Abstract
Objective
To characterize further the relationship between smoking history and Parkinson disease (PD) risk by considering temporal and qualitative features of smoking exposure, including duration, average intensity, and recentness, as well as the relative importance of smoking during different periods of life.
Methods
We prospectively assessed incident PD from 1992 to 2001 among 79,977 women and 63,348 men participating in the Cancer Prevention Study II Nutrition Cohort, according to their cigarette smoking status and lifetime smoking histories.
Results
During follow-up, 413 participants had definite or probable PD confirmed by their treating neurologists or medical record review. Compared with never smokers, former smokers had a relative risk (RR) of 0.78 (95% CI 0.64 to 0.95) and current smokers had an RR of 0.27 (95% CI 0.13 to 0.56). On average, participants with more years smoked, more cigarettes per day, older age at quitting smoking, and fewer years since quitting smoking had lower PD risk. The relative risks and trends did not vary significantly by sex. The cumulative incidence of PD was lowest among participants who quit smoking at later ages. A 30% to 60% decreased risk of PD was apparent for smoking as early as 15 to 24 years before symptom onset, but not for smoking 25 or more years before onset.
Conclusions
The lower risk of Parkinson disease among current and former smokers varied with smoking duration, intensity, and recentness. The dependence of this association on the timing of smoking during life is consistent with a biologic effect.
The lower risk of Parkinson disease (PD) among current and former smokers is documented in numerous retrospective studies but in only a few prospective investigations.1–3 Few groups have published results from studies in women,2 and the relative importance of smoking duration, total lifetime dose, average intensity, age at quitting, years since quitting, and smoking during different periods of life remains uncertain. Clarifying these issues would provide additional evidence for or against the association with smoking being causal. If it is causal, screening tobacco constituents for neuroprotective effects would be important. Here we present results on the association between cigarette smoking and incident PD from the Cancer Prevention Study II Nutrition Cohort, a large prospective study of women and men with detailed lifetime smoking histories.
Methods
Study participants
The participants in this study are members of the Cancer Prevention Study II Nutrition Cohort, a prospective study of 97,786 women and 86,404 men in the United States.4 The Nutrition Cohort is a subset of the Cancer Prevention Study II, which began in 1982.5 Participants joined the Nutrition Cohort by responding to a mailed baseline questionnaire in 1992 and completed subsequent questionnaires in 1997, 1999, and 2001. The 2001 questionnaire included a question about the lifetime occurrence of PD. Our cohort for analysis comprised 79,977 women and 63,348 men who returned the 2001 questionnaire and had no PD symptoms or suspected PD at baseline in 1992. Participants were followed from baseline until the onset of PD symptoms or the end of follow-up on September 30, 2001, whichever occurred first. The Human Subjects Committee at the Harvard School of Public Health and Institutional Review Board at Emory University approved the research protocol.
Exposure assessment
Participants reported their cigarette smoking status (never, former, or current) and smoking exposure history in 1992 and updated their smoking status in 1997. We characterized former smokers by their age at starting smoking, number of years smoked, number of pack-years smoked (years smoked times number of packs per day), number of cigarettes smoked per day, age at quitting smoking, and number of years since quitting smoking. We evaluated potential confounding by several variables that could have affected the association between smoking and PD, including age, sex, race, coffee drinking, alcohol use, nonsteroidal anti-inflammatory drug use, supplementary vitamin intake, pesticide exposure, education, physical activity, and body mass index. These variables were ascertained at baseline in 1992, except for race, coffee drinking, pesticide exposure, education, and height (for body mass index calculation), which were collected through the 1982 questionnaire of the Cancer Prevention Study II.
Case ascertainment
PD cases were first identified by self-report in the 2001 questionnaire and then followed for diagnostic confirmation, as previously described.6 Briefly, cases were confirmed either if the treating neurologists (or internists if the neurologists did not respond) reported a diagnosis of clinically definite or probable PD, or if there was evidence in the medical records of at least two of four cardinal signs (with one being rest tremor or bradykinesia), a progressive course, and the absence of unresponsiveness to L-dopa or other features suggesting an alternative diagnosis, as assessed by a movement disorders specialist (M.A.S.) who reviewed the records blind to exposure. Of 840 participants who reported a diagnosis of PD at any time in the past, 677 provided informed consent for contacting the treating neurologists, and 588 were confirmed. We excluded 175 confirmed cases with symptom onset before the baseline survey, retaining 413 with symptom onset after baseline. Of these 413 incident cases, 67.6% were confirmed by the treating neurologists or movement disorders specialists, 21.1% by the review of medical records, and 11.4% by the treating internists or family physicians. Most analyses in this report are based on confirmed incident cases using the date of first symptoms of PD as the primary outcome. For estimation of the age- and sex-specific incidence of PD, we included as cases 160 additional participants who self-confirmed their PD diagnosis but did not permit access to their detailed medical information for confirmation of the diagnosis. Permitting release of medical information was not associated with baseline smoking status (χ2df = 2 = 3.2; p = 0.2).
