Abstract
Within the mammalian host, Leishmania donovani is an obligatory intracellular protozoan parasite that resides and multiplies exclusively in the phagolysosomes of macrophages. Leishmania control relies primarily on chemotherapy, with the mainstay being pentavalent antimony (SbV) complexed to carbohydrates in the form of sodium stibogluconate (Pentostam) or meglumine antimoniate (Glucantime). The mode of action of SbV is still not known precisely. To explore the effect of SbV on macrophage gene expression, a microarray analysis was performed using Affymetrix focus arrays to compare gene expression profiles in noninfected and L. donovani-infected THP-1 monocytic cells treated or not treated with sodium stibogluconate. Under our experimental conditions, SbV changed the expression of a few host genes, and this was independent of whether cells were infected or not infected with Leishmania. Leishmania infection had a greater effect on the modulation of host gene expression. Statistical analyses have indicated that the expression of eight genes was modified by at least twofold upon SbV treatment, with six genes upregulated and two genes downregulated. One gene whose expression was affected by SbV was the heme oxygenase gene HMOX-1, and this change was observed both in the monocytic cell line THP-1 and in primary human monocyte-derived macrophages. Another pathway that was affected was the glutathione biosynthesis pathway, where the expression of the glutamate-cysteine ligase modifier subunit was increased upon SbV treatment. Our analysis has suggested that, under our experimental conditions, the expression of a few genes is altered upon SbV treatment, and some of these encoded proteins may be implicated in the yet-to-be-defined mode of action of SbV.
Leishmania is a protozoan parasite found as a motile flagellated promastigote in the sand fly vector and as a round nonflagellated amastigote inside the phagolysosome of the macrophage. Cells of the macrophage lineage (i.e., macrophages, monocytes, and dendritic cells) are the exclusive host cells of this parasite species (28). The mainstay for leishmaniasis treatment has been pentavalent antimony (SbV) complexed to carbohydrates in the form of sodium stibogluconate (Pentostam) or meglumine antimoniate (Glucantime) (25). Although these compounds have been in use since 1940, their mode(s) of action is still not known precisely. Recent findings suggested that antimonial drugs interfere with trypanothione metabolism by extruding thiols outside the Leishmania cell and by inhibiting trypanothione reductase (43). Trypanothione is a glutathione (GSH) spermidine conjugate and the main thiol of these parasites (14). It has been suggested that SbV is a prodrug which needs to be converted to SbIII to be active (10, 29). The main site of conversion (within macrophages or parasites) and the mechanism by which this is achieved are unclear. Moreover, the precise molecular target of antimonials is unknown, but it is likely that the macrophages either directly or indirectly contribute to the anti-Leishmania activity of SbV. Interestingly, recent results have demonstrated that SbV can selectively inhibit protein tyrosine phosphatases (Src homology 2 domain-containing tyrosine phosphatase 1 and 2) in vitro and augment cytokine-induced signaling and responses in hematopoietic cell lines (31), suggesting a role of phosphatases and, possibly, other signal transduction pathways in the SbV-induced killing of Leishmania. Recent studies have shown that sodium stibogluconate and alpha interferon synergize to abrogate alpha interferon resistance in various human cancer cell lines by activating STAT1 (46). Also, SbV can trigger the activation of phosphoinositide 3-kinase, protein kinase C, and mitogen-activated protein kinases. Coupled with the activation of the microbicidal mechanisms of macrophages, this ultimately leads to the elimination of the intracellular Leishmania donovani parasites (23). We also showed recently that SbV alters the activity of the mitogen-activated protein kinase/extracellular signal-related kinase (ERK) and other signal transduction pathways (3). Additional studies have reported that SbV could induce a protective effect in vivo against the downregulation of phagocyte defense activities mediated by Leishmania. This property appears to be mediated at least by a direct interaction between SbV and phagocytes, resulting in the priming of their ability to generate reactive oxygen species (ROS) in response to various stimuli and to enhance the activity of NADPH oxidase, the enzyme generating superoxides (33).
DNA microarrays have been extensively used to study Leishmania-macrophage interactions (5, 7, 11, 34), and to further understand the possible contribution of the macrophage to the mode of action of SbV against Leishmania, we have studied genes differentially expressed in THP-1 cells treated with SbV by using DNA microarray studies. Our analysis has suggested that, under our experimental conditions, the expression of a few genes is altered upon SbV treatment and that some of these encoded proteins may be involved in the mode of action of SbV.
MATERIALS AND METHODS
Parasite culture and characterization.
The L. donovani field isolate 9551 (sensitive to SbV treatment) derived from a kala-azar patient has been described previously (19, 41). Promastigotes were maintained in SDM79 medium (4a) supplemented with 10% heat-inactivated fetal calf serum at 25°C. Growth assays of promastigotes were done as described previously (30). For intracellular amastigote assays, parasites transfected with firefly luciferase (35) were used at a 10:1 ratio to infect the human leukemia monocyte cell line THP-1 as described and validated previously (39). Drug susceptibility assays were performed as described previously (13, 35, 39), and values expressed as relative light units.
Isolation and culture of THP-1 cells and monocyte-derived macrophages (MDMs).
Briefly, the cell line THP-1 was cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mM glutamate, 100 international units of penicillin/ml, and 100 μg of streptomycin/ml. THP-1 cells in the log phase of growth were differentiated by incubation for 2 days in medium containing 20 ng of phorbol myristate acetate/ml, which caused the cells to become adherent. These cells were infected with Leishmania species (39).
Human peripheral blood mononuclear cells (PBMCs) were isolated from healthy blood donors by density-gradient centrifugation on Ficoll-Hypaque gradients. Monocytes were purified by adherence to plastic in RPMI 1640 supplemented with 10% fetal bovine serum, penicillin, and streptomycin. Briefly, PBMCs (6 × 106 cells) were first seeded into six-well plates; after 2 h, nonadherent cells were removed by several washes with warmed phosphate-buffered saline (PBS). Freshly isolated monocytes were allowed to differentiate into MDMs in complete RPMI 1640 supplemented with human recombinant macrophage colony stimulating factor (100 ng/ml) for 6 days before, when applicable, being treated with sodium stibogluconate at 200 μg/ml.
RNA extraction and array processing. Experimental setup.
