Abstract
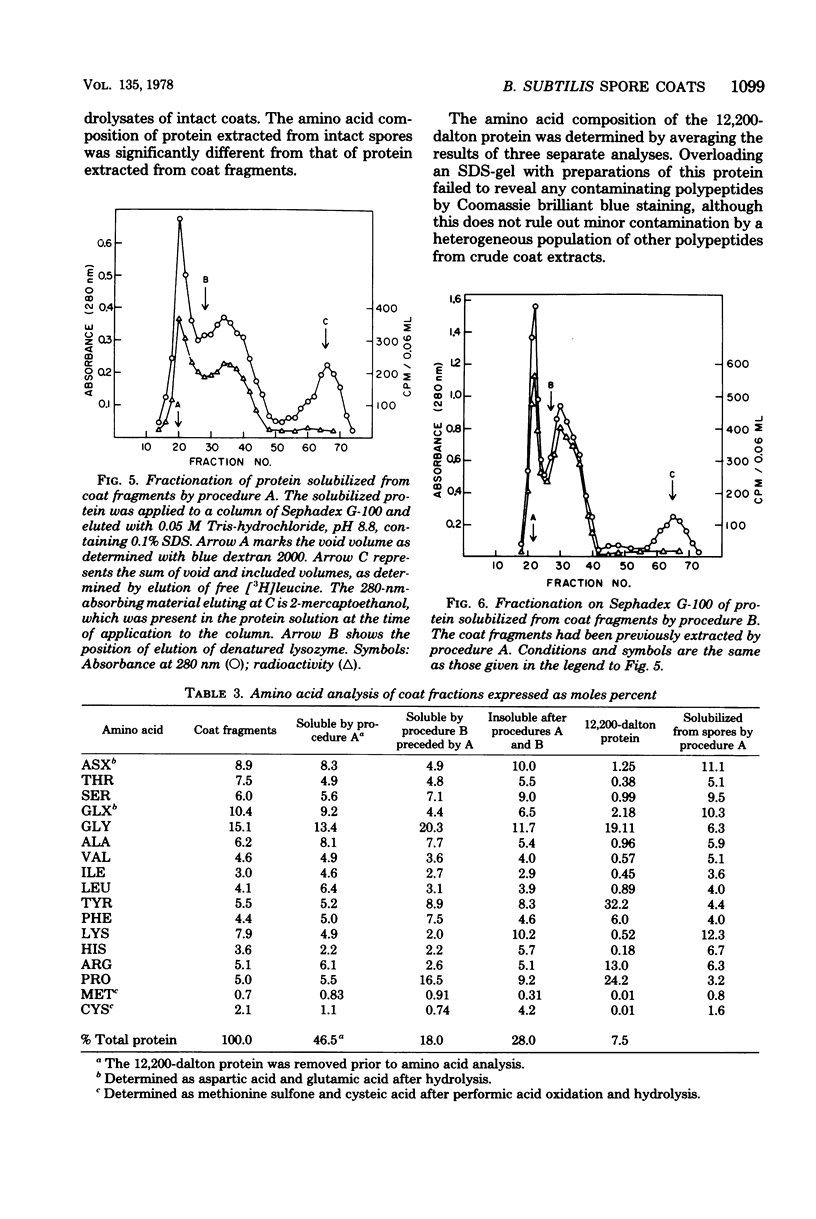
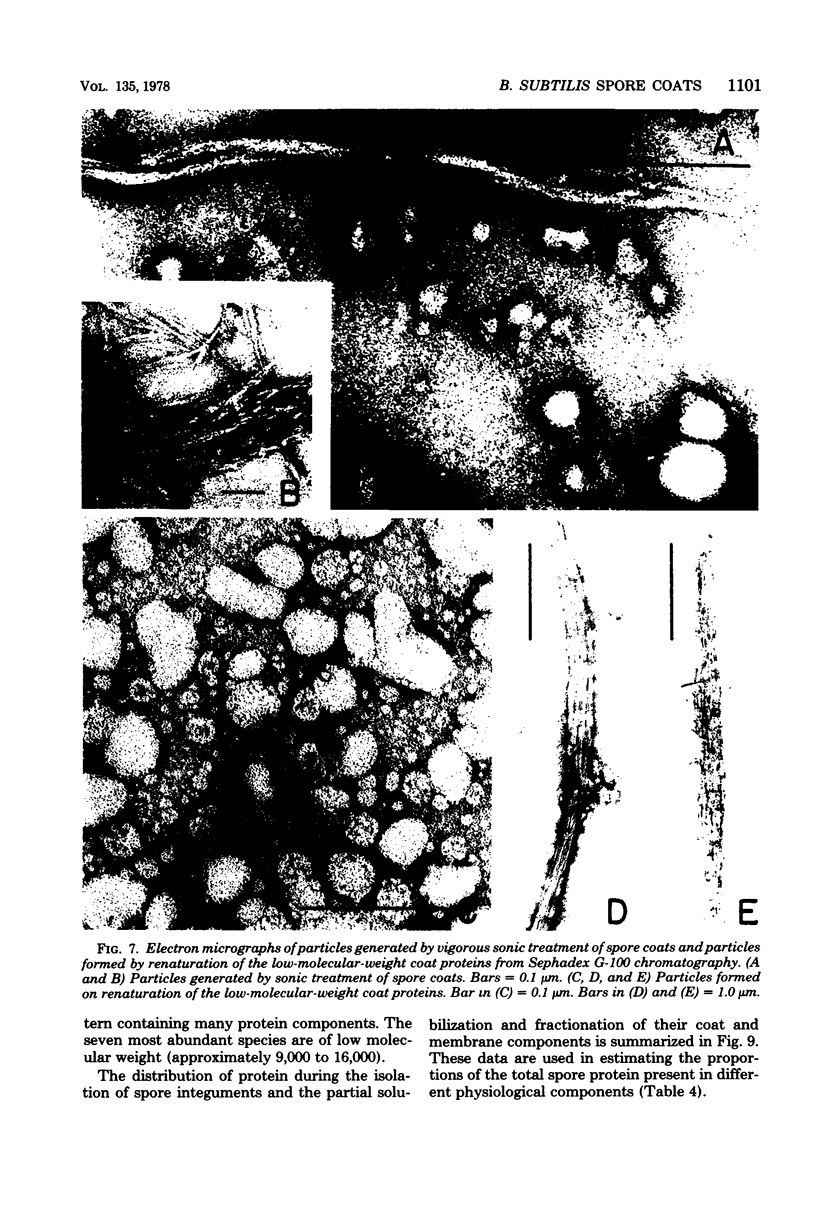
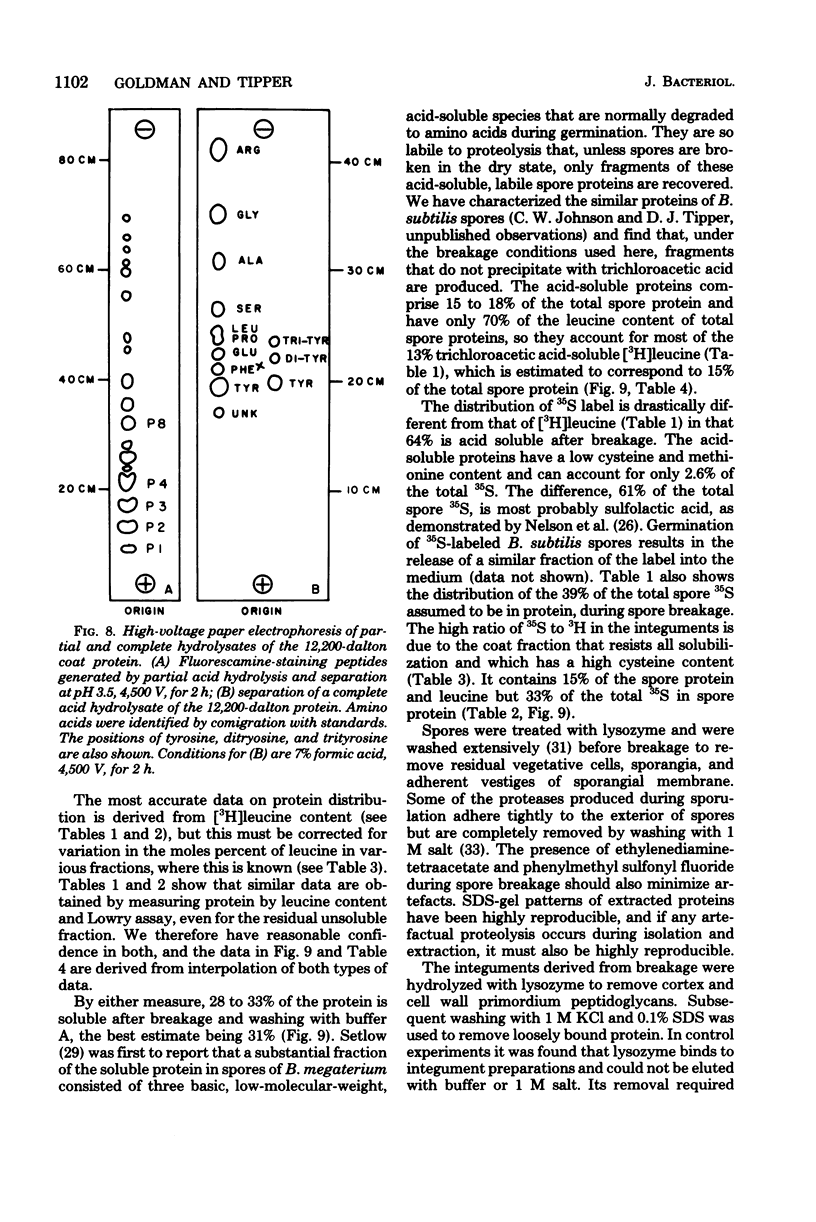
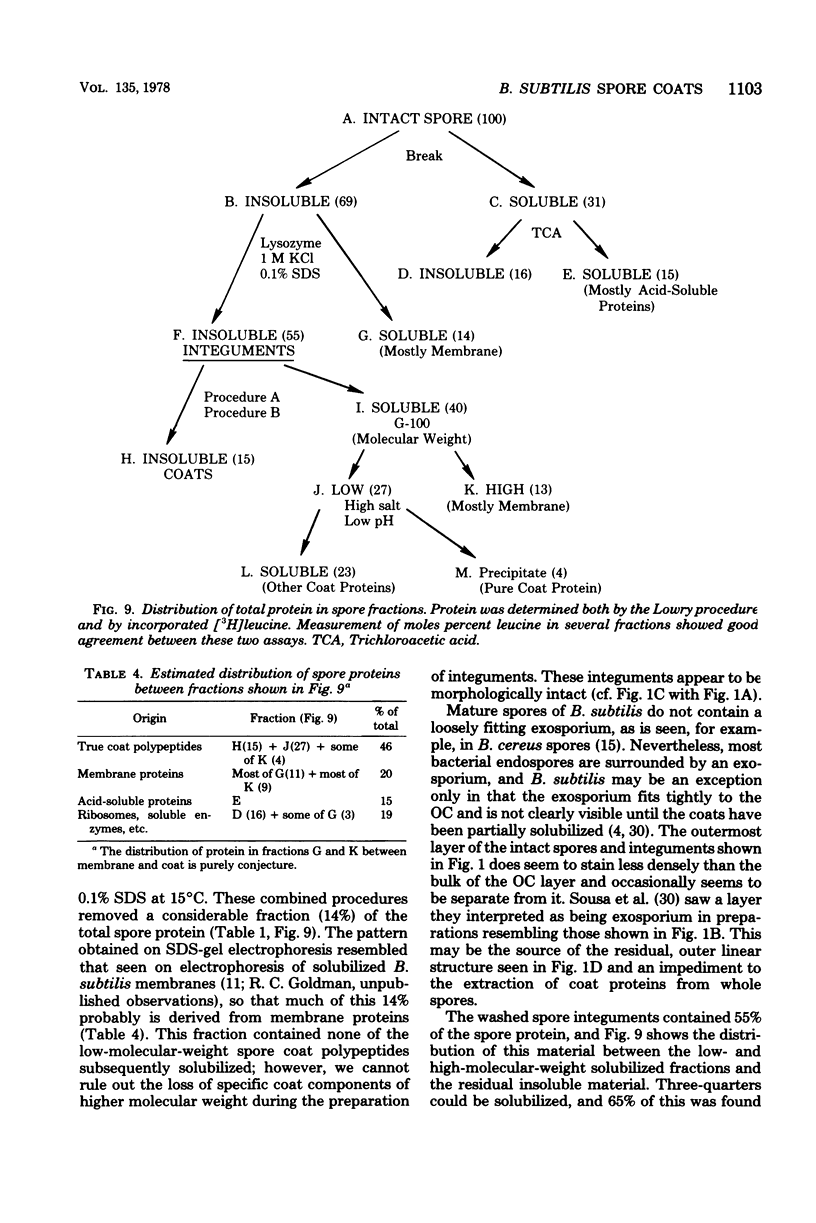
Extensively washed, dormant spores of Bacillus subtilis were disrupted with glass beads in buffer at pH 7 in the presence of protease inhibitors. Approximately 31% of the total spore protein was soluble, and another 14% was removed from the insoluble fraction by hydrolysis with lysozyme and washing with 1 M KCl and 0.1% sodium dodecyl sulfate. The residual spore integuments comprised 55% of the total spore proteins and consisted of coats and residual membrane components. Treatment of integuments with sodium dodecyl sulfate and reducing agents at pH 10 solubilized 40% of the total spore protein. Seven low-molecular-weight polypeptide components of this solubilized fraction comprised 27% of the total spore protein. They are not normal membrane components and reassociated to form fibrillar