Statistical analyses
We calculated sex– and age group–specific PD incidence rates (per 100,000 person-years) among never smokers and ever smokers by dividing the number of PD cases by the total number of person-years in each group. Using never smokers as the reference group, we calculated relative risks (RRs) of PD for former and current smokers, adjusting for sex and 5-year age groups by the Mantel–Haenszel method. We also calculated age- and sex-adjusted RRs and trend tests for former smokers by categories of age at starting smoking (<15, 15 to 19, 20 to 24, ≥ 25 years), years smoked (<10, 10 to 19, 20 to 29, ≥ 30), pack-years smoked (<10, 10 to 24, 25 to 44, ≥ 45), cigarettes per day (<10, 10 to 19, 20 to 29, ≥ 30), age at quitting smoking (<35, 35 to 44, 45 to 54, 55 to 64, ≥ 65), and years since quitting smoking (<10, 10 to 19, 20 to 29, ≥ 30). More than 37% of ever smokers reported one or more period of quitting for 6 months or longer. In secondary analyses, we therefore recalculated smoking duration by subtracting 6 months for each report of temporary quitting. We used Cox proportional hazards models stratified on age and sex to obtain RRs and trend tests adjusted for additional variables and to obtain mutual adjustments for pairs of smoking history variables. In addition to sex-adjusted RRs, we calculated RRs for women and men separately.
To determine how smoking during particular time periods of life was related to PD risk, we plotted the cumulative incidence of PD for women and men separately, through age 80 years, stratified by the age at quitting smoking. We also used a series of Cox proportional hazards models to estimate the RRs for smoking during specific 10-year intervals before PD symptom onset. In the first model, we focused on the interval 0 to 9 years before PD symptom onset, and in each subsequent model, we shifted the interval of interest 5 years earlier (i.e., 5 to 14 years before, 10 to 19 years before, 15 to 24 years before, and so on until 35 to 44 years before). In each model, the RR for smoking during the interval of interest was adjusted for all previous and subsequent pack-years of smoking. Using this series of RRs, we examined the differences in PD risk for very recent smoking vs smoking in the distant past, in the context of the lifetime smoking history.
Results
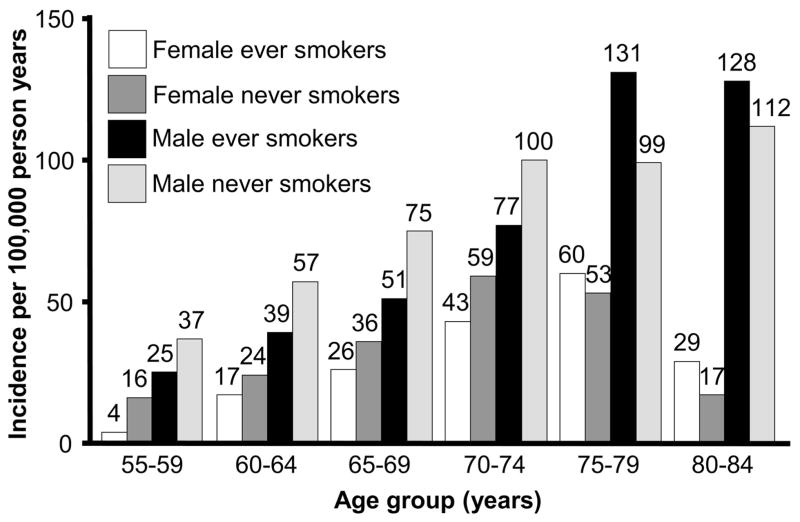
Baseline characteristics of the Nutrition Cohort have been reported previously.4 More than 98% of the participants were between ages 50 and 79 years at baseline, with more than half between ages 60 and 69 years. The majority of cohort participants were white (97%), most were currently married (89%), and many had graduated from college (38%). During follow-up, 413 participants, including 405 with valid baseline smoking data (142 women and 263 men), were confirmed to have clinically definite or probable PD with the onset of first symptoms occurring after the 1992 survey date and up to the study cutoff date. Confirmed cases were predominantly white (99%), and 48% were college graduates. The mean age at onset was 70.0 years in women and 70.5 years in men. Rest tremor was documented in 75% of cases, bradykinesia in 83%, rigidity in 73%, and postural instability in 42%. The cumulative incidence of PD through age 80 years was 0.6% in women and 1.2% in men. When we included the cases for which diagnostic confirmation could not be obtained, the estimated cumulative incidence through age 80 years was 0.9% in women and 1.7% in men. Age-specific incidence rates for PD were lower for ever smokers than for never smokers through age 74 years in both sexes (figure 1). Compared with never smokers, the risk of PD was 22% lower in former smokers and 73% lower in current smokers (table). These results were not substantially altered when we adjusted for race, coffee drinking, alcohol use, nonsteroidal anti-inflammatory drug use, supplementary vitamin intake, pesticide exposure, education, physical activity, and body mass index, and did not vary significantly by sex.