The RNAs were first isolated from THP-1 cells infected or not infected with L. donovani 9951 (sensitive to SbV) treated or not treated with SbV (200 μg/ml) after 4 days (THP-1; THP-1 and SbV; THP-1 and strain 9551; and THP-1, SbV, and strain 9551). RNAs were prepared by using an RNeasy mini kit (Qiagen) and treated with DNase according to the manufacturer's protocol. RNA quality was controlled spectrophotometrically and by bioanalyzer analysis. Similar amounts of RNA from each condition were labeled and hybridized to a GeneChip human genome focus array containing over 8,500 verified human sequences from the NCBI RefSeq database, as described at http://www.affymetrix.com/products/arrays/specific/focus.affx. For each condition, we performed one biological replicate that was hybridized to a second set of microarrays.
Microarray preparation.
Total RNA from each sample was used to prepare biotinylated target RNA, as described by the manufacturer's protocol. Briefly, 5 μg of RNA was used to generate first-strand cDNA by using a T7-linked oligo(dT) primer. After second-strand synthesis, in vitro transcription was performed with biotinylated UTP and CTP (Enzo Diagnostics), resulting in approximately 100-fold linear amplification of RNA. Spike controls were added to 15 μg of fragmented cRNA before overnight hybridization. Arrays were then washed and stained with streptavidin-phycoerythrin before being scanned on a GeneChip scanner 3000 (Affymetrix).
Microarray quality control.
After the scanning, array images were assessed by eye to confirm scanner alignment and the absence of significant bubbles or scratches on the chip surface. The 3′/5′ ratios for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and beta-actin were confirmed to be within acceptable limits (1.00 to 1.85 and 1.04 to 2.238, respectively), and BioB spike controls were found to be present, along with BioC, BioD, and CreX controls. When scaled to a target intensity of 100 (using Affymetrix MAS 5.0 array analysis software), the scaling factors for all arrays were within acceptable limits (0.410 to 0.627), as were the backgrounds, Q values, and mean intensities.
Real-time PCR analysis of gene expression in THP-1 cells.
RNAs were first isolated from THP-1 cells infected or not infected with L. donovani 9951 (sensitive to SbV) treated or not treated with SbV (200 μg/ml) after 4 days (THP-1; THP-1 and SbV; THP-1 and strain 9551; and THP-1, SbV, and strain 9551). Primers for the EDNRB, HMOX-1, GCLM, SEPW-1, NT5C2, MAGEB2, and MRC1 genes were designed using Primer 3 software (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi) (see Table S1 in the supplemental material). Primers for the GAPDH control have been described by others (26). cDNA synthesis and real-time PCR on a Rotor-Gene 6000 from Corbett Life Science were performed with three independent RNA preparations. The relative amount of PCR product generated from each primer set was determined based on the threshold cycle value and amplification efficiencies and was normalized by dividing the values by the relative amount of the GAPDH gene used as a control. Real-time PCR analysis of the MX1 and G1P2 genes was performed by the gene quantification core laboratory of the Centre de Génomique de Québec (https://genome.ulaval.ca/qrtpcr).
THP-1 cells (6 × 106) were harvested by centrifugation at 2,500 × g, washed twice in HEPES-NaCl buffer, and resuspended and disrupted in an equal volume of boiling 2× lysis buffer (125 mM Tris-HCl, pH 6.8, 4% sodium dodecyl sulfate, 3% β-mercaptoethanol, 17.5% glycerol, 5 mM Na3VO4, 20 μg/ml leupeptin, 20 μg/ml aprotinin, 0.0025% bromophenol blue, 1× protease inhibition cocktail). The samples were boiled for 10 min and vortexed, and total cellular proteins (from 6 × 106 cells) were separated on a 15% sodium dodecyl sulfate-polyacrylamide gel and transferred onto a nitrocellulose membrane. The membrane was then incubated in 1× PBS, 5% nonfat milk, 0.2% Tween, and a 1:1,000 dilution of a monoclonal anti-human HMOX-1 antibody (StressGen) for 90 min. The membrane was washed with 1× PBS containing 0.1% Tween 20, incubated with a 1:10,000 dilution of horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (Bio-Rad, Hercules, CA) in 1× PBS plus 5% nonfat milk for 1 h, and washed again with PBS. The results were visualized using a chemiluminescence detection kit (Pierce).
Thiol analysis.
Thiols were derivatized with monobromobimane, separated by high-pressure liquid chromatography, and quantitated as described previously (15).
Statistical analyses.
The means of the quantitative reverse transcription-PCR (qRT-PCR) values or thiol measurements were compared by using Student's t test or a single analysis of variance, followed by Tukey's or Dunnett's multiple comparison when more than two means were considered. P values of less than 0.05 were deemed statistically significant. Computations were carried out using Microsoft Excel software.
RESULTS
Modulation of gene expression induced by sodium stibogluconate in THP-1 cells.
Sodium stibogluconate (SbV-containing drug) is the first-line drug against infections caused by the protozoan parasite Leishmania. Its mode of action is unknown, but it is likely to act as a prodrug. Recent evidence suggests interaction with host signaling proteins (3, 23, 31, 33, 46). DNA microarrays have been useful for determining the mode of action of a number of drugs (4, 27, 42, 47). Consequently, we have used DNA microarrays to assess any putative SbV-mediated changes in gene expression in the macrophage host cell in the hope that it may be informative on the mode of action of SbV. We used SbV at 200 μg/ml, a concentration that kills sensitive L. donovani isolates but is not toxic to THP-1 cells (41).
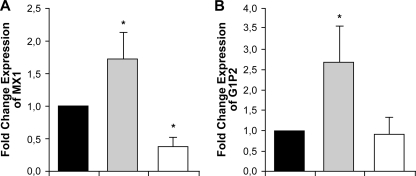
Macrophages, infected or not infected with the clinical isolate L. donovani 9551, were treated with SbV. RNAs from cells under the four different conditions (THP-1; THP-1 and SbV; THP-1 and strain 9551; THP-1, 9551, and SbV) were isolated and hybridized to a human genome focus array as described in Materials and Methods. While the focus of this study is on the mode of action of SbV, as part of a control, we also monitored changes in host gene expression upon Leishmania infection. Statistical analyses using the Agilent GeneSpring software package have revealed that, after having arbitrarily set up a cutoff of twofold differential expression, the expression of 44 genes (24 upregulated and 20 downregulated) was changed in THP-1 cells upon infection with strain 9951 of L. donovani (see Table S2 in the supplemental material). The expression of most of these genes was not modulated by SbV treatment alone (see Table S2 in the supplemental material). Several of these genes whose expression is modulated upon Leishmania infection are part of the gamma interferon pathway, like the genes encoding MX1 (human interferon-regulated resistance GTP-binding protein MxA) and G1P2 (alpha interferon-inducible protein [clone IFI-15K]) (see Table S2 in the supplemental material). qRT-PCR experiments confirmed the expression data derived from the DNA microarray experiments for the MX1 and G1P2 genes (Fig. 1). Often, SbV treatment of cells infected with Leishmania altered the modulation of gene expression induced by Leishmania (see Table S2 in the supplemental material). This was also confirmed by real-time PCR of the two studied host genes that are part of the gamma interferon pathway (Fig. 1).
