Abstract
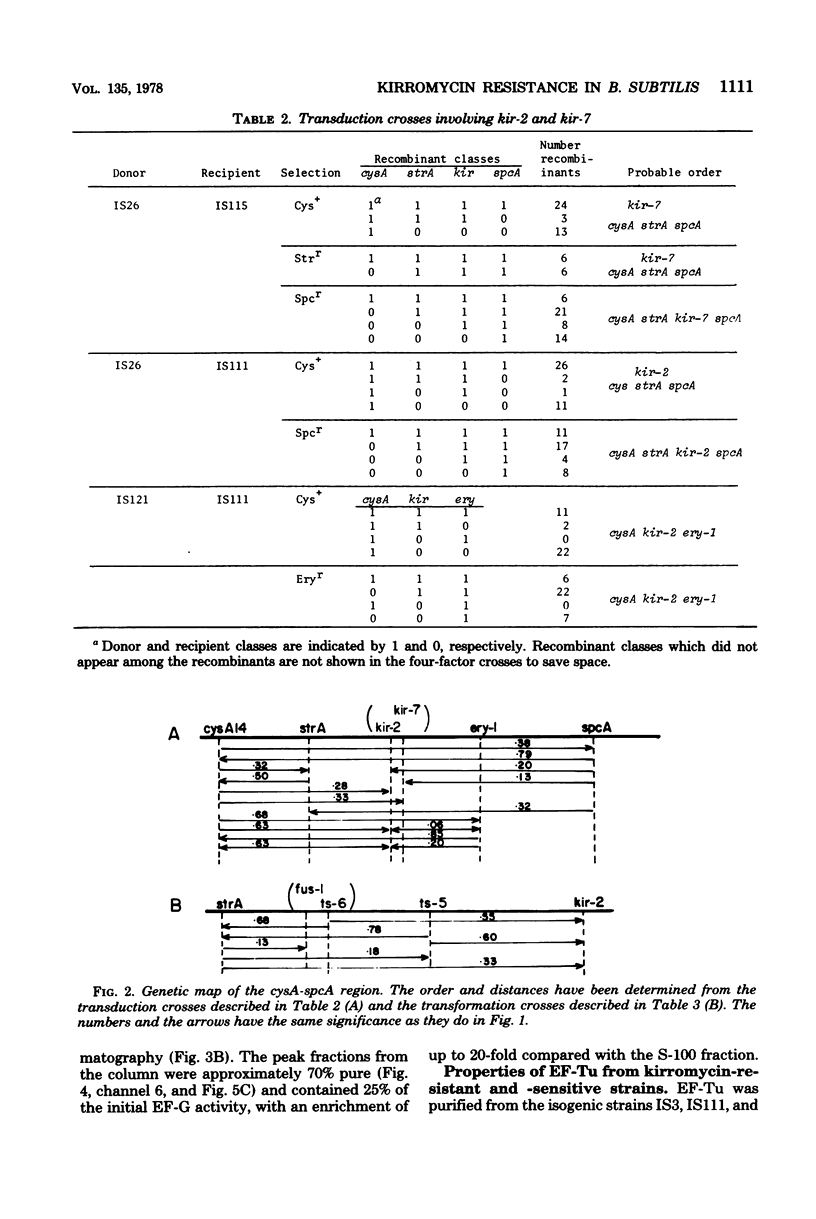
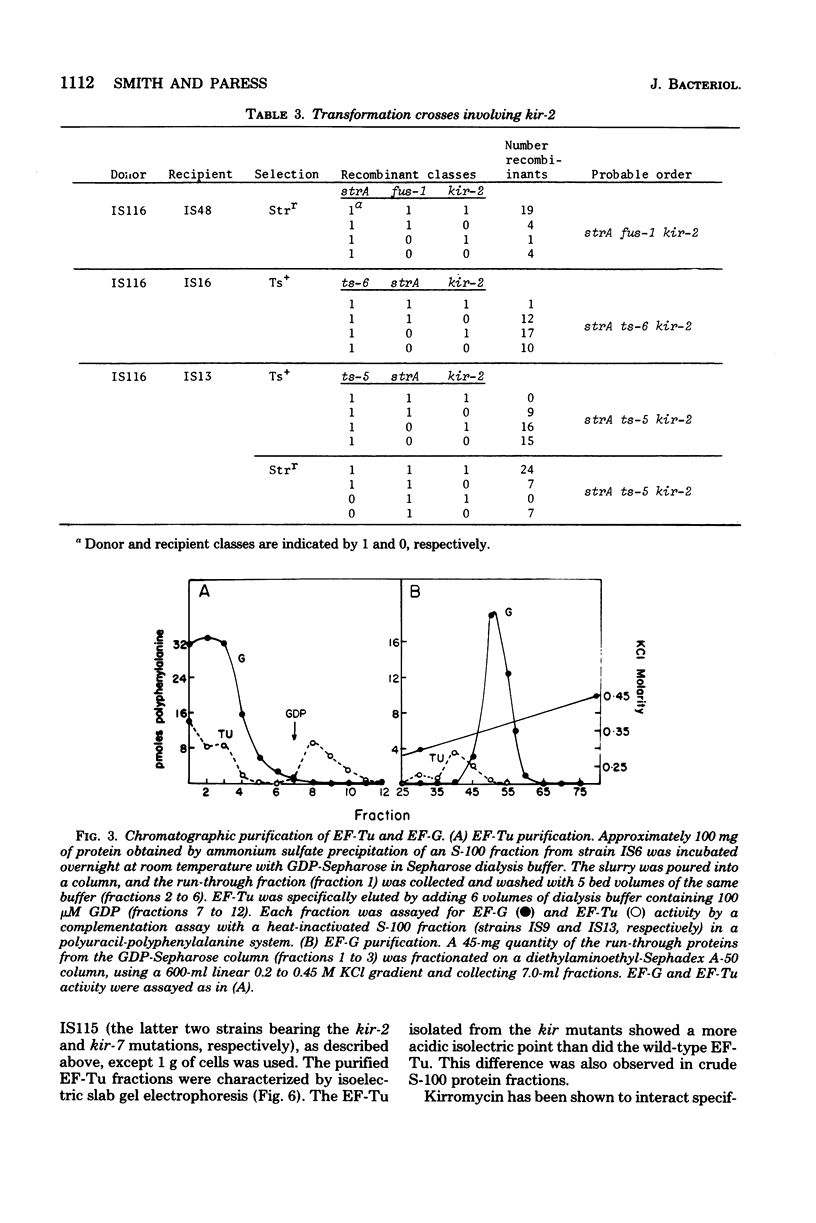
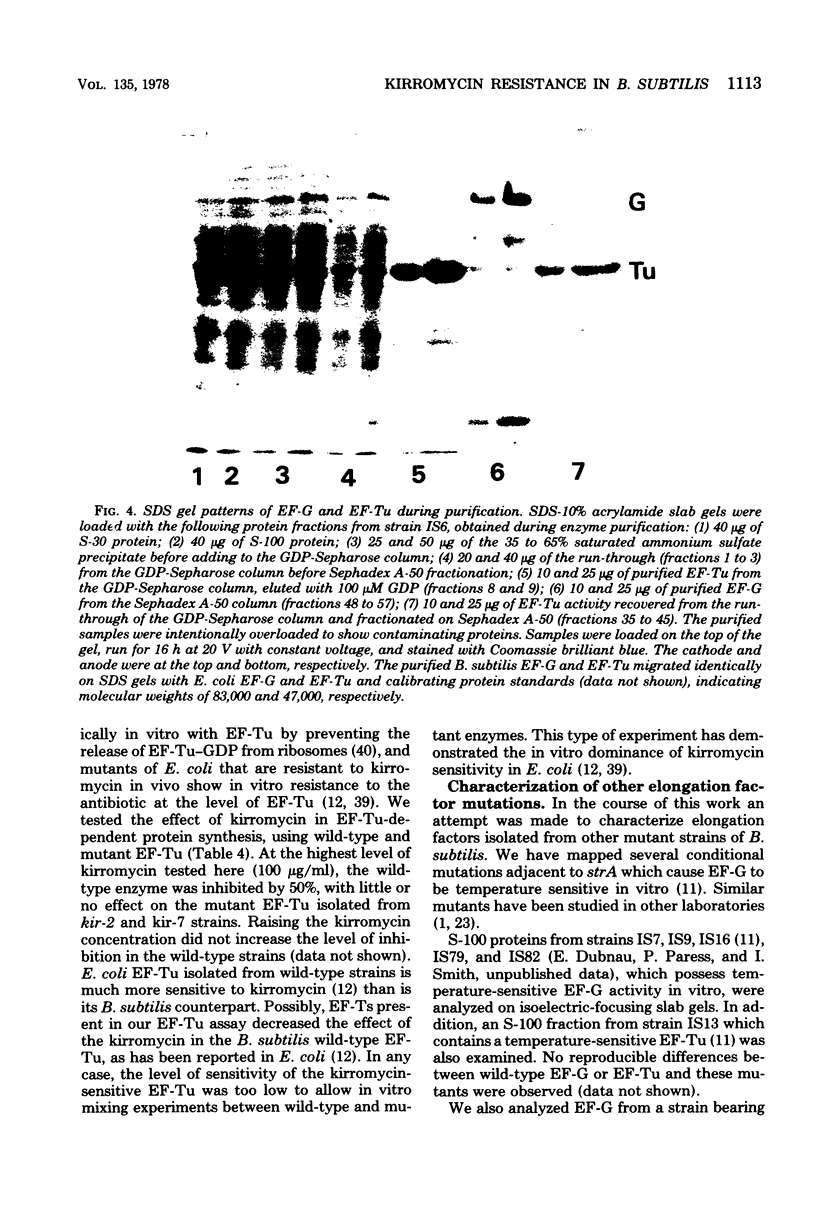
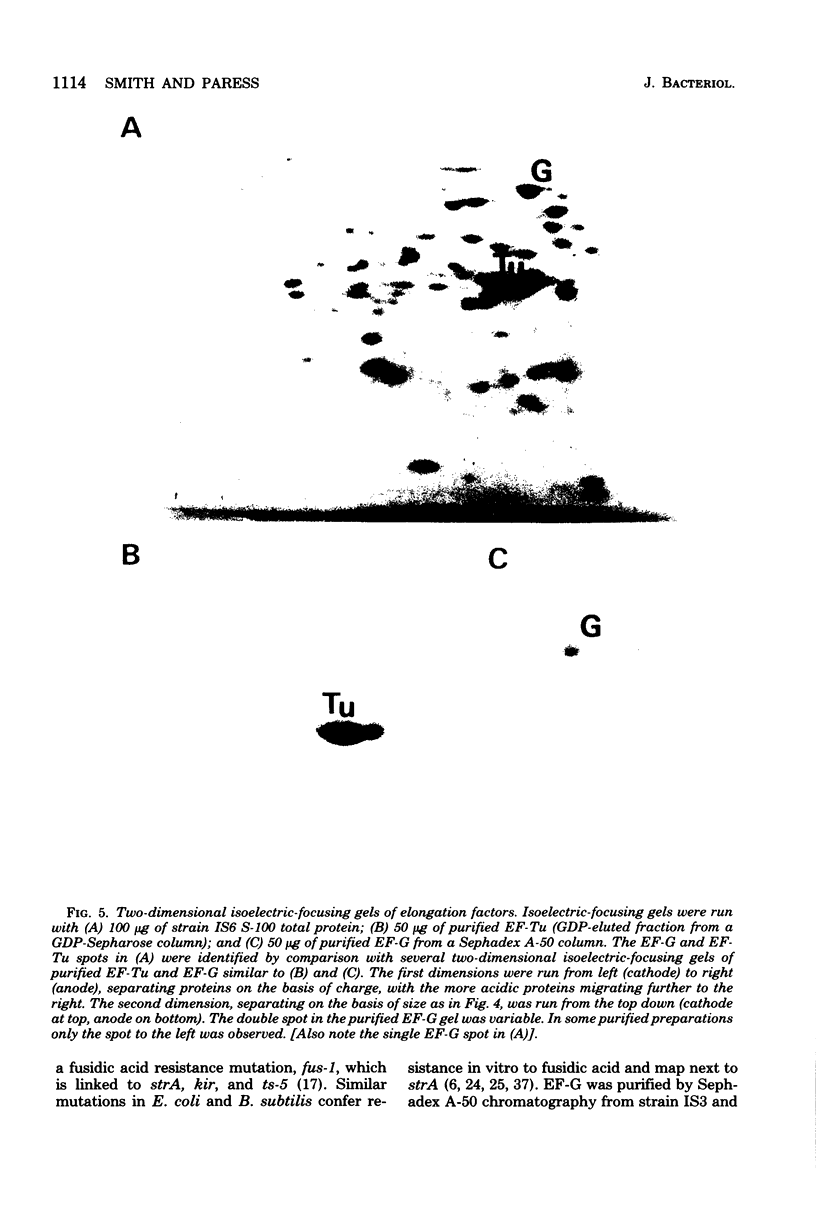
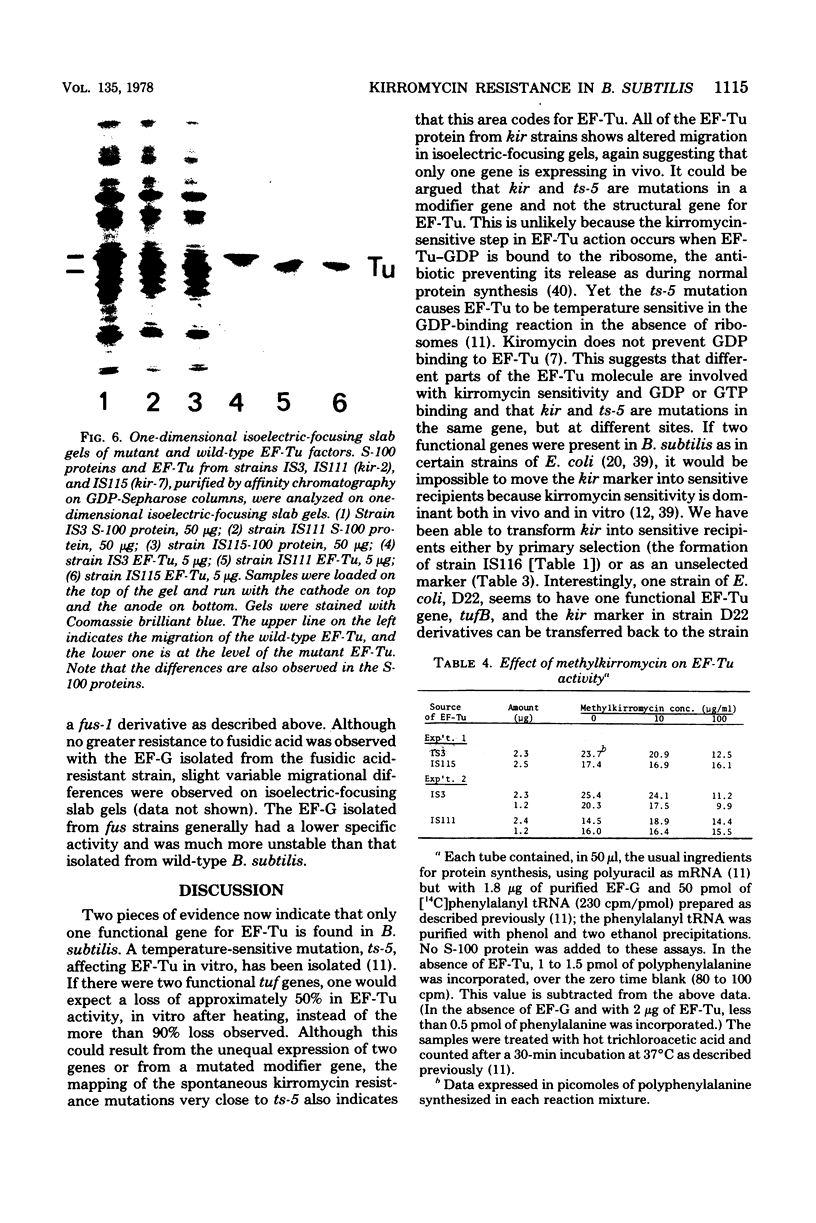
Spontaneous mutations causing resistance to the EF-Tu-specific antibiotic kirromycin have been isolated and mapped in Bacillus subtilis. Three-factor transductional and transformational crosses have placed the kir locus proximal to ery-1 and distal to strA (rpsL) and several mutations affecting elongation factors EF-G and EF-Tu, in the order: cysA strA [fus-1/ts-6(EF-G)] [ts-5(EF-Tu)] kir ery-1 spcA. Purified EF-Tu from mutant strains is more resistant to kirromycin as measured by in vitro protein synthesis and also shows a more acidic isoelectric point than wild-type EF-Tu. This indicates that the kir locus is the genetic determinant (tuf) for EF-Tu and that there is a single active gene for this enzyme in B. subtilis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharonowitz Y., Ron E. Z. A temperature sensitive mutant in Bacillus subtilis with an altered elongation factor G. Mol Gen Genet. 1972;119(2):131–138. doi: 10.1007/BF00269132. [DOI] [PubMed] [Google Scholar]
- Ames G. F., Nikaido K. Two-dimensional gel electrophoresis of membrane proteins. Biochemistry. 1976 Feb 10;15(3):616–623. doi: 10.1021/bi00648a026. [DOI] [PubMed] [Google Scholar]
