Abstract
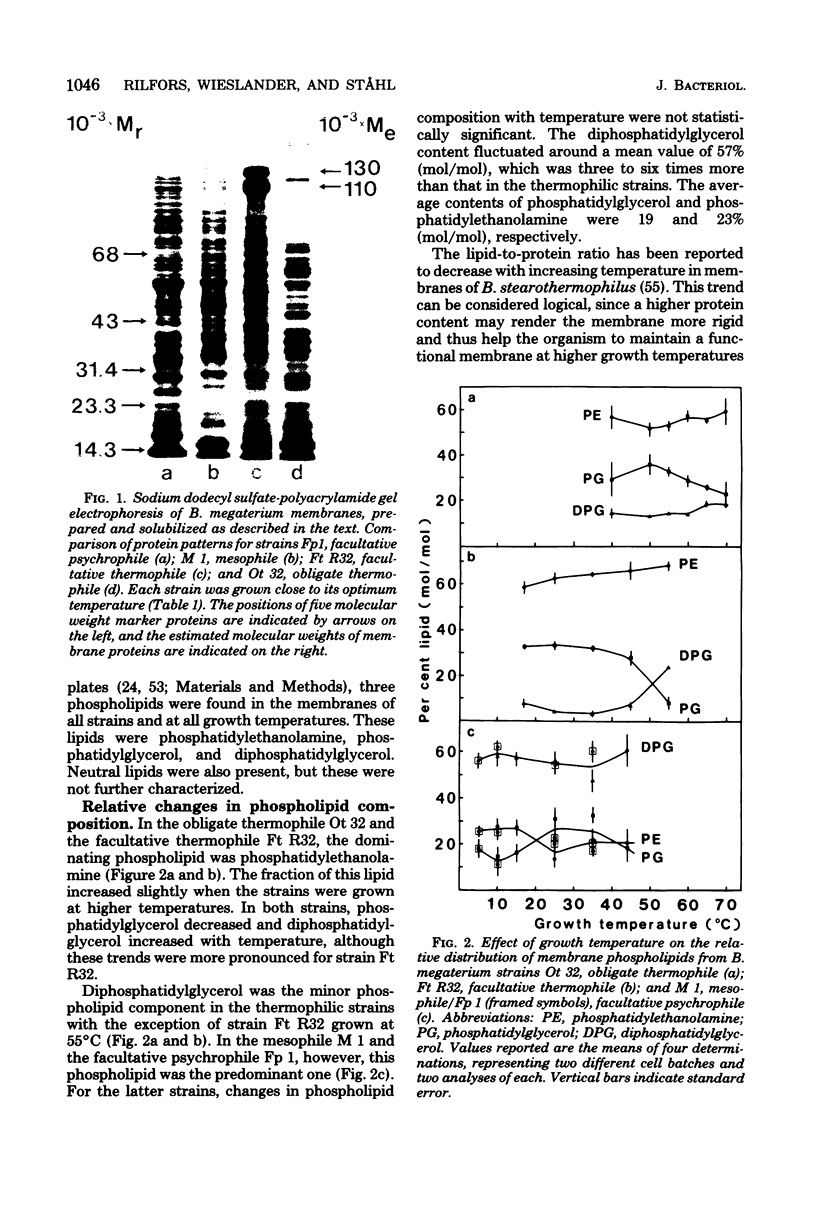
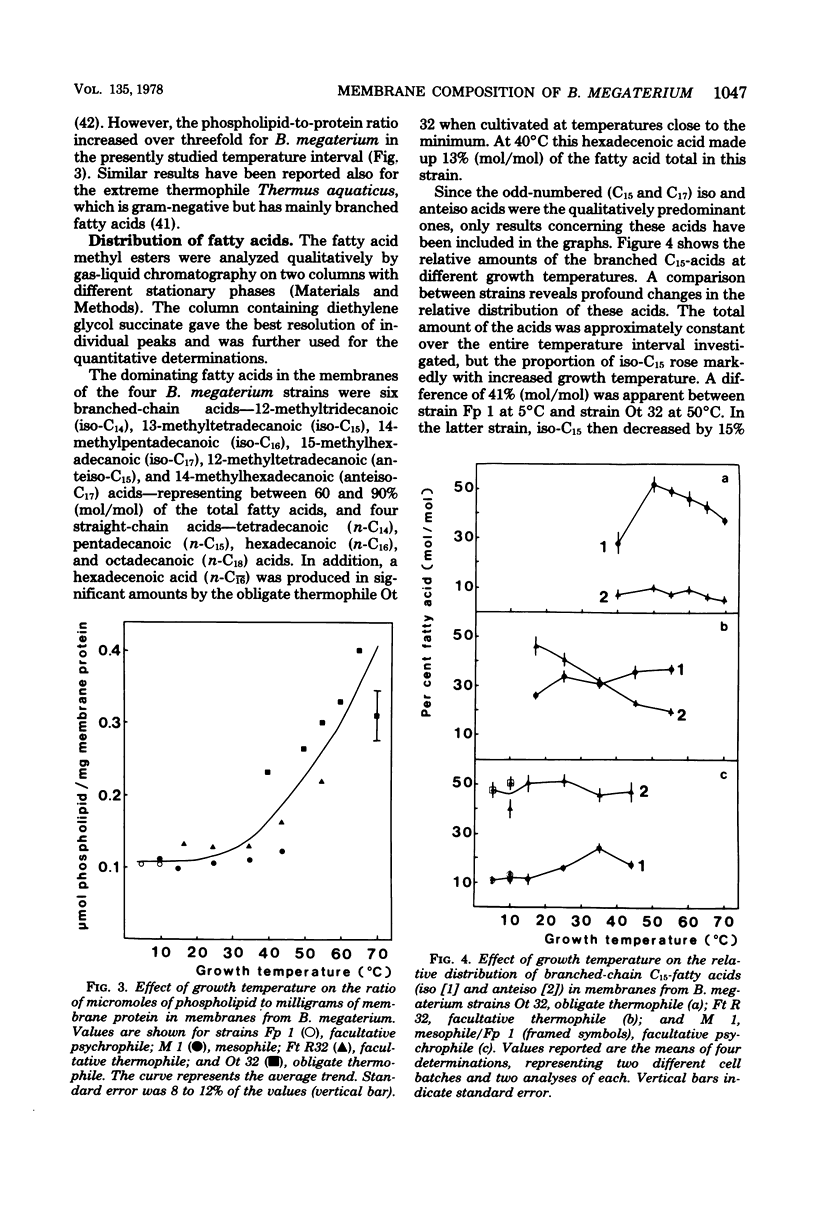
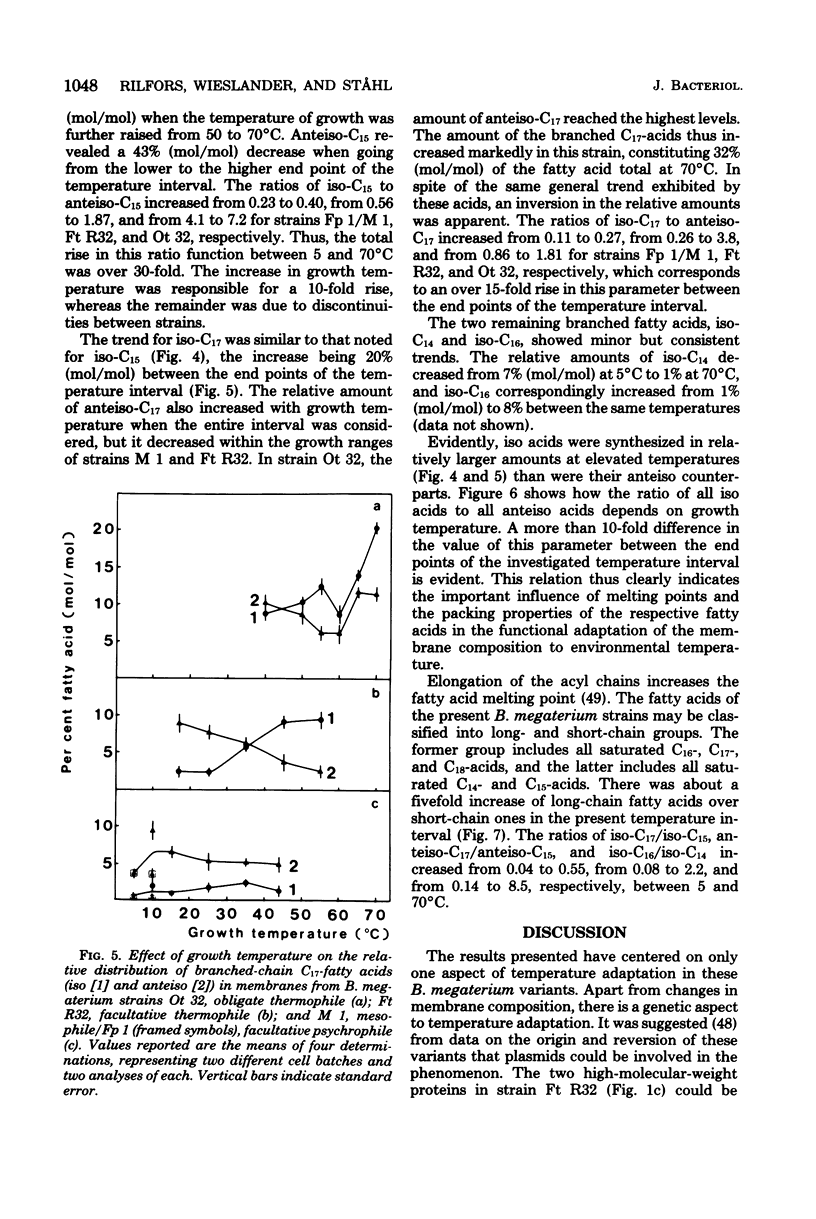
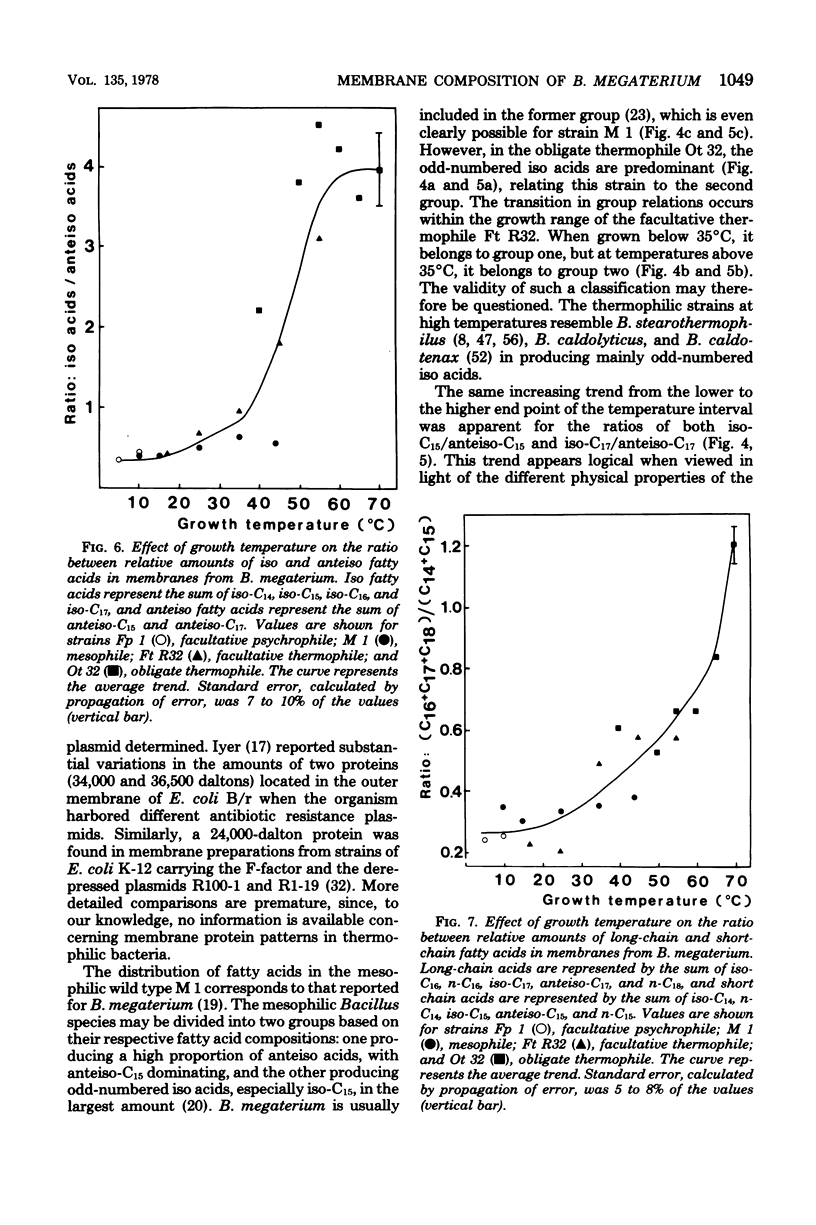
Membranes were prepared from four temperature range variants of Bacillus megaterium: one obligate thermophile, one facultative thermophile, one mesophile, and one facultative psychrophile, covering the temperature interval between 5 and 70 degrees C. The following changes in membrane composition were apparent with increasing growth temperatures: (i) the relative amount of iso fatty acids increased and that of anteiso acids decreased, the ratio of iso acids to anteiso acids being 0.34 at 5 degrees C and 3.95 at 70 degrees C, and the pair iso/anteiso acids thus seemed to parallel the pair saturated/unsaturated acids in their ability to regulate membrane fluidity; (ii) the relative/unsaturated acids in their ability to regulate membrane fluidity; (ii) the relative amount of long-chain acids (C16 to C18) increased fivefold over that of short-chain acids (C14 and C15) between 5 and 70 degrees C; (iii) the relative amount of phosphatidylethanolamine increased, and this phospholipid accordingly dominated in the thermophilic strains, whereas diphosphatidylglycerol was predominant in the two other strains; and (iv) the ratio of micromoles of phospholipid to milligrams of membrane protein increased three-fold between 5 and 70 degrees C. Moreover, a quantitative variation in membrane proteins was evident between the different strains. Briefly, membrane phospholipids with higher melting points and packing densities appeared to be synthesized at elevated growth temperatures.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bausum H. T., Matney T. S. Boundary Between Bacterial Mesophilism and Thermophilism. J Bacteriol. 1965 Jul;90(1):50–53. doi: 10.1128/jb.90.1.50-53.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertsch L. L., Bonsen P. P., Kornberg A. Biochemical studies of bacterial sporulation and germination. XIV. Phospholipids in Bacillus megaterium. J Bacteriol. 