Figure 1.
Incidence of Parkinson disease by age group, sex, and smoking status in the Cancer Prevention Study II Nutrition Cohort, 1992 to 2001.
Table.
Relative risks of the onset of Parkinson disease according to smoking status in the Cancer Prevention Study II Nutrition Cohort, 1992 to 2001*
| Sex | Smoking status | No. of cases | Person-years | RR (95% CI) | p Trend† |
|---|---|---|---|---|---|
| Both sexes | Never smokers | 201 | 556,616 | 1.00 (reference) | |
| Former smokers | 196 | 572,353 | 0.78 (0.64–0.95) | ||
| Years of smoking | |||||
| <10 | 36 | 105,329 | 0.89 (0.62–1.27) | ||
| 10–19 | 62 | 140,122 | 1.08 (0.81–1.44) | ||
| 20–29 | 47 | 137,753 | 0.73 (0.52–1.02) | ||
| ≥ 30 | 45 | 167,718 | 0.56 (0.40–0.78) | 0.002 | |
| Cigarettes per day | |||||
| <10 | 53 | 136,239 | 1.05 (0.77–1.42) | ||
| 10–19 | 47 | 137,959 | 0.80 (0.58–1.10) | ||
| 20–29 | 58 | 167,006 | 0.74 (0.55–1.01) | ||
| ≥ 30 | 35 | 120,125 | 0.58 (0.39–0.84) | 0.009 | |
| Years since quitting | |||||
| ≥ 30 | 94 | 198,644 | 1.00 (0.78–1.28) | ||
| 20–29 | 58 | 145,319 | 0.93 (0.69–1.25) | ||
| 10–19 | 32 | 124,726 | 0.61 (0.42–0.90) | ||
| <10 | 11 | 88,886 | 0.33 (0.18–0.60) | 0.0004 | |
| Current smokers | 8 | 79,295 | 0.27 (0.13–0.56) | ||
| Women‡ | Never smokers | 94 | 372,976 | 1.00 (reference) | |
| Former smokers | 46 | 251,299 | 0.76 (0.53–1.07) | ||
| Years of smoking | |||||
| <10 | 12 | 54,883 | 1.00 (0.55–1.81) | ||
| 10–19 | 18 | 63,861 | 1.24 (0.75–2.05) | ||
| 20–29 | 5 | 55,089 | 0.37 (0.15–0.90) | ||
| ≥ 30 | 11 | 68,602 | 0.59 (0.31–1.10) | 0.02 | |
| Cigarettes per day | |||||
| <10 | 24 | 86,856 | 1.12 (0.72–1.76) | ||
| 10–19 | 11 | 68,911 | 0.64 (0.34–1.20) | ||
| 20–29 | 10 | 59,797 | 0.70 (0.37–1.34) | ||
| ≥ 30 | 1 | 30,926 | 0.14 (0.02–0.99) | 0.01 | |
| Years since quitting | |||||
| ≥ 30 | 24 | 80,263 | 1.12 (0.71–1.75) | ||
| 20–29 | 13 | 61,722 | 0.92 (0.52–1.63) | ||
| 10–19 | 6 | 57,147 | 0.44 (0.19–1.01) | ||
| <10 | 3 | 45,452 | 0.29 (0.09–0.91) | 0.007 | |
| Current smokers | 2 | 44,031 | 0.20 (0.05–0.83) | ||
| Men§ | Never smokers | 107 | 183,640 | 1.00 (reference) | |
| Former smokers | 150 | 321,055 | 0.79 (0.62–1.02) | ||
| Years of smoking | |||||
| <10 | 24 | 50,446 | 0.83 (0.54–1.30) | ||
| 10–19 | 44 | 76,260 | 1.02 (0.72–1.45) | ||
| 20–29 | 42 | 82,664 | 0.86 (0.60–1.23) | ||
| ≥ 30 | 34 | 99,116 | 0.54 (0.37–0.81) | 0.02 | |
| Cigarettes per day | |||||
| <10 | 29 | 49,383 | 0.99 (0.65–1.49) | ||
| 10–19 | 36 | 69,048 | 0.87 (0.60–1.27) | ||
| 20–29 | 48 | 107,209 | 0.76 (0.54–1.07) | ||
| ≥ 30 | 34 | 89,199 | 0.66 (0.45–0.97) | 0.11 | |
| Years since quitting | |||||
| ≥ 30 | 70 | 118,381 | 0.95 (0.70–1.29) | ||
| 20–29 | 45 | 83,597 | 0.93 (0.66–1.32) | ||
| 10–19 | 26 | 67,578 | 0.69 (0.45–1.05) | ||
| <10 | 8 | 43,434 | 0.35 (0.17–0.71) | 0.01 | |