- Arai K. I., Kawakita M., Kaziro Y. Studies on polypeptide elongation factors from Escherichia coli. II. Purification of factors Tu-guanosine diphosphate, Ts, and Tu-Ts, and crystallization of Tu-guanosine diphosphate and Tu-Ts. J Biol Chem. 1972 Nov 10;247(21):7029–7037. [PubMed] [Google Scholar]
- Bernardi A., Leder P. Protein biosynthesis in Escherichia coli. Purification and characteristics of a mutant G factor. J Biol Chem. 1970 Sep 10;245(17):4263–4268. [PubMed] [Google Scholar]
- Chinali G., Wolf H., Parmeggiani A. Effect of kirromycin on elongation factor Tu. Location of the catalytic center for ribosome-elongation-factor-Tu GTPase activity on the elongation factor. Eur J Biochem. 1977 May 2;75(1):55–65. doi: 10.1111/j.1432-1033.1977.tb11503.x. [DOI] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Davidoff-Abelson R., Smith I. Transformation and transduction in Bacillus subtilis: evidence for separate modes of recombinant formation. J Mol Biol. 1969 Oct 28;45(2):155–179. doi: 10.1016/0022-2836(69)90097-7. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Goldthwaite C., Smith I., Marmur J. Genetic mapping in Bacillus subtilis. J Mol Biol. 1967 Jul 14;27(1):163–185. doi: 10.1016/0022-2836(67)90358-0. [DOI] [PubMed] [Google Scholar]