1969 Apr;98(1):75–81. doi: 10.1128/jb.98.1.75-81.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan M., Virmani Y. P., Himes R. H., Akagi J. M. Spin-labeling studies on the membrane of a facultative thermophilic bacillus. J Bacteriol. 1973 Jan;113(1):322–328. doi: 10.1128/jb.113.1.322-328.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Vagelos P. R. Metabolism and function of the membrane phospholipids of Escherichia coli. Biochim Biophys Acta. 1972 Feb 14;265(1):25–60. doi: 10.1016/0304-4157(72)90018-4. [DOI] [PubMed] [Google Scholar]
- Cullis P. R., De Kruyff B. 31P NMR studies of unsonicated aqueous dispersions of neutral and acidic phospholipids. Effects of phase transitions, p2H and divalent cations on the motion in the phosphate region of the polar headgroup. Biochim Biophys Acta. 1976 Jul 1;436(3):523–540. doi: 10.1016/0005-2736(76)90438-7. [DOI] [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- Dancey G. F., Shapiro B. M. Specific phospholipid requirement for activity of the purified respiratory chain NADH dehydrogenase of Escherichia coli. Biochim Biophys Acta. 1977 May 25;487(2):368–377. doi: 10.1016/0005-2760(77)90013-3. [DOI] [PubMed] [Google Scholar]
- Daron H. H. Fatty acid composition of lipid extracts of a thermophilic Bacillus species. J Bacteriol. 1970 Jan;101(1):145–151. doi: 10.1128/jb.101.1.145-151.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Siervo A. J. Alterations in the phospholipid composition of Escherichia coli B during growth at different temperatures. J Bacteriol. 1969 Dec;100(3):1342–1349. doi: 10.1128/jb.100.3.1342-1349.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esfahani M., Rudkin B. B., Cutler C. J., Waldron P. E. Lipid-protein interactions in membranes: interaction of phospholipids with respiratory enzymes of Escherichia coli membrane. J Biol Chem. 1977 May 25;252(10):3194–3198. [PubMed] [Google Scholar]
- Esko J. D., Gilmore J. R., Glaser M. Use of a fluorescent probe to determine the viscosity of LM cell membranes with altered phospholipid compositions. Biochemistry. 1977 May 3;16(9):1881–1890. doi: 10.1021/bi00628a019. [DOI] [PubMed] [Google Scholar]
