Abstract
A novel class of nonnucleoside hepatitis C virus (HCV) polymerase inhibitors characterized by a dihydropyrone core was identified by high-throughput screening. Crystallographic studies of these compounds in complex with the polymerase identified an allosteric binding site close to the junction of the thumb and finger domains, approximately 30 Å away from the catalytic center. AG-021541, a representative compound from this series, displayed measurable in vitro antiviral activity against the HCV genotype 1b subgenomic replicon with a mean 50% effective concentration of 2.9 μM. To identify mutations conferring in vitro resistance to AG-021541, resistance selection was carried out using HCV replicon cells either by serial passages in increasing concentrations of AG-021541 or by direct colony formation at fixed concentrations of the compound. We identified several amino acid substitutions in the AG-021541-binding region of the polymerase, including M423(T/V/I), M426T, I482(S/T), and V494A, with M423T as the predominant change observed. These mutants conferred various levels of resistance to AG-021541 and structurally related compounds but remained sensitive to interferon and HCV polymerase inhibitors known to interact with the active site or other allosteric sites of the protein. In addition, dihydropyrone polymerase inhibitors retained activity against replicons that contain signature resistance changes to other polymerase inhibitors, including S282T, C316N, M414T, and P495(S/L), indicating their potential to be used in combination therapies with these polymerase inhibitors. AG-021541-resistant replicon cell lines provide a valuable tool for mechanism-of-action studies of dihydropyrone polymerase inhibitors. The clinical relevance of in vitro resistance to HCV polymerase inhibitors remains to be investigated.
Hepatitis C virus (HCV) has emerged as one of most important etiological factors for blood-transmitted chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma (34, 38). The infection becomes persistent in about 85% of infected individuals, despite the presence of a strong humoral and cellular immune response (3). Currently, about 4.5 million individuals in the United States and more than 170 million worldwide are infected with HCV, which represents an important public health problem. A combination of pegylated forms of alpha interferon (IFN-α) and ribavirin is the only therapy available against HCV, but the success rate observed in individuals infected with genotype 1, which is the most prevalent genotype in the United States and worldwide, is only about 40% to 50% (7, 8, 25). In addition, IFN-α therapy is associated with significant side effects including fatigue, headache, myalgia, fever, nausea, and insomnia in more than 30% of patients. Ribavirin also causes hemolytic anemia in 10% to 20% of patients (22, 36). Consequently, there remains a significant unmet medical need for more effective and safer HCV therapy.
The HCV genome is a single-stranded, positive-sense RNA of approximately 9.6 kb (5). The genomic RNA encodes a polyprotein that is processed by host and viral proteases into at least 10 structural and nonstructural (NS) proteins. Most of the HCV NS proteins are required for viral RNA replication (1). The NS5B protein, encoding the viral RNA-dependent RNA polymerase, is a key component of the HCV RNA replication complex (14). Due to its apparent sequence and structural differences from human DNA and RNA polymerases, the HCV RNA polymerase is considered an attractive target for antiviral drug discovery. In addition to nucleoside analogs (2) and pyrophosphate mimics (37) that target the active site, a number of structurally diverse nonnucleoside polymerase inhibitors have been reported (13). They were shown to interact with at least four distinct allosteric sites by a combination of crystallographic analysis and in vitro resistance studies (11, 13).
One of the major factors limiting the efficacy of virus-specific inhibitors against retroviruses and many other RNA viruses has been the emergence of drug-resistant variants. Similar to most RNA viruses, HCV has a high degree of genetic variability as a result of mutations that occur during viral RNA replication due to the absence of an intrinsic repair mechanism associated with the HCV RNA-dependent RNA polymerase. Consequently, HCV exists in vivo as a population of heterogeneous, albeit closely related, genomes known as quasispecies, which contain a quantitatively predominant “master” genome and a multitude of minor genomes representing variable proportions of the total population. The heterogeneous nature of HCV has significant biological consequences and clinical implications, including the response of patients to antiviral therapy and resistance development.
In vitro resistance studies of various HCV inhibitors, including NS3 protease (20, 21, 24, 41, 44) and NS5B polymerase inhibitors (10, 11, 15, 17, 27, 30, 39, 40, 43), identified resistance mutations in the corresponding viral target regions, some of which have also been observed in subsequent clinical studies. A recent report indicated that resistance mutations observed in vitro were also developed in vivo after a 14-day monotherapy treatment with an NS3 protease inhibitor, VX-950, and correlated strongly with clinical outcome (33). A nonnucleoside polymerase inhibitor, HCV-796, achieved a peak reduction in viral load of >1 log on day 4, but the reduction dropped to approximately 0.7 log on day 14 (4) as a result of the emergence of resistance (42). These results highlight the importance of conducting in vitro resistance studies, which could provide important insights into resistance development in future clinical trials.
By high-throughput screening and structure-based drug design, we identified a novel class of nonnucleoside HCV polymerase inhibitors characterized by a dihydropyrone core (18, 19). Crystallographic studies indicated that these compounds bind to the polymerase at an allosteric site close to the junction of the thumb and finger domains (designated as the thumb-base site), approximately 30 Å away from the enzyme's catalytic center (23). AG-021541, a representative compound from this series, displayed measurable in vitro antiviral activity against the HCV subgenomic replicon. To further confirm the binding site for AG-021541 and determine its in vitro resistance profile, replicon cell lines resistant to AG-021541 were selected either by in vitro serial passages in increasing concentrations or by direct colony formation at fixed concentrations of the compound. Resistance mutations were identified and introduced back into the wild-type (wt) replicon for the determination of replication fitness and resistance phenotype. Identification of resistance mutations confirmed that AG-021541 targets the HCV RNA polymerase at the thumb-base allosteric site. Further evaluation of polymerase inhibitors against replicons that contain some of the resistance mutations identified to date demonstrated that dihydropyrone inhibitors have nonoverlapping resistance profiles with other polymerase inhibitors that target different regions of the enzyme. In vitro resistance study is a useful tool to confirm target, mechanism of action, and binding specificity of various inhibitors and also allows rapid preclinical evaluation of resistance development that may have future clinical implications.
MATERIALS AND METHODS
Compounds.
AG-021541 and compounds A and B were synthesized at Pfizer Global Research and Development (La Jolla, CA). NM107 (32), HCV-796 (11), A-782759 (27), and Boerhinger Ingelheim's benzimidazole compound C (15) were prepared by external vendors according to literature procedures. Individual compounds were dissolved in dimethyl sulfoxide (DMSO) to a concentration of 100 mM and diluted to appropriate concentrations in growth medium. IFN-α (Sigma, St. Louis, MO) was dissolved in phosphate-buffered saline to a stock concentration of 105 IU/ml and diluted to appropriate concentrations in growth medium.
Cells and plasmids.
Huh7 and Huh7.5 cells, obtained from Apath LLC (St. Louis, MO), were propagated in Dulbecco's modified Eagle medium (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (HyClone, Logan, UT), 0.1 mM nonessential amino acid (Invitrogen), 100 units/ml of penicillin (Invitrogen), and 100 μg/ml of streptomycin sulfate (Invitrogen).
The dicistronic selectable replicon BB7, containing the HCV genes NS3-NS5B derived from the Con1 strain of genotype 1b (1b-Con1), was licensed from Apath LLC. To improve its replication efficiency, the S2204I adaptive mutation in BB7 was reverted to the wt amino acid while three different adaptive mutations, E1202G, T1280I, and S2197P, were introduced into BB7 to construct the BB7M4 replicon (9). The replicon was further modified to include a humanized Renilla luciferase (hRLuc) gene and the autocleavage site of the foot-and-mouth disease virus 2A protease so that HCV RNA replication could be monitored by a reporter assay (9). The reporter replicon construct was electroporated into Huh7 cells to generate a reporter replicon cell line, DSR. Subsequently, the firefly luciferase (FLuc) reporter gene under the control of a cytomegalovirus promoter was transfected into DSR cells to generate a dual-reporter stable cell line DDR, which allowed for simultaneous monitoring of antiviral activity and cytotoxicity of inhibitors (9).
