) subtilin fragment 1-29, m/z 3,010 Da; (D) subtilin fragment 2-29, m/z 2,824 Da. The observed molecular ion at m/z 2,466 Da is due to an internal reference, human adrenocorticotropic hormone fragment 18-39.
MIC determinations.
MIC determinations were carried out by an agar diffusion assay (16, 27, 42) against L. lactis MG1614.
Synthesis and purification of lipid II.
Synthesis and purification of the lipid-bound cell wall precursor were performed as described previously (7). Briefly, lipid II was synthesized in vitro using membrane preparations of Micrococcus luteus ATCC 4698. Membranes were isolated from lysozyme-treated cells by centrifugation (40,000 × g), washed twice in 50 mM Tris-HCl-10 mM magnesium chloride (pH 7.5), and stored under liquid nitrogen until use. The analytical assay was performed in a total volume of 150 μl containing 400 to 800 μg of membrane protein, 10 nmol of undecaprenylphosphate, 100 nmol of UDP-N-acetylmuramyl pentapeptide (UDP-MurNAc-PP), and 100 nmol of UDP-N-acetylglucosamine (UDP-GlcNAc) in 100 mM Tris-HCl, 5 mM MgCl2 (pH 8.0), and 1% (wt/vol) Triton X-100. UDP-MurNAc-PP was purified as described previously (32). For purification of milligram quantities of lipid II, the analytical procedure was scaled up by a factor of 250. Reaction mixtures were incubated for 1 h at 30°C, and lipids were extracted with the same volume of n-butanol-6 M pyridine-acetate (2:1 [vol/vol]; pH 4.2). Purification of lipid II was performed on a DEAE-cellulose column and eluted with a linear gradient of chloroform-methanol-water (2:3:1 [vol/vol/vol]) to chloroform-methanol-600 mM ammonium bicarbonate (2:3:1 [vol/vol/vol]). Lipid II-containing fractions were identified by thin-layer chromatography (60F254 silica plates; Merck) using chloroform-methanol-water-ammonia (88:48:10:1) as the solvent (35). Spots were visualized by using iodine vapor. The concentration of purified lipid II was determined as inorganic phosphate after treatment with perchloric acid (3).
Preparation of unilamellar vesicles.
Large unilamellar vesicles for CF efflux experiments were prepared by extrusion through fixed pore-size membranes (8, 26). Lipids, DOPC-DPOG (3:1 molar ratio) with or without 0.1 mol% lipid II (referring to the total amount of phospholipids), were mixed in chloroform-methanol (1:2 [vol/vol]), and the solvent was removed by rotary evaporation. The lipid films were left under vacuum (0.1 mbar) for 2 h to remove trace amounts of solvents. Dry lipid films were hydrated for 1 h in 50 mM CF, 50 mM sodium chloride, and 10 mM HEPES (pH 7.4) by vortexing. The lipid suspension was frozen and thawed 10 times and then extruded through 400-nm-pore-size polycarbonate filters (11 times). Nonencapsulated CF was removed by gel filtration on a PD-10 column equilibrated with 100 mM sodium chloride and 10 mM HEPES (pH 7.4) buffer. Lipid concentration was quantified from inorganic phosphate (3).
CF leakage assay.
The CF-loaded vesicles were diluted in 100 mM sodium chloride and 10 mM HEPES (pH 7.4) buffer to the appropriated concentration for the leakage assay. After the addition of peptide, the increase in fluorescence intensity was measured at 515 nm (excitation at 490 nm) on an RF-5301 spectrophotometer (Shimadzu, Duisburg, Germany) at 20°C. Peptide-induced leakage was documented relative to the total amount of marker release after disintegration of the vesicles by the addition of 10 μl of 20% Triton X-100.
NMR experiments.
Samples for NMR experiments were prepared by codissolving DOPC and DOPG (3:1 molar ratio) and 4% molar of the corresponding cell wall precursor in a chloroform-methanol mixture (1:1 [vol/vol]). The solvent was removed under vacuum, and the dry lipid films were hydrated in subtilin-containing buffer (10 mM acetate [pH 5.0]). Multilamellar vesicles were obtained by fivefold freeze-thawing between liquid nitrogen and a 50°C water bath. The samples were pelleted by 30 min of centrifugation at 19,000 × g, and the pellet was loaded into a 4-mm magic angle spinning (MAS) NMR rotor.
