Abstract
Combination therapy could be of use for the treatment of fungal infections, especially those caused by drug-resistant fungi. However, the methods and approaches used for data generation and result interpretation need further optimizing. The fractional inhibitory concentration index (FICI) is the most commonly used method, but it has several drawbacks in characterizing antifungal drug interaction. Alternatively, some new methods can be used such as the ΔE model (difference between the predicted and measured fungal growth percentages) and the response surface approach, which uses the concentration-effect relationship over the whole concentration range instead of just the MIC. In the present study, in vitro interactions between tacrolimus (FK506) and three azoles—fluconazole (FLC), itraconazole (ITR), and voriconazole (VRC)-against Candida albicans were evaluated by the checkerboard microdilution method and time-killing test. The intensity of the interactions was determined by visual reading and the spectrophotometric method in a checkerboard assay, and the nature of the interactions was assessed by nonparametric models of FICI and ΔE. Colony counting and colorimetric viable detection methods (2,3-bis {2-methoxy-4-nitro-5-[(sulfenylamino) carbonyl]-2H-tetrazolium hydroxide} [XTT] reduction test) were used for evaluating the combination antifungal effects over time. Synergistic and indifferent effects were found for the combination of FK506 and azoles against azole-sensitive strains, while strong synergy was found against azole-resistant strains analyzed by FICI. The ΔE model gave more consistent results with FICI. The positive interactions were also confirmed by the time-killing test. Our findings suggest a potential role for combination therapy with calcineurin pathway inhibitors and azoles to augment activity against resistant C. albicans.
The incidence of invasive fungal infections has increased significantly in the last several decades, paralleling the increase in the number of immunocompromised patients (15, 34). Candida infections represent an increasing challenge for clinicians. The epidemiology of the last decade shows that these infections are continuously increasing (21), especially in patients with compromised immune systems. Candida albicans is the causative agent of most candidiasis (33).
Azoles are a widely applied class of antifungal agents, and fluconazole (FLC) has been shown to be as effective as amphotericin B in the treatment of candidemia in nonneutropenic patients (42). Since amphotericin B is toxic in its conventional form and very expensive in its new lipidic forms, azole antifungal agents are currently used as first-line drugs (13) because of their excellent oral bioavailability, stable parenteral formulation, and especially their low toxicity. However, with the increasing clinical use of azole, resistance is emerging in clinical isolates from immunocompromised patients. In addition, azole is only fungistatic; this characteristic probably contributes to the development of resistance. The emergence of C. albicans strains with decreased susceptibility complicates the management of these infections (9, 29). Therefore, new approaches for treating these infections are warranted.
Combination therapy is one approach that can be used to improve the efficacy of antimicrobial therapy for difficult-to-treat infections (1). Attempts have been made to cope with treatment failures either by combining different antifungals or by combining antifungals with nonantifungals (1, 2, 20, 21, 24, 26, 33). However, assessing the nature and intensity of drug interactions is still a debated issue. The observed in vitro interaction of two agents depends on different methodology for data generation and different approaches for data analysis, resulting in variable as well as controversial conclusions (5, 14, 37). In the present study, we investigated the combined effects of three azoles and FK506 against C. albicans by the checkerboard microdilution method and the time-killing test. New methods and interpretation models such as the spectrophotometric method and the ΔE model were employed in comparison with the traditional methods of MIC visual reading and fractional inhibitory concentration index (FICI) combination interpretation. The colorimetric method was compared with colony counting in a time-killing study.
MATERIALS AND METHODS
Strains.
Ten clinical isolates of C. albicans were tested in this study, including five azole-susceptible isolates (CA5, CA8, CA12, CA14, and CA129) and five azole-resistant isolates (CA10, CA15, CA16, CA135, and CA137). All the strains were isolates from patients with invasive candidiasis from our hospital and were confirmed according to standard mycological methods (3, 12, 35) by the Microbiological Research Laboratory, the Center of Health Research and Epidemic Prevention, Shandong Province. Their susceptibilities to azoles were tested according to CLSI (Clinical and Laboratory Standards Institute, formerly NCCLS) method M27-A2 (27). In addition, Candida parapsilosis (ATCC 22019) and Candida albicans (ATCC 10231) were used as quality controls. All the isolates were stored at −70°C.
Medium.
The medium used for the broth microdilution method was RPMI 1640 (pH 7.0; with l-glutamine but without sodium bicarbonate; GIBCO BRL, Life Technologies, Woerden, The Netherlands) supplemented with dextrose to a final concentration of 2% and 0.165 M morpholinepropanesulfonic acid (MOPS; Sigma-Aldrich Chemie GmbH, Steinheim, Germany); the pH of the medium was adjusted with 0.1 M NaOH to 7.0 ± 0.1 at 22°C. The medium used for the colony counting was Sabouraud dextrose agar (Tian He Microbiological Agent Co. Ltd., Hang Zhou, China).
Inoculum preparation.
Each isolate from deep-frozen stock cultures had been grown for 7 days on Sabouraud dextrose agar at room temperature and was then subcultured on the same medium for at least three generations at 35°C. Cells were collected with a stick and suspended in 0.9% NaCl water. After thorough mixing, the turbidity of the suspension was compared with the isolate density standard (National Institute for Drug and Biological Products Identification, China). Then, each suspension was diluted in RPMI 1640 to obtain two times the final inoculum size of 2.5 × 103 CFU/ml. The inoculum size was verified by determination of the number of viable CFU after serial dilutions of the inoculum were plated onto Sabouraud dextrose agar.
Drugs and stock solution preparation.
The three azoles used in the present study were FLC, itraconazole (ITR), and voriconazole (VRC). FLC was kindly provided by Cheng Chuang Pharmaceutical Co., Ltd., China; ITR was bought from Xian-Jansen Pharmaceutical Co., Ltd., China; and VRC was obtained from Chengdu Qihe Pharmaceutical Co., Ltd., China. FK506 was obtained from the National Institute for the Control of Pharmaceutical and Biological Products, China. A 2,560-μg/ml stock solution was prepared for FLC by dissolving the powder in distilled water, while a 25,600-μg/ml stock solution for the other tested drugs was prepared by dissolving ITR and VRC in dimethyl sulfoxide and FK506 in methanol. The stock solutions were stored at −70°C until use.
Drug susceptibility testing.