In vitro selection of AG-021541-resistant replicon cells.
For serial passage experiments in increasing concentrations of AG-021541, DSR cells were seeded in T-25 flasks at approximately 30% confluence in medium containing 250 μg/ml of G418 and AG-021541 at a starting concentration of 0.2 times the 90% effective concentration (0.2× EC90) (3 μM). The cells were cultured until they reached 90 to 100% confluence and were maintained at the same compound concentration for three passages. At the fourth passage, the compound concentration was increased by twofold to 0.4× EC90 (6 μM). The cells were maintained at 0.4× EC90 for three passages and subjected to another twofold increase in the AG-021541 concentration to 0.8× EC90 (12 μM). This selection process was repeated until the compound concentration reached 6.4× EC90 (96 μM). The concentration of AG-021541 could not be increased further due to apparent cytotoxicity at the next higher concentration, 12.8× EC90 (192 μM). Cells grown at 0.8× EC90, 1.6× EC90, and 3.2× EC90 of AG-021541 were seeded at a density of 2.5 × 104, 5 × 104, and 1 ×105 in 10-cm dishes and subjected to colony formation at 1.6× EC90, 3.2× EC90, and 6.4× EC90, respectively. The cell growth medium containing appropriate concentrations of AG-021541 was changed weekly. Resistant colonies were isolated after approximately 2 to 3 weeks of selection.
For resistance selection at fixed concentrations of AG-021541, DSR cells were seeded at densities of 5 × 104, 1 × 105, and 2.5 × 105 in 10-cm plates for direct colony formation. The cells were grown in 3× EC90 and 6× EC90 (45 and 90 μM) of AG-021541 with weekly changes of selection medium containing 250 μg/ml of G418. Colonies were isolated after approximately 3 weeks of selection. To determine the frequency of resistance generation, a separate plate for each cell density and compound concentration was set up in parallel for crystal violet staining and counting of colonies.
At each AG-021541 concentration used for colony formation, 10 colonies were isolated and expanded to cell lines, which were then tested for replication fitness, followed by resistance phenotype determination. All lines that showed a relative change in EC50 of >10-fold in susceptibility assays were subjected to population sequencing of the entire NS5B region after reverse transcription-PCR (RT-PCR) amplification of the replicon RNA isolated from these cell lines. Clonal sequencing was also performed on a selected number of resistant cell lines. As a negative control, the replicon cells were cultured in compound-free medium in parallel with those cultured in the presence of compounds for resistance selection. Population sequencing was also performed on the entire NS5B region amplified by RT-PCR of the replicon RNA isolated from these control cell lines.
Colony formation assay.
In vitro transcripts were generated from the replicon cDNA constructs using a T7 Megascript kit (Ambion, Austin, TX) according to the manufacturer's protocol. Two micrograms of the replicon in vitro transcripts was added to 400 μl of Huh7 cells at 2 × 107 cells/ml in phosphate-buffered saline. The cells were electroporated in a 0.4-cm gap cuvette with a Bio-Rad Gene Pulser II Electroporator at 270 V and 950 μF. Cells were resuspended in 10 ml of Dulbecco's modified Eagle medium and seeded in 10-cm plates at various dilutions. After 3 weeks of selection in 250 μg/ml of G418, colonies were stained with crystal violet and counted. The number of CFU was determined as the number of colonies formed per microgram of RNA used in electroporation.
Luciferase reporter assay.
HCV RNA replication was monitored by the hRLuc reporter activity in dicistronic replicon cell lines with a single (DSR) or dual reporter (DDR). In DDR cells, cytotoxicity was measured simultaneously by the FLuc reporter activity. A total of 2 × 104 DSR or DDR cells were seeded in 96-well plates in the absence of selection antibiotics. Compounds were tested at half-log serial dilutions over a range of concentrations with appropriate solvent controls (compound free). Cells were incubated with compounds for 3 days at 37°C. For DDR, reporter activities were determined using a Dual Luciferase Reporter kit (Promega, Pittsburgh, PA) following the manufacturer's instructions in a Perkin Elmer 1450 MicroBeta Jet (Waltham, MA). For DSR, hRLuc activity was determined using a Renilla Luciferase Assay System (Promega) following the manufacturer's instructions. The EC50 was calculated as the concentration of compound that effected a decrease in viral RNA replication (as measured by hRLuc activity or quantitative PCR) in compound-treated cells to 50% of that produced in compound-free cells. The 50% cytotoxic concentration (CC50) of compound was calculated as the amount that decreased host cell viability (as measured by FLuc activity) in compound-treated cells to 50% of that produced in compound-free cells. The values were determined by nonlinear regression analyses to a four-parameter fit.
Transient replication assay.
Ten micrograms of replicon transcripts was electroporated into 8 × 106 Huh7.5 cells in a 0.4-cm cuvette using a Bio-Rad Gene Pulser II Electroporator (Hercules, CA) at 220 V and 950 μF. Electroporated cells were resuspended in growth medium without selection antibiotics, plated in 96-well black plates with clear bottoms at 6 × 104 cells per well, and allowed to adhere for 4 h at 37°C. Compounds were tested at half-log serial dilutions over a range of concentrations with appropriate DMSO controls (compound free). After incubation for 3 days at 37°C, hRLuc activity was determined using the Renilla Luciferase Assay System according to the manufacturer's protocol in the Perkin Elmer 1450 MicroBeta Jet.
Introduction of resistance mutations into the wt replicon and the wt enzyme.
Resistance mutations were introduced into the wt replicon by site-directed mutagenesis using a Quikchange mutagenesis strategy according to the manufacturer's protocol (Stratagene, La Jolla, CA). To facilitate subcloning of fragments in the NS5B region, an StuI restriction enzyme site was first introduced into the subgenomic reporter replicon in the 3′ untranslated region (6) by site-directed mutagenesis. The primers used for mutagenesis were 5′-GGACCTAAACACTCCAGGCCAATAGGCCTTCCTG-3′ and 5′-CAGGAAGGCCTATTGGCCTGGAGTGTTTAGGTCC-3′ (nucleotide changes are underlined). The primer sequences used to introduce resistance mutations were the following: 5′-CCCACCTTGTGGGCAAGGACGATCCTGATGACTCATTTC-3′ and 5′-GAAATGAGTCATCAGGATCGTCCTTGCCCACAAGGTGGG-3′ for M423T; 5′-CCCACCTTGTGGGCAAGGATCATCCTGATGACTCATTTC-3′ and 5′-GAAATGGTCATCAGGATGATCCTTGCCCACAAGGTGGG-3′ for M423I; 5′-CCCACCTTGTGGGCAAGGGTGATCCTGATGACTCATTTC-3′ and 5′-GAAATGAGTCATCAGGATCACCCTTGCCCACAAGGTGGG-3′ for M423V; 5′-TGGGCAAGGATGATCCTGACGACTCATTTCTTCTCCATC-3′ and 5′-GATGGAGAAGAAATGAGTCGTCAGGATCATCCTTGCCCA-3′ for M426T; 5′-AGTTACTCTCCAGGTGAGACCAATAGG GTGGCTTCATGC-3′ and 5′-GCATGAAGCCACCCTATTGGTCTCACCTGGAGAGTAACT-3′ for I482T; 5′-TGCCTCAGGAAACTTGGGGCACCGCCCTTGCGAGTCTGG-3′ and 5′-CCAGACTCGCAAGGGCGGTGCCCCAAGTTTCCTGAGGCA-3′ for V494A; 5′-GACTGCACGATGCTCGTAAACGGAGACGACCTTGTCGTT-3′ and 5′-AACGACAAGGTCGTCTCCGTTTACGAGCATCGTGCAGTC-3′ for C316N; 5′-TATCGCCGGTGCCGCGCGACCGGTGTACTGACGACCAGC-3′ and 5′-GCTGGTCGTCAGTACACCGGTCGCGCGGCACCGGCGATA-3′ for S282T; 5′-TGGCTAGGCAACATCATCACGTATGCGCCCACCTTGTGG-3′ and 5′-CCACAAGGTGGGCGCATACGTGATGATGTTGCCTAGCCA-3′ for M414T; 5′-CTCAGGAAACTTGGGGTACTGCCCTTGCGAGTCTGGAGA-3′ and 5′-TCTCCAGACTCGCAAGGGCAGTACCCCAAGTTTCCTGAG-3′ for P495L; and 5′-CTCAGGAAACTTGGGGTATCGCCCTTGCGAGTCTGGAGA-3′ and 5′-TCTCCAGACTCGCAAGGGCGATACCCCAAGTTTCCTGAG-3′ for P495S. All mutations were confirmed by sequencing.