| Current smokers | 6 | 35,263 | 0.31 (0.14–0.71) | ||
Adjusted for age in 5-year categories; results for both sexes combined also adjusted for sex.
Trend tests do not include never smokers.
Smoking status was missing for 10,473 person-years, including 5 cases. Additionally, for former smokers, years of smoking was missing for 8,864 person-years, cigarettes per day for 4,809 person-years, and years since quitting for 6,715 person-years.
Smoking status was missing for 4,710 person-years, including 3 cases. Additionally, for former smokers, years of smoking was missing for 12,569 person-years, including 6 cases; cigarettes per day for 6,216 person-years, including 3 cases; and years since quitting for 8,064 person-years, including 1 case.
RR = relative risk.
In age- and sex-adjusted analyses, more years smoked, more cigarettes per day, and more recent smoking (fewer years since quitting) were all associated with lower PD risk. (table) The results for smoking duration did not change appreciably when we accounted for periods of temporarily quitting smoking for 6 months or longer. More pack-years smoked also predicted a lower risk of PD (≥ 45 pack-years vs never smoking, RR 0.55; 95% CI 0.35 to 0.86; ptrend across pack-year categories = 0.0008). Sex-specific age-adjusted models and models with additional covariate adjustments produced similar results. For former smokers, when we included cigarettes per day and years smoked in the same model, the trend for cigarettes per day was no longer significant, but the trend for years smoked was still significant. Similarly, when we modeled cigarettes per day and years since quitting together, the trend for cigarettes per day nearly lost significance, whereas the trend for age at quitting remained significant.
When stratified by the age at quitting smoking, the cumulative incidence of PD through age 80 years was lower for participants who quit smoking at later ages, in both women (figure 2) and men (figure 3). As shown in figure 4, the reduced PD risk among smokers was strongest for participants who smoked during the interval 0 to 9 years before symptom onset, independent of pack-years smoked in earlier intervals (smoked during interval vs did not smoke during interval, RR 0.42; 95% CI 0.26 to 0.68). Participants who smoked during the interval 0 to 9 years before symptom onset included those who smoked up to the onset of symptoms (current smokers) or nearly so (recent quitters). The risk reduction persisted for smoking as early as 15 to 24 years before symptom onset, with adjustment for previous and more recent pack-years smoked, but was not apparent for smoking during earlier intervals (figure 4). Results including as cases individuals who reported a PD diagnosis but did not give permission to review their medical records were virtually identical.
Figure 2.
