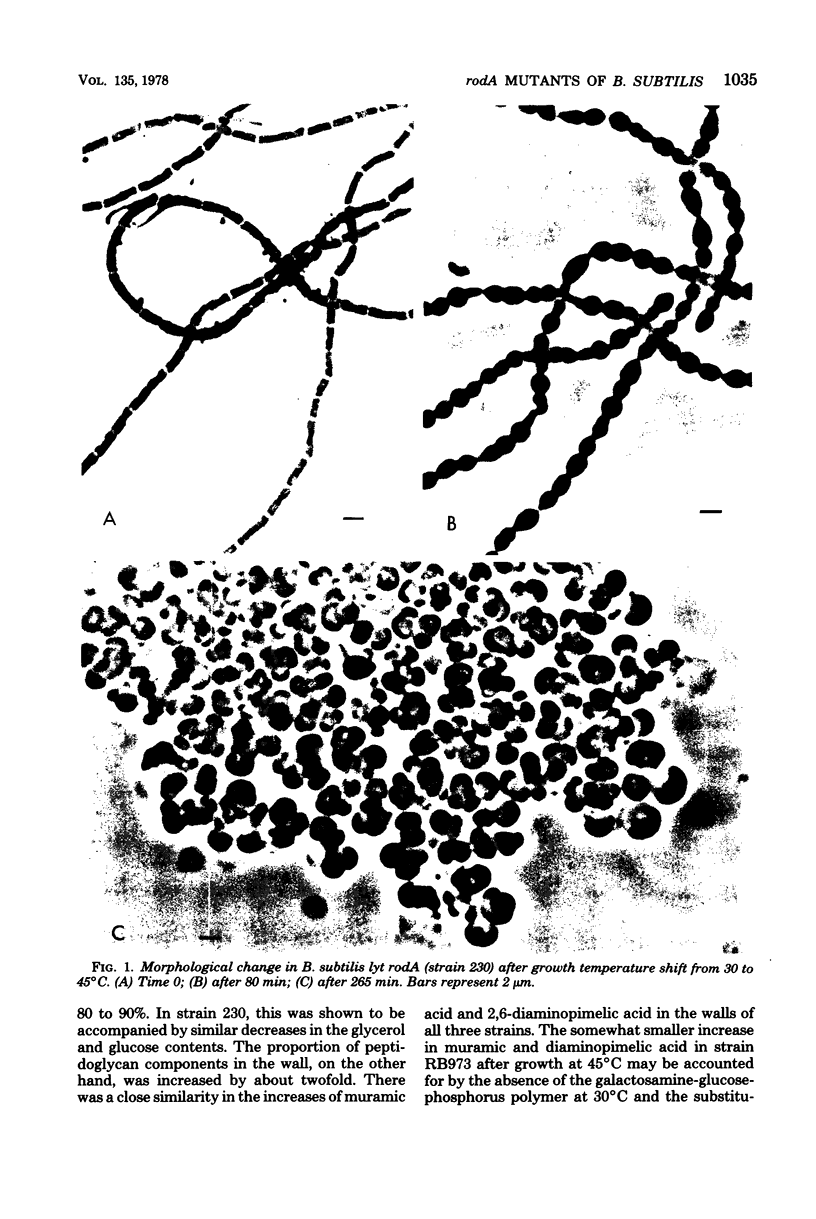
Abstract
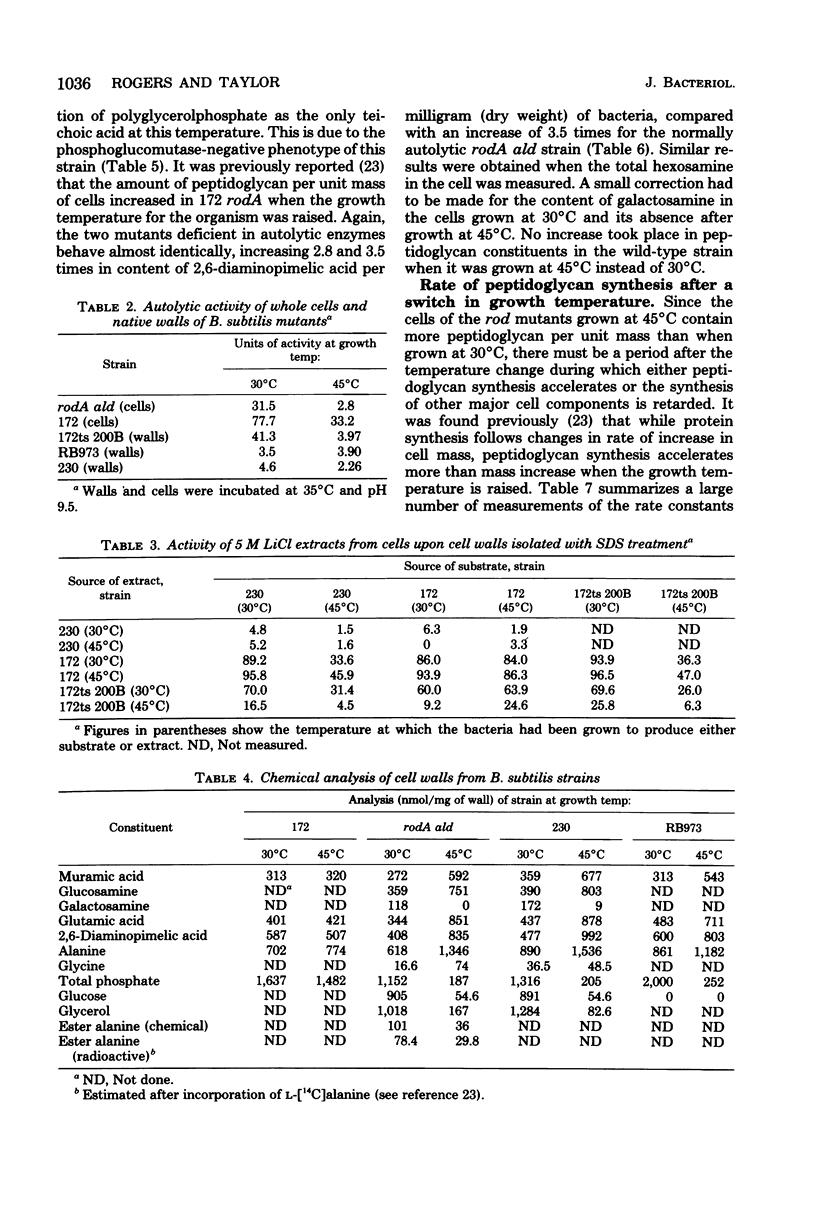
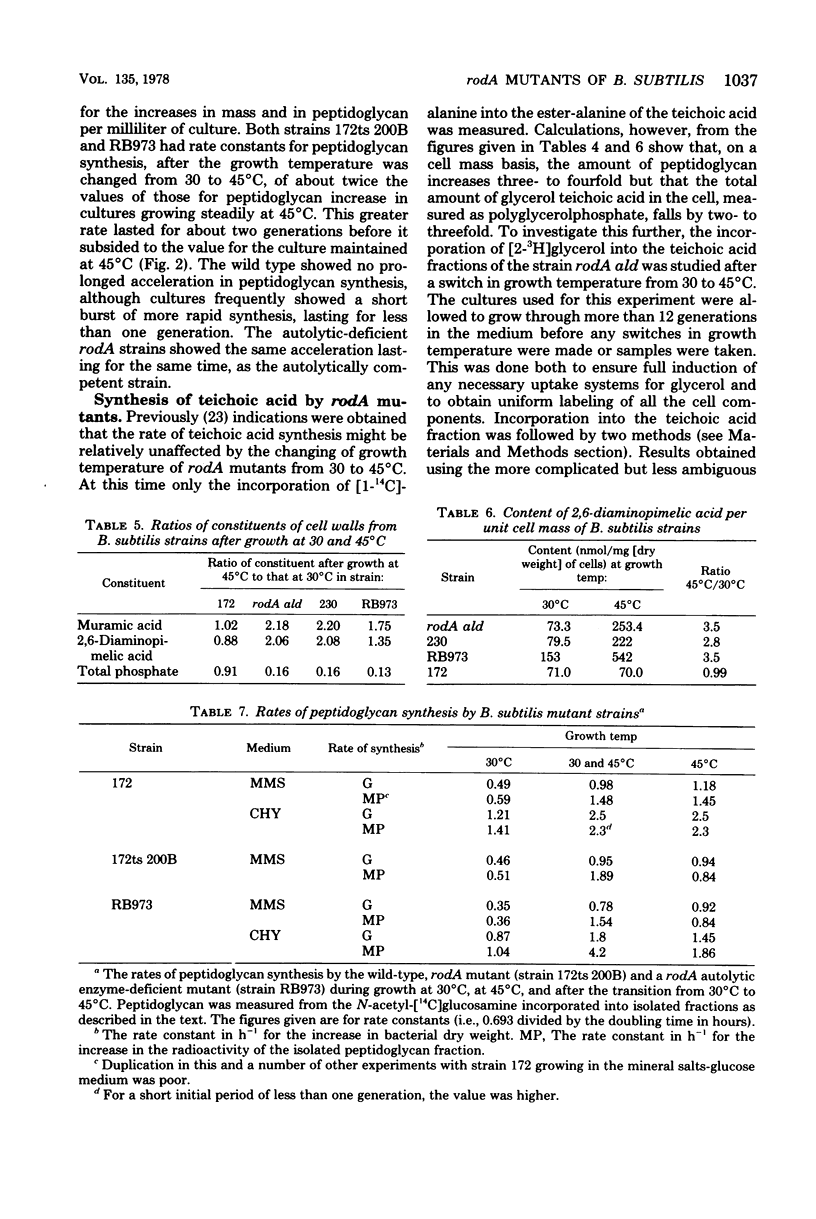
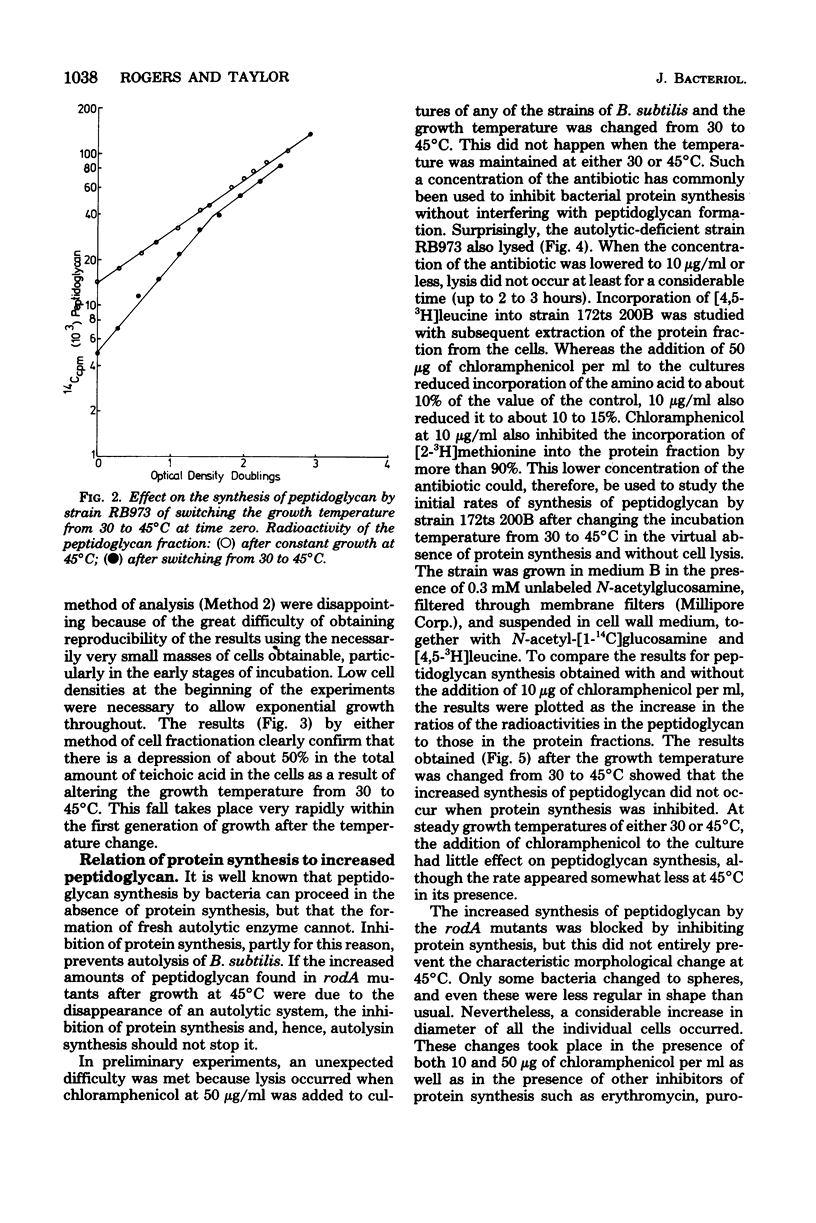
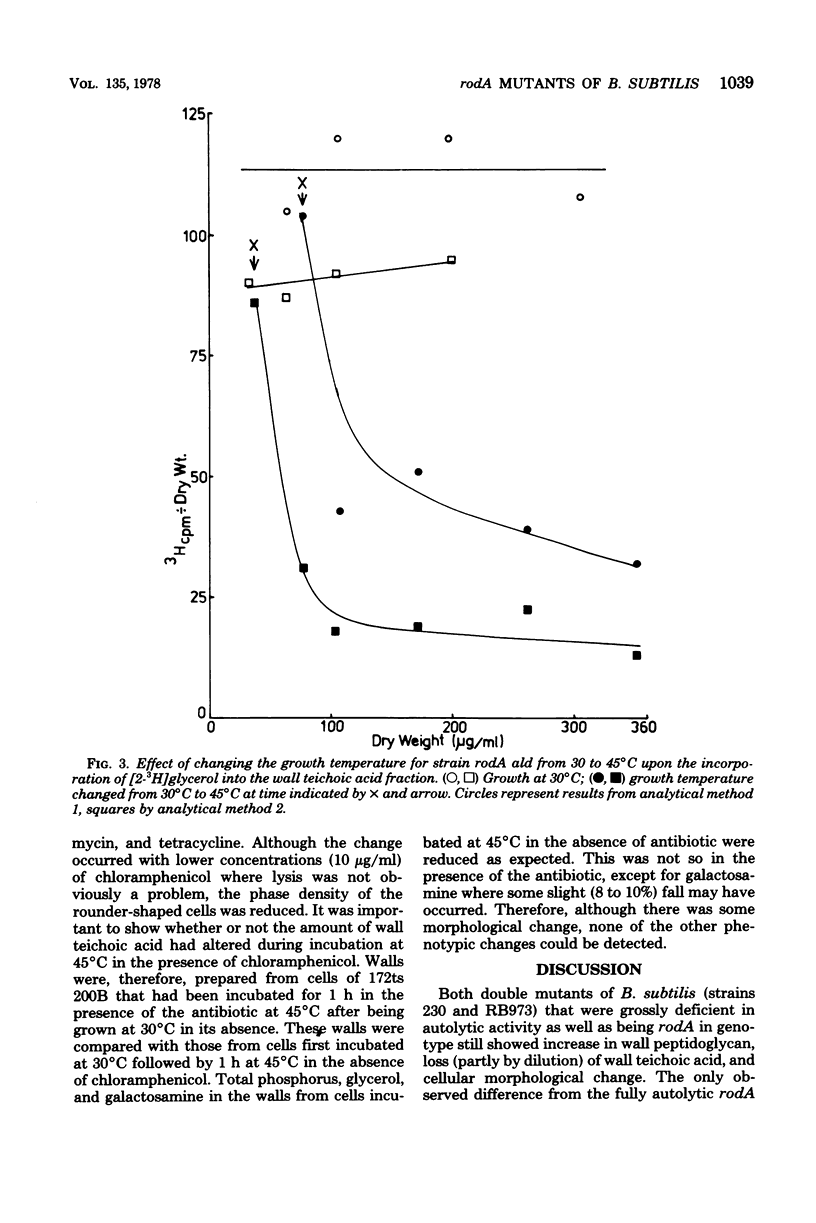
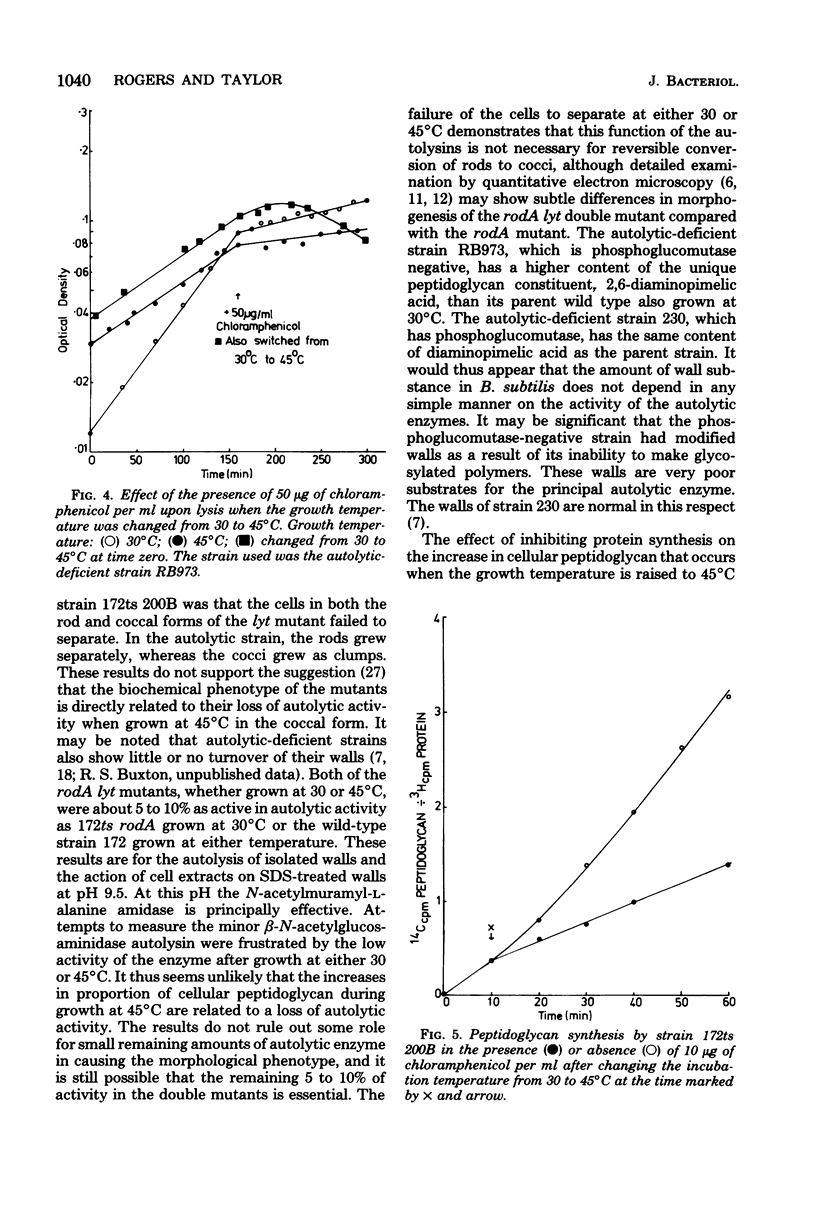
The biochemical phenotype of rodA mutants was not affected by the simultaneous presence in double mutants of the lyt gene which makes them 90 to 95% deficient in autolysin action. The only morphological effect of this deficiency on the expression of the rod gene was that both the rod and the coccal forms of the mutant failed to separate and grew as long chains of cells. Inhibition of protein synthesis stopped the increase in peptidoglycan that occurred when the growth temperature for the mutants was raised to 45 degrees C. These observations support the idea that a derepression of peptidoglycan synthesis occurs at this temperature. The increased amount of cellular peptidoglycan at the higher growth temperature is not likely to be the result of the concomitant switching off of autolytic enzyme action.