The susceptibilities to all tested drugs were studied by the broth microdilution method according to the CLSI standard M27-A2 (27). The test was carried out in 96-well flat-bottomed microtitration plates. In order to choose the appropriate range of concentrations for different drugs against strains with different susceptibilities, the MICs of individual drugs were determined in an exploratory study for each strain. After agitation for 15 s, the plates were incubated at 35°C without shaking, and readings were performed following 48 h of incubation by both visual reading and optical density (OD) determination with a spectrophotometer. For the visual reading, growth was graded on a scale from 0 to 5. For the OD determination, each plate was shaken for 5 min and the OD values at 492 nm of each well were read with a microtiter plate reader (Thermolabsystems Multiskan MK3). The MIC80 was defined as the lowest drug concentration that resulted in an 80% decrease in absorbance compared with that of the control (no drug). All experiments were performed in triplicate.
Interactions between FK506 and azoles.
The interaction between FK506 and three azoles against five susceptible C. albicans strains and five resistant C. albicans strains was tested by a microdilution checkerboard technique. Drug dilutions were prepared to obtain four times the final concentration. A total of 50 μl of each concentration of azoles was added to columns 2 to 12, and then 50 μl of FK506 was added to rows A to G. In the wells of column 1, 50 μl of the medium containing 1% of the corresponding azole solvent was added, and in wells of row H, 50 μl of the medium containing 1% of the FK506 solvent was added. Thus, row H and column 1 contained only the azole and FK506, respectively, and the well at the intersection of row H and column 1 (well H1) was the drug-free well that served as the growth control. The final drug concentrations after the addition of 100 μl of inoculum ranged from 0.125 to 128 μg/ml for FLC, 0.008 to 8 μg/ml for ITR and VRC, and 0.002 to 0.125 μg/ml for FK506, and the final inoculum size was 2.5 × 103 CFU/ml. Plates were incubated at 35°C for 48 h. Then visual reading of MICs was performed, and OD values were measured at 492 nm. The value of background OD was subtracted from that of each well. Each isolate was tested in triplicate on different days. The percentage of growth in each well was calculated as the OD of each well/OD of the drug-free well after subtracting the background OD obtained from microorganism-free microtiter plates processed in the same manner as the inoculated plates.
Drug interaction interpretation.
In order to assess the nature of the in vitro interactions between the three azoles and FK506 against each isolate, the data obtained by the spectrophotometric method were analyzed using two models, FICI and ΔE, that have been used to characterize antifungal drug interactions (24, 48).
Many models and approaches have been described for the assessment of in vitro drug interactions and extensively reviewed (14, 38). The assumption of no interaction has a central position in these debates, since synergy and antagonism are defined as departures from this. Thus, when the observed effects are more or less than the effect predicted from the no-interaction theory, synergy or antagonism, respectively, is claimed. Among the various no-interaction theories, the Loewe additivity (LA) and Bliss independence (BI) theories are the two major competitor candidates for referenced models (14, 37-39). FICI and ΔE are nonparametric models based on LA theory and BI theory, respectively, while the response surface approach is a parametric model based on LA theory which has been proven to be inadequate for this study because one of the combined agents has little or no antifungal activity (1). In the FICI model, based on LA theory, concentrations of the drugs, alone or in combination, which produce the same effect are prepared, while in the BI-based ΔE model, the estimates of the combined effects based on the effects of the individual drugs were compared with those obtained from the experiment.
FICI.
Based on LA theory, the FICI method, a nonparametric model, was defined as the following equation: FICI = FICA + FICB = CAcomb/MICAalone + CBcomb/MICBalone, where MICAalone and MICBalone are the MICs of drugs A and B when acting alone and CAcomb and CBcomb are concentrations of drugs A and B at the isoeffective combinations, respectively (24, 48). Off-scale MICs were converted to the next highest or the next lowest doubling concentration. Among all FICIs calculated for each data set, the FICImin was reported as the FICI in all cases unless the FICImax was higher than 4; in these cases, the FICImax was reported as the FICI.
The interpretation of the FICI was as follows: a FICI value of ≤0.5 revealed synergy, a value of 1 to 4 revealed indifference, and a value of >4 represented antagonism (30).
ΔE model.
The model of difference in fungal growth percentage was based on BI theory, which is described by the equation Ii = (IA + IB) − (IA × IB), where Ii is the predicted percentage of inhibition of the theoretical combination of drug A and B, and IA and IB are the experimental percentages of inhibition of each drug acting alone, respectively. Since I is equal to 1 − E, where E is the percentage of growth, and by substitution into the former equation, the following equation is derived: Ei = EA × EB, where Ei is the predicted percentage of growth of the theoretical combination of drug A and drug B, respectively, and EA and EB are the experimental percentages of growth of each drug action alone, respectively. Interaction is described by the difference ΔE between the predicted and the measured percentages of growth at various concentrations (ΔE = Epredicted − Emeasured). By the nonparametric approach described by Prichard et al. (39, 40), EA and EB are obtained directly from the experimental data. Because of the nature of interaction, testing with microtiter plates and a twofold dilution of either drug results in a ΔE for each drug combination. In each of the three independent experiments, the observed percentages of growth obtained from the experimental data were subtracted from the predicted percentages, and then the average difference of three experiments was calculated. When the average difference as well as its 95% confidence interval among the three replicates was positive, statistically significant synergy was claimed; when the difference as well as its 95% confidence interval was negative, significant antagonism was claimed. In any other case, BI was concluded.
To summarize the interaction surface, the sums of the percentages of all statistically significant synergistic ([summ]SYN) and antagonistic ([summ]ANT) interactions were calculated. Interactions with <100% statistically significant interactions were considered weak, interactions with 100% to 200% statistically significant interactions were considered moderate, and interactions with >200% statistically significant interactions were considered strong, as described previously (24). In addition, the numbers of statistically significant synergistic and antagonistic combinations among all tested drug combinations were calculated for each strain.
Time-killing test.