Cross-polarization MAS NMR (23, 24) experiments were performed on a Varian VNMR 400 spectrometer equipped with a 4-mm MAS T3 probe (Varian, Palo Alto, CA). A single 119-kHz pulse was used to excite the entire 31P nuclear population, and the free induction decay was acquired under 54-kHz SPINAL proton decoupling (19) with an 8-s interpulse delay to minimize sample heating. Cross-polarization MAS experiments, during which 105-kHz proton excitation was followed by 1 ms of 65-kHz contact field and 55-kHz SPINAL proton decoupling during acquisition and an 8-s interpulse delay, were used to excite only relatively immobile 31P nuclei. Between 256 and 16,384 transients were averaged. Phosphorus-31 spectra were referenced externally to 0 ppm for phosphoric acid (85%).
RESULTS
Leakage assay.
The ability of subtilin to affect membrane integrity was investigated in the presence of lipid II. CF-loaded unilamellar vesicles of DOPC-DOPG containing 0.1 mol% target molecule and DOPC-DOPG vesicles without a cell wall precursor were incubated with a range of subtilin concentrations. No leakage was observed from vesicles without lipid II treated with either subtilin or N-succinylated subtilin, whereas profound leakage was observed from lipid II-containing vesicles.
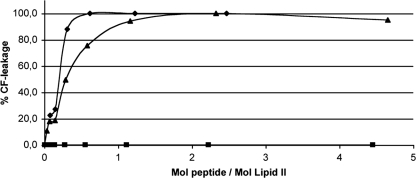
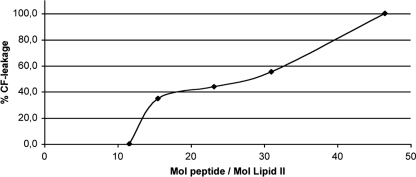
Leakage from lipid II-containing vesicles increased with increasing subtilin concentration (Fig. 3). The range over which subtilin causes partial leakage is quite narrow, which is consistent with the formation of a single pore per vesicle, sufficient to cause full leakage. Higher concentrations of N-succinylated subtilin were required to induce leakage (Fig. 4), suggesting that the peptide has much reduced pore-forming ability. To compare the activities of subtilin and N-succinylated subtilin, we calculated the ratio between lipid II and the peptide. The values showed that N-succinylated subtilin is 75-fold less active than subtilin in the presence of lipid II. Two phases of CF release can be distinguished in Fig. 3 and 4. Partial leakage (roughly 20%) at low subtilin concentrations may reflect transient binary poration, which follows target recognition and depends on asymmetric distribution of the antibiotic on the outer leaflet of the membrane. Higher antibiotic/target ratios lead to full release of fluorophore, most likely via cooperative formation of more stable oligomeric binary pores. The latter process may involve a secondary binding site on lipid II, as we have observed for nisin (4).
FIG. 3.
Activity of subtilin and subtilin fragments (⧫, subtilin; ▴, subtilin 1-29; ▪, subtilin 2-29) against DOPC-DOPG vesicles with 0.1 mol% lipid II. Peptides induce a leakage of CF from the vesicles.
FIG. 4.
Activity of N-succinylated subtilin against DOPC-DOPG vesicles with 0.1 mol% lipid II. Peptides induce a leakage of CF from the vesicles.
Digestion study.
Solid-state NMR data from nisin pointed to a direct involvement of the N terminus of nisin in target recognition (4). We investigated the role N-terminal tryptophan plays in target recognition by subtilin in vitro from leakage assays and in vivo against L. lactis 1614. Chymotryptic digestion of N-succinylated subtilin was carried out to remove the N-terminal tryptophan, and subtilin was treated similarly as a control. The reaction proceeds to completion in the N-succinylated peptide but is impossible in native subtilin due to the inability of the endoprotease to process N-terminal tryptophan residues. The proteolysis is slow and takes 72 h to complete. The final product of the N-succinylated subtilin reaction is fragment 2-29, while that of the subtilin reaction is fragment 1-29. Each of the fragments produced by these digestions was purified by HPLC and characterized by MALDI-TOF MS.