- Dubnau E., Pifko S., Sloma A., Cabane K., Smith I. Conditional mutations in the translational apparatus of Bacillus subtils. Mol Gen Genet. 1976 Aug 10;147(1):1–12. doi: 10.1007/BF00337929. [DOI] [PubMed] [Google Scholar]
- Fischer E., Wolf H., Hantke K., Parmeggiani A. Elongation factor Tu resistant to kirromycin in an Escherichia coli mutant altered in both tuf genes. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4341–4345. doi: 10.1073/pnas.74.10.4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gast W. H., Kabsch W., Wittinghofer A., Leberman R. Crystals of a large tryptic peptide (fragment A) of elongation factor EF-Tu from Escherichia coli. FEBS Lett. 1977 Feb 15;74(1):88–90. doi: 10.1016/0014-5793(77)80759-x. [DOI] [PubMed] [Google Scholar]
- Geiser M., Gordon J. Two chromatographically separable forms of Escherichia coli elongation factor Tu. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1140–1144. doi: 10.1073/pnas.75.3.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldthwaite C., Dubnau D., Smith I. Genetic mapping of antibiotic resistance in markers Bacillus subtilis. Proc Natl Acad Sci U S A. 1970 Jan;65(1):96–103. doi: 10.1073/pnas.65.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldthwaite C., Smith I. Genetic mapping of aminoglycoside and fusidic acid resistant mutations in Bacillus subtilis. Mol Gen Genet. 1972;114(3):181–189. doi: 10.1007/BF01788887. [DOI] [PubMed] [Google Scholar]