- Esser A. F., Souza K. A. Correlation between thermal death and membrane fluidity in Bacillus stearothermophilus. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4111–4115. doi: 10.1073/pnas.71.10.4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnerty W. R., Makula R. A. Microbial lipid metabolism. CRC Crit Rev Microbiol. 1975 Oct;4(1):1–40. doi: 10.3109/10408417509105485. [DOI] [PubMed] [Google Scholar]
- Fulco A. J. The effect of temperature on the formation of delta 5-unsaturated fatty acids by bacilli. Biochim Biophys Acta. 1967 Dec 5;144(3):701–703. doi: 10.1016/0005-2760(67)90065-3. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Iyer R. Plasmid mediated alterations in composition and structure of envelopes of Escherichia coli B/r. Biochim Biophys Acta. 1977 Oct 17;470(2):258–272. doi: 10.1016/0005-2736(77)90105-5. [DOI] [PubMed] [Google Scholar]
- Jergil B., Ohlsson R. Phosphorylation of proteins in rat liver. Endogenous phosphorylation and dephosphorylation of proteins from smooth and rough endoplasmic reticulum and free ribosomes. Eur J Biochem. 1974 Jul 1;46(1):13–25. doi: 10.1111/j.1432-1033.1974.tb03592.x. [DOI] [PubMed] [Google Scholar]
- Kaneda T. Factors affecting the relative ratio of fatty acids in Bacillus cereus. Can J Microbiol. 1971 Feb;17(2):269–275. doi: 10.1139/m71-045. [DOI] [PubMed] [Google Scholar]
- Kaneda T. Fatty acids in the genus Bacillus. I. Iso- and anteiso-fatty acids as characteristic constituents of lipids in 10 species. J Bacteriol. 1967 Mar;93(3):894–903. doi: 10.1128/jb.93.3.894-903.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda T. Fatty acids of the genus Bacillus: an example of branched-chain preference. Bacteriol Rev. 1977 Jun;41(2):391–418. doi: 10.1128/br.41.2.391-418.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda T. Major occurrence of cis-delta 5 fatty acids in three psychrophilic species of Bacillus. Biochem Biophys Res Commun. 1971 Apr 16;43(2):298–302. doi: 10.1016/0006-291x(71)90752-2. [DOI] [PubMed] [Google Scholar]