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Boylan R. J., Mendelson N. H., Brooks D., Young F. E. Regulation of the bacterial cell wall: analysis of a mutant of Bacillus subtilis defective in biosynthesis of teichoic acid. J Bacteriol. 1972 Apr;110(1):281–290. doi: 10.1128/jb.110.1.281-290.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan R. J., Mendelson N. H. Initial characterization of a temperature-sensitive rod--mutant of Bacillus subtilis. J Bacteriol. 1969 Dec;100(3):1316–1321. doi: 10.1128/jb.100.3.1316-1321.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. C., Wilson C. R., Lukehart S., Young F. E., Shiflett M. A. Analysis of autolysins in temperature-sensitive morphological mutants of Bacillus subtilis. J Bacteriol. 1976 Jan;125(1):166–173. doi: 10.1128/jb.125.1.166-173.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett I. D., Higgins M. L. Study of pole assembly in Bacillus subtilis by computer reconstruction of septal growth zones seen in central, longitudinal thin sections of cells. J Bacteriol. 1978 Feb;133(2):959–971. doi: 10.1128/jb.133.2.959-971.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein J. E., Rogers H. J. Autolytic enzyme-deficient mutants of Bacillus subtilis 168. J Bacteriol. 1976 Sep;127(3):1427–1442. doi: 10.1128/jb.127.3.1427-1442.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C. W., Rogers H. J. Characterization of Bacillus licheniformis 6346 mutants which have altered lytic enzyme activities. J Bacteriol. 1974 May;118(2):358–368. doi: 10.1128/jb.118.2.358-368.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C. W., Wyrick P. B., Ward J. B., Rogers H. J. Effect of phosphate limitation on the morphology and wall composition of Bacillus licheniformis and its phosphoglucomutase-deficient mutants. J Bacteriol. 1973 Feb;113(2):969–984. doi: 10.1128/jb.113.2.969-984.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Study of cycle of cell wall assembly in Streptococcus faecalis by three-dimensional reconstructions of thin sections of cells. J Bacteriol. 