FIG. 1.
Leishmania- and SbV-mediated changes in gene expression in THP-1 cells as analyzed by real-time qRT-PCR. RNAs derived from THP-1 cells (black bars) infected with L. donovani 9551 (gray bars) and infected cells treated with sodium stibogluconate (200 μg/ml) (white bars) were subjected to qRT-PCR for the MX1 (A) and G1P2 (B) genes, two genes whose expression was modulated by Leishmania infections as determined by DNA microarrays (see Table S2 in the supplemental material). Changes in expression were normalized to GAPDH expression. The amount of mRNA expression was expressed as the relative change standardized to the expression in control cells in each experiment. Each value represents the mean of the results of three experiments. *, P < 0.05 compared to the control without sodium stibogluconate.
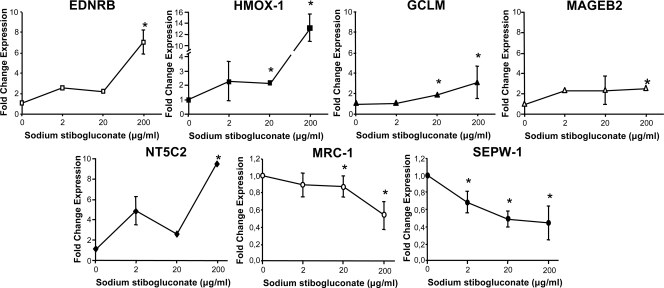
The main thrust of this study was to find SbV-modulated genes. SbV changed the expression of fewer genes, and this was independent of whether THP-1 cells were infected or not infected with Leishmania (Table 1). Indeed, analyses of microarray data have indicated that a total of eight genes were at least twofold differentially expressed after treatment, with six genes upregulated and two genes downregulated. The confirmation of the different expression levels of seven genes was done by real-time qRT-PCR (Fig. 2). We were unable to amplify the RNA of the APOC1 gene starting from THP-1-derived RNA. The qRT-PCR experiments confirmed the expression data derived from the DNA microarray experiments for the seven other genes (Fig. 2).
TABLE 1.
Modulation of gene expression in THP-1 cells by sodium stibogluconate (SbV-containing drug)a
| Protein | Gene symbol | GenBank accession no. | Change (fold) in gene expression mediated by:
|
Function | ||
|---|---|---|---|---|---|---|
| Leishmania | SbV | Leishmania and SbV | ||||
| Selenoprotein W, 1 | SEPW1 | NM_003009 | 0.77 | 0.37 | 0.36 | Unknown |
| Mannose receptor, C type 1 | MRC1 | NM_002438 | 0.65 | 0.41 | 0.42 | Receptor-mediated endocytosis |
| 5′-Nucleotidase, cytosolic II | NT5C2 | NM_012229 | 1.39 | 2.04 | 1.64 | 5′-Nucleotidase activity |
| Apolipoprotein C-I | APOC1 | NM_001645 | 1.77 | 2.07 | 2.32 | Lipid metabolic process |
| Heme oxygenase (decycling) 1 | HMOX1 | NM_002133 | 0.42 | 2.11 | 2.16 | Heme oxygenase (decyclizing) activity |
| Glutamate-cysteine ligase, modifier subunit | GCLM | NM_002061 | 0.91 | 2.20 | 1.94 | Response to drug/oxidative stress |
| Melanoma antigen family B, 2 | MAGEB2 | NM_002364 | 1.51 | 2.37 | 2.19 | Protein binding |
| Endothelin receptor type B | EDNRB | NM_000115 | 0.64 | 4.77 | 4.50 | G-protein signaling, coupled to IP3 second messenger (phospholipase C activating) |
Modulation of gene expression in THP-1 cells was measured using an Affymetrix human genome focus array after 4 days of incubation with 200 μg/ml of SbV.
FIG. 2.
SbV-mediated changes in gene expression in THP-1 cells as analyzed by real-time qRT-PCR. RNAs derived from THP-1 cells treated with sodium stibogluconate (200 μg/ml) were subjected to qRT-PCR for the genes that were differentially expressed as determined by DNA microarrays (Table 1). Changes in expression were normalized to GAPDH expression levels. The amount of mRNA expression was expressed as the relative change standardized to the expression in control cells in each experiment. Each value represents the mean of the results of three experiments. *, P < 0.05 compared to untreated cells.
SbV modulation of gene expression.
The microarray experiment was done with 200 μg/ml of SbV, as this kills sensitive Leishmania cells while not being toxic for macrophages, but we wished to test whether lower concentrations of SbV could also modulate the expression of these genes. This was tested by qRT-PCR using the seven aforementioned genes. For all genes that were upregulated, we found a maximum induction of gene expression in THP-1 cells at 200 μg/ml of sodium stibogluconate (Fig. 2). The induction in gene expression was clear at the highest concentration of sodium stibogluconate tested, and only a modest increase was seen at lower sodium stibogluconate concentrations, although, for some genes, the changes were statistically significant (Fig. 2). Possibly due to the dynamic range of each technique, the increase was often greater with qRT-PCR than with the microarrays. For example, HMOX-1 expression with 200 μg/ml of sodium stibogluconate was increased ∼13-fold as determined by qPCR, compared to a 2.1-fold increase determined by microarray (Table 1). We also observed a maximal effect at 200 μg/ml of SbV for downregulated genes, but some effect was observed at 20 μg/ml of SbV (Fig. 2).
