Human p40phox was expressed, purified and crystallized. Diffraction data were collected to a resolution of 3.0 Å.
Keywords: p40phox, NADPH oxidase
Abstract
p40phox is a cytosolic component of the phagocyte NADPH oxidase, which is responsible for production of the superoxide that kills invasive microorganisms. Full-length p40phox was expressed in Escherichia coli, purified and crystallized by the sitting-drop vapour-diffusion method at 293 K using polyethylene glycol 20 000 as a precipitant. Diffraction data were collected to 3.0 Å resolution at 100 K using synchrotron radiation. The crystal belongs to space group C2221, with unit-cell parameters a = 146.27, b = 189.81, c = 79.88 Å. This crystal was estimated to contain two or three protein molecules per asymmetric unit from the acceptable range of volume-to-weight ratio values.
1. Introduction
The phagocyte NADPH oxidase plays an important role in killing invasive microorganisms by generating superoxide. NADPH oxidase is a multisubunit enzyme comprised of a membrane-bound cytochrome b 558 (p22phox and gp91phox), Rac and three cytosolic regulatory subunits, p47phox, p67phox and p40phox (Babior, 1999 ▶; Sumimoto et al., 2005 ▶). In the resting state, the cytosolic subunits Rac and cytochrome b 558 exist separately. When activated, the cytosolic subunits and Rac are tethered to the membrane, where they associate with cytochrome b 558 and form the active NADPH oxidase complex (Babior et al., 2002 ▶; Vignais, 2002 ▶; Sumimoto et al., 2005 ▶).
p40phox is a multidomain protein of 39 030 Da, consisting of PX, SH3 and PB1 domains (Wientjes et al., 1993 ▶; Ponting, 1996 ▶; Ito et al., 2001 ▶). The PX domain of p40phox specifically interacts with phosphatidylinositol 3-phosphate [PtdIns(3)P; Ago et al., 2001 ▶] and the PB1 domain of p40phox interacts with that of p67phox (Nakamura et al., 1998 ▶). It is known that p40phox enhances membrane translocation of p67phox and p47phox in stimulated cells, thereby facilitating superoxide generation (Kuribayashi et al., 2002 ▶). The binding ability of the PX domain of p40phox to PtdIns(3)P is thought to play an important role in this process. Although the structures of each of the domains of p40phox have already been determined (Bravo et al., 2001 ▶; Wilson et al., 2003 ▶; Massenet et al., 2005 ▶), its full-length structure has not been reported so far. In this report, we describe the expression, purification and crystallization of full-length p40phox.
2. Protein expression, purification and crystallization
Full-length (residues 1–339) human p40phox was expressed in Escherichia coli as described previously (Hashida et al., 2004 ▶). Briefly, full-length p40phox was cloned between the NcoI and EcoRI sites of pProEX HTb to express p40phox as a 6×His-tagged protein. The plasmid pT-Trx was a generous gift from Dr S. Ishii (Laboratory of Molecular Genetics, The Institute of Physical and Chemical Research, RIKEN, Japan; Yasukawa et al., 1995 ▶). These plasmids were co-transformed in E. coli BL21 (DE3) and overexpressed. The cells were disrupted by sonication at 277 K in PBS with 0.5 mM AEBSF. The protein was applied onto an Ni–NTA column (Qiagen) and was washed with 25 mM Tris–HCl pH 7.4, 500 mM NaCl and 5 mM imidazole. The bound protein was eluted with 25 mM Tris–HCl pH 7.4, 500 mM NaCl and 250 mM imidazole. Fractions containing proteins were purified on a Superdex 75 gel-filtration column (GE Healthcare) and eluted with 25 mM Tris–HCl pH 8.0, 150 mM NaCl. The amino-terminal His tag of p40phox was removed by incubation with TEV protease for 12 h at 298 K. This solution was applied onto a SourceQ column (GE Healthcare) and eluted using a gradient of 0–100 mM NaCl in running buffer (20 mM Tris–HCl pH 8.0). Further purification was carried out on a Superdex 75 gel-filtration column eluted with 25 mM Tris–HCl pH 8.0 and 150 mM NaCl. After addition of dithiothreitol (DTT) to a final concentration of 40 mM to prevent protein oxidization, the purified protein was concentrated to about 10 mg ml−1 for crystallization screening.