structures resembling spore coat fragments. The residual insoluble material (15% of the total spore protein) was rich in cysteine and was probably also derived from the spore coats. A solubilized coat polypeptide of molecular weight 12,200 has been purified in good yield (4 to 5% of the total spore protein). Five amino acids account for 92% of its total amino acid residues: glycine, 19%; tyrosine, 31%; proline, 23%; arginine, 13%; and phenylalanine, 6%.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN S. O. THE CROSS-LINKS IN RESILIN IDENTIFIED AS DITYROSINE AND TRITYROSINE. Biochim Biophys Acta. 1964 Oct 9;93:213–215. doi: 10.1016/0304-4165(64)90289-2. [DOI] [PubMed] [Google Scholar]
- Andreeva N. S., Esipova N. G., Millionova M. I., Rogulenkova V. N., Tumanian V. G. Sinteticheskie reguliarnye politripeptidy i belki kollagenovogo klassa. Biofizika. 1970 Mar-Apr;15(2):198–205. [PubMed] [Google Scholar]
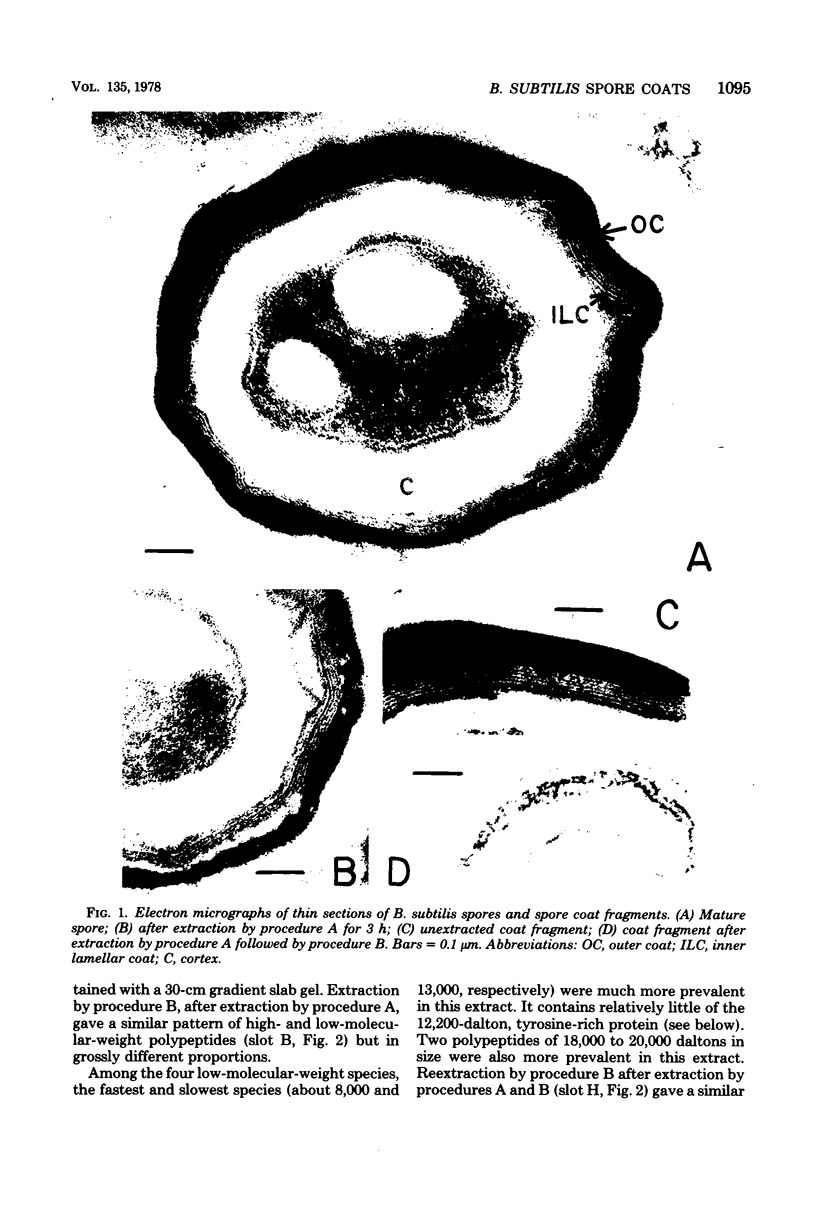