We performed an in vitro time-killing test to investigate the activity of the calcineurin inhibitor FK506 with and without azoles against one susceptible strain, CA14, and one resistant strain, CA10, at the starting inoculum of 105 CFU/ml. The concentrations were 10 μg/ml for FLC, 1 μg/ml for ITR and VRC, and 0.016 μg/ml for FK506 (the concentrations of all drugs were within the range achievable in human plasma). A drug-free sample served as a growth control. Dimethyl sulfoxide comprised <1% of the total test volume. At predetermined time points (0, 6, 12, 24, and 48 h after incubation at 35°C), (i) a 100-μl aliquot was removed from every solution and serially diluted in RPMI 1640. A 100-μl aliquot from each dilution was streaked on the Sabouraud dextrose agar plate. Colony counts were performed after incubation at 35°C for 48 h. (ii) At the same time, the XTT test was performed to detect the cell viability after different treatments according to the method described previously (16, 41). Briefly, at each time point, a 100-μl aliquot was removed from every treatment mixture and transferred to a well of a 96-well microplate, and then a 100-μl aliquot of XTT-menadione solution was added. (XTT was purchased from Sigma. Prior to each assay, XTT was dissolved in a saturated solution at 0.5 g/liter in Ringer's lactate. The solution was filter sterilized through an 0.22-μm filter, and then 100 mM menadione in acetone was added to a final concentration of 10 μM.) The plate was then incubated in the dark for up to 2 h at 35°C. After that, the absorbance of the XTT reduction product, formazan, was read at 492 nm for each well with a microtiter plate reader (Thermolabsystems Multiskan MK3). All experiments were conducted in triplicate, and the results were reported as mean values. Thus, growth- and metabolism-inhibitory effects of the drugs alone and in combination were observed based on the results of colony counting and spectrophotometric methods.
For antifungal interactions tested by the time-killing method, the following criteria were used: synergism and indifference were defined as a decrease of ≥2 log10 and <2 log10 CFU/ml with respect to the most active drug, and antagonism was defined as an increase of ≥2 log10 CFU/ml with respect to the least active drug (43).
The correlation between viable cell counts determined by colony counting and OD value by XTT-menadione colorimetric readings was studied for the OD data transformed before interpretation of results.
RESULTS
Drug susceptibility alone and in combination.
According to the criteria of CLSI M27-A2, five susceptible and five resistant isolates were selected for this study. The MICs of two control strains, ATCC 22019 and ATCC 10231, were within the range provided by CLSI. The results for the tested drug alone and in combination against the tested 10 isolates are summarized in Table 1. By itself, FK506 has little or no effect on the tested strains. However, when it was combined with azoles, a potent effect was revealed, especially on the resistant strains. In terms of MIC80, the MICs of either individual agent were reduced by one to two dilutions against the susceptible strains, while remarked reductions were observed for three azoles against resistant strains when combined with FK506. The MICs of azoles against the resistant strains became almost the same as those against the susceptible strains. The result from the visual reading has no obvious difference from those from the OD reading (data not shown).
TABLE 1.
Susceptibilities of C. albican strains against azoles alone and in combination with FK506 obtained by the spectrophotometric methoda
| Drug in combination and strain | Median MIC (range) of drug (μg/ml)
|
|||
|---|---|---|---|---|
| Alone
|
In combination
|
|||
| Azole | FK506 | Azole | FK506 | |
| FLC | ||||
| Susceptible | ||||
| CA5 | 0.25 (0.25-0.5) | 512 | 0.25 (0.125-0.5) | 0.002 (0.002) |
| CA8 | 0.25 (0.125-0.5) | 512 | 0.125 (0.125-0.25) | 0.002 (0.002) |
| CA12 | 4 (2-4) | 512 | 2 (1-2) | 0.008 (0.004-0.008) |
| CA14 | 0.5 (0.25-0.5) | 512 | 0.25 (0.25-1) | 0.002 (0.002-0.004) |
| CA129 | 0.5 (0.5-1) | 512 | 0.25 (0.25-0.5) | 0.008 (0.004-0.016) |
| Resistant | ||||
| CA10 | 256 (256-512) | 512 | 0.25 (0.125-0.5) | 0.063 (0.016-0.125) |
| CA15 | 128 (64-128) | 512 | 0.5 (0.25-0.5) | 0.008 (0.004-0.016) |
| CA16 | 64 (64-128) | 512 | 0.5 (0.25-2) | 0.008 (0.008-0.016) |
| CA135 | 64 (64-128) | 512 | 0.25 (0.125-0.5) | 0.004 (0.004-0.008) |
| CA137 | 64 (64-128) | 512 | 0.5 (0.25-0.5) | 0.002 (0.002-0.008) |
| ITR | ||||
| Susceptible | ||||
| CA5 | 0.031 (0.031-0.063) | 512 | 0.031 (0.031-0.063) | 0.063 (0.016-0.063) |
| CA8 | 0.016 (0.008-0.031) | 512 | 0.008 (0.008-0.016) | 0.002 (0.002) |
| CA12 | 0.063 (0.063-0.125) | 512 | 0.031 (0.031-0.063) | 0.125 (0.063-0.125) |
| CA14 | 0.063 (0.031-0.125) | 512 | 0.031 (0.031-0.125) | 0.002 (0.002) |
| CA129 | 0.063 (0.063-0.125) | 512 | 0.031 (0.031-0.063) | 0.063 (0.063-0.125) |
| Resistant | ||||
| CA10 | 128 (64-128) | 512 | 0.031 (0.031-0.063) | 0.125 (0.063-0.125) |
| CA15 | 32 (16-64) | 512 | 0.063 (0.031-0.125) | 0.063 (0.031-0.125) |
| CA16 | 32 (16-64) | 512 | 0.016 (0.008-0.031) | 0.125 (0.125) |
| CA135 | 16 (16-32) | 512 | 0.016 (0.016-0.031) | 0.063 (0.031-0.125) |
| CA137 | 16 (16-32) | 512 | 0.063 (0.031-0.063) | 0.008 (0.008-0.031) |
| VRC | ||||
| Susceptible | ||||
| CA5 | 0.016 (0.016-0.031) | 512 | 0.016 (0.016-0.031) | 0.002 (0.002) |
| CA8 | 0.008 (0.008-0.016) | 512 | 0.016 (0.008-0.031) | 0.002 (0.002) |
| CA12 | 0.031 (0.008-0.031) | 512 | 0.016 (0.008-0.031) | 0.008 (0.004-0.016) |
| CA14 | 0.008 (0.004-0.016) | 512 | 0.008 (0.008) | 0.002 (0.002) |
| CA129 | 0.016 (0.016-0.031) | 512 | 0.008 (0.008-0.016) | 0.008 (0.008-0.125) |
| Resistant | ||||
| CA10 | 64 (32-64) | 512 | 0.008 (0.008-0.016) | 0.063 (0.031-0.063) |
| CA15 | 8 (4-16) | 512 | 0.008 (0.008-0.016) | 0.125 (0.063-0.125) |
| CA16 | 8 (4-8) | 512 | 0.008 (0.008-0.016) | 0.031 (0.031-0.063) |
| CA135 | 8 (4-8) | 512 | 0.016 (0.008-0.031) | 0.031 (0.016-0.063) |
| CA137 | 8 (4-16) | 512 | 0.008 (0.008-0.016) | 0.016 (0.008-0.016) |
Strains were categorized as susceptible or resistant according to the interpretive breakpoints of CLSI criteria for FLC (≤8 and ≥64 μg/ml, respectively), ITR (≤0.1 and ≥1 μg/ml, respectively), and VRC (≤1 and ≥4 μg/ml, respectively) (11). For azole-susceptible isolates, when drugs were used in combination, MICs of azoles showed no apparent changes, while for azole-resistant strains, MICs of azoles decreased markedly when drugs were used in combination.