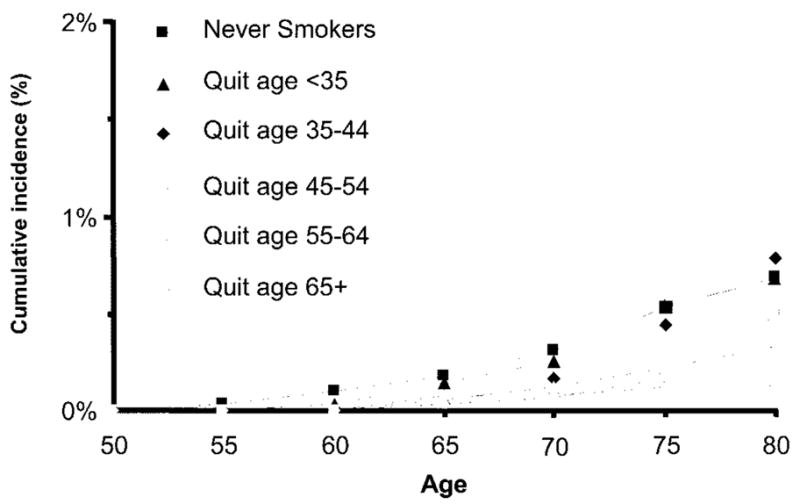
Cumulative incidence of Parkinson disease through age 80 years by age at quitting smoking for women in the Cancer Prevention Study II Nutrition Cohort, 1992 to 2001. The category “quit age 65+” includes current smokers.
Figure 3.
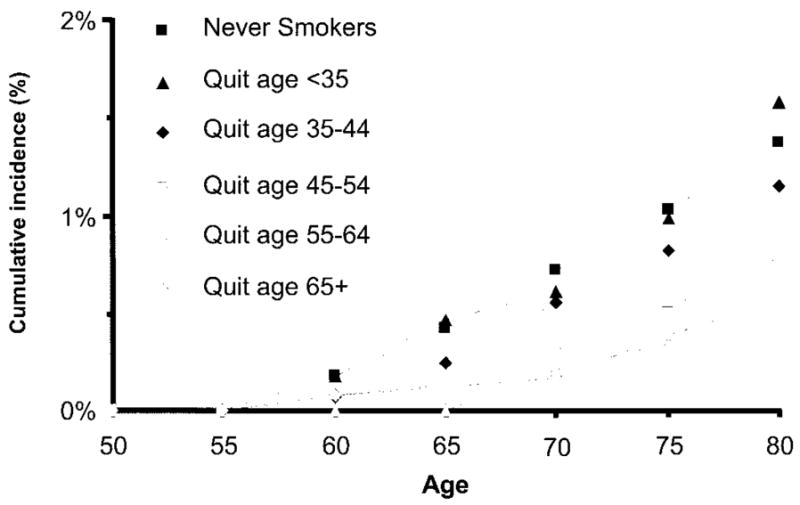
Cumulative incidence of Parkinson disease through age 80 years by age at quitting smoking for men in the Cancer Prevention Study II Nutrition Cohort, 1992 to 2001. The category “quit age 65+” includes current smokers.
Figure 4.
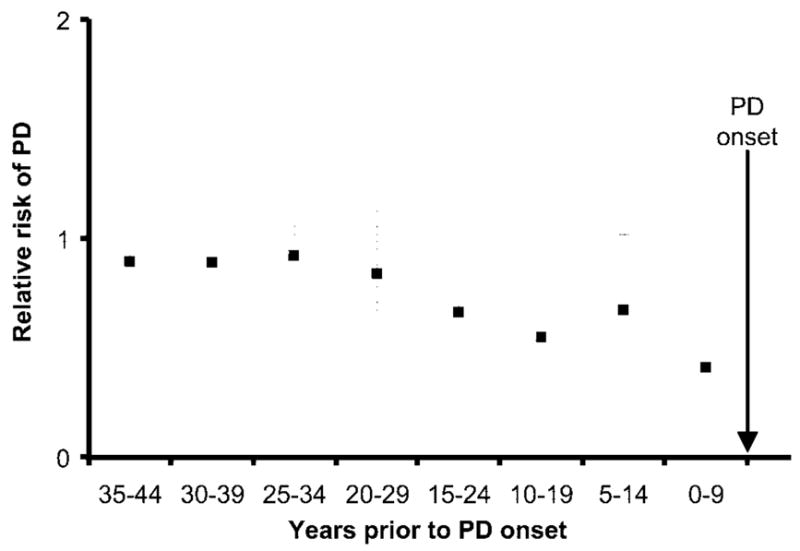
Relative risks of Parkinson disease (PD) for smoking during 10-year periods before disease onset in the Cancer Prevention Study II Nutrition Cohort, 1992 to 2001. The relative risk for smoking during each period was adjusted for age, sex, and pack-years of smoking occurring before and after that period.