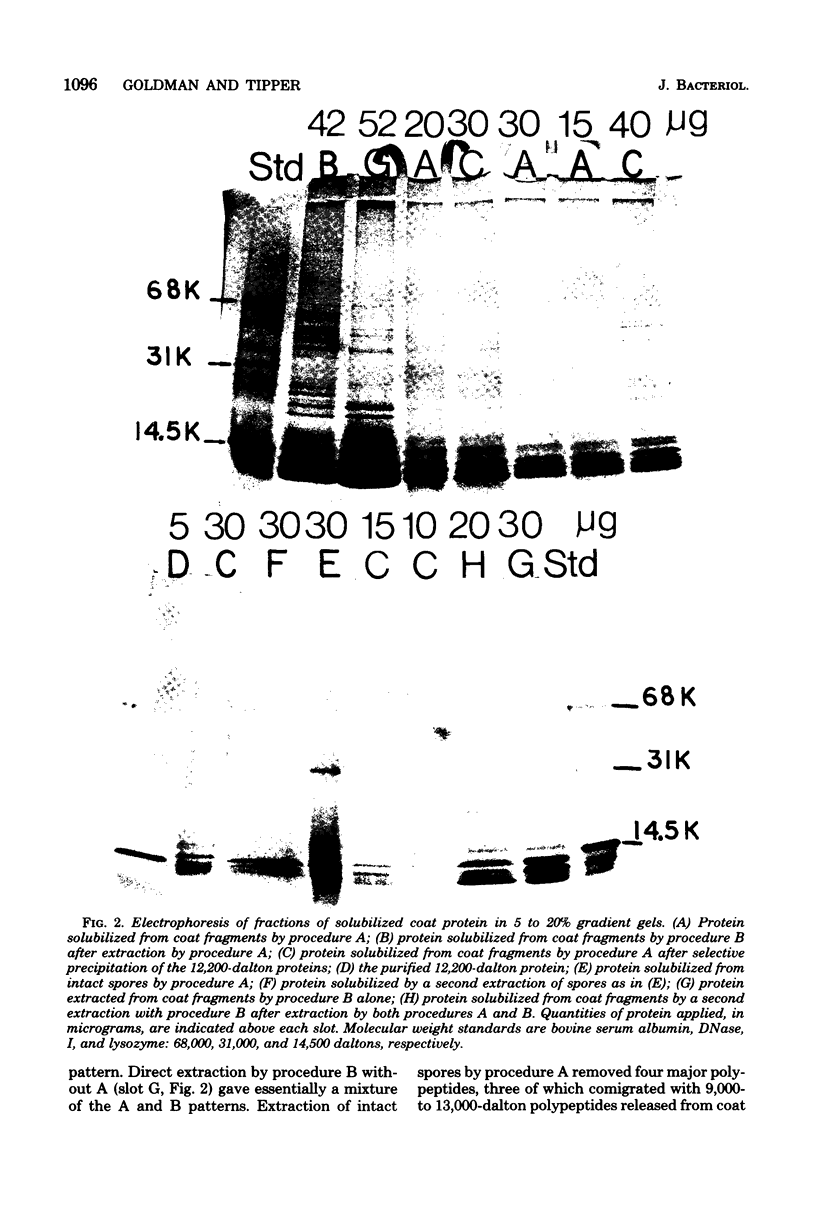
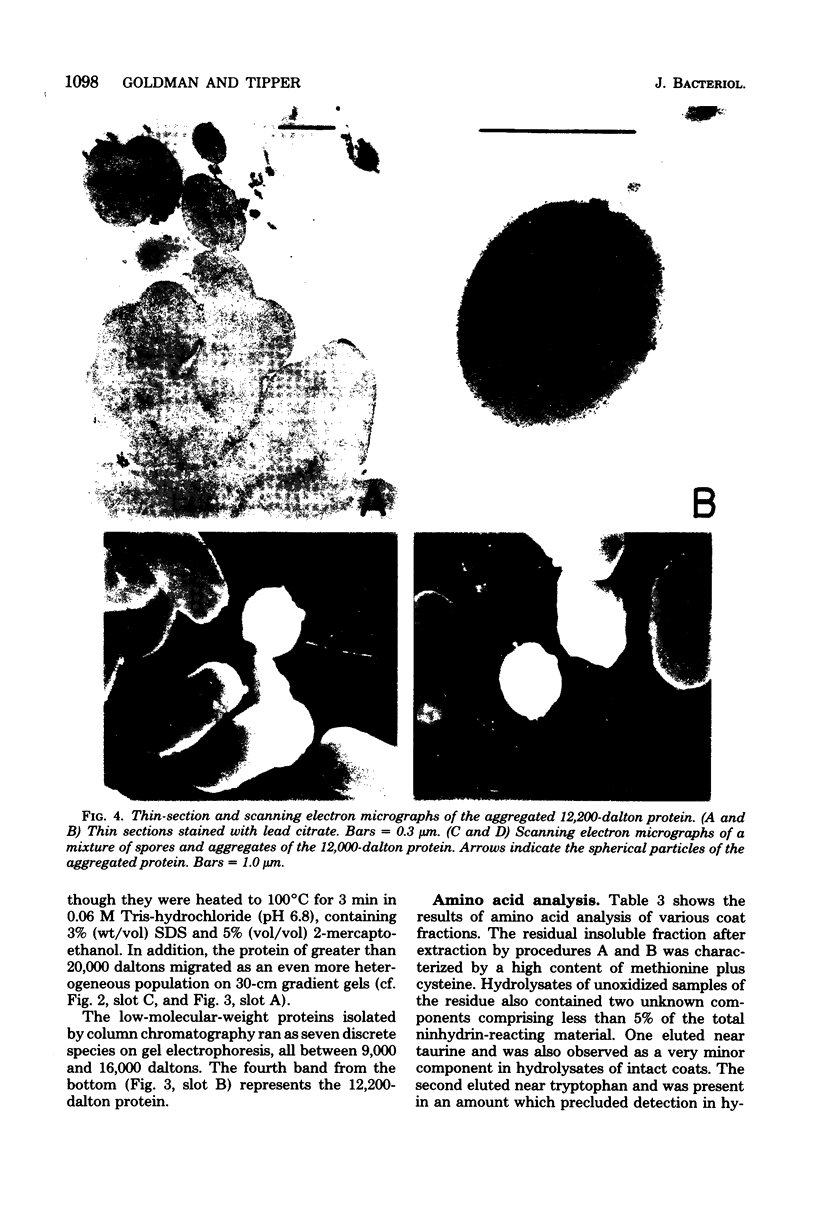
- Aronson A. I., Fitz-James P. Structure and morphogenesis of the bacterial spore coat. Bacteriol Rev. 1976 Jun;40(2):360–402. doi: 10.1128/br.40.2.360-402.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOHAK Z. N-EPSILON-(DL-2-AMINO-2-CARBOXYETHYL)-L-LYSINE, A NEW AMINO ACID FORMED ON ALKALINE TREATMENT OF PROTEINS. J Biol Chem. 1964 Sep;239:2878–2887. [PubMed] [Google Scholar]
- Balassa G. Biochemical genetics of bacterial sporulation. I. Unidirectional pleiotropic interactions among genes controlling sporulation in Bacillus subtilis. Mol Gen Genet. 1969;104(1):73–103. [PubMed] [Google Scholar]
- Cheng Y. S., Aronson A. I. Alterations of spore coat processing and protein turnover in a Bacillus cereus mutant with a defective postexponential intracellular protease. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1254–1258. doi: 10.1073/pnas.74.3.