The percentages of fungal growth for each combination were calculated by comparing the OD of the drug-containing well with that of the drug-free well after subtracting the background OD obtained from the microorganism-free well. The interaction between FLC and FK506 against azole-resistant strain CA10 is presented in Table 2.
TABLE 2.
Checkerboard showing the effect of each combination of FLC and FK506 on the spectrophotometrically measured growth of drug-resistant C. albicans CA10a
| FK506 concn (μg/ml) | % Growth at FLC concn (μg/ml):
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | |
| 0.125 | 95 | 25 | 7 | 6 | 3 | 12 | 5 | 2 | 3 | 0 | 0 | 0 |
| 0.063 | 93 | 27 | 11 | 13 | 18 | 13 | 13 | 12 | 7 | 1 | 0 | 5 |
| 0.031 | 93 | 36 | 23 | 19 | 17 | 12 | 16 | 18 | 15 | 16 | 13 | 14 |
| 0.016 | 96 | 38 | 23 | 21 | 24 | 20 | 18 | 19 | 19 | 18 | 16 | 18 |
| 0.008 | 96 | 60 | 39 | 34 | 25 | 22 | 30 | 28 | 24 | 25 | 21 | 39 |
| 0.004 | 99 | 67 | 42 | 38 | 29 | 32 | 46 | 34 | 35 | 30 | 27 | 45 |
| 0.002 | 97 | 76 | 61 | 55 | 50 | 48 | 44 | 38 | 35 | 36 | 28 | 44 |
| 0 | 100 | 75 | 63 | 53 | 56 | 54 | 53 | 55 | 50 | 48 | 48 | 47 |
The isoeffective combinations on the basis of which the FICIs were calculated are shown in bold. Among all the FICIs calculated based on the isoeffective combinations, FICImax was <4, and so FICImin (0.0006) was reported as the FICI. The combination with the lowest FICI is shown by underlining.
Interpretation of drug interactions.
The results of the checkerboard analysis interpreted by the nonparametric methods based on both the LA and BI theories are summarized in Table 3. In the checkerboard microtiter plate format, strong synergism was concluded in all five resistant isolates analyzed by both models, FICI and ΔE, and the two models correlated very well. However, different interpretations were obtained for the five susceptible isolates. Synergisms were observed in two strains by FICI and in one strain by ΔE for FK506-FLC and FK506-ITR combinations, and indifference was observed in other strains, while for the FK506-VRC combination, synergism was observed in one strain by FICI and in two strains by ΔE; others revealed indifference. The ΔE value of each well was the average of triplicate results. When the ΔE value was positive, synergy was concluded, and higher ΔE values suggested stronger synergistic interactions. [summ]SYN and [summ]ANT were the sums of the percentages for all statistically significant synergistic and antagonistic interactions among a whole checkerboard combination with different concentrations. The interaction was considered weak, moderate, and strong synergism when the [summ]SYN was <100%, 100 to 200%, and >200%, respectively.
TABLE 3.
In vitro interaction between FK506 and azoles
| Drug in combination, strain, and median | Nonparametric methoda
|
||||
|---|---|---|---|---|---|
| FICI
|
ΔE model
|
||||
| Mean (range) | Interpretation | ΣSYN (n) | ΣANT (n) | Interpretation | |
| FLC | |||||
| Susceptible | |||||
| CA5 | 2.00 (1.00-2.00) | IND | 16 (7) | −51 (11) | IND |
| CA8 | 1.00 (0.5-1.00) | IND | 31 (16) | −43.2 (33) | IND |
| CA12 | 0.5 (0.25-0.50) | SYN | 103.5 (23) | −45.3 (9) | SYN |
| CA14 | 1.00 (0.50-2.00) | IND | 16.8 (7) | −33.9 (21) | IND |
| CA129 | 0.50 (0.25-0.50) | SYN | 65.6 (8) | −36 (16) | IND |
| Median | 1.00 | 31 | −43.2 | ||
| Resistant | |||||
| CA10 | 0.00 (0.00) | SYN | 1,032 (38) | −55.7 (11) | SYN |
| CA15 | 0.00 (0.00) | SYN | 962.7 (34) | −28.4 (8) | SYN |
| CA16 | 0.00 (0.00-0.03) | SYN | 1,322.4 (49) | −13.5 (14) | SYN |
| CA135 | 0.00 (0.00) | SYN | 956 (42) | −33.4 (10) | SYN |
| CA137 | 0.00 (0.00) | SYN | 1,154.3 (52) | −15.6 (6) | SYN |
| Median | 0.00 | 1,032 | −28.4 | ||
| ITR | |||||
| Susceptible | |||||
| CA5 | 1.00 (0.50-1.00) | IND | 11.8 (4) | −38.4 (15) | IND |
| CA8 | 2.00 (0.50-2.00) | IND | 13.7 (4) | −78.4 (21) | IND |
| CA12 | 0.50 (0.25-0.50) | SYN | 137.6 (29) | −49.7 (16) | SYN |
| CA14 | 1.00 (0.50-1.00) | IND | 33.7 (13) | −54.8 (23) | IND |
| CA129 | 0.25 (0.25-0.50) | SYN | 98.1 (54) | −53 (9) | IND |
| Median | 1.00 | 33.7 | −53 | ||
| Resistant | |||||
| CA10 | 0.00 (0.00) | SYN | 1,384.2 (56) | −33.4 (10) | SYN |
| CA15 | 0.00 (0.00) | SYN | 1,618 (49) | −56.1 (13) | SYN |
| CA16 | 0.00 (0.00)) | SYN | 1,142 (57) | −43.8 (7) | SYN |
| CA135 | 0.00 (0.00) | SYN | 1,512.3 (32) | −4.7 (2) | SYN |
| CA137 | 0.00 (0.00) | SYN | 1,035.2 (38) | −38.1 (13) | SYN |
| Median | 0.00 | 1,384.2 | −38.1 | ||
| VRC | |||||
| Susceptible | |||||
| CA5 | 1.00 (1.00-2.00) | IND | 42.9 (6) | −64.3 (11) | IND |
| CA8 | 1.00 (0.50-2.00) | IND | 91.3 (22) | −6.5 (3) | SYN |
| CA12 | 1.00 (0.50-1.00) | IND | 100.6 (26) | −5.7 (5) | SYN |
| CA14 | 1.00 (0.50-2.00) | IND | 35.4 (21) | −83.2 (18) | IND |
| CA129 | 0.25 (0.25-0.50) | SYN | 89.1 (27) | −32.5 (12) | IND |
| Median | 1.00 | 89.1 | −32.5 | ||
| Resistant | |||||
| CA10 | 0.00 (0.00) | SYN | 1,024 (62) | −3.4 (1) | SYN |
| CA15 | 0.00 (0.00) | SYN | 857.8 (42) | −33.5 (13) | SYN |
| CA16 | 0.00 (0.00) | SYN | 912.1 (44) | −15.6 (6) | SYN |
| CA135 | 0.00 (0.00-0.01) | SYN | 1,328 (52) | −97.4 (23) | SYN |
| CA137 | 0.00 (0.00) | SYN | 764.2 (34) | −28.4 (15) | SYN |
| Median | 0.00 | 912.1 | 28.4 | ||
SYN, synergism; ANT, antagonism; IND, indifference. For the FICI model, synergy was defined as a FICI of ≤0.5, antagonism was defined as a FICI of >4.0, and indifference was defined as a FICI of >0.5 to 4 (i.e., no interaction). For the ΔE model, ΣSYN and ΣANT were the sums of the percentages of all statistically significant synergistic and antagonistic interactions. Interactions with <100% statistically significant interactions were considered weak synergism, those with 100 to 200% statistically significant interactions were considered moderate, and those with >200% statistically significant interactions were considered strong.