- Goldthwaite C., Smith I. Physiological characterization of antibiotic resistant mutants of Bacillus subtilis. Mol Gen Genet. 1972;114(3):190–204. doi: 10.1007/BF01788888. [DOI] [PubMed] [Google Scholar]
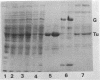
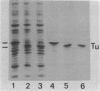
- Jacobson G. R., Rosenbusch J. P. Affinity purification of elongation factors Tu and Ts. FEBS Lett. 1977 Jul 1;79(1):8–10. doi: 10.1016/0014-5793(77)80338-4. [DOI] [PubMed] [Google Scholar]
- Jacobson G. R., Rosenbusch J. P. Limited proteolysis of elongation factor Tu from Escherichia coli, Multiple intermediates. Eur J Biochem. 1977 Jul 15;77(2):409–417. doi: 10.1111/j.1432-1033.1977.tb11681.x. [DOI] [PubMed] [Google Scholar]
- Jaskunas S. R., Fallon A. M., Nomura M. Identification and organization of ribosomal protein genes of Escherichia coli carried by lambdafus2 transducing phage. J Biol Chem. 1977 Oct 25;252(20):7323–7336. [PubMed] [Google Scholar]
- Jaskunas S. R., Lindahl L., Nomura M. Identification of two copies of the gene for the elongation factor EF-Tu in E. coli. Nature. 1975 Oct 9;257(5526):458–462. doi: 10.1038/257458a0. [DOI] [PubMed] [Google Scholar]