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS A. J., SIZER I. W. The oxidation of tyramine, tyrosine, and related compounds by peroxidase. J Biol Chem. 1959 Jun;234(6):1611–1614. [PubMed] [Google Scholar]
- Goldman R. C. Membrane protein alterations during the early stages of sporulation of Bacillus subtilis. J Supramol Struct. 1976;5(4):457–473. doi: 10.1002/jss.400050405. [DOI] [PubMed] [Google Scholar]
- Hiragi Y. Physical, chemical and morphological studies of spore coat of Bacillus subtilis. J Gen Microbiol. 1972 Aug;72(1):87–99. doi: 10.1099/00221287-72-1-87. [DOI] [PubMed] [Google Scholar]
- Holt S. C., Leadbetter E. R. Comparative ultrastructure of selected aerobic spore-forming bacteria: a freeze-etching study. Bacteriol Rev. 1969 Jun;33(2):346–378. doi: 10.1128/br.33.2.346-378.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M., Foster J. W. Chemical and electron microscope studies on fractions prepared from coats of Bacillus spores. J Gen Microbiol. 1967 May;47(2):257–271. doi: 10.1099/00221287-47-2-257. [DOI] [PubMed] [Google Scholar]
- Kottel R. H., Bacon K., Clutter D., White D. Coats from Myxococcus xanthus: characterization and synthesis during myxospore differentiation. J Bacteriol. 1975 Oct;124(1):550–557. doi: 10.1128/jb.124.1.550-557.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee P. L. Single-column system for accelerated amino acid analysis of physiological fluids using five lithium buffers. Biochem Med. 1974 Jun;10(2):107–121. doi: 10.1016/0006-2944(74)90013-1. [DOI] [PubMed] [Google Scholar]
- Leighton T. J., Doi R. H. The stability of messenger ribonucleic acid during sporulation in Bacillus subtilis. J Biol Chem. 1971 May 25;246(10):3189–3195. [PubMed] [Google Scholar]
- Leighton T. Sporulation-specific translational discrimination in Bacillus subtilis. J Mol Biol. 1974 Jul 15;86(4):855–863. doi: 10.1016/0022-2836(74)90358-1. [DOI] [PubMed] [Google Scholar]