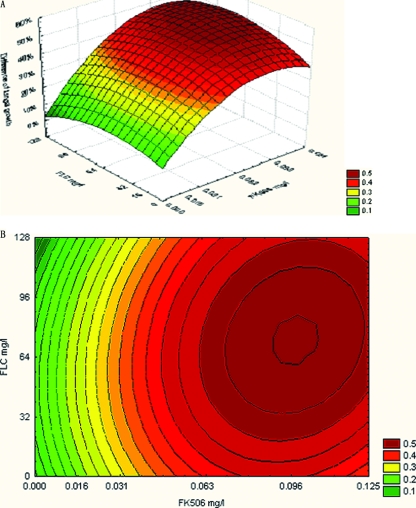
In addition, the values obtained for each combination based on BI theory were used to construct a three-dimensional plot. Thus, a surface plot was obtained by use of a three-dimensional plot, with ΔE depicted on the z axis (Fig. 1). The contour plot was also constructed in order to visualize the drug concentrations that produce an interaction.
FIG. 1.
The three-dimensional (A) and contour (B) plots of interaction between FLC and FK506 against resistant C. albicans CA10 based on the ΔE model. The difference between the predicted and the measured percentage of fungal growth (ΔE = Epredicted − Emeasured) is shown on the z axis. Epredicted = EA × EB, where EA and EB were the experimental percentages of growth of each drug action alone, respectively. Emeasured is the percentage of fungal growth when drugs were used in combination. The ΔE value of each well was the average of triplicate results. Higher ΔE values suggested stronger synergistic interactions between FLC and FK506.
Time-killing curves.
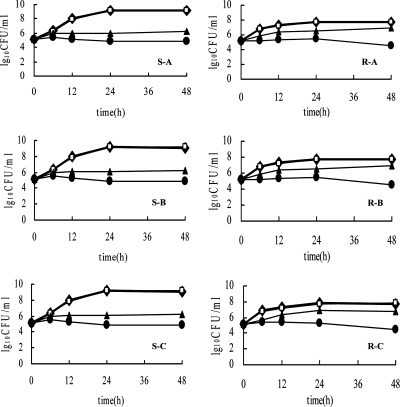
Positive interactions were confirmed by time-kill curves. The OD value obtained from the XTT reduction assay was transformed to a colony count according to the standard curve (Fig. 2). The intensity and nature of interactions between FK506 and azoles by time-kill study are shown in Table 4, and the representative plots of the log10 CFU/ml versus time for FK506 alone or in combination with three azoles are depicted in Fig. 3. In general, indifference was displayed for all three combinations against the susceptible strains, while synergism was revealed for the resistant strain. The result by metabolism inhibition (XTT reduction assay) displayed no difference from the interpretation based on colony counting except for the combination of ITR plus FK506 as shown in Table 4. Figure 3 revealed that FK506 itself can hardly affect the growth at 0.016 μg/ml after 48 h, but the antifungal activity of azoles against the resistant strain was dramatically enhanced by addition of it. Given the initial inoculum of 105 CFU/ml, the combination yielded a 2.37-log10-CFU/ml decrease compared with 10 μg/ml FLC alone (Fig. 3, R-A), a 2.46-log10-CFU/ml decrease compared with 1 μg/ml ITR alone (Fig. 3, R-B), and a 2.26-log10-CFU/ml decrease compared with 1 μg/ml VRC alone at 48 h (Fig. 3, R-C).
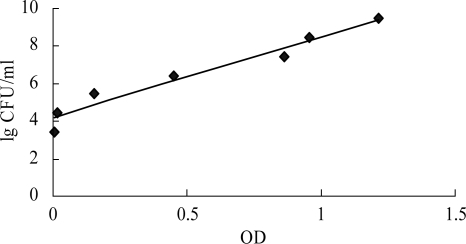
FIG. 2.
Correlation between viable cell counts determined by colony counting and OD values determined by XTT-menadione colorimetric readings. Results are expressed as Y = 4.2610x + 4.2087, with a correlation coefficient r of 0.9622.
TABLE 4.
Intensity and nature of interactions between FK506 and azoles determined by time-kill study
| Drug combined with FK506 | Colony counting
|
XTT methodc
|
||||||
|---|---|---|---|---|---|---|---|---|
| CA14 colony counta | Interpretationb | CA10 colony counta | Interpretationb | CA14 colony counta | Interpretationb | CA10 colony counta | Interpretationb | |
| FLC | 1.98 | IND | 2.03 | SYN | 1.51 | IND | 2.37 | SYN |
| ITR | 2.13 | SYN | 2.12 | SYN | 1.40 | IND | 2.46 | SYN |
| VRC | 1.49 | IND | 2.15 | SYN | 1.37 | IND | 2.26 | SYN |
Colony counts are decreases in colony numbers [log(48 h)] compared with the value for the most active drug.
SYN, synergism; ANT, antagonism; IND, indifference.