Figure 3 summarizes the effects of subtilin, N-terminally and C-terminally truncated subtilin 2-29, and C-terminally truncated subtilin 1-29 on the integrity of lipid II-containing membranes. C-terminal truncation reduced by an order of magnitude the ability of subtilin to release CF from lipid II-containing vesicles compared to that of the full peptide subtilin 1-32. Such a reduction in activity may have arisen from a diminished ability of the C terminus of subtilin 1-29 to insert into the membrane after target recognition and may indicate a more cooperative mechanism of translocation, in which higher peptide concentrations are required. In contrast, loss of the N-terminal tryptophan in subtilin 2-29 completely prevented the peptide from disrupting membrane vesicles over the tested concentration range, which shows that N-terminal Trp plays a pivotal role in membrane permeabilization by subtilin.
The ability of subtilin, N-succinylated subtilin, and subtilin fragments 1-29 and 2-29 to inhibit growth in L. lactis MG1614 was assessed by using disk diffusion antimicrobial assays. Quantitative analysis was carried out to determine the MIC as described previously (18), and the sensitivity of L. lactis MG1614 to subtilin, N-succinylated subtilin, and subtilin fragments 1-29 and 2-29 was determined. The inhibition values (i.e., the MICs in micrograms/milliliter) were as follows: subtilin, 1.13; (N-succinyl-Trp) subtilin, 19.16; subtilin 1-29, 11.49; and subtilin 2-29, no activity (>50). The MICs of subtilin and N-succinylated subtilin are, respectively, 1 and 19 μg/ml against L. lactis MG1614, a finding in agreement with published values (14).
Chymotrypsin treatment of subtilin, which removes the C-terminal residues Ile30-Dha31-Lys32 and leads to the truncated peptide subtilin 1-29, resulted in a 10-fold reduction in its activity in vivo compared to that of subtilin. Leakage assays in the presence of subtilin 1-29 showed an almost unchanged ability of this fragment to disrupt lipid II-containing membranes. In contrast, truncation of the N-terminal tryptophan, which occurs during chymotryptic digestion of N-succinylated subtilin in addition to the loss of C-terminal residues 30 to 32 and produces fragment 2-29, leads to a complete loss of activity against L. lactis MG1614.
Previously, we reported that the pyrophosphate moiety plays a key role in the target recognition by nisin (4). While nisin-lipid II complexes breach bilayer membranes (9), membrane integrity is maintained in the presence of complexes between antibiotic and polyisoprenyl pyrophosphates. In order to detect these complexes, we developed an assay based on solid state 31P MAS NMR, which is very sensitive to changes in molecular mobility. We applied this technique to investigate the interactions between subtilin and geranylgeranyl pyrophosphate in membranes. Since we observed no direct involvement of the isoprenoid chain in target recognition (4, 7), we chose geranylgeranyl pyrophosphate, a shorter-chain derivative than undecaprenyl pyrophosphate, as a readily available target molecule.
High-resolution solid-state 31P MAS NMR spectra from model membranes, composed of DOPC-DOPG at a 3:1 molar ratio and containing 4% geranylgeranyl pyrophosphate, are shown in Fig. 5. In the absence of subtilin (Fig. 5A) the phosphate moiety possesses a high degree of motional freedom. The phosphates of DOPC and DOPG show motionally averaged chemical shift anisotropy values reduced from the ca. 200 ppm of immobile phosphates to ∼40 ppm.
FIG. 5.