- Linn T., Greenleaf A. L., Losick R. RNA polymerase from sporulating Bacillus subtilis. Purification and properties of a modified form of the enzyme containing two sporulation polypeptides. J Biol Chem. 1975 Dec 25;250(24):9256–9261. [PubMed] [Google Scholar]
- Losick R., Sonenshein A. L. Change in the template specificity of RNA polymerase during sporulation of Bacillus subtilis. Nature. 1969 Oct 4;224(5214):35–37. doi: 10.1038/224035a0. [DOI] [PubMed] [Google Scholar]
- Mendez E., Lai C. Y. Reaction of peptides with fluorescamine on paper after chromatography or electrophoresis. Anal Biochem. 1975 May 12;65(1-2):281–292. doi: 10.1016/0003-2697(75)90511-4. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P. Protein metabolism during germination of Bacillus megaterium spores. II. Degradation of pre-existing and newly synthesized protein. J Biol Chem. 1975 Jan 25;250(2):631–637. [PubMed] [Google Scholar]
- Sousa J. C., Silva M. T., Balassa G. An exosporium-like outer layer in Bacillus subtilis spores. Nature. 1976 Sep 2;263(5572):53–54. doi: 10.1038/263053a0. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Kornberg A. Biochemical studies of bacterial sporulation and germination. VI. Origin of spore core and coat proteins. J Biol Chem. 1968 Sep 10;243(17):4588–4599. [PubMed] [Google Scholar]
- Steinert P. M., Idler W. W., Zimmerman S. B. Self-assembly of bovine epidermal keratin filaments in vitro. J Mol Biol. 1976 Dec 15;108(3):547–567. doi: 10.1016/s0022-2836(76)80136-2. [DOI] [PubMed] [Google Scholar]
- Tesone C., Torriani A. Protease associated with spores of Bacillus cereus. J Bacteriol. 1975 Oct;124(1):593–594. doi: 10.1128/jb.124.1.593-594.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper D. J., Johnson C. W., Ginther C. L., Leighton T., Wittmann H. G. Erythromycin resistant mutations in Bacillus subtilis cause temperature sensitive sporulation. Mol Gen Genet. 1977 Jan 18;150(2):147–159. doi: 10.1007/BF00695395. [DOI] [PubMed] [Google Scholar]