The data from the XTT reduction assay were calculated according to the equation Y = 4.2610x + 4.2087.
FIG. 3.
Time-kill curves of azoles and FK506 alone and in combination against azole-susceptible strain (S) CA14 and azole-resistant strain (R) CA10. The strains at starting inocula of 105 CFU/ml were exposed to in vivo-achievable concentrations of 10 μg/ml FLC (A), 1 μg/ml ITR (B), and 1 μg/ml VRC (C) alone and in combination with 0.016 μg/ml FK506. At 0, 6, 12, 24, and 48 h, aliquots were removed from each test tube to examine the cell metabolism ability by XTT reduction assay. Symbols: ⧫, growth control; □, FK506; ▴, azole; •, azole plus FK506. The OD value obtained by the XTT test was transformed to viable cells according to the equation Y = 4.2610x + 4.2087.
DISCUSSION
Transplant recipients are one of the highest-risk patient groups to develop fungal infection, and it is this group of patients who are generally already receiving calcineurin inhibitors as part of their immunosuppressive regimen. FK506 is a new immunosuppressant used clinically in the prevention of allograft rejection. It can suppress T-cell-proliferative responses and inhibit calcineurin, a Ca2+-calmodulin-dependent protein phosphatase that is important in cell signaling (6, 19). The calcineurin pathway has been shown to be critical in fungal survival and stress responses in several fungi (32, 44), including in vitro antifungal activity against Saccharomyces cerevisiae, C. albicans, Cryptococcus neoformans, and Aspergillus fumigatus (7, 8, 31).
Previous study has shown that FK506 and caspofungin exhibited a clear in vitro positive interaction against A. fumigatus (45, 46) and a potent fungicidal synergism with the ergosterol biosynthesis inhibitors fenpropimorph and terbinafine against C. albicans determined by microdilution checkerboard assay and analyzed by FICI (33). The authors of another report showed that FK506 has a synergism effect with azole (fluconazole, ketoconazole, and itraconazole) against azole-resistant C. albican strains by performing the time-killing test and evaluating the impact on azole accumulation (20). However, no formal MIC study was performed, and no study was reported on the interaction of FK506 and VRC.
In this study we evaluated comprehensive interactions between FK506 and three azoles (FLC, ITR, and VRC) against newly clinically isolated C. albicans strains by different methods and models. The interactions were evaluated with the checkerboard microdilution assay based on antifungal growth, which is assessed either visually by grading turbidity or spectrophotometrically by measuring OD. MIC80 was used as an endpoint, and in order to make the results from the two methods comparable, the fungal growth was graded 0 to 5 for the visual reading. The results were interpreted by two nonparametric models, FICI, based on LA, and ΔE, based on BI no-interaction theories. The time-killing test was performed by colony counting and XTT reduction assay.
As we know, different results may be obtained by different methods. In recent years, considerable progress has been made in the methods of evaluating drug interactions. Spectrophotometric and colorimetric methods have been developed in recent years. They can quantify the fungal growth more precisely and are able to detect small changes in metabolic activity of fungi (22, 23). Based on these methods, the effects of antifungal drugs either on the fungal biomass or on the metabolic status of fungi can be measured. Our results revealed that the data obtained by the spectrophotometric method correlate well with the data from visual reading. Furthermore, the method is easier to perform and the results can be interpreted by more new models.
FICI is the most frequently used model to interpret the interaction between antifungal drugs. However, the interpretation of FICI in defining synergy or antagonism is a problem in itself. Several different definitions have been described in the literature (4, 25, 28), but there is no consensus on which definition to use in interaction studies. According to the work of Odds (30), for the interpretation of a single FICI, a value of >4 is usually considered antagonism, and a value of ≤0.5 is usually considered synergy. In this study we interpreted the results according to the definition of Odds. However, because we performed triplicate experiments for each strain studied, we interpreted the results of all replicates as one outcome, thereby constraining the interexperimental error. Thus, when the results of all three replicates were concordant, synergy or antagonism was defined if FICIs were below 1 or above 1, respectively. In all other cases interactions were defined as indifference (47). Ease of use, simplicity, and feasibility of performance make FICI still the method of choice for analyses of drug-drug interactions, although it has some disadvantages.
The ΔE model is the nonparametric method based on BI theory. When it was used in assessing the interaction between FK506 and three azoles, the percentages of fungal growth were derived from the experimental data directly, and good agreement was found with the FICI model, particularly against azole-resistant strains. Therefore, the ΔE model, developed in recent years, is a useful approach in analyzing the nature of interactions between different drugs based on a checkerboard method.
Time-kill curves can provide growth kinetic information over time and give a more detailed picture of the effect of drug combinations on cell viability (26). This method is capable of detecting differences in the rate and extent of antifungal activity over time and has been widely used in recent years (10, 18, 36). For fungal growth evaluation, besides visual reading of turbidity and OD measurement, growth can be determined colorimetrically using indicator substances that are reduced to colored products by viable microorganisms (23). XTT is a new yellow tetrazolium salt which can be converted by mitochondrial dehydrogenases of viable fungi to a water-soluble orange formazan product, which can be measured spectrophotometrically at 492 nm (17). Therefore, we performed the time-kill experiment by the XTT assay and compared it with colony counting. The data from time-kill curves based on cell colony counting and metabolism-inhibitory effects confirmed the positive interactions observed in the checkerboard test. The potency of all the tested azoles can be enhanced by combining them with FK506 against C. albicans, especially against the resistant isolates. The data from the XTT test correlate well with the colony counting results.
In conclusion, our study revealed that the activity of azole antifungal agents that target ergosterol biosynthesis can be enhanced by FK506, an inhibitor of the calcineurin signaling pathway, especially against azole-resistant C. albicans in vitro. The results confirmed those earlier findings by using a normal MIC test with different interpretation models and a time-kill study to demonstrate combined effects against C. albicans and suggested differences in the combination effects against sensitive and resistant isolates. In addition, the isolates that we tested were newly isolated from clinical sources and were resistant to all three tested azoles, so our results also expanded on the potent effect of FK506 with VRC against resistant C. albicans. This result provides an alternative approach by which to overcome antifungal drug resistance. However, the potential of using this combination therapy in vivo warrants investigation, and further study is needed to determine the underlying mechanism of the synergistic action.
Acknowledgments
This work was supported by a grant (2005GG4402036) from Science and Technology of Shandong Province, China.
Footnotes
Published ahead of print on 3 December 2007.