Three out of the seven genes, those for endothelin receptor type B (EDNRB), heme oxygenase-1 (HMOX-1), and the glutamate-cysteine ligase modifier subunit (GCLM), have been selected for further analysis. These three genes were chosen either because they were the most highly differentially expressed (EDNRB) or because data in the literature suggested a possible role in the mode of action of SbV. Indeed, host thiols (potentially provided by GCLM) have been proposed to be involved in the reduction of SbV (37), and HMOX-1 expression was found to be modulated by SbV treatment (12). Sodium stibogluconate is one of the two main SbV-containing drugs used to treat Leishmania, the other one being meglumine antimoniate. The same induction in gene expression was observed with 200 μg/ml of meglumine antimoniate, and this drug was found to be a potent inducer of the expression of both the EDNRB and HMOX-1 genes of THP-1 cells, with increases of more than 10-fold as determined by qRT-PCR (see Fig. S1 in the supplemental material).
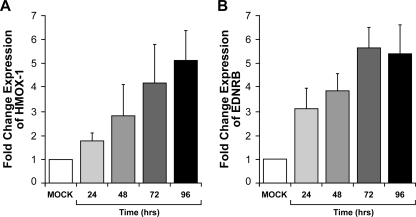
For measuring SbV activity against Leishmania, long incubation times are required (39). SbV-mediated alterations in some signaling pathways were found to be a slow process (3). This explains why we opted to investigate gene expression modulation at 96 h posttreatment. Nonetheless, we were interested in testing whether SbV-mediated changes in gene expression could occur more rapidly. RNA was extracted from THP-1 cells treated with sodium stibogluconate (200 μg/ml) after different incubation times (24, 48, 72, and 96 h). qRT-PCR analyses revealed that HMOX-1 and EDNRB mRNA levels increased with time of treatment, reaching their highest levels after 72 h for EDNRB and 96 h for HMOX-1 (Fig. 3A and B).
FIG. 3.
Time-dependent SbV-mediated changes of gene expression in THP-1 cells as analyzed by real-time qRT-PCR. RNAs derived at different times from 0 to 96 h from THP-1 cells treated with sodium stibogluconate (200 μg/ml) were subjected to qRT-PCR with primers for the HMOX-1 (A) and EDNRB (B) genes. Changes were normalized to GAPDH expression levels. The amount of mRNA expression was expressed as the relative change standardized to the expression in control cells in each experiment. Each value represents the mean of the results of three experiments. The P value was <0.004 compared to mock treatment for all values. “Mock” represents the control without sodium stibogluconate (ratio of GAPDH expression in the presence and absence of sodium stibogluconate at 96 h).
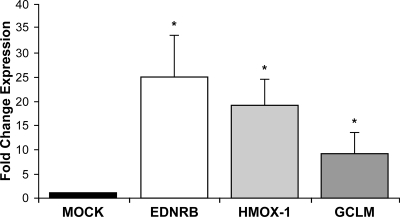
The SbV-induced changes in gene expression described so far in this study were observed in phorbol myristate acetate-differentiated THP-1 cells. It was of interest to test whether SbV could also modulate gene expression in a different, more relevant, cellular context. For this purpose, monocytes derived from PBMCs were allowed to differentiate into macrophages. The expression of HMOX-1, EDNRB, and GCLM was increased in MDMs of two independent donors upon sodium stibogluconate (200 μg/ml) incubation (Fig. 4). The increases for those three genes in the MDMs were greater than the increases observed in the THP-1 cells (Fig. 2 and 4).
FIG. 4.
SbV alteration in gene expression in MDMs as determined by real-time qRT-PCR. The changes in expression levels of EDNRB, HMOX-1, and GCLM were analyzed by real-time RT-PCR of RNA derived from MDMs treated with sodium stibogluconate (200 μg/ml). Changes were normalized to GAPDH expression levels. The amount of mRNA was expressed as the relative change standardized to the expression in control cells in each experiment. Each value represents the mean of the results of two experiments with MDMs from two different donors. *, P < 0.03 compared to mock treatment. “Mock” represents the control without sodium stibogluconate (ratio of GAPDH expression in the presence and absence of sodium stibogluconate).
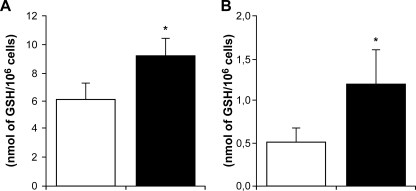
We attempted to correlate changes in gene expression with either protein production or function. Antibodies against the products of EDNRB and HMOX-1 are commercially available, but the EDNRB antibody was not able to detect a protein in our THP-1 cell line (results not shown), and this was not investigated further. In contrast, the anti-HMOX-1 antibody recognized a protein of 32 kDa (the predicted size of HO-1) in THP-1 cells, and the production of this protein increased 9-fold ± 1.4-fold (mean ± standard deviation) upon SbV treatment (see Fig. 5, lanes 1 and 3). GCLM codes for the modulator subunit of the rate-limiting enzyme of the GSH biosynthesis pathway, and it is possible that its overexpression upon SbV treatment could lead to an increase in GSH biosynthesis. This was tested by measuring thiols by high-pressure liquid chromatography, and indeed, we found an increased level of GSH in both THP-1 cells and MDMs treated with SbV after 96 h (Fig. 6A and B).
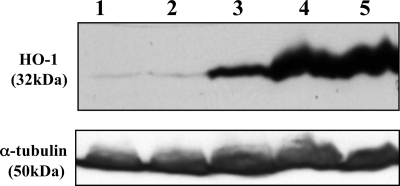
FIG. 5.
Heme oxygenase (HO-1) expression in THP-1 cells. Western analysis directed against HO-1 of total proteins from THP-1 cells. Lanes: 1, THP-1; 2, THP-1 and NaOH (0.2 μM); 3, THP-1 and sodium stibogluconate (200 μg/ml); 4, THP-1 and ZnPP IX (5 μM); 5, THP-1, sodium stibogluconate (200 μg/ml), and ZnPP IX (5 μM). All incubations lasted 4 days. A Western blot using an antitubulin (α-tubulin) antibody was used as an internal control for gel loading. The experiment was repeated three times, and a representative result is shown.
FIG. 6.
Quantitation of intracellular GSH in SbV-treated macrophages. GSH levels of THP-1 cells (A) or MDMs (B) were measured as described in Materials and Methods. For THP-1 cells, the averages of the results of a triplicate experiment, which was repeated two times with similar results, are shown, and for MDMs, the averages of the results for cells from two donors are shown. White bars, untreated cells; black bars, cells treated with 200 μg/ml of sodium stibogluconate for 96 h. *, P < 0.02.