1976 Sep;127(3):1346–1358. doi: 10.1128/jb.127.3.1346-1358.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L. Three-dimensional reconstruction of whole cells of Streptococcus faecalis from thin sections of cells. J Bacteriol. 1976 Sep;127(3):1337–1345. doi: 10.1128/jb.127.3.1337-1345.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R. C. Autolysis of isolated cell walls of Bacillus licheniformis N.C.T.C. 6346 and Bacillus subtilis Marburg Strain 168. Separation of the products and characterization of the mucopeptide fragments. Biochem J. 1970 Oct;119(5):849–860. doi: 10.1042/bj1190849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltje J. V., Tomasz A. Specific recognition of choline residues in the cell wall teichoic acid by the N-acetylmuramyl-L-alanine amidase of Pneumococcus. J Biol Chem. 1975 Aug 10;250(15):6072–6076. [PubMed] [Google Scholar]
- Karamata D., McConnell M., Rogers H. J. Mapping of rod mutants of Bacillus subtilis. J Bacteriol. 1972 Jul;111(1):73–79. doi: 10.1128/jb.111.1.73-79.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVVY G. A., MCALLAN A. The N-acetylation and estimation of hexosamines. Biochem J. 1959 Sep;73:127–132. doi: 10.1042/bj0730127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauck J., Chan L., Glaser L. Turnover of the cell wall of Gram-positive bacteria. J Biol Chem. 1971 Mar 25;246(6):1820–1827. [PubMed] [Google Scholar]
- Pooley H. M. Layered distribution, according to age, within the cell wall of bacillus subtilis. J Bacteriol. 1976 Mar;125(3):1139–1147. doi: 10.1128/jb.125.3.1139-1147.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley H. M. Turnover and spreading of old wall during surface growth of Bacillus subtilis. J Bacteriol. 1976 Mar;125(3):1127–1138. doi: 10.1128/jb.125.3.1127-1138.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J., Forsberg C. W. Role of autolysins in the killing of bacteria by some bactericidal antibiotics. J Bacteriol. 1971 Dec;108(3):1235–1243. doi: 10.1128/jb.108.3.1235-1243.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J., Thurman P. F., Buxton R. S. Magnesium and anion requirements of rodB mutants of Bacillus subtilis. J Bacteriol. 1976 Feb;125(2):556–564. doi: 10.1128/jb.125.2.556-564.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J., Thurman P. F., Taylor C., Reeve J. N. Mucopeptide synthesis by rod mutants of Bacillus subtilis. J Gen Microbiol. 1974 Dec;85(2):335–349. doi: 10.1099/00221287-85-2-335. [DOI] [PubMed] [Google Scholar]
- Rogers H. J., Thurman P. F. Temperature-sensitive nature of the rodB maturation in Bacillus subtilis. J Bacteriol. 1978 Jan;133(1):298–305. doi: 10.1128/jb.133.1.298-305.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. G. Synchronous cultures of Bacillus subtilis obtained by filtration with glass fiber filters. J Bacteriol. 1973 Nov;116(2):736–740. doi: 10.1128/jb.116.2.736-740.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Westphal M. Abnormal autolytic enzyme in a pneumococus with altered teichoic acid composition. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2627–2630. doi: 10.1073/pnas.68.11.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]