REFERENCES
- 1.Afeltra, J., R. G. Vitale, J. W. Mouton, and P. E. Verweij. 2004. Potent synergistic in vitro interaction between nonantimicrobial membrane-active compounds and itraconazole against clinical isolates of Aspergillus fumigatus resistant to itraconazole. Antimicrob. Agents Chemother. 48:1335-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barchiesi, F., L. F. Di Francesco, P. Compagnucci, D. Arzeni, A. Giacometti, and C. Scalise. 1998. In-vitro interaction of terbinafine with amphotericin B, fluconazole and itraconazole against clinical isolates of Candida albicans. J. Antimicrob. Chemother. 41:59-65. [DOI] [PubMed] [Google Scholar]
- 3.Baumgartner, C., A. Freydiere, and Y. Gile. 1996. Direct identification and recognition of yeast species from clinical material by using Albicans ID and CHROMagar Candida plates. J. Clin. Microbiol. 34:454-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berenbaum, M. C. 1978. A method for testing for synergy with any number of agents. J. Infect. Dis. 137:122-130. [DOI] [PubMed] [Google Scholar]
- 5.Berenbaum, M. C. 1989. What is synergy? Pharmacol. Rev. 41:93-141. [PubMed] [Google Scholar]
- 6.Cardenas, M. E., M. C. Cruz, M. Del Poeta, N. Chung, J. R. Perfect, and J. Heitman. 1999. Antifungal activities of antineoplastic agents: Saccharomyces cerevisiae as a model system to study drug action. Clin. Microbiol. Rev. 12:583-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruz, M. C., M. Del Poeta, P. Wang, R. Wenger, G. Zenke, V. F. Quesniaux, N. R. Movva, J. R. Perfect, M. E. Cardenas, and J. Heitman. 2000. Immunosuppressive and nonimmunosuppressive cyclosporine analogs are toxic to the opportunistic fungal pathogen Cryptococcus neoformans via cyclophilin-dependent inhibition of calcineurin. Antimicrob. Agents Chemother. 44:143-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Del Poeta, M., M. C. Cruz, M. E. Cardenas, J. R. Perfect, and J. Heitman. 2000. Synergistic antifungal activities of bafilomycin A1, fluconazole, and the pneumocandin MK-0991/caspofungin acetate (L-743,873) with calcineurin inhibitors FK506 and L-685,818 against Cryptococcus neoformans. Antimicrob. Agents Chemother. 44:739-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denning, D. W., G. G. Baily, and S. V. Hood. 1997. Azole resistance in Candida. Eur. J. Clin. Microbiol. Infect. Dis. 16:261-280. [DOI] [PubMed] [Google Scholar]
- 10.Ernst, E. J., E. E. Roling, C. R. Petzold, D. J. Keele, and M. E. Klepser. 2002. In vitro activity of micafungin (FK-463) against Candida spp.: microdilution, time-kill, and postantifungal-effect studies. Antimicrob. Agents Chemother. 46:3846-3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espinel-Ingroff, A., F. Barchiesi, M. Cuenca-Estrella, A. Fothergill, M. A. Pfaller, M. Rinaldi, J. L. Rodriguez-Tudela, and P. E. Verweij. 2005. Comparison of visual 24-hour and spectrophotometric 48-hour MICs to CLSI reference microdilution MICs of fluconazole, itraconazole, posaconazole, and voriconazole for Candida spp.: a collaborative study. J. Clin. Microbiol. 43:4535-4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fricker-Hidalgo, H., O. Vandapel, M. A. Duchesne, M. A Mazoyer, D. Monget, B. Lardy, B. Lebeau, J. Freney, P. Ambroise-Thomas, and R. Grillot. 1996. Comparison of the new API Candida system to the ID 32C system for the identification of clinically important yeast species. J. Clin. Microbiol. 34:1846-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graybill, J. R. 1997. Editorial response: can we agree on the treatment of candidiasis? Clin. Infect. Dis. 25:60-62. [DOI] [PubMed] [Google Scholar]
- 14.Greco, W. R., G. Bravo, and J. C. Parsons. 1995. The search for synergy: a critical review from a response surface perspective. Pharmacol. Rev. 47:331-385. [PubMed] [Google Scholar]
- 15.Hibberd, P. L., and R. H. Rubin. 1994. Clinical aspects of fungal infection in organ transplant recipients. Clin. Infect. Dis. 19(Suppl.):S33-S40. [DOI] [PubMed] [Google Scholar]
- 16.Klepser, M. E., E. J. Ernst, R. E. Lewis, M. E. Ernst, and M. A. Pfaller. 1998. Influence of test conditions on antifungal time-kill curve results: proposal for standardized methods. Antimicrob. Agents Chemother. 42:1207-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuhn, D. M., J. Chandra, P. K. Mukherjee, and M. A. Ghannoum. 2002. Comparison of biofilms formed by Candida albicans and Candida parapsilosis on bioprosthetic surfaces. Infect. Immun. 70:878-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis, R. E., D. J. Diekema, S. A. Messer, M. A. Pfaller, and M. E. Klepser. 2002. Comparison of Etest, chequerboard dilution and time-kill studies for the detection of synergy or antagonism between antifungal agents tested against Candida species. J. Antimicrob. Chemother. 49:345-351. [DOI] [PubMed] [Google Scholar]
- 19.Liu, J., J. D. Farmer, Jr., W. S. Lane, J. Friedman, I. Weissman, and S. L. Schreiber. 1991. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 66:807-815. [DOI] [PubMed] [Google Scholar]
- 20.Maesaki, S., P. Marichal, M. A. Hossain, D. Sanlard, H. V. Bossche, and S. Kohno. 1998. Synergic effects of tacrolimus and azole antifungal agents against azole-resistant Candida albicans strains. J. Antimicrob. Chemother. 42:747-753. [DOI] [PubMed] [Google Scholar]
- 21.Marchetti, O., P. Moreillon, M. P. Glauser, J. Bille, and D. Sanglard. 2000. Potent synergism of the combination of fluconazole and cyclosporine in Candida albicans. Antimicrob. Agents Chemother. 44:2373-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meletiadis, J., J. F. Meis, J. W. Mouton, J. P. Donnelly, and P. E. Verweij. 2000. Comparison of NCCLS and 3-(4,5-dimethyl-2-thiazyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) methods of in vitro susceptibility testing of filamentous fungi and development of a new simplified method. J. Clin. Microbiol. 38:2949-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meletiadis, J., J. W. Mouton, J. F. Meis, B. A. Bouman, P. J. Donnelly, P. E. Verweij, and EUROFUNG Network. 2001. Comparison of spectrophotometric and visual reading of NCCLS method and evaluation of a colorimetric method based on reduction of a soluble tetrazolium salt, 2,3-bis {2-methoxy-4-nitro-5-[(sulfenylamino) carbonyl]-2H-tetrazolium hydroxide}, for antifungal susceptibility testing of Aspergillus species. J. Clin. Microbiol. 39:4256-4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meletiadis, J., J. W. Mouton, J. F. G. M. Meis, and P. E. Verweij. 2003. In vitro drug interaction modeling of combinations of azoles with terbinafine against clinical Scedosporium prolificans isolates. Antimicrob. Agents Chemother. 47:106-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moody, J. A. 1992. Synergism testing: broth microdilution checkerboard and broth macrodilution methods, p. 1-28. In H. D. Isenberg (ed.), Clinical microbiology procedures handbook. American Society for Microbiology, Washington, DC.