Construction and expression of the M423T polymerase protein.
M423T was introduced into the 1b-Con1 NS5BΔ21 construct using a Quikchange Mutagenesis Kit (Stratagene) and primers 5′-CACCTTGTGGGCAAGGACGATCCTGATGACTCATTTC-3′ and 5′-GAAATGAGTCATCAGGATCGTCCTTGCCCACAAGGTG-3′. Protein expression and purification were performed as described previously (23).
HCV polymerase biochemical assay.
The recombinant HCV polymerase was tested for its ability to perform primer/template-directed transcription. The reaction mixture contained 20 mM Tris-HCl (pH 7.0), 10 mM MgCl2, 1 mM dithiothreitol, 0.05% (vol/vol) Tween 20, annealed primer-template [60 nM biotin-dT17 primer and 5 nM poly(rA) template], 20 mM NaCl (from primer-template), 2.5 μM UTP, 0.6 μCi of [α-33P]UTP, 1% (vol/vol) DMSO, and a 30 nM concentration of the wt or mutant M423T 1b-Con1 NS5BΔ21 polymerase. Reactions were initiated by the addition of enzyme, incubated for 30 min at 30°C, and stopped by the addition of 18 mM EDTA. The polynucleotide products were collected by microfiltration on Nunc Silent Screen Biodyne B nitrocellulose microfiltration plates and rinsed, and radioactivity was quantified using a Storm 840 Phosphorimager (Perkin Elmer). The specific activity of the M423T mutant was not significantly different from that of the wt polymerase (data not shown). Compounds were tested in duplicates at twofold dilutions over a range of concentrations where inhibition was expected. The concentration at which 50% inhibition was obtained was determined by fitting of the data to an equation for noncompetitive tight-binding inhibition using KaleidaGraph.
X-ray crystallographic structure determination.
Tetragonal crystals of the AG-021541 in complex with the quadruple mutant (K114R L47Q F101Y V59D) of 1b-BK NS5BΔ21 were obtained as described previously (23). X-ray diffraction data were collected at −177°C on beam line 7.1 at the Stanford Synchrotron Radiation Laboratory to a resolution of 1.78 Å. Data were processed with Denzo/Scalepack (31). Statistics for the data over 30 to 1.78 Å (1.84 to 1.78 Å) were as follows: completeness, 99.9% (100.0); Rmerge, 5.0 (45.9); <I/σI>, 39.5 (4.6); and redundancy for the unique 61,909 reflections, 8.19. The structure was solved by difference Fourier methods using the ARP/wARP algorithm (28), which was employed to provide the initial automatically rebuilt model for further fitting with XFIT (26) and refinement with REFMAC (29). The final model of the protein-ligand complex containing 4,330 protein (residues 1 to 562) and 38 ligand atoms in addition to 521 water molecules was refined to an Rwork of 19.22% (58,710 reflections with F > 0) and Rfree of 21.90% (for 3,133 reflections; 5%) in the 30- to 1.78-Å resolution range. The average isotropic temperature factor was 25.2 Å2, 25.0 Å2, and 36.2 Å2 for protein, ligand, and water atoms, respectively. The model has good geometry with a root mean square deviation for bond lengths and angles of 0.014 Å and 1.406°, respectively, and all residues lie in the allowed Ramachandran regions according to PROCHECK (16).
RESULTS
In vitro antiviral activity and cytotoxicity of AG-021541.
The ability of AG-021541 to inhibit HCV RNA replication was evaluated against subgenomic replicons derived from the 1b-Con1 strain in replicon assays using the luciferase reporter endpoint. AG-021541 demonstrated measurable antiviral activity against the 1b-Con1 replicon, with mean EC50 and EC90 values of 2.9 and 15 μM, respectively, and no apparent cytotoxicity up to 100 μM. The inhibition of HCV RNA replication by AG-021541 was confirmed by a direct measurement of the replicon RNA levels using quantitative RT-PCR.
In vitro selection of AG-021541-resistant replicon cell lines by serial passages.
To select for AG-021541-resistant mutants, the HCV subgenomic reporter replicon cells, DSR (9), were serially passaged in 250 μg/ml of G418 and increasing concentrations of AG-021541 starting at 0.2× EC90 (3 μM). The cells under selection were passed when they reached 90 to 100% confluence and were maintained for three passages before the compound concentration was doubled. A decrease in the rate of cell growth followed by a gradual recovery was observed when the AG-021541 concentration was increased to 0.8× EC90, indicating a possible breakthrough in the growth of resistant replicon cells. No apparent change in growth rate was observed when the selection pressure was further increased to 6.4× EC90. Selection was not continued at higher concentrations of AG-021541 due to significant cytotoxicity observed in the presence of 12.8× EC90 (192 μM) of the compound.
After resistance breakthrough was observed at 0.8× EC90 of AG-021541, colony formation was started at the next higher concentration, 1.6× EC90, with cells from passage 7 ( Table 1). As the serial passages continued in 1.6× and 3.2× EC90 of AG-021541, colony formation was performed at 3.2× and 6.4× EC90 with cells from passages 10 and 13, respectively. Individual AG-021541-resistant colonies were formed and isolated after approximately 2 to 3 weeks of selection. Altogether, the lengths of time required from the start of selection to resistant colony formation of cells treated with 1.6×, 3.2×, and 6.4× EC90 of AG-021541 were 41, 50, and 59 days, respectively (Table 1).
TABLE 1.
Serial passages and colony formation of replicon cells in increasing concentrations of AG-021541a
| Passage nos. | AG-21541 concn (μM) | EC90 factor | Concn for colony formation (EC90 factor) | No. of days to obtain colonies |
|---|---|---|---|---|
| 1-3 | 3 | 0.2 | ND | |
| 4-6 | 6 | 0.4 | ND | |
| 7-9 | 12 | 0.8 | 1.6 | 41 |
| 10-12 | 24 | 1.6 | 3.2 | 50 |
| 13-15 | 48 | 3.2 | 6.4 | 59 |
| 16-18 | 96 | 6.4 | 12.8 | No colony |
| 19-21 | 192 | 12.8 | ND |
The replicon cells were cultured in the starting concentration of 0.2× EC90 of AG-021541. The cells were cultured until they reached 90 to 100% confluence (an average of 3 days), and the compound concentration was doubled after every three passages until cytotoxicity became apparent at 12.8× EC90. Cells grown at 0.8× EC90, 1.6× EC90, and 3.2× EC90 of AG-021541 were subjected to colony formation at 1.6× EC90, 3.2× EC90, and 6.4× EC90, respectively. ND, not done.
In vitro selection of AG-021541-resistant replicon cell lines at fixed concentrations.