Effect of EDNRB and HMOX-1 antagonists on SbV-mediated gene expression.
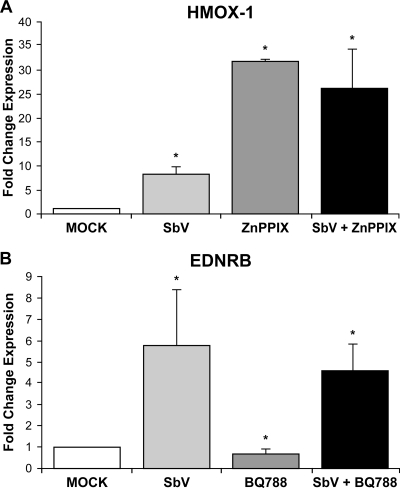
SbV changed the expression of a selected number of genes, and to further understand how EDNRB and HMOX-1 expression was induced by SbV, we treated cells with antagonists of these two gene products. Several molecules that alter the expression of HMOX-1 have been developed (reviewed in references 1 and 32), and zinc protoporphyrin IX (ZnPP IX), a known inhibitor (18), was tested. Surprisingly, ZnPP IX increased the HMOX-1 RNA level by 30-fold (Fig. 7A), and this also correlated with an increase in HMOX-1 protein (Fig. 5, lane 3). Coincubation of cells with ZnPP IX and SbV did not further increase RNA or protein levels (Fig. 5, lane 5; Fig. 7A). BQ788 is an antagonist of EDNRB (16), and incubation with BQ788 (Calbiochem) indeed resulted in a decrease in the mRNA expression of the EDNRB gene (0.63-fold; P, 0.009) compared to its level in the THP-1 mock control (Fig. 7B). However, treatment of cells with BQ788 could not significantly modulate the SbV-mediated change in EDNRB gene expression (Fig. 7B). As expected from the expression data shown in Fig. 7A and B, Leishmania infection and susceptibility to SbV were unchanged in THP-1 cells treated with either ZnPP IX or BQ788 (results not shown).
FIG. 7.
Modulation of HMOX-1 and EDNRB mRNA expression in THP-1 cells. RNA expression of HMOX-1 (A) and EDNRB (B) was measured by qRT-PCR in the THP-1 cell line after 4 days of treatment with SbV (200 μg/ml) and ZnPP IX (5 μM) or BQ788 (10 μM), respectively. Changes were normalized with GAPDH expression levels. The amount of mRNA was expressed as a relative change standardized to expression in control cells in each experiment. Each value represents the mean of the results of three experiments. *, P < 0.05. “Mock” represents the control without sodium stibogluconate (ratio of GAPDH expression in the presence and absence of sodium stibogluconate and one of the two inhibitors).
DISCUSSION
Antimonials have been used for decades against leishmaniasis, but their mode of action is still not completely understood. However, there is a general consensus that SbV is converted to SbIII (reviewed in references 10 and 29). The metal reduction could occur either in the macrophage or the intracellular parasite or in both, and it is likely that the macrophage either directly or indirectly is important for this conversion. Moreover, SbV was shown to modulate host signaling pathways (3, 23, 31), which could also contribute to the antileishmanial activity of SbV. Therefore, we chose to use DNA microarrays in order to investigate the potential role of the macrophage in the mode of action of sodium stibogluconate. Indeed, it has been shown that RNA expression profiling is a useful technique to look at the mode of action of drugs (4, 27, 42, 47). Several studies have also dealt with changes of host gene expression upon infection with Leishmania spp. (5, 7, 11, 34), and we indeed also observed changes in host gene expression upon Leishmania infection (see Table S2 in the supplemental material). Several of the genes (but not all) listed in Table S2 in the supplemental material were also pinpointed by others, including the two genes for which results are shown in Fig. 1 (7). Interestingly, SbV treatment of Leishmania infections decreases the effect of Leishmania-induced expression (Fig. 1; see Table S2 in the supplemental material). This may suggest that modulation of gene expression depends on dividing parasites, and this clearly warrants further investigations.
Under the experimental conditions that we have used, the expression of a few genes was significantly modulated by SbV (Table 1), and this was independent of whether the macrophages were infected or not infected with Leishmania parasites (Table 1). SbV drugs used against Leishmania have been shown to be potent inhibitors of a family of protein phosphatases which modulates signaling pathways and cytokine responses (31). Stimulation of ERK-1 by SbV and alterations in intracellular signaling pathways have also been described (3, 23). Under our experimental conditions, none of these signaling pathways were found to be altered using focus DNA microarrays. This observation is not surprising given that the majority of signal transduction pathways are modulated by posttranscriptional events (e.g., phosphorylation/dephosphorylation). Thus, other global approaches, such as proteomic screens, may highlight SbV-mediated changes in signaling pathways.
The few genes whose expression was modulated by SbV suggested that SbV induced some oxidative stress and that this may contribute to the anti-Leishmania activity. Indeed, there are already links between SbV and the production of ROS. For example, the activation of ERKs by SbV is known to lead to the production of ROS (23), and this is consistent with the observation that SbV can prime the phagocyte respiratory burst (33). It should be noted, however, that a short exposure (4 h) of THP-1 cells to SbV did not lead to the production of ROS but that incubation with SbIII for 4 h did in fact lead to ROS production (20, 44). If SbV is slowly converted to SbIII inside macrophages, it may take longer to see an increase in SbV-mediated ROS production. Indeed, we found that modulation of gene expression was maximal at 96 h (Fig. 3), and this parallels the rather slow activity of SbV against Leishmania cells. It is noteworthy that susceptibility testing is routinely carried out after 4 to 5 days of exposure to SbV (39). Interestingly, all genes pinpointed as differentially expressed in THP-1 cells by using focus arrays were also differentially expressed in MDMs (Fig. 4). The increase in SbV-mediated changes in gene expression was even greater in MDMs, suggesting that, if we had used this system initially, we might have detected more genes responding to SbV. However, one caveat with MDMs, in comparison to established cell lines, is the heterogeneity in gene expression between donors which could have complicated the analysis of the data. Since SbV is converted to SbIII, it will be interesting eventually to monitor SbIII-mediated modulation in host gene expression. Since SbIII is much more toxic than SbV to host cells, it may lead, however, to the expression of several genes related to the stress response not directly involved in the mode of action of SbV.