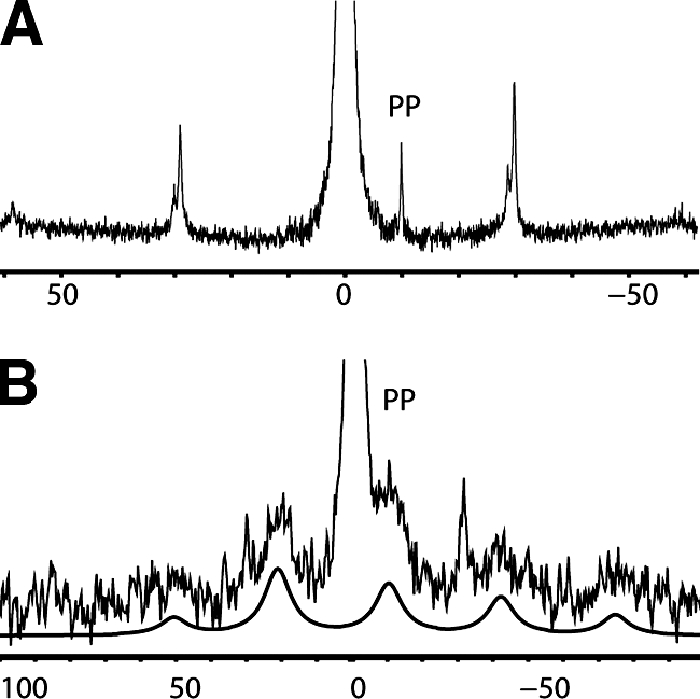
Phosphorus-31 NMR spectra of lipid bilayers containing geranylgeranyl pyrophosphate. (A) Spectrum acquired using single-pulse excitation, followed by proton decoupling under 4.7 kHz MAS; (B) spectrum from membranes treated with subtilin and acquired by using tangent ramp cross-polarization, followed by proton decoupling under 5 kHz MAS. A numerical fit to the pyrophosphate spectrum is shown below the spectrum in panel B to guide the eye.
Under 5-kHz MAS, this is seen as single, low-intensity rotational sidebands. The absence of rotational sidebands from the resonance at −10 ppm in Fig. 5A suggests high mobility in the pyrophosphate region and effective chemical shift anisotropy significantly smaller than 40 ppm. Therefore, cross-polarization experiments were ineffective and a single-pulse excitation was used.
After addition of subtilin, the motional characteristics of the pyrophosphates changed dramatically in a way very similar to that described during the action of nisin (4). Cross-polarization was used, yielding a broad center band bracketed by a number of rotational sidebands (Fig. 5B). Interestingly, in contrast to nisin, subtilin was much less able to restrict pyrophosphate dynamics, and a lower number of sidebands was observed. To enhance the relative contribution from immobile pyrophosphates, a shorter mixing time of 1 ms was used instead of the longer mixing times of 5 to 10 ms, required for optimal excitation of partially mobile phosphates in DOPC and DOPG. This allowed us to select the spectral contribution of the low-fraction pyrophosphates from the abundant DOPC and DOPG phosphates. The center band arising from the pyrophosphates in the complex was significantly broader than the sharp resonance we observed from subtilin-free membranes, which indicates, most likely, a nonunique mode of attachment of subtilin on the pyrophosphate.
DISCUSSION
Subtilin had been described as a pore-forming lantibiotic that causes dissipation of transmembrane proton motive force and release of cytoplasmic solutes from Staphylococcus simulans and B. subtilis cells and from membrane vesicles (33). Early work also showed that subtilin caused in vitro inhibition of cell wall biosynthesis (34). However, information on the molecular determinants of subtilin-mediated membrane permeabilization and the role of cell wall intermediates is not available. Similarities with nisin allow for important comparisons both in function and in target recognition. We use a combination of biochemical, microbiological, and physicochemical techniques to investigate the recognition interface between subtilin and membrane-associated cell wall intermediates.
Previous studies have shown that nisin in low concentrations can induce rapid efflux of CF from multilamellar liposomes when supplemented with 0.1 mol% lipid II. In the absence of lipid II, such liposomes were not affected by nisin concentrations as high as 1.25 μM (12). We have analyzed the interactions between subtilin and pyrophosphate-containing mature cell wall intermediates as well as the effects of subtilin digestion on its ability to induce leakage from CF-loaded vesicles. We found subtilin capable of permeabilizing lipid II-containing membrane vesicles at concentrations very similar to those at which nisin lysis was observed (6, 9). Following the analogy with nisin, we speculated that C-terminal truncation would not result in major change in activity (15). Indeed, the ability of subtilin 1-29, lacking C-terminal residues Ile30-Dha31-Lys32, to disrupt lipid membranes containing lipid II was almost unaffected.