Discussion
We conducted a prospective study of cigarette smoking and PD in a large population of women and men in the United States. The risk of PD was lowest among participants with the longest duration of smoking, the greatest lifetime dose of smoking, the highest average daily intensity of smoking, and the most recent smoking. The duration and recentness of smoking were more relevant to PD risk than the average daily amount of smoking. Examination of PD risk according to the age at quitting smoking and smoking behavior during 10-year intervals before symptom onset showed that the timing of smoking during life was an important component of the relationship between smoking and PD. Smoking up to or nearly up to the onset of symptoms was most strongly associated with decreased PD risk, and smoking as early as 20 years in the past was also associated with lower PD risk after adjusting for earlier and more recent smoking. Overall, women had a lower incidence of PD than men, but both sexes had similar RRs and trends for smoking history variables.
Several aspects of our study support the validity of the results. We examined a large population, assessed lifetime smoking histories before symptom onset, identified and rigorously confirmed incident PD cases, and considered adjustments for multiple risk factors. We also recognize limitations of our data. Our results could have been influenced by misdiagnosis or underdiagnosis of PD. Some level of diagnostic error by the treating physicians, who confirmed the majority of the cases, is probably inevitable. However, the accuracy of PD diagnosis has been reported to be 90% by neurologists7 and 99% by movement disorders specialists.8 Also, because some participants reporting PD did not permit us to contact their neurologists or access their medical records, our confirmation of PD cases was incomplete. However, sensitivity analyses including the participants who did not release medical information as cases in our models did not materially change the association between smoking and PD risk, suggesting that bias from this source was minimal.
Studies of PD mortality or prevalent PD cases have been criticized as being biased toward spuriously showing that smokers have a reduced risk of PD due to various methodologic problems. However, most potential biases, including increased early mortality among smokers, should not influence the results of prospective cohort studies based on incident PD cases.1,2 Further, mortality among PD patients has been shown to be similar for smokers and non-smokers.9 Debate continues over whether the reduced risk of PD among smokers is due to a protective effect of smoking.2,10,11 Some researchers support the alternative that a low novelty-seeking or low sensation-seeking personality, appearing early in life as a correlate of PD, protects against developing or maintaining a smoking habit, and thus the apparent protective effect of smoking against PD arises.12,13 However, in a recent study, although PD cases had higher sensation seeking scores than controls, adjusting for sensation seeking barely influenced the strong, significant association between higher levels of smoking and lower risk of PD.14 Another issue regarding causality, as discussed by others, is whether protection against PD by smoking persists at later ages.15 Our results suggest that smoking may not reduce the risk of PD among older subjects (i.e., age 75 years and older), but because of small numbers, a longer follow-up will be needed to confirm this finding.
A biologic explanation of protection against PD by smoking should accommodate dose dependency, duration dependency, and a decline in protection after quitting smoking.10,16 Possible mechanisms and candidate molecules have been reviewed,17,18 but evidence supporting any specific mechanism or chemical remains inconclusive. The observation that smokeless tobacco users also have a lower risk of PD19,20 suggests that the most likely candidates are not compounds generated by combustion, but rather constituents of the tobacco leaves.
Acknowledgments
The authors thank the participants in the Cancer Prevention Study II Nutrition Cohort.
Supported by a grant to Dr. Ascherio from the National Institute of Neurologic Disorders and Stroke (NS048517) and in part by the Intramural Research Program of the NIH, the National Institute of Environmental Health Sciences.
Footnotes
Disclosure: The authors report no conflicts of interest.