One gene whose expression was greatly modulated by SbV was HMOX-1, and this was observed both in THP-1 cells (Fig. 2) and in MDMs (Fig. 4). HMOX-1 is the gene coding for the HO-1 enzyme that catalyzes the rate-limiting step of heme degradation into biliverdin, carbon monoxide, and iron (reviewed in reference 36). The HMOX-1 gene is activated under a number of cellular stresses, and it has been shown to be important in dealing with oxidative stress (reviewed in reference 1). Significantly, SbIII and, to a lesser extent, SbV were shown to induce HO-1 (12). The HMOX-1 gene is under the control of several transcription factors and pathways (reviewed in reference 2). The ERK pathway was found to be necessary for metal (arsenic) HO-1 induction (9) and, since SbV was shown to induce the ERK pathway (23), it is possible that it is through this pathway that HO-1 expression is increased. ZnPP IX is an HO-1 inhibitor. In our hands, however, ZnPP IX was shown to be a potent inducer of HMOX-1 gene and protein expression (Fig. 7A; Fig. 5, lane 4). This induction effect has also been observed by others (45). This is explained by ZnPP IX-induced binding of nuclear proteins to the HMOX-1 regulatory regions (45). Attempts to look at whether ZnPP IX could modulate the activity of SbV against Leishmania have been inconclusive (results not shown), possibly because of the complexity of HMOX-1 regulation. Future studies are nonetheless warranted to see whether HO-1 is involved in the mode of action of SbV.
Another pathway that was highlighted was that of GSH biosynthesis, where the expression of the glutamate-cysteine ligase modifier subunit (GCLM) was increased. This is the noncatalytic subunit of the rate-limiting enzyme in GSH biosynthesis (the catalytic gene GCLC was not present on the focus array), but its increase may nonetheless indicate that GSH levels could be important for the SbV mode of action. It has been shown that an increase in GCLM expression could lead to an increase of GSH (8). Indeed, GSH reduces SbV to SbIII nonenzymatically (37), and the activity of SbV was associated with the manipulation of both host and parasite GSH levels by the parasite (6). Thiol levels are known to be important for the parasite itself, where SbIII decreases the level of intracellular GSH (21, 43), and an increased level of thiols is associated with resistance to antimonials (15, 22, 24). Similarly, a short exposure of host cells to SbIII depleted the cells of GSH (44), whereas longer exposure can lead to increased GSH (40). We showed that total GSH levels are increased in SbV-treated cells (Fig. 6), concomitant with an increase in GCLM expression, both in THP-1 cells (Fig. 2) and in MDMs (Fig. 4). It is possible that, during the initial contact, SbIII (or SbV being converted to SbIII) reacts with cellular GSH and this complex is effluxed outside the cell, hence reducing cellular thiol levels. To compensate for this loss in GSH levels, the cell increases the synthesis of GSH upon chronic metal exposure.
Another gene that was extensively upregulated by SbV was the endothelin receptor type B gene (EDNRB). This receptor, upon endothelin binding, promotes vasodilatation (reviewed in reference 38). The expression of EDNRB was increased by SbV both in THP-1 cells (Fig. 2) and in MDMs (Fig. 4). Inhibitors of EDNRB are available, and we used BQ788. BQ788 was able to decrease the expression of EDNRB (Fig. 7B), but the effect was minimal, and we could not observe any effect of BQ788 on the antileishmanial activity of SbV (results not shown). High expression of EDNRB is associated with hypertension (38), and one may need to be careful if SbV is used to treat leishmaniasis in individuals suffering from hypertension.
In addition to EDNRB, GCLM, and HMOX-1, the expression of five other genes (three upregulated and two downregulated) was modulated by SbV (Table 1). The modulation in the expression of these genes in THP-1 cells was confirmed by real-time PCR (Fig. 2). The three other genes that were upregulated corresponded to the cytosolic 5′-nucleotidase type II (NT5C2), a melanoma antigen (MAGEB2) of unknown function, and apolipoprotein C-I (APOC1). For the latter, every primer design failed to detect the specific mRNA in THP-1 cells. It is not clear whether the regulation of these genes has a role to play in SbV activity. Two genes were downregulated, those coding for the membrane receptor MRC1 and for the selenoprotein SEPW1 (Fig. 2). It has been suggested that SEPW1 is a target for the heavy metal MeHg (methylmercury) and that its expression is dependent on GSH levels (17), although further work will be required to determine whether any of these downregulated genes are related to SbV's mode of action. Indeed, the transcriptomic approach used here has been useful for the detection of changes in gene expression mediated by SbV, and future work should determine how these changes could modulate the efficacy of SbV drugs against leishmaniasis.
Supplementary Material
Acknowledgments
This work was funded in part by the CIHR group and operating grants to M.O. K.E.F. is a strategic training fellow of the strategic training program in microbial resistance, a partnership of the CIHR Institute of Infection and Immunity and the Fonds de Recherche en Santé du Québec. M.I. is the recipient of a CIHR studentship. M.J.T. holds the Canada research chair in human immuno-retrovirology (senior level). M.O. is a Burroughs Wellcome fund scholar in molecular parasitology and holds the Canada research chair in antimicrobial resistance.