Direct involvement of the N-terminal Ile1 of nisin in pyrophosphate recognition (4) prompted an enzymatic deletion study in subtilin, which was not possible in nisin. We removed successfully the N-terminal tryptophan and compared the ability of the resulting subtilin fragment 2-29 to that of 1-29. The effect was dramatic, and we did not observe any membrane-disrupting action of subtilin 2-29. Even a minor modification of the peptide, which is seen in the naturally occurring N-succinylated variant, led to a noticeable, ∼20-fold reduction in lytic activity. These results emphasize the pivotal role the N terminus plays in the action of subtilin and in target recognition, as we have seen in nisin (4).
The pore-forming ability of subtilin is intimately linked to its structure. The study of the antimicrobial activity of the fragments of subtilin has highlighted the pivotal role of the N-terminal amino acid of subtilin in pore formation. In fact, removal of the three last amino acids of the C terminus affects the activity of the lantibiotic but does not affect its ability to form pores, and the modification of the first amino acid at the N terminus results in a decrease of the antimicrobial activity. N-succinylated subtilin has an MIC 20 times higher than that of subtilin, and subtilin fragment 2-29 presents no activity. This reduction of subtilin's antimicrobial activity, which follows the removal of the N-tryptophan, points to a mechanism of lipid II recognition that involves the formation of a complex between tryptophan and lipid II.
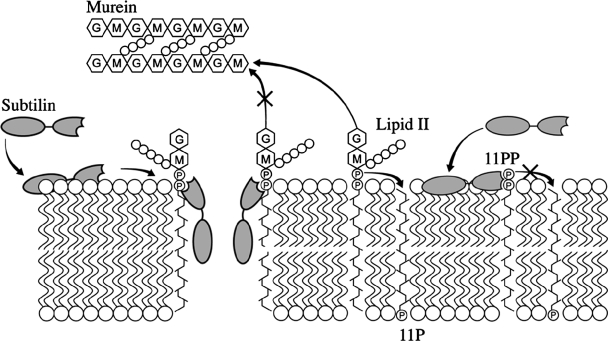
Our 31P MAS NMR results show the ability of subtilin to engage pyrophosphate-containing intermediates into stable complexes (Fig. 6). In addition to disrupting lipid II-containing membranes, this points to its role as a cell wall inhibitor. Pyrophosphate targeting is essential to the high activity of subtilin and nisin. This specific mechanism is essential to the selective targeting of bacteria, which are unique in presenting pyrophosphate derivatives on the exterior surfaces of their membranes. In contrast, pyrophosphates are not found on the outer surfaces of eukaryotic plasma membranes.
FIG. 6.
Proposed mechanism of pyrophosphate-mediated target engagement by the lanthionine antibiotic subtilin. Membrane breach occurs in a lipid II-mediated fashion and cell wall synthesis is impeded through engagement of pyrophosphate-containing intermediates into complexes.
During its action the lanthionine antibiotic subtilin relies on engaging membrane-bound bacterial cell wall intermediates lipid II and bactoprenyl pyrophosphate. The high potency that arises from such a target-mediated action can be connected to its significantly higher efficacy by comparison to membrane-perturbing bactericidal peptides from defense systems of higher organisms, which act via nonspecific mechanisms. Akin to nisin, subtilin in the presence of lipid II disrupts the barrier function of lipid bilayers even at 20 nM. The ability of subtilin to interfere with the cell wall and its biosynthetic pathway by interacting with molecular intermediates strongly suggests the possibility of other antibacterial effects, which follow membrane breach. These may include aberrant morphogenesis and abnormal cell division, detachment of cell wall from the plasma membrane, and minicell formation, as seen during the action of nisin (30).