- Kaziro Y., Inoue-Yokosawa N., Kawakita M. Studies on polypeptide elongation factor from E. coli. I. Crystalline factor G. J Biochem. 1972 Oct;72(4):853–863. doi: 10.1093/oxfordjournals.jbchem.a129980. [DOI] [PubMed] [Google Scholar]
- Kimura A., Muto A., Osawa S. Control of stable RNA synthesis in a temperature-sensitive mutant of elongation factor G of Bacillus subtilis. Mol Gen Genet. 1974 May 31;130(3):203–214. doi: 10.1007/BF00268800. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Kobayashi K., Kobayashi Y. Isolation and characterization of fusidic acid-resistant, sporulation-defective mutants of Bacillus subtilis. J Bacteriol. 1977 Oct;132(1):262–269. doi: 10.1128/jb.132.1.262-269.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwano M., Schlessinger D. G factor mutants of Escherichia coli: map location and properties. Biochem Biophys Res Commun. 1971 Feb 5;42(3):441–444. doi: 10.1016/0006-291x(71)90390-1. [DOI] [PubMed] [Google Scholar]
- Laursen R. A., Nagarkatti S., Miller D. L. Amino acid sequence of elongation factor Tu. Characterization and alignment of the cyanogen bromide fragments and location of the cysteine residues. FEBS Lett. 1977 Aug 1;80(1):103–106. doi: 10.1016/0014-5793(77)80416-x. [DOI] [PubMed] [Google Scholar]