- Kaneda T. Positional preference of fatty acids in phospholipids of Bacillus cereus and its relation to growth temperature. Biochim Biophys Acta. 1972 Oct 5;280(2):297–305. doi: 10.1016/0005-2760(72)90097-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larson T. J., Hirabayshi T., Dowhan W. Phosphatidylglycerol biosynthesis in Bacillus licheniformis Resolution of membrane-bound enzymes by affinity chromatography on cytidinediphospho-sn-1,2-diacylglycerol Sepharose. Biochemistry. 1976 Mar 9;15(5):974–979. doi: 10.1021/bi00650a005. [DOI] [PubMed] [Google Scholar]
- Martin C. E., Hiramitsu K., Kitajima Y., Nozawa Y., Skriver L., Thompson G. A. Molecular control of membrane properties during temperature acclimation. Fatty acid desaturase regulation of membrane fluidity in acclimating Tetrahymena cells. Biochemistry. 1976 Nov 30;15(24):5218–5227. doi: 10.1021/bi00669a004. [DOI] [PubMed] [Google Scholar]
- McClare C. W. An accurate and convenient organic phosphorus assay. Anal Biochem. 1971 Feb;39(2):527–530. doi: 10.1016/0003-2697(71)90443-x. [DOI] [PubMed] [Google Scholar]
- McElhaney R. N., Souza K. A. The relationship between environmental temperature, cell growth and the fluidity and physical state of the membrane lipids in Bacillus stearothermophilus. Biochim Biophys Acta. 1976 Sep 7;443(3):348–359. doi: 10.1016/0005-2736(76)90455-7. [DOI] [PubMed] [Google Scholar]
- Minkley E. G., Jr, Ippen-Ihler K. Identification of a membrane protein associated with expression of the surface exclusion region of the F transfer operon. J Bacteriol. 1977 Mar;129(3):1613–1622. doi: 10.1128/jb.129.3.1613-1622.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnikin D. E., Abdolrahimzadeh H. Thin-layer chromatography of bacterial lipids on sodium acetate-impregnated silica gel. J Chromatogr. 1971 Dec 23;63(2):452–454. doi: 10.1016/s0021-9673(01)85672-7. [DOI] [PubMed] [Google Scholar]