References
- 1.Morens DM, Grandinetti A, Davis JW, Ross GW, White LR, Reed D. Evidence against the operation of selective mortality in explaining the association between cigarette smoking and reduced occurrence of idiopathic Parkinson disease. Am J Epidemiol. 1996;144:400–404. doi: 10.1093/oxfordjournals.aje.a008941. [DOI] [PubMed] [Google Scholar]
- 2.Hernan MA, Zhang SM, Rueda-deCastro AM, Colditz GA, Speizer FE, Ascherio A. Cigarette smoking and the incidence of Parkinson’s disease in two prospective studies. Ann Neurol. 2001;50:780–786. doi: 10.1002/ana.10028. [DOI] [PubMed] [Google Scholar]
- 3.Paganini-Hill A. Risk factors for Parkinson’s disease: the Leisure World Cohort Study. Neuroepidemiology. 2001;20:118–124. doi: 10.1159/000054770. [DOI] [PubMed] [Google Scholar]
- 4.Calle EE, Rodriguez C, Jacobs EJ, et al. The American Cancer Society Cancer Prevention Study II Nutrition Cohort: rationale, study design, and baseline characteristics. Cancer. 2002;94:2490–2501. doi: 10.1002/cncr.101970. [DOI] [PubMed] [Google Scholar]
- 5.Garfinkel L. Selection, follow-up, and analysis in the American Cancer Society prospective studies. Natl Cancer Inst Monogr. 1985;67:49–52. [PubMed] [Google Scholar]
- 6.Ascherio A, Chen H, Weisskopf MG, et al. Pesticide exposure and risk for Parkinson’s disease. Ann Neurol. 2006;60:197–203. doi: 10.1002/ana.20904. [DOI] [PubMed] [Google Scholar]
- 7.Hughes AJ, Daniel SE, Lees AJ. Improved accuracy of clinical diagnosis of Lewy body Parkinson’s disease. Neurology. 2001;57:1497–1499. doi: 10.1212/wnl.57.8.1497. [DOI] [PubMed] [Google Scholar]
- 8.Hughes AJ, Daniel SE, Ben-Shlomo Y, Lees AJ. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain. 2002;125:861–870. doi: 10.1093/brain/awf080. [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Zhang SM, Schwarzschild MA, Hernan MA, Ascherio A. Survival of Parkinson’s disease patients in a large prospective cohort of male health professionals. Mov Disord. 2006;21:1002–1007. doi: 10.1002/mds.20881. [DOI] [PubMed] [Google Scholar]
- 10.Morens DM, Grandinetti A, Reed D, White LR, Ross GW. Cigarette smoking and protection from Parkinson’s disease: false association or etiologic clue? Neurology. 1995;45:1041–1051. doi: 10.1212/wnl.45.6.1041. [DOI] [PubMed] [Google Scholar]
- 11.Marder K, Logroscino G. The ever-stimulating association of smoking and coffee and Parkinson’s disease. Ann Neurol. 2002;52:261–262. doi: 10.1002/ana.10315. [DOI] [PubMed] [Google Scholar]
- 12.Shahi GS, Moochhala SM. Smoking and Parkinson’s disease: a new perspective. Rev Environ Health. 1991;9:123–136. doi: 10.1515/reveh.1991.9.3.123. [DOI] [PubMed] [Google Scholar]
- 13.Menza MA, Golbe LI, Cody RA, Forman NE. Dopamine-related personality traits in Parkinson’s disease. Neurology. 1993;43:505–508. doi: 10.1212/wnl.43.3_part_1.505. [DOI] [PubMed] [Google Scholar]
- 14.Evans AH, Lawrence AD, Potts J, et al. Relationship between impulsive sensation seeking traits, smoking, alcohol and caffeine intake, and Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2006;77:317–321. doi: 10.1136/jnnp.2005.065417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tzourio C, Rocca WA, Breteler MM, et al. Smoking and Parkinson’s disease: an age-dependent risk effect? The EUROPARKINSON Study Group Neurology. 1997;49:1267–1272. doi: 10.1212/wnl.49.5.1267. [DOI] [PubMed] [Google Scholar]
- 16.Logroscino G. The role of early life environmental risk factors in Parkinson disease: what is the evidence? Environ Health Perspect. 2005;113:1234–1238. doi: 10.1289/ehp.7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ross GW, Petrovitch H. Current evidence for neuroprotective effects of nicotine and caffeine against Parkinson’s disease. Drugs Aging. 2001;18:797–806. doi: 10.2165/00002512-200118110-00001. [DOI] [PubMed] [Google Scholar]
- 18.Quik M. Smoking, nicotine and Parkinson’s disease. Trends Neurosci. 2004;27:561–568. doi: 10.1016/j.tins.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Benedetti MD, Bower JH, Maraganore DM, et al. Smoking, alcohol, and coffee consumption preceding Parkinson’s disease: a case-control study. Neurology. 2000;55:1350–1358. doi: 10.1212/wnl.55.9.1350. [DOI] [PubMed] [Google Scholar]
- 20.O’Reilly EJ, McCullough ML, Chao A, et al. Smokeless tobacco use and the risk of Parkinson’s disease mortality. Mov Disord. 2005;20:1383–1384. doi: 10.1002/mds.20587. [DOI] [PubMed] [Google Scholar]