- Maehr H., Leach M., Yarmchuk L., Stempel A. Antibiotic X-5108. V. Structures of antibiotic X-5108 and mocimycin. J Am Chem Soc. 1973 Dec 12;95(25):8449–8450. doi: 10.1021/ja00806a043. [DOI] [PubMed] [Google Scholar]
- Morell P., Smith I., Dubnau D., Marmur J. Isolation and characterization of low molecular weight ribonucleic acid species from Bacillus subtilis. Biochemistry. 1967 Jan;6(1):258–265. doi: 10.1021/bi00853a040. [DOI] [PubMed] [Google Scholar]
- Nomura M. Organization of bacterial genes for ribosomal components: studies using novel approaches. Cell. 1976 Dec;9(4 Pt 2):633–644. doi: 10.1016/0092-8674(76)90127-6. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Orrego C., Kerjan P., Manca de Nadra M. C., Szulmajster J. Ribonucleic acid polymerase in a thermosensitive sporulation mutant (ts-4) of Bacillus subtilis. J Bacteriol. 1973 Nov;116(2):636–647. doi: 10.1128/jb.116.2.636-647.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa S. Gene locus of a 30s ribosomal protein S20 of Bacillus subtilis. Mol Gen Genet. 1976 Feb 27;144(1):49–51. doi: 10.1007/BF00277303. [DOI] [PubMed] [Google Scholar]
- Osawa S., Takata R., Tanaka K., Tamaki M. Chloramphenicol resistant mutants of Bacillus subtilis. Mol Gen Genet. 1973 Dec 20;127(2):163–173. doi: 10.1007/BF00333664. [DOI] [PubMed] [Google Scholar]