Resistance selection was also performed at fixed concentrations of AG-021541. The HCV subgenomic reporter replicon cell line, DSR, was plated at different cell densities and subjected to direct colony formation in 3× EC90 and 6× EC90 of AG-021541. Individual colonies were observed after 3 to 4 weeks of selection and isolated for expansion into cell lines. The rates of resistance emergence, determined as the percentages of DSR replicon cells that formed AG-021541-resistant colonies, were 0.3% and 0.1% for resistance selection carried out at 3× EC90 and 6× EC90 of AG-021541, respectively, suggesting that mutations that result in a higher level resistance exist at a lower percentage.
Phenotypic and genotypic determination of AG-021541-resistant cell lines.
AG-021541-resistant colonies derived from either serial passaging or direct colony formation were expanded to cell lines in appropriate concentrations of the compound and subjected to replication fitness analysis using the luciferase reporter assay. A decrease in median luciferase activity of resistant cell lines, representing a reduction in replication fitness, was observed with an increase in the AG-021541 concentration at which these cell lines were selected (Table 2). Resistant cell lines with hRLuc activity above 200 relative light units (RLU) were carried further for phenotypic determinations. When tested against AG-021541, these cell lines displayed various levels of resistance, but no change in susceptibility to IFN was observed (Table 2 and Fig. 1). For serial passages, all of the resistant cell lines selected at the final concentration of 1.6× EC90 of AG-021541 demonstrated less than 60-fold resistance, with a median relative change in the EC50 of 33-fold. When the selection pressure increased to 3.2× EC90, 38% (three out of eight) of lines showed at least 100-fold resistance to AG-021541, which increased to 57% (four out of seven) when the selection pressure was further increased to 6.4× EC90 (Fig. 1). There was also a gradual increase in the median EC50 values against AG-021541 with the increase in selection pressure (Table 2). Similarly, the resistant cell lines selected by direct colony formation also showed a positive correlation between the level of resistance and selection pressure, with all lines grown at 3× EC90 showing less than 50-fold resistance and four out of five lines selected at 6× EC90 showing more than 100-fold resistance to AG-021541 (Fig. 1).
TABLE 2.
Replication fitness and resistance phenotype of AG-021541-resistant replicon cell linesa
| Selection method and AG-21541 concn | No. of cell lines | Range (median) of fold change in EC50
|
Median RLuc activity | |
|---|---|---|---|---|
| AG-021541 | IFN | |||
| Serial passage | ||||
| 1.6× EC90 | 9 | 6-58 (33) | 0.8-2.4 (1.5) | 133,000 |
| 3.2× EC90 | 8 | 9-150 (62) | 0.6-1.4 (0.9) | 51,000 |
| 6.4× EC90 | 7 | 37-104 (100) | 0.4-3.6 (1.1) | 12,000 |
| Direct colony formation | ||||
| 3× EC90 | 6 | 28-47 (36) | 0.4-1.9 (1.4) | 526,000 |
| 6× EC90 | 5 | 27-246 (128) | 1.3-2.0 (1.5) | 16,000 |
Stable cell lines obtained at various concentrations of AG-021541 either by serial passages or direct colony formation were evaluated for their replication fitness and susceptibility to AG-021541 and IFN in the reporter replicon assay. Cells were exposed to compounds for 3 days before RLuc activity was determined.
FIG. 1.
Phenotypic determination of AG-021541-resistant cell lines. Susceptibility of AG-021541-resistant cell lines, derived from serial passaging (A) or direct colony formation (B), to AG-021541 and IFN was determined in the replicon assay using hRLuc reporter activity as the endpoint. Cells were seeded in 96-well plates at 2 × 104/well and cultured for 3 days in the presence of AG-021541 or IFN before hRLuc activity was determined. The bars show the relative change in EC50 values of AG-021541 or IFN in individual AG-021541-resistant cell lines compared to the wt DSR cell line. The values represent the averages of duplicates in a single assay.
After confirmation of resistance, replicon RNA was isolated from AG-021541-resistant cell lines, amplified by RT-PCR, and subjected to population sequencing to determine the genotypic changes in the NS5B region of the replicon. Clonal sequencing was also performed on a selected number of resistant cell lines to confirm the results of population sequencing and to establish linkage (data not shown). The replicons in these cell lines were shown to harbor one to three mutations that resulted in amino acid changes in the polymerase (Table 3). The methionine-to-threonine change at residue 423 (M423T) appeared as the predominant amino acid change, which was present in 57% (20 out of 35) of all resistant cell lines and in cell lines selected across different concentrations of AG-021541. Furthermore, changes at amino acid M423, including M423V and M423I, were observed in 71% (25 out of 35) of resistant cell lines (Table 3). Besides M423T, all other amino acid changes existed at a much lower frequencies (<10%). Every resistant cell line contained at least one change at an amino acid that was shown to be part of the inhibitor-binding pocket, which includes M423, M426, I482, and V494 (23). There was no apparent difference in mutational profiles generated by either serial passaging or direct colony formation in the 35 resistant replicon cell lines analyzed. Although the sample size was too small to establish correlations between selection pressure and mutational profile, it is worth noting that while the M423T mutation was observed across all selection groups, M426T, I482T, and V494A were present only in resistant cell lines generated at lower concentrations (1.6× and 3× EC90) of the compound (Table 3). No change in the NS5B sequence was observed in the replicon cells that were cultured in parallel in compound-free medium.
TABLE 3.
Genotypic changes of AG-021541-resistant replicon cell lines
| Amino acid change(s) | No. of cell lines with amino acid change(s) by selection method and AG-021541 concna
|
||||
|---|---|---|---|---|---|
| Serial passage
|
Direct colony formation
|
||||
| 1.6× EC90 | 3.2× EC90 | 6.4× EC90 | 3× EC90 | 6× EC90 | |
| M423T | 2 | 6 | 2 | 2 | 4 |
| M423T, A421V | 1 | ||||
| M423T, K535N | 1 | 1 | |||
| M423T, T19P | 1 | ||||
| M423V | 1 | 1 | 1 | ||
| M423V, V147I | 1 | ||||
| M423I | 1 | ||||
| M426T | 3 | ||||
| I482S | 1 | ||||
| I482S, A421V | 1 | ||||
| I482S, I432V, Y587C | 1 | ||||
| I482T | 1 | ||||
| I482T, K81N | 1 | ||||
| V494A | 1 | ||||
| V494A, K50R | 1 | ||||
| Total no. of lines | 9 | 8 | 7 | 6 | 5 |
RNA was extracted from stable cell lines obtained at various concentrations of AG-021541 either by serial passages or direct colony formation and subjected to RT-PCR amplification and population sequencing of the NS5B region.
Phenotypic characterization of AG-021541 resistance mutations.
The predominant resistance change, M423T, was introduced back into the wt replicon, from which a stable cell line was generated for the determination of its resistance phenotype. M423T conferred an 87-fold increase in resistance to AG-021541, but no change in susceptibility to IFN was observed (Table 4). The replication fitness of the M423T replicon was reduced to 1.8 × 105 RLU, which was less than 50% of that of the wt replicon (5.2 × 105 RLU), as indicated by the hRLuc reporter activity. However, the colony-forming efficiency of the M423T replicon was comparable to the wt replicon in the colony formation assay (data not shown), suggesting that the mutant replicon replicates at a lower level but is still sufficient to support colony formation. When compounds A and B, two additional polymerase inhibitors that are structurally related to AG-021541, were tested in the M423T replicon cell line, both showed a significant reduction in antiviral activity, suggesting that their interactions with the polymerase are also primarily mediated by M423 (Table 4). Similarly, the 1b-Con1 polymerase enzyme that contained the M423T substitution also displayed resistance to AG-021541 and the structurally related compounds in the enzymatic assay (Table 4). The relative resistance of the mutant enzyme to these compounds was shown to be consistent with that of the mutant replicon. However, the levels of resistance of the mutant enzyme to the compounds were approximately an order of magnitude lower than those observed for the mutant replicon. This could be a result of differences between the biochemical and cell-based assay systems, including the presence of HCV proteins (polymerase alone versus replication complex) and replication capacity (nonreplicative versus replicative).