Footnotes
Published ahead of print on 10 December 2007.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Abraham, N. G., and A. Kappas. 2005. Heme oxygenase and the cardiovascular-renal system. Free Radic. Biol. Med. 39:1-25. [DOI] [PubMed] [Google Scholar]
- 2.Alam, J., and J. L. Cook. 2007. How many transcription factors does it take to turn on the heme oxygenase-1 gene? Am. J. Respir. Cell Mol. Biol. 36:166-174. [DOI] [PubMed] [Google Scholar]
- 3.Barat, C., C. Zhao, M. Ouellette, and M. J. Tremblay. 2007. HIV-1 replication is stimulated by sodium stibogluconate, the therapeutic mainstay in the treatment of leishmaniasis. J. Infect. Dis. 195:236-245. [DOI] [PubMed] [Google Scholar]
- 4.Brazas, M. D., and R. E. Hancock. 2005. Using microarray gene signatures to elucidate mechanisms of antibiotic action and resistance. Drug Discov. Today 10:1245-1252. [DOI] [PubMed] [Google Scholar]
- 4a.Brun, R., and M. Schönenberger. 1979. Cultivation and in vitro cloning of procyclic culture forms of Trypanosoma brucei in a semi-defined medium. Acta Trop. 36:289-292. [PubMed] [Google Scholar]
- 5.Buates, S., and G. Matlashewski. 2001. General suppression of macrophage gene expression during Leishmania donovani infection. J. Immunol. 166:3416-3422. [DOI] [PubMed] [Google Scholar]
- 6.Carter, K. C., S. Hutchison, F. L. Henriquez, D. Légaré, M. Ouellette, C. W. Roberts, and A. B. Mullen. 2006. Resistance of Leishmania donovani to sodium stibogluconate is related to the expression of host and parasite γ-glutamylcysteine synthetase. Antimicrob. Agents Chemother. 50:88-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaussabel, D., R. T. Semnani, M. A. McDowell, D. Sacks, A. Sher, and T. B. Nutman. 2003. Unique gene expression profiles of human macrophages and dendritic cells to phylogenetically distinct parasites. Blood 102:672-681. [DOI] [PubMed] [Google Scholar]
- 8.Chen, Y., H. G. Shertzer, S. N. Schneider, D. W. Nebert, and T. P. Dalton. 2005. Glutamate cysteine ligase catalysis: dependence on ATP and modifier subunit for regulation of tissue glutathione levels. J. Biol. Chem. 280:33766-33774. [DOI] [PubMed] [Google Scholar]
- 9.Cooper, K. L., K. J. Liu, and L. G. Hudson. 2007. Contributions of reactive oxygen species and mitogen-activated protein kinase signaling in arsenite-stimulated hemeoxygenase-1 production. Toxicol. Appl. Pharmacol. 218:119-127. [DOI] [PubMed] [Google Scholar]
- 10.Croft, S. L., S. Sundar, and A. H. Fairlamb. 2006. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 19:111-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dogra, N., C. Warburton, and W. R. McMaster. 2007. Leishmania major abrogates gamma interferon-induced gene expression in human macrophages from a global perspective. Infect. Immun. 75:3506-3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drummond, G. S., and A. Kappas. 1981. Potent heme-degrading action of antimony and antimony-containing parasiticidal agents. J. Exp. Med. 153:245-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El Fadili, K., N. Messier, P. Leprohon, G. Roy, C. Guimond, N. Trudel, N. G. Saravia, B. Papadopoulou, D. Legare, and M. Ouellette. 2005. Role of the ABC transporter MRPA (PGPA) in antimony resistance in Leishmania infantum axenic and intracellular amastigotes. Antimicrob. Agents Chemother. 49:1988-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fairlamb, A. H., and A. Cerami. 1992. Metabolism and functions of trypanothione in the Kinetoplastida. Annu. Rev. Microbiol. 46:695-729. [DOI] [PubMed] [Google Scholar]
- 15.Haimeur, A., C. Brochu, P. Genest, B. Papadopoulou, and M. Ouellette. 2000. Amplification of the ABC transporter gene PGPA and increased trypanothione levels in potassium antimonyl tartrate (SbIII) resistant Leishmania tarentolae. Mol. Biochem. Parasitol. 108:131-135. [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa, K., M. Ihara, K. Noguchi, T. Mase, N. Mino, T. Saeki, T. Fukuroda, T. Fukami, S. Ozaki, T. Nagase, et al. 1994. Biochemical and pharmacological profile of a potent and selective endothelin B-receptor antagonist, BQ-788. Proc. Natl. Acad. Sci. USA 91:4892-4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim, Y. J., Y. G. Chai, and J. C. Ryu. 2005. Selenoprotein W as molecular target of methylmercury in human neuronal cells is down-regulated by GSH depletion. Biochem. Biophys. Res. Commun. 330:1095-1102. [DOI] [PubMed] [Google Scholar]
- 18.Labbe, R. F., H. J. Vreman, and D. K. Stevenson. 1999. Zinc protoporphyrin: a metabolite with a mission. Clin. Chem. 45:2060-2072. [PubMed] [Google Scholar]
- 19.Lira, R., S. Sundar, A. Makharia, R. Kenney, A. Gam, E. Saraiva, and D. Sacks. 1999. Evidence that the high incidence of treatment failures in Indian kala-azar is due to the emergence of antimony-resistant strains of Leishmania donovani. J. Infect. Dis. 180:564-567. [DOI] [PubMed] [Google Scholar]
- 20.Mann, K. K., K. Davison, M. Colombo, A. L. Colosimo, Z. Diaz, A. M. Padovani, Q. Guo, P. J. Scrivens, W. Gao, S. Mader, and W. H. Miller, Jr. 2006. Antimony trioxide-induced apoptosis is dependent on SEK1/JNK signaling. Toxicol. Lett. 160:158-170. [DOI] [PubMed] [Google Scholar]
- 21.Mehta, A., and C. Shaha. 2006. Mechanism of metalloid-induced death in Leishmania spp.: role of iron, reactive oxygen species, Ca2+, and glutathione. Free Radic. Biol. Med. 40:1857-1868. [DOI] [PubMed] [Google Scholar]
- 22.Mittal, M. K., S. Rai, Ashutosh, Ravinder, S. Gupta, S. Sundar, and N. Goyal. 2007. Characterization of natural antimony resistance in Leishmania donovani isolates. Am. J. Trop. Med. Hyg. 76:681-688. [PubMed] [Google Scholar]
- 23.Mookerjee Basu, J., A. Mookerjee, P. Sen, S. Bhaumik, P. Sen, S. Banerjee, K. Naskar, S. K. Choudhuri, B. Saha, S. Raha, and S. Roy. 2006. Sodium antimony gluconate induces generation of reactive oxygen species and nitric oxide via phosphoinositide 3-kinase and mitogen-activated protein kinase activation in Leishmania donovani-infected macrophages. Antimicrob. Agents Chemother. 50:1788-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mukherjee, A., P. K. Padmanabhan, S. Singh, G. Roy, I. Girard, M. Chatterjee, M. Ouellette, and R. Madhubala. 2007. Role of ABC transporter MRPA, gamma-glutamylcysteine synthetase and ornithine decarboxylase in natural antimony-resistant isolates of Leishmania donovani. J. Antimicrob. Chemother. 59:204-211. [DOI] [PubMed] [Google Scholar]