- Mosley G. A., Card G. L., Koostra W. L. Effect of calcium and anaerobiosis on the thermostability of Bacillus stearothermophilus. Can J Microbiol. 1976 Apr;22(4):468–474. doi: 10.1139/m76-073. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Oshima M., Miyagawa A. Comparative studies on the fatty acid composition of moderately and extremely thermophilic bacteria. Lipids. 1974 Jul;9(7):476–480. doi: 10.1007/BF02534274. [DOI] [PubMed] [Google Scholar]
- Overath P., Schairer H. U., Stoffel W. Correlation of in vivo and in vitro phase transitions of membrane lipids in Escherichia coli. Proc Natl Acad Sci U S A. 1970 Oct;67(2):606–612. doi: 10.1073/pnas.67.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson P. H., Lennarz W. J. Studies on the membranes of bacilli. I. Phospholipid biosynthesis. J Biol Chem. 1971 Feb 25;246(4):1062–1072. [PubMed] [Google Scholar]
- Ray P. H., White D. C., Brock T. D. Effect of temperature on the fatty acid composition of Thermus aquaticus. J Bacteriol. 1971 Apr;106(1):25–30. doi: 10.1128/jb.106.1.25-30.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Samuni A. Effect of proteins on the motion of spin-labeled fatty acids in mycoplasma membranes. Biochim Biophys Acta. 1973 Feb 27;298(1):32–38. doi: 10.1016/0005-2736(73)90006-0. [DOI] [PubMed] [Google Scholar]
- Shaw N. The detection of lipids on thin-layer chromatograms with the periodate-Schiff reagents. Biochim Biophys Acta. 1968 Oct 22;164(2):435–436. doi: 10.1016/0005-2760(68)90171-9. [DOI] [PubMed] [Google Scholar]
- Shen P. Y., Coles E., Foote J. L., Stenesh J. Fatty acid distribution in mesophilic and thermophilic strains of the genus Bacillus. J Bacteriol. 1970 Aug;103(2):479–481. doi: 10.1128/jb.103.2.479-481.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinensky M. Homeoviscous adaptation--a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc Natl Acad Sci U S A. 1974 Feb;71(2):522–525. doi: 10.1073/pnas.71.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza K. A., Kostiw L. L., Tyson B. J. Alterations in normal fatty acid composition in a temperature-sensitive mutant of a thermophilic bacillus. Arch Microbiol. 1974 Apr 19;97(2):89–102. doi: 10.1007/BF00403049. [DOI] [PubMed] [Google Scholar]