TABLE 4.
Susceptibility of the M423T mutant replicon and polymerase to AG-021541 and structurally related compoundsa
The susceptibility of the M423T replicon cell line to AG-021541 and related compounds was evaluated in the reporter replicon assay. Cells were exposed to compounds for 3 days before RLuc activity was determined. The susceptibility of the M423T mutant polymerase to AG-021541 and related compounds was evaluated in the HCV polymerase biochemical assay as described in Materials and Methods. Results represent the mean ± standard deviation (4 experiments) or individual values (1 to 2 experiments). Values from each experiment represent the averages of duplicates or triplicates. FC, fold change; ND, not determined; IC50, 50% inhibitory concentration.
b IU/ml.
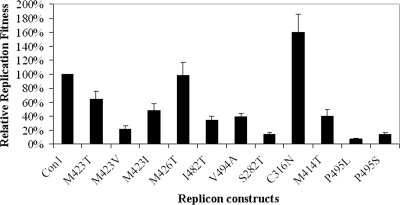
In addition to M423T, a number of minor AG-021541-resistant mutations affecting amino acids located in the AG-021541-binding site, including M423I, M423V, M426T, I482T, and V494A, were engineered individually into the wt replicon and characterized in a transient reporter replicon assay that allowed rapid screening for replication-competent resistance mutants and determination of their resistance phenotypes. The mutant replicon RNA was electroporated into Huh7.5 cells and incubated for 3 days in the presence or absence of compounds. The reporter signal was compared to that of the cells transfected with the wt replicon RNA to determine the fitness of the replicons and their susceptibility to compounds. Except for the M426T replicon, replicons containing single amino acid changes, M423T, M423V, M423I, or V494A, showed a 40 to 80% loss of hRLuc reporter activity compared to the wt replicon, indicating a reduction in replication fitness (Fig. 2). The extent of reduction in replication activity of the M423T replicon observed in transient replication assays was consistent with that of the M423T stable cell line. Changes from M to T, V, or I at amino acid 423 of the polymerase conferred more than a 31-fold increase in resistance to AG-021541, while changes at other amino acids in the binding pocket (M426T, I482T, or V494A) resulted in a lower level of resistance, ranging from 4.5- to 7.0-fold (Table 5). These results are consistent with the observation that M426T, I482T, and V494A were present only in replicon cell lines generated at lower concentrations of AG-021541 (Table 3). The difference in the levels of resistance between changes at M423 and other residues was also observed for compound B (Table 5), indicating that M423 is a key residue involved in the interaction between dihydropyrone compounds and the polymerase. Regardless of their replication fitness or level of resistance to AG-021541, all of the resistant replicons remained sensitive to IFN (Table 5). Furthermore, the resistance changes at the thumb-base site did not affect the activity of other HCV polymerase inhibitors known to interact with the active site (Idenix Pharmaceuticals NM107) or other allosteric sites (Wyeth/Viropharma HCV-796, Abbott A-782759, and Boehringer Ingelheim benzimidazole compound C) of the polymerase protein (Table 5).
FIG. 2.
Replication fitness of replicons containing resistance mutations to various HCV polymerase inhibitors. Huh7.5 cells were electroporated with in vitro transcripts of resistant replicons, seeded in 96-well plates at 6 × 104 cells/well, and cultured for 3 days before hRLuc activity was determined. The bars show the replication fitness of mutant replicons relative to that of the wt 1b-Con1 replicon (indicated as 100%). Standard deviations are shown as error bars.
TABLE 5.
Susceptibility of resistant mutant replicons to AG-021541 and other HCV inhibitors in the transient assay
| Replicon construct | Fold change in EC50 relative to wt replicon with the indicated inhibitora:
|
||||||
|---|---|---|---|---|---|---|---|
| IFN | AG-021541 | Compound B | NM107 | HCV-796 | A-782759 | Compound C | |
| M423T | 1.0 | >31 | 5625 | 0.8 | 1.2 | 0.7 | 1.3 |
| M423V | 1.0 | >44 | 781 | 1.4 | 0.9 | 0.7 | 0.2 |
| M423I | 1.0 | >44 | 179 | 1.4 | 0.9 | 1.4 | 1.4 |
| M426T | 0.9 | 4.5 | 4.0 | 0.8 | 0.9 | 1.2 | 1.6 |
| I482T | 1.1 | 7.0 | 5.6 | 1.4 | 0.5 | 0.3 | 1.1 |
| V494A | 0.4 | 6.9 | 11 | 0.7 | 0.2 | 0.6 | 0.2 |
| S282T | 1.2 | 1.2 | 0.9 | 18 | 1.1 | 1.7 | 0.6 |
| C316N | 0.5 | 0.9 | 0.8 | 0.5 | 9.0 | 1.5 | 0.5 |
| M414T | 0.7 | 0.6 | 0.7 | 0.8 | 0.6 | 169 | 0.3 |
| P495L | 1.4 | 2.5 | 3.5 | 1.0 | 0.7 | 8.7 | 16 |
| P495S | 1.1 | 0.5 | 0.6 | 1.0 | 0.5 | 2.9 | 13 |
The susceptibility of AG-021541-resistant replicons to IFN and various HCV polymerase inhibitors was evaluated in the reporter replicon assay. Cells were exposed to compounds for 3 days before RLuc activity was determined. Results showing at least fourfold increases in the EC50 are indicated in boldface.
To further demonstrate the activity of AG-021541 against replicons containing changes resistant to other polymerase inhibitors, signature resistance mutations within other binding sites, including S282T, C316N, M414T, P495L, and P495S, were introduced into the wt replicon. Similar to AG-021541-resistant changes, most of the single-amino-acid substitutions resulted in a reduction in replication fitness. In contrast, C316N, which is present in 40% of the genotype 1b clinical isolates based on sequences from the GenBank and Los Alamos databases, increased the replication fitness by 1.6-fold. While S282T, C316N, M414T, and P495(S/L) conferred a significant level of resistance to NM107, HCV-796, A-782759, and compound C, respectively, their susceptibility to AG-021541 and compound B remained unchanged. Our results support the potential use of dihydropyrones in combination therapies with polymerase inhibitors that target other distinct binding sites on the HCV polymerase (Table 5).
DISCUSSION
One of the major issues in the development of antiviral therapy is the emergence of drug-resistant variants. In vitro resistance studies have been widely used for human immunodeficiency virus (HIV) inhibitors and more recently for HCV inhibitors to identify mutations that are likely present in vivo after antiviral therapy. In the present study, in vitro resistance studies of AG-021541 by either serial passages or direct colony formation in the HCV subgenomic replicon cells identified amino acid changes at the thumb-base allosteric site of the viral polymerase, including M423(T/V/I), M426T, I482(S/T), and V494A. The predominant mutation, M423T, conferred an 87-fold reduction in susceptibility to AG-021541 but no change in susceptibility to IFN. Introduction of M423T and other minor resistance changes into the wt replicon resulted in reduced replication fitness (with the exception of M426T) and various levels of resistance to AG-021541 and its structurally related compounds. However, no cross-resistance was observed between AG-021541 and polymerase inhibitors that interact with other binding sites of the enzyme, suggesting its potential use in combination therapies with other inhibitors targeting different regions of the polymerase for the treatment of HCV.