We have investigated here the interactions between lanthionine antibiotic subtilin and pyrophosphate cell wall intermediates by fluorescence leakage assays and solid-state NMR spectroscopy. We have shown that subtilin forms complexes with membrane-associated pyrophosphate-containing cell wall intermediates and that it permeabilizes lipid membranes in a lipid II-dependent fashion. The activity of N-terminally modified subtilin variants was assessed in vivo and in vitro, and we conclude that N-terminal engagement of the target is pivotal to antibiotic function. In contrast, removal of the three C-terminal residues led to a comparatively small change in subtilin's antimicrobial activity and in its ability to breach membranes. Pyrophosphate recognition and engagement of geranylgeranyl pyrophosphate into stable complexes were shown to take place in lipid membranes, which suggests the ability of subtilin to inhibit cell wall synthesis.
Acknowledgments
We thank Nikki Horn, Biotechnology and Biological Sciences Research Council (BBSRC) IFR, Norwich, United Kingdom, for providing the bacterial strains and Alex Morgan for preparing the illustrations. Mass spectroscopy was done by the Biopolymer Synthesis and Analysis Unit at the School of Biomedical Sciences, University of Nottingham.
Support for this study was provided by the BBSRC of the United Kingdom through grants B20039 and BB/C510924/1 to B.B. The contribution from Varian, Inc., toward the NMR instrument is gratefully acknowledged.
Footnotes
Published ahead of print on 12 November 2007.
REFERENCES
- 1.Abee, T., F. M. Rombouts, J. Hugenholtz, G. Guihard, and L. Letellier. 1994. Mode of action of nisin-Z against Listeria monocytogenes Scott, a grown at high and low temperatures. Appl. Environ. Microbiol. 60:1962-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barna, J. C. J., and D. H. Williams. 1984. The structure and mode of action of glycopeptide antibiotics of the vancomycin group. Annu. Rev. Microbiol. 38:339-357. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett, G. R. 1959. Phosphorus assay in column chromatography. J. Biol. Chem. 234:466-468. [PubMed] [Google Scholar]
- 4.Bonev, B. B., E. Breukink, E. Swiezewska, B. De Kruijff, and A. Watts. 2004. Targeting extracellular pyrophosphates underpins the high selectivity of nisin. FASEB J. 18:1862-1869. [DOI] [PubMed] [Google Scholar]
- 5.Bonev, B. B., W. C. Chan, B. W. Bycroft, G. C. K. Roberts, and A. Watts. 2000. Interaction of the lantibiotic nisin with mixed lipid bilayers: a P-31 and H-2 NMR study. Biochemistry 39:11425-11433. [DOI] [PubMed] [Google Scholar]
- 6.Breukink, E., B. B. Bonev, I. Wiedemann, H. G. Sahl, A. Watts, and B. de Kruijff. 2001. Specific interaction of the lantibiotic nisin with lipid II leads to highly efficient pore formation. Biophys. J. 80:7. [Google Scholar]
- 7.Breukink, E., H. E. van Heusden, P. J. Vollmerhaus, E. Swiezewska, L. Brunner, S. Walker, A. J. R. Heck, and B. de Kruijff. 2003. Lipid II is an intrinsic component of the pore induced by nisin in bacterial membranes. J. Biol. Chem. 278:19898-19903. [DOI] [PubMed] [Google Scholar]
- 8.Breukink, E., C. van Kraaij, R. A. Demel, R. J. Siezen, O. P. Kuipers, and B. de Kruijff. 1997. The C-terminal region of nisin is responsible for the initial interaction of nisin with the target membrane. Biochemistry 36:6968-6976. [DOI] [PubMed] [Google Scholar]
- 9.Breukink, E., I. Wiedemann, C. van Kraaij, O. P. Kuipers, H. G. Sahl, and B. de Kruijff. 1999. Use of the cell wall precursor lipid II by a pore-forming peptide antibiotic. Science 286:2361-2364. [DOI] [PubMed] [Google Scholar]