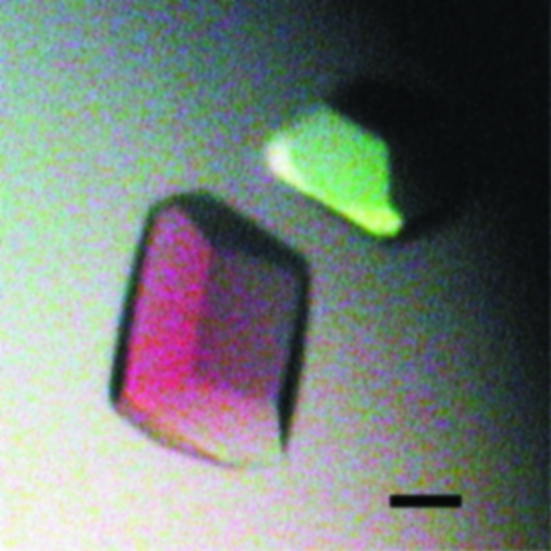
The crystallization of p40phox was performed using the sitting-drop vapour-diffusion method at 293 K. In each trial, a sitting drop of 1 µl purified protein solution was mixed with 1 µl reservoir solution and equilibrated against 100 µl reservoir solution. Initial screening was performed using Crystal Screen, Crystal Screen 2 (Hampton Research) and Wizard I and II (Emerald Biostructures), but no crystals were obtained. However, gelatinous precipitates, which are known to be a solid phase of the protein close to crystals (Bergfors, 2001 ▶), were observed in drops where sodium cacodylate was used as a buffer. Since sodium cacodylate seemed to be important, it was added to a final concentration of 10 mM to the purified p40phox solution as an additive and grid screening (PEG 20 000 concentrations of 5, 10, 15, 20, 25, 30% versus a pH range of 3.6 to 9.6) was performed. Small crystals of p40phox were obtained in reservoir solution containing 5% PEG 20 000, 200 mM Bis-Tris–HCl pH 6.5. To obtain larger crystals, a microseeding technique was applied. Small crystals were transferred into an Eppendorf tube containing a Seed Bead (Hampton Research) and 50 µl stabilization buffer (8% PEG 8000, 100 mM sodium citrate pH 6.4, 10 mM sodium cacodylate, 10 mM DTT) and were vortexed to produce microseeds. PEG 8000 was used in the stabilization solution instead of PEG 20 000 because it was less viscous and easier to handle. The sample was then briefly centrifuged and serially diluted (tenfold to 108-fold) in the same stabilization buffer. Microseeds were introduced by adding 0.2 µl of each diluted solution to 2 µl of newly prepared drops (consisting of 5% PEG 20 000, 200 mM Bis-Tris–HCl pH 6.5). Crystals appeared at a dilution rate of 103-fold to 104-fold and grew in 5 d to maximal dimensions of 0.3 × 0.2 × 0.2 mm (Fig. 1 ▶).
Figure 1.
Crystals of p40phox. The black scale bar is 100 µm in length.
3. Data collection and processing
Crystals were immersed in a cryoprotectant (25% glycerol, 200 mM acetate pH 5.6, 5% DMSO) for several seconds and then flash-cooled and maintained under nitrogen gas at 100 K during data collection. Diffraction data were collected using an ADSC Quantum 210 charge-coupled device detector on beamline PF-AR NW12 at the Photon Factory (Tsukuba, Japan). Data collection was performed with a total oscillation range of 180° with a step of 1.0° for each exposure time of 10 s and a wavelength of 1.00 Å. Diffraction data were processed using the HKL-2000 program suite (Otwinowski & Minor, 1997 ▶). The data-collection statistics are summarized in Table 1 ▶. The crystals belong to space group C2221, with unit-cell parameters a = 146.27, b = 189.81, c = 79.88 Å. The crystal diffracted to 3 Å, after which the resolution limit abruptly declined. The acceptable range of the volume-to-weight ratio (V M) values (Matthews, 1968 ▶) indicates that the crystal contains two (V M = 3.54