Similar mutational profiles were observed in resistant cell lines selected by either serial passaging or direct colony formation (Table 3). Interestingly, the M426T, I482T, and V494A changes, which were observed only in resistant cell lines selected at lower concentrations of AG-021541, conferred lower levels of resistance. Our results are consistent with the hypothesis that the nature of resistance mutations was determined by the inhibitor concentration at which the resistant cell lines were selected rather than the selection process. Since serial passaging is more complex and labor-intensive, direct colony formation at fixed concentrations appeared to be a more efficient alternative and has been used in many HCV in vitro resistance studies reported in the literature. Increasing the number of concentrations used in resistance selection may increase the chance of identifying the full spectrum of resistance mutations that are associated with various levels of exposure to a given HCV inhibitor in vivo.
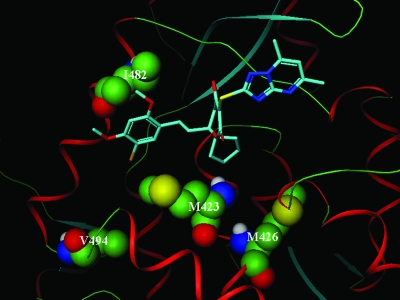
At least one change in each resistant cell line selected in our study was found at the amino acid residues M423, M426, I482, and V494 in the polymerase protein (Table 3). The cocrystal structure of AG-021541 and the 1b-NS5BΔ21(RQYD) polymerase showed that all of these residues lie in the long cleft that defines the thumb-base noncompetitive inhibitor binding site, as previously described (23) (Fig. 3). Of these amino acid residues, M423 and I482 make direct contacts with the inhibitor. In addition, the cocrystal structure reveals a very tight complementary fit of the cyclopentyl group of AG-021541 into a pocket (A-pocket) of the protein that is formed by the concerted movement of M423 and L497 upon inhibitor binding (23) (Fig. 3). The movement of L497 also creates an additional pocket (B-pocket) that accommodates the dimethoxychlorophenyl group. This tight complementary fit of the cyclopentyl group into the A-pocket prohibits residues with branched beta-carbons at position 423, such as threonine, valine, and isoleucine, thus explaining the high levels of resistance observed for the M423(T/V/I) substitution. On the other hand, a more modest level of resistance is expected for I482(S/T), a nonpolar-to-polar residue change in the B-pocket at a position where hydrophobic contacts to the dimethoxychlorophenyl group are required. Taken together, these structural observations are consistent with the results from our in vitro resistance study indicating that, of the amino acid changes at M426, I482, V494, and M423, the changes at M423 exert the most effects on inhibitor binding (Table 5). Compounds A and B share many structural features similar to AG-021541, including the dihydropyrone core, the cyclopentyl ring, and the triazolopyrimidine motif, which accounts for the observed cross-resistance of the AG-021541-resistant replicons to these compounds (Table 5). Among the changes that occurred at residue 423, replacement of methionine with threonine conferred the highest level of resistance to compound B. A close examination of the binding pocket revealed that this substitution introduced a polar hydroxyl group in the otherwise hydrophobic pocket, thus significantly altering the hydrophobic interaction between the protein and the cyclopentyl group of AG-021541. Changes at residues M423, M426, and I482 were previously observed in in vitro resistance studies of compounds containing pyranoindole or thiophene-2-carboxylic acid cores, both of which bind to a similar region in the thumb domain of the polymerase as the dihydropyrone inhibitors analyzed in this study (10, 17).
FIG. 3.
Binding of AG-021541 in the thumb-base allosteric site of the HCV polymerase. The X-ray cocrystal structure of AG-021541 and the 1b-BK NS5BΔ21 quadruple mutant protein (K114R L47Q F101Y V59D) is shown, with protein backbone in a ribbon structure and the key amino acid residues that form the AG-021541-binding site (M423, M426, I482, and V494) highlighted in a space-filling model. The carbon, sulfur, nitrogen, and oxygen atoms are depicted in green, yellow, blue, and red, respectively.
The baseline prevalence of the resistance substitutions observed in this study in naturally occurring HCV isolates cannot be accurately determined due to the lack of adequate clonal sequences of the HCV genome. Based on the available sequences from the GenBank and Los Alamos HCV databases, M423 is conserved in >99% of the HCV isolates reported, suggesting a significant role for the region in the polymerase where M423 lies during HCV replication and possibly explaining the lower replication efficiency of replicons with changes at this position. The only exception is that I423 is the wt amino acid found in the genotype 5 polymerase sequences, which may be one of the mechanisms underlying the reduced sensitivity of genotype 5 to this class of inhibitors (unpublished observations).
In the present study, multiple mutational patterns were observed in response to in vitro selection of AG-021541. As previously observed for other HCV nonnucleoside polymerase inhibitors and protease inhibitors, single-amino-acid substitutions were shown to confer high levels of resistance to dihydropyrone inhibitors, which cannot be easily overcome by increasing drug concentrations in vivo. Similar characteristics were also observed for HIV nonnucleoside reverse transcriptase inhibitors (NNRTIs), in which single mutations in the NNRTI-binding pocket resulted in high levels of resistance to one or more of the NNRTIs (35). Therefore, as in the case of treatment for HIV infection, monotherapy with HCV polymerase or protease inhibitors is unlikely to lead to effective and lasting suppression of HCV RNA replication. However, all of the resistance changes evaluated in this study remained fully susceptible to IFN and polymerase inhibitors targeting regions distinct from the AG-021541-binding site. The lack of cross-resistance to these inhibitors provides a rationale for combining the dihydropyrone polymerase inhibitors with nonpolymerase inhibitors or other polymerase inhibitors that target a different region of the enzyme in future HCV therapies. In vitro resistance studies of the Abbott benzothiadiazine nonnucleoside polymerase inhibitor, A-782759, and the Boehringer Ingelheim protease inhibitor, BILN-2061, indicated that the frequency of resistance to the combination was significantly lower than that to each compound alone (27), consistent with the notion that combination therapy with two or more small-molecule inhibitors may also afford an advantage in overcoming resistance development. In clinical trials, the Viropharma/Wyeth nonnucleoside polymerase inhibitor HCV-796 was shown to induce a 2- to 3-log reduction in viral load in combination therapy with IFN compared to a 1.4-log drop when administered as a monotherapy for 14 days (4). Furthermore, clinical results from Vertex showed that a 14-day treatment with the combination of VX-950 and IFN effectively suppressed development of resistance variants, which were observed in VX-950 monotherapy, and viral load was undetectable in all patients for a 12-week follow-up period (12). It is expected that future combination therapies will include IFN-sparing regimens, involving two or more HCV inhibitors with nonoverlapping resistance profiles, for the successful treatment of HCV infections.
Due to the lack of a robust infectious culture system for genotype 1 HCV, in vitro resistance studies of HCV inhibitors have been carried out in the replicon system, in which competition from the wt replicon is limited. As a result, resistance changes identified in vitro that show significantly reduced replication fitness may not be observed prominently in vivo. The A156(V/T) amino acid changes observed in in vitro resistance studies of VX-950 were seen only transiently during monotherapy treatment as a result of impaired replication fitness (33). Regardless, in vitro resistance studies have proven to be a powerful tool not only for determining the target and mechanism of action of anti-HCV compounds but also for predicting the identities of resistance mutations in HCV-infected patients after inhibitor exposure. Furthermore, the development of successful therapies targeting viral proteins requires an understanding of the nature of resistance variants and their fitness. Understanding the structural basis for inhibitor resistance will help in the design of more efficacious therapies. These studies will allow for the future identification of inhibitors with antiviral activity against replicons that contain clinically relevant drug-resistant mutations.
Acknowledgments
We acknowledge all members of the HCV polymerase project team at Pfizer Global Research and Development, La Jolla Laboratories.
Footnotes
Published ahead of print on 10 December 2007.