- 10.Brotz, H., G. Bierbaum, A. Markus, E. Molitor, and H. G. Sahl. 1995. Mode of action of the lantibiotic mersacidin: inhibition of peptidoglycan biosynthesis via a novel mechanism. Antimicrob. Agents Chemother. 39:714-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brotz, H., G. Bierbaum, P. E. Reynolds, and H. G. Sahl. 1997. The lantibiotic mersacidin inhibits peptidoglycan biosynthesis at the level of transglycosylation. Eur. J. Biochem. 246:193-199. [DOI] [PubMed] [Google Scholar]
- 12.Brotz, H., M. Josten, I. Wiedemann, U. Schneider, F. Gotz, G. Bierbaum, and H. G. Sahl. 1998. Role of lipid-bound peptidoglycan precursors in the formation of pores by nisin, epidermin, and other lantibiotics. Mol. Microbiol. 30:317-327. [DOI] [PubMed] [Google Scholar]
- 13.Bugg, T. D. H., and C. T. Walsh. 1992. Intracellular steps of bacterial cell wall peptidoglycan biosynthesis: enzymology, antibiotics, and antibiotic resistance. Nat. Prod. Rep. 9:199-215. [DOI] [PubMed] [Google Scholar]
- 14.Chan, W. C., B. W. Bycroft, M. L. Leyland, L. Y. Lian, and G. C. K. Roberts. 1993. A novel posttranslational modification of the peptide antibiotic subtilin: isolation and characterization of a natural variant from Bacillus subtilis ATCC 6633. Biochem. J. 291:23-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan, W. C., M. Leyland, J. Clark, H. M. Dodd, L. Y. Lian, M. J. Gasson, B. W. Bycroft, and G. C. K. Roberts. 1996. Structure-activity relationships in the peptide antibiotic nisin: antibacterial activity of fragments of nisin. FEBS Lett. 390:129-132. [DOI] [PubMed] [Google Scholar]
- 16.Cooper, K. E. 1955. Theory of antibiotic inhibition zones in agar media. Nature 176:510-511. [DOI] [PubMed] [Google Scholar]
- 17.Delves-Broughton, J., P. Blackburn, R. J. Evans, and J. Hugenholtz. 1996. Applications of the bacteriocin, nisin. Antonie van Leeuwenhoek 69:193-202. [DOI] [PubMed] [Google Scholar]
- 18.Finn, R. K. 1959. Theory of agar diffusion methods for bioassay. Anal. Chem. 31:975-977. [Google Scholar]
- 19.Fung, B. M., A. K. Khitrin, and K. Ermolaev. 2000. An improved broadband decoupling sequence for liquid crystals and solids. J. Magn. Reson. 142:97-101. [DOI] [PubMed] [Google Scholar]
- 20.Gasson, M. J. 1983. Plasmid complements of Streptococcus lactis Ncdo-712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 154:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gross, E., H. H. Kiltz, and E. Nebelin. 1973. Subtilin, VI: structure of subtilin. Hoppe-Seyler's Zeitschr. Physiol. Chemie 354:810-812. [PubMed] [Google Scholar]
- 22.Guder, A., I. Wiedemann, and H. G. Sahl. 2000. Posttranslationally modified bacteriocins: the lantibiotics. Biopolymers 55:62-73. [DOI] [PubMed] [Google Scholar]
- 23.Haeberlen, U., and J. S. Waugh. 1969. Spin-lattice relaxation in periodically perturbed systems. Phys. Rev. 185:420-425. [Google Scholar]
- 24.Hartmann, S. R., and E. L. Hahn. 1962. Nuclear double resonance in rotating frame. Phys. Rev. 128:2042-2045. [Google Scholar]
- 25.Hasper, H. E., N. E. Kramer, J. L. Smith, J. D. Hillman, C. Zachariah, O. P. Kuipers, B. de Kruijff, and E. Breukink. 2006. An alternative bactericidal mechanism of action for lantibiotic peptides that target lipid II. Science 313:1636-1637. [DOI] [PubMed] [Google Scholar]
- 26.Hope, M. J., M. B. Bally, G. Webb, and P. R. Cullis. 1985. Production of large unilamellar vesicles by a rapid extrusion procedure: characterization of size distribution, trapped volume and ability to maintain a membrane-potential. Biochim. Biophys. Acta 812:55-65. [DOI] [PubMed] [Google Scholar]