- 25.Murray, H. W., J. Berman, C. R. Davies, and N. G. Saravia. 2005. Advances in leishmaniasis. Lancet 366:1561-1577. [DOI] [PubMed] [Google Scholar]
- 26.Neuvians, T. P., I. Gashaw, C. G. Sauer, C. von Ostau, S. Kliesch, M. Bergmann, A. Hacker, and R. Grobholz. 2005. Standardization strategy for quantitative PCR in human seminoma and normal testis. J. Biotechnol. 117:163-171. [DOI] [PubMed] [Google Scholar]
- 27.Ng, W. L., K. M. Kazmierczak, G. T. Robertson, R. Gilmour, and M. E. Winkler. 2003. Transcriptional regulation and signature patterns revealed by microarray analyses of Streptococcus pneumoniae R6 challenged with sublethal concentrations of translation inhibitors. J. Bacteriol. 185:359-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olivier, M., D. J. Gregory, and G. Forget. 2005. Subversion mechanisms by which Leishmania parasites can escape the host immune response: a signaling point of view. Clin. Microbiol. Rev. 18:293-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ouellette, M., J. Drummelsmith, and B. Papadopoulou. 2004. Leishmaniasis: drugs in the clinic, resistance and new developments. Drug Resist. Updates 7:257-266. [DOI] [PubMed] [Google Scholar]
- 30.Ouellette, M., F. Fase-Fowler, and P. Borst. 1990. The amplified H circle of methotrexate-resistant leishmania tarentolae contains a novel P-glycoprotein gene. EMBO J. 9:1027-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pathak, M. K., and T. Yi. 2001. Sodium stibogluconate is a potent inhibitor of protein tyrosine phosphatases and augments cytokine responses in hemopoietic cell lines. J. Immunol. 167:3391-3397. [DOI] [PubMed] [Google Scholar]
- 32.Prawan, A., J. K. Kundu, and Y. J. Surh. 2005. Molecular basis of heme oxygenase-1 induction: implications for chemoprevention and chemoprotection. Antioxid. Redox Signal. 7:1688-1703. [DOI] [PubMed] [Google Scholar]
- 33.Rais, S., A. Perianin, M. Lenoir, A. Sadak, D. Rivollet, M. Paul, and M. Deniau. 2000. Sodium stibogluconate (Pentostam) potentiates oxidant production in murine visceral leishmaniasis and in human blood. Antimicrob. Agents Chemother. 44:2406-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez, N. E., H. K. Chang, and M. E. Wilson. 2004. Novel program of macrophage gene expression induced by phagocytosis of Leishmania chagasi. Infect. Immun. 72:2111-2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roy, G., C. Dumas, D. Sereno, Y. Wu, A. K. Singh, M. J. Tremblay, M. Ouellette, M. Olivier, and B. Papadopoulou. 2000. Episomal and stable expression of the luciferase reporter gene for quantifying Leishmania spp. infections in macrophages and in animal models. Mol. Biochem. Parasitol. 110:195-206. [DOI] [PubMed] [Google Scholar]
- 36.Ryter, S. W., J. Alam, and A. M. Choi. 2006. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol. Rev. 86:583-650. [DOI] [PubMed] [Google Scholar]
- 37.Santos Ferreira, C., P. S. Martins, C. Demicheli, C. Brochu, M. Ouellette, and F. Frezard. 2003. Thiol-induced reduction of antimony(V) into antimony(III): a comparative study with trypanothione, cysteinyl-glycine, cysteine and glutathione. Biometals 16:441-446. [DOI] [PubMed] [Google Scholar]
- 38.Schneider, M. P., E. I. Boesen, and D. M. Pollock. 2007. Contrasting actions of endothelin ET(A) and ET(B) receptors in cardiovascular disease. Annu. Rev. Pharmacol. Toxicol. 47:731-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sereno, D., G. Roy, J. L. Lemesre, B. Papadopoulou, and M. Ouellette. 2001. DNA transformation of Leishmania infantum axenic amastigotes and their use in drug screening. Antimicrob. Agents Chemother. 45:1168-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snawder, J. E., M. A. Tirmenstein, P. I. Mathias, and M. Toraason. 1999. Induction of stress proteins in rat cardiac myocytes by antimony. Toxicol. Appl. Pharmacol. 159:91-97. [DOI] [PubMed] [Google Scholar]
- 41.Vergnes, B., B. Gourbal, I. Girard, S. Sundar, J. Drummelsmith, and M. Ouellette. 2007. A proteomics screen implicates HSP83 and a small kinetoplastid calpain-related protein in drug resistance in Leishmania donovani clinical field isolates by modulating drug-induced programmed cell death. Mol. Cell. Proteomics 6:88-101. [DOI] [PubMed] [Google Scholar]
- 42.Wilson, M., J. DeRisi, H. H. Kristensen, P. Imboden, S. Rane, P. O. Brown, and G. K. Schoolnik. 1999. Exploring drug-induced alterations in gene expression in Mycobacterium tuberculosis by microarray hybridization. Proc. Natl. Acad. Sci. USA 96:12833-12838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wyllie, S., M. L. Cunningham, and A. H. Fairlamb. 2004. Dual action of antimonial drugs on thiol redox metabolism in the human pathogen Leishmania donovani. J. Biol. Chem. 279:39925-39932. [DOI] [PubMed] [Google Scholar]
- 44.Wyllie, S., and A. H. Fairlamb. 2006. Differential toxicity of antimonial compounds and their effects on glutathione homeostasis in a human leukaemia monocyte cell line. Biochem. Pharmacol. 71:257-267. [DOI] [PubMed] [Google Scholar]
- 45.Yang, G., X. Nguyen, J. Ou, P. Rekulapelli, D. K. Stevenson, and P. A. Dennery. 2001. Unique effects of zinc protoporphyrin on HO-1 induction and apoptosis. Blood 97:1306-1313. [DOI] [PubMed] [Google Scholar]
- 46.Yi, T., M. K. Pathak, D. J. Lindner, M. E. Ketterer, C. Farver, and E. C. Borden. 2002. Anticancer activity of sodium stibogluconate in synergy with IFNs. J. Immunol. 169:5978-5985. [DOI] [PubMed] [Google Scholar]
- 47.Zembutsu, H., Y. Ohnishi, T. Tsunoda, Y. Furukawa, T. Katagiri, Y. Ueyama, N. Tamaoki, T. Nomura, O. Kitahara, R. Yanagawa, K. Hirata, and Y. Nakamura. 2002. Genome-wide cDNA microarray screening to correlate gene expression profiles with sensitivity of 85 human cancer xenografts to anticancer drugs. Cancer Res. 62:518-527. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.