- Ståhl S., Olsson O. Temperature range variants of Bacillus megaterium. Arch Microbiol. 1977 Jun 20;113(3):221–229. doi: 10.1007/BF00492029. [DOI] [PubMed] [Google Scholar]
- Tsukagoshi N., Fox C. F. Abortive assembly of the lactose transport system in Escherichia coli. Biochemistry. 1973 Jul 17;12(15):2816–2822. doi: 10.1021/bi00739a007. [DOI] [PubMed] [Google Scholar]
- Weerkamp A., Heinen W. Effect of temperature on the fatty acid composition of the extreme thermophiles, Bacillus caldolyticus and Bacillus caldotenax. J Bacteriol. 1972 Jan;109(1):443–446. doi: 10.1128/jb.109.1.443-446.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieslander A., Rilfors L. Qualitative and quantitative variations of membrane lipid species in Acholeplasma laidlawii A. Biochim Biophys Acta. 1977 Apr 18;466(2):336–346. doi: 10.1016/0005-2736(77)90229-2. [DOI] [PubMed] [Google Scholar]
- Willecke K., Pardee A. B. Fatty acid-requiring mutant of bacillus subtilis defective in branched chain alpha-keto acid dehydrogenase. J Biol Chem. 1971 Sep 10;246(17):5264–5272. [PubMed] [Google Scholar]
- Wisdom C., Welker N. E. Membranes of Bacillus stearothermophilus: factors affecting protoplast stability and thermostability of alkaline phosphatase and reduced nicotinamide adenine dinucleotide oxidase. J Bacteriol. 1973 Jun;114(3):1336–1345. doi: 10.1128/jb.114.3.1336-1345.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M., Walker H. W., Lillard D. A. Fatty acids from vegetative cells and spores of Bacillus stearothermophilus. J Bacteriol. 1970 Jun;102(3):877–878. doi: 10.1128/jb.102.3.877-878.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Kamp JA O. P., Houtsmuller U. M., van Deenen L. L. On the phospholipids of Bacillus megaterium. Biochim Biophys Acta. 1965 Oct 4;106(2):438–441. doi: 10.1016/0005-2760(65)90059-7. [DOI] [PubMed] [Google Scholar]
- den Kamp JA O. P., van Iterson W., van Deenen L. L. Studies of the phospholipids and morphology of protoplasts of Bacillus megaterium. Biochim Biophys Acta. 1967;135(5):862–884. doi: 10.1016/0005-2736(67)90056-9. [DOI] [PubMed] [Google Scholar]