- Smith I., Smith H. Location of the SPO2 attachment site and the bryamycin resistance marker on the Bacillus subtilis chromosome. J Bacteriol. 1973 Jun;114(3):1138–1142. doi: 10.1128/jb.114.3.1138-1142.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Tanaka N., Kawano G., Kinoshita T. Chromosomal location of a fusidic acid resistant marker in Escherichia coli. Biochem Biophys Res Commun. 1971 Feb 5;42(3):564–567. doi: 10.1016/0006-291x(71)90408-6. [DOI] [PubMed] [Google Scholar]
- Trowsdale J., Sheflett M., Hoch J. A. New cluster of ribosomal genes in Bacillus subtilis with regulatory role in sporulation. Nature. 1978 Mar 9;272(5649):179–181. doi: 10.1038/272179a0. [DOI] [PubMed] [Google Scholar]
- Wolf H., Chinali G., Parmeggiani A. Mechanism of the inhibition of protein synthesis by kirromycin. Role of elongation factor Tu and ribosomes. Eur J Biochem. 1977 May 2;75(1):67–75. doi: 10.1111/j.1432-1033.1977.tb11504.x. [DOI] [PubMed] [Google Scholar]
- van de Klundert J. A., den Turk E., Borman A. H., van der Meide P. H., Bosch L. Isolation and characterization of a mocimycin resistant mutant of Escherichia coli with an altered elongation factor EF-Tu. FEBS Lett. 1977 Sep 15;81(2):303–307. doi: 10.1016/0014-5793(77)80540-1. [DOI] [PubMed] [Google Scholar]