REFERENCES
- 1.Bartenschlager, R., M. Frese, and T. Pietschmann. 2004. Novel insights into hepatitis C virus replication and persistence. Adv. Virus Res. 63:71-180. [DOI] [PubMed] [Google Scholar]
- 2.Carroll, S. S., and D. B. Olsen. 2006. Nucleoside analog inhibitors of hepatitis C virus replication. Infect. Disord. Drug Targets 6:17-29. [DOI] [PubMed] [Google Scholar]
- 3.Cerny, A., and F. V. Chisari. 1999. Pathogenesis of chronic hepatitis C: immunological features of hepatic injury and viral persistence. Hepatology 30:595-601. [DOI] [PubMed] [Google Scholar]
- 4.Chandra, P., D. Raible, D. Harper, J. Speth, S. Villano, and G. Bichier. 2006. Antiviral activity of the non-nucleoside polymerase inhibitor, HCV-796, in patients with chronic hepatitis C virus: preliminary results from a randomized, double-blind, placebo-controlled, ascending multiple dose study, abstr. 1. Abstr. Dig. Dis. Week, Los Angeles, CA, 21 to 24 May 2006.
- 5.Choo, Q. L., K. H. Richman, J. H. Han, K. Berger, C. Lee, C. Dong, C. Gallegos, D. Coit, R. Medina-Selby, P. J. Barr, et al. 1991. Genetic organization and diversity of the hepatitis C virus. Proc. Natl. Acad. Sci. USA 88:2451-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friebe, P., and R. Bartenschlager. 2002. Genetic analysis of sequences in the 3′ nontranslated region of hepatitis C virus that are important for RNA replication. J. Virol. 76:5326-5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fried, M. W., M. L. Shiffman, K. R. Reddy, C. Smith, G. Marinos, F. L. Goncales, Jr., D. Haussinger, M. Diago, G. Carosi, D. Dhumeaux, A. Craxi, A. Lin, J. Hoffman, and J. Yu. 2002. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 347:975-982. [DOI] [PubMed] [Google Scholar]
- 8.Hadziyannis, S. J., H. Sette, Jr., T. R. Morgan, V. Balan, M. Diago, P. Marcellin, G. Ramadori, H. Bodenheimer, Jr., D. Bernstein, M. Rizzetto, S. Zeuzem, P. J. Pockros, A. Lin, and A. M. Ackrill. 2004. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann. Intern. Med. 140:346-355. [DOI] [PubMed] [Google Scholar]
- 9.Hao, W., K. J. Herlihy, N. J. Zhang, S. A. Fuhrman, C. Doan, A. K. Patick, and R. Duggal. 2007. Development of a novel dicistronic reporter-selectable hepatitis C virus replicon suitable for high-throughput inhibitor screening. Antimicrob. Agents Chemother. 51:95-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howe, A. Y., H. Cheng, I. Thompson, S. K. Chunduru, S. Herrmann, J. O'Connell, A. Agarwal, R. Chopra, and A. M. Del Vecchio. 2006. Molecular mechanism of a thumb domain hepatitis C virus nonnucleoside RNA-dependent RNA polymerase inhibitor. Antimicrob. Agents Chemother. 50:4103-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howe, A. Y. M., H. Cheng, S. Johann, S. Mullen, S. K. Chunduru, D. C. Young, J. Bard, and R. Chopra. 2006. Identification and characterization of HCV replicon variants with reduced susceptibility to HCV-796, abstr. 165. Abstr. 13th International Meeting on Hepatitis C Virus and Related Viruses, Cairns, Australia, 27 to 31 August, 2006.
- 12.Kieffer, T. L., C. Sarrazin, J. S. Miller, M. W. Welker, N. Forestier, H. W. Reesink, A. D. Kwong, and S. Zeuzem. 2007. Telaprevir and pegylated interferon alpha-2a inhibit wild-type and resistant genotype 1 hepatitis C virus replication in patients. Hepatology 46:631-639. [DOI] [PubMed] [Google Scholar]
- 13.Koch, U., and F. Narjes. 2006. Allosteric inhibition of the hepatitis C virus NS5B RNA dependent RNA polymerase. Infect. Disord. Drug Targets 6:31-41. [DOI] [PubMed] [Google Scholar]
- 14.Kolykhalov, A. A., K. Mihalik, S. M. Feinstone, and C. M. Rice. 2000. Hepatitis C virus-encoded enzymatic activities and conserved RNA elements in the 3′ nontranslated region are essential for virus replication in vivo. J. Virol. 74:2046-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kukolj, G., G. A. McGibbon, G. McKercher, M. Marquis, S. Lefebvre, L. Thauvette, J. Gauthier, S. Goulet, M. A. Poupart, and P. L. Beaulieu. 2005. Binding site characterization and resistance to a class of non-nucleoside inhibitors of the hepatitis C virus NS5B polymerase. J. Biol. Chem. 280:39260-39267. [DOI] [PubMed] [Google Scholar]
- 16.Laskowski, R. J., M. W. MacArthur, D. S. Moss, and J. M. Thornton. 1993. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26:283-291. [Google Scholar]
- 17.Le Pogam, S., H. Kang, S. F. Harris, V. Leveque, A. M. Giannetti, S. Ali, W. R. Jiang, S. Rajyaguru, G. Tavares, C. Oshiro, T. Hendricks, K. Klumpp, J. Symons, M. F. Browner, N. Cammack, and I. Najera. 2006. Selection and characterization of replicon variants dually resistant to thumb- and palm-binding nonnucleoside polymerase inhibitors of the hepatitis C virus. J. Virol. 80:6146-6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li, H., A. Linton, J. Tatlock, J. Gonzalez, A. Borchardt, M. Abreo, T. Jewell, L. Patel, M. Drowns, S. Ludlum, M. Goble, M. Yang, J. Blazel, R. Rahavendran, H. Skor, S. Shi, C. Lewis, and S. Fuhrman. 2007. Allosteric inhibitors of hepatitis C polymerase: discovery of potent and orally bioavailable carbon-linked dihydropyrones. J. Med. Chem. 50:3969-3972. [DOI] [PubMed] [Google Scholar]
- 19.Li, H., J. Tatlock, A. Linton, J. Gonzalez, A. Borchardt, P. S. Dragovich, T. Jewell, T. Prins, R. Zhou, J. Blazel, H. E. Parge, R. A. Love, M. J. Hickey, C. Doan, S. T. Shi, R. Duggal, C. Lewis, and S. A. Fuhrman. 2006. Identification and structure-based optimization of novel dihydropyrones as potent HCV RNA polymerase inhibitors. Bioorg. Med. Chem. Lett. 16:4834-4838. [DOI] [PubMed] [Google Scholar]
- 20.Lin, C., C. A. Gates, B. G. Rao, D. L. Brennan, J. R. Fulghum, Y. P. Luong, J. D. Frantz, K. Lin, S. Ma, Y. Y. Wei, R. B. Perni, and A. D. Kwong. 2005. In vitro studies of cross-resistance mutations against two hepatitis C virus serine protease inhibitors, VX-950 and BILN 2061. J. Biol. Chem. 280:36784-36791. [DOI] [PubMed] [Google Scholar]
- 21.Lin, C., K. Lin, Y. P. Luong, B. G. Rao, Y. Y. Wei, D. L. Brennan, J. R. Fulghum, H. M. Hsiao, S. Ma, J. P. Maxwell, K. M. Cottrell, R. B. Perni, C. A. Gates, and A. D. Kwong. 2004. In vitro resistance studies of hepatitis C virus serine protease inhibitors, VX-950 and BILN 2061: structural analysis indicates different resistance mechanisms. J. Biol. Chem. 279:17508-17514. [DOI] [PubMed] [Google Scholar]