- 27.Housewright, R. D., R. J. Henry, and S. Berkman. 1948. A microbiological method for the assay of subtilin. J. Bacteriol. 55:545-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu, S. T. D., E. Breukink, E. Tischenko, M. A. G. Lutters, B. de Kruijff, R. Kaptein, A. Bonvin, and N. A. J. van Nuland. 2004. The nisin-lipid II complex reveals a pyrophosphate cage that provides a blueprint for novel antibiotics. Nat. Struct. Mol. Biol. 11:963-967. [DOI] [PubMed] [Google Scholar]
- 29.Hurst, A. 1981. Nisin. Adv. Appl. Microbiol. 27:85-123. [Google Scholar]
- 30.Hyde, A. J., J. Parisot, A. McNichol, and B. B. Bonev. 2006. Nisin-induced changes in Bacillus morphology suggest a paradigm of antibiotic action. Proc. Natl. Acad. Sci. USA 103:19896-19901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jansen, E. F., and D. J. Hirschmann. 1944. Subtilin, an antibacterial product of Bacillus subtilis: culturing conditions and properties. Arch. Biochem. 4:297-304. [Google Scholar]
- 32.Kohlrausch, U., and J. V. Holtje. 1991. One-step purification procedure for UDP-N-acetylmuramyl-peptide murein precursors from Bacillus cereus. FEMS Microbiol. Lett. 78:253-258. [DOI] [PubMed] [Google Scholar]
- 33.Kordel, M., F. Schuller, and H. G. Sahl. 1989. Interaction of the pore forming-peptide antibiotics pep 5, nisin and subtilin with non-energized liposomes. FEBS Lett. 244:99-102. [DOI] [PubMed] [Google Scholar]
- 34.Linnett, P. E., and J. L. Strominger. 1973. Additional antibiotic inhibitors of peptidoglycan synthesis. Antimicrob. Agents Chemother. 4:231-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rick, P. D., G. L. Hubbard, M. Kitaoka, H. Nagaki, T. Kinoshita, S. Dowd, V. Simplaceanu, and C. Ho. 1998. Characterization of the lipid-carrier involved in the synthesis of enterobacterial common antigen (ECA) and identification of a novel phosphoglyceride in a mutant of Salmonella typhimurium defective in ECA synthesis. Glycobiology 8:557-567. [DOI] [PubMed] [Google Scholar]
- 36.Rogers, L. A., and E. O. Whittier. 1928. Limiting factors in the lactic fermentation. J. Bacteriol. 16:211-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sahl, H.-G. 1991. Pore formation in bacterial membranes by cationic lantibiotics, p. 347-358. In G. Jung and H. G. Sahl (ed.), Nisin and novel lantibiotics. ESCOM, Leiden, The Netherlands.
- 38.Sahl, H. G., and G. Bierbaum. 1998. Lantibiotics: biosynthesis and biological activities of uniquely modified peptides from gram-positive bacteria. Annu. Rev. Microbiol. 52:41-79. [DOI] [PubMed] [Google Scholar]
- 39.Scheffers, D. J., and M. G. Pinho. 2005. Bacterial cell wall synthesis: new insights from localization studies. Microbiol. Mol. Biol. Rev. 69:585-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schuller, F., R. Benz, and H. G. Sahl. 1989. The peptide antibiotic subtilin acts by formation of voltage-dependent multi-state pores in bacterial and artificial membranes. Eur. J. Biochem. 182:181-186. [DOI] [PubMed] [Google Scholar]
- 41.Stone, K. J., and J. l. Strominger. 1971. Mechanism of action of bacitracin: complexation with metal ion and C55-isoprenyl pyrophosphate. Proc. Natl. Acad. Sci. USA 68:3223-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tagg, J. R., and A. R. McGiven. 1971. Assay system for bacteriocins. Appl. Microbiol. 21:943-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tipper, D. J., and J. L. Stroming. 1968. Biosynthesis of peptidoglycan of bacterial cell walls. 12. Inhibition of cross-linking by penicillins and cephalosporins: studies in Staphylococcus aureus in vivo. J. Biol. Chem. 243:3169-3179. [PubMed] [Google Scholar]
- 44.van Heijenoort, J. 2001. Recent advances in the formation of the bacterial peptidoglycan monomer unit. Nat. Prod. Rep. 18:503-519. [DOI] [PubMed] [Google Scholar]