- 26.Mukherjee, P. K., D. J. Sheehan, C. A. Hitchcock, and M. A. Ghannoum. 2005. Combination treatment of invasive fungal infections. Clin. Microbiol. Rev. 18:163-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard. NCCLS document M27-A2. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 28.Nguyen, M. H., F. Barchiesi, D. A. McGough, V. L. Yu, and M. G. Rinaldi. 1995. In vitro evaluation of combination of fluconazole and flucytosine against Cryptococcus neoformans var. neoformans. Antimicrob. Agents Chemother. 39:1691-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Odds, F. C. 1996. Resistance of clinically important yeasts to antifungal agents. Int. J. Antimicrob. Agents 6:145-147. [DOI] [PubMed] [Google Scholar]
- 30.Odds, F. C. 2003. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 52:1. [DOI] [PubMed] [Google Scholar]
- 31.Odom, A., M. Del Poeta, J. R. Perfect, and J. Heitman. 1997. The immunosuppressant FK506 and its nonimmunosuppressive analog L-685,818 are toxic to Cryptococcus neoformans by inhibition of a common target protein. Antimicrob. Agents Chemother. 41:156-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Odom, A., S. Muir, E. Lim, D. L. Toffaletti, J. R. Perfect, and J. Heitman. 1997. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 16:2576-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Onyewu, C., J. R. Blankenship, M. Del Poeta, and J. Heitman. 2003. Ergosterol biosynthesis inhibitors become fungicidal when combined with calcineurin inhibitors against Candida albicans, Candida glabrata, and Candida krusei. Antimicrob. Agents Chemother. 47:956-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paya, C. V. 1993. Fungal infections in solid-organ transplantation. Clin. Infect. Dis. 16:677-688. [DOI] [PubMed] [Google Scholar]
- 35.Pfaller, M. A., A. Houston, and S. Coffman. 1996. Application of CHROMagar Candida for rapid screening of clinical specimens for Candida albicans, Candida tropicalis, Candida krusei, and Candida (Torulopsis) glabrata. J. Clin. Microbiol. 34:58-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfaller, M. A., D. J. Sheehan, and J. H. Rex. 2004. Determination of fungicidal activities against yeasts and molds: lessons learned from bactericidal testing and the need for standardization. Clin. Microbiol. Rev. 17:268-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polak, A. 1999. The past, present and future of antimycotic combination therapy. Mycoses 42:355-370. [DOI] [PubMed] [Google Scholar]
- 38.Prichard, M. N., and C. Shipman, Jr. 1990. A three-dimensional model to analyze drug-drug interactions. Antivir. Res. 14:181-205. [DOI] [PubMed] [Google Scholar]
- 39.Prichard, M. N., L. E. Prichard, and C. Shipman, Jr. 1993. Strategic design and three-dimensional analysis of antiviral drug combinations. Antimicrob. Agents Chemother. 37:540-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prichard, M. N., L. E. Prichard, W. A. Baguley, M. R. Nassiri, and C. Shipman, Jr. 1991. Three-dimensional analysis of the synergistic cytotoxicity of ganciclovir and zidovudine. Antimicrob. Agents Chemother. 35:1060-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramage, G., K. V. Walle, B. L. Wickes, and J. L. López-Ribot. 2001. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 45:2475-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rex, J. H., J. E. Bennett, A. M. Sugar, P. G. Pappas, C. M. van der Horst, J. E. Edwards, R. G. Washburn, W. M. Scheld, A. W. Karchmer, A. P. Dine, M. J. Levenstein, and C. D. Webb. 1994. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. N. Engl. J. Med. 331:1325-1330. [DOI] [PubMed] [Google Scholar]
- 43.Sahuquillo Arce, J. M., E. Colombo Gainza, A. Gil Brusola, R. Ortiz Estevez, E. Canton, and M. Gobernado. 2006. In vitro activity of linezolid in combination with doxycycline, fosfomycin, levofloxacin, rifampicin and vancomycin against methicillin-susceptible Staphylococcus aureus. Rev. Esp. Quimioter. 19:252-257. [PubMed] [Google Scholar]
- 44.Sanglard, D., F. Ischer, O. Marchetti, J. Entenza, and J. Bille. 2003. Calcineurin A of Candida albicans: involvement in antifungal tolerance, cell morphogenesis and virulence. Mol. Microbiol. 48:959-976. [DOI] [PubMed] [Google Scholar]
- 45.Steinbach, W. J., W. A. Schell, J. R. Blankenship, C. Onyewu, J. Heitman, and J. R. Perfect. 2004. In vitro interactions between antifungals and immunosuppressants against Aspergillus fumigatus. Antimicrob. Agents Chemother. 48:1664-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steinbach, W. J., N. Singh, J. L. Miller, D. K. Benjamin, Jr., W. A. Schell, J. Heitman, and J. R. Perfect. 2004. In vitro interactions between antifungals and immunosuppressants against Aspergillus fumigatus isolates from transplant and nontransplant patients. Antimicrob. Agents Chemother. 48:4922-4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Te Dorsthorst, D. T. A., P. E. Verweij, J. F. G. M. Meis, N. C. Punt, and J. W. Mouton. 2004. In vitro interactions between amphotericin B, itraconazole, and flucytosine against 21 clinical Aspergillus isolates determined by two drug interaction models. Antimicrob. Agents Chemother. 48:2007-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Te Dorsthorst, D. T., P. E. Verweij, J. Meletiadis, M. Bergervoet, N. C. Punt, J. F. Meis, and J. W. Mouton. 2002. In vitro interaction of flucytosine combined with amphotericin B or fluconazole against thirty-five yeast isolates determined by both the fractional inhibitory concentration index and the response surface approach. Antimicrob. Agents Chemother. 46:2982-2989. [DOI] [PMC free article] [PubMed] [Google Scholar]