- 22.Lindahl, K., L. Stahle, A. Bruchfeld, and R. Schvarcz. 2005. High-dose ribavirin in combination with standard dose peginterferon for treatment of patients with chronic hepatitis C. Hepatology 41:275-279. [DOI] [PubMed] [Google Scholar]
- 23.Love, R. A., H. E. Parge, X. Yu, M. J. Hickey, W. Diehl, J. Gao, H. Wriggers, A. Ekker, L. Wang, J. A. Thomson, P. S. Dragovich, and S. A. Fuhrman. 2003. Crystallographic identification of a noncompetitive inhibitor binding site on the hepatitis C virus NS5B RNA polymerase enzyme. J. Virol. 77:7575-7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu, L., T. J. Pilot-Matias, K. D. Stewart, J. T. Randolph, R. Pithawalla, W. He, P. P. Huang, L. L. Klein, H. Mo, and A. Molla. 2004. Mutations conferring resistance to a potent hepatitis C virus serine protease inhibitor in vitro. Antimicrob. Agents Chemother. 48:2260-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manns, M. P., J. G. McHutchison, S. C. Gordon, V. K. Rustgi, M. Shiffman, R. Reindollar, Z. D. Goodman, K. Koury, M. Ling, and J. K. Albrecht. 2001. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 358:958-965. [DOI] [PubMed] [Google Scholar]
- 26.McRee, D. E. 1992. XtalView: a visual protein crystallographic system for X11/Xview. J. Mol. Graph. 10:44-47. [Google Scholar]
- 27.Mo, H., L. Lu, T. Pilot-Matias, R. Pithawalla, R. Mondal, S. Masse, T. Dekhtyar, T. Ng, G. Koev, V. Stoll, K. D. Stewart, J. Pratt, P. Donner, T. Rockway, C. Maring, and A. Molla. 2005. Mutations conferring resistance to a hepatitis C virus (HCV) RNA-dependent RNA polymerase inhibitor alone or in combination with an HCV serine protease inhibitor in vitro. Antimicrob. Agents Chemother. 49:4305-4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris, R. J., A. Perrakis, and V. S. Lamzin. 2002. ARP/wARP's model-building algorithms. I. The main chain. Acta Crystallogr. D 58:968-975. [DOI] [PubMed] [Google Scholar]
- 29.Murshudov, G. N., A. A. Vagin, and E. J. Dodson. 1997. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D 53:240-255. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen, T. T., A. T. Gates, L. L. Gutshall, V. K. Johnston, B. Gu, K. J. Duffy, and R. T. Sarisky. 2003. Resistance profile of a hepatitis C virus RNA-dependent RNA polymerase benzothiadiazine inhibitor. Antimicrob. Agents Chemother. 47:3525-3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Otwinoski, Z., and W. Minor. 1977. Processing X-ray Diffraction data collected in oscillation mode. Methods Enzymol. 276:307-326. [DOI] [PubMed] [Google Scholar]
- 32.Pierra, C., A. Amador, S. Benzaria, E. Cretton-Scott, M. D'Amours, J. Mao, S. Mathieu, A. Moussa, E. G. Bridges, D. N. Standring, J. P. Sommadossi, R. Storer, and G. Gosselin. 2006. Synthesis and pharmacokinetics of valopicitabine (NM283), an efficient prodrug of the potent anti-HCV agent 2′-C-methylcytidine. J. Med. Chem. 49:6614-6620. [DOI] [PubMed] [Google Scholar]
- 33.Sarrazin, C., T. L. Kieffer, D. Bartels, B. Hanzelka, U. Muh, M. Welker, D. Wincheringer, Y. Zhou, H. M. Chu, C. Lin, C. Weegink, H. Reesink, S. Zeuzem, and A. D. Kwong. 2007. Dynamic hepatitis C virus genotypic and phenotypic changes in patients treated with the protease inhibitor telaprevir. Gastroenterology 132:1767-1777. [DOI] [PubMed] [Google Scholar]
- 34.Seeff, L. B. 1995. Natural history of viral hepatitis, type C. Semin. Gastrointest. Dis. 6:20-27. [PubMed] [Google Scholar]
- 35.Shulman, N., and M. Winters. 2003. A review of HIV-1 resistance to the nucleoside and nucleotide inhibitors. Curr. Drug Targets Infect. Disord. 3:273-281. [DOI] [PubMed] [Google Scholar]
- 36.Sulkowski, M. S. 2003. Anemia in the treatment of hepatitis C virus infection. Clin. Infect. Dis. 37(Suppl. 4):S315—S322. [DOI] [PubMed] [Google Scholar]
- 37.Summa, V., A. Petrocchi, V. G. Matassa, M. Taliani, R. Laufer, R. De Francesco, S. Altamura, and P. Pace. 2004. HCV NS5b RNA-dependent RNA polymerase inhibitors: from alpha, gamma-diketoacids to 4,5-dihydroxypyrimidine- or 3-methyl-5-hydroxypyrimidinonecarboxylic acids. Design and synthesis. J. Med. Chem. 47:5336-5339. [DOI] [PubMed] [Google Scholar]
- 38.Thomas, D. L., and L. B. Seeff. 2005. Natural history of hepatitis C. Clin. Liver Dis. 9:383-398, vi. [DOI] [PubMed] [Google Scholar]
- 39.Tomei, L., S. Altamura, L. Bartholomew, A. Biroccio, A. Ceccacci, L. Pacini, F. Narjes, N. Gennari, M. Bisbocci, I. Incitti, L. Orsatti, S. Harper, I. Stansfield, M. Rowley, R. De Francesco, and G. Migliaccio. 2003. Mechanism of action and antiviral activity of benzimidazole-based allosteric inhibitors of the hepatitis C virus RNA-dependent RNA polymerase. J. Virol. 77:13225-13231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomei, L., S. Altamura, L. Bartholomew, M. Bisbocci, C. Bailey, M. Bosserman, A. Cellucci, E. Forte, I. Incitti, L. Orsatti, U. Koch, R. De Francesco, D. B. Olsen, S. S. Carroll, and G. Migliaccio. 2004. Characterization of the inhibition of hepatitis C virus RNA replication by nonnucleosides. J. Virol. 78:938-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tong, X., R. Chase, A. Skelton, T. Chen, J. Wright-Minogue, and B. A. Malcolm. 2006. Identification and analysis of fitness of resistance mutations against the HCV protease inhibitor SCH 503034. Antiviral Res. 70:28-38. [DOI] [PubMed] [Google Scholar]
- 42.Villano, S., A. Howe, D. Raible, D. Harper, J. Speth, and G. Bichier. 2006. Analysis of HCV NS5B genetic variants following monotherapy with HCV-796, a non-nucleoside polymerase inhibitor, in treatment-naïve HCV-infected patients. Hepatology 44:607A-608A. [Google Scholar]
- 43.Wang, M., K. K. Ng, M. M. Cherney, L. Chan, C. G. Yannopoulos, J. Bedard, N. Morin, N. Nguyen-Ba, M. H. Alaoui-Ismaili, R. C. Bethell, and M. N. James. 2003. Non-nucleoside analogue inhibitors bind to an allosteric site on HCV NS5B polymerase. Crystal structures and mechanism of inhibition. J. Biol. Chem. 278:9489-9495. [DOI] [PubMed] [Google Scholar]
- 44.Yi, M., X. Tong, A. Skelton, R. Chase, T. Chen, A. Prongay, S. L. Bogen, A. K. Saksena, F. G. Njoroge, R. L. Veselenak, R. B. Pyles, N. Bourne, B. A. Malcolm, and S. M. Lemon. 2006. Mutations conferring resistance to SCH6, a novel hepatitis C virus NS3/4A protease inhibitor. Reduced RNA replication fitness and partial rescue by second-site mutations. J. Biol. Chem. 281:8205-8215. [DOI] [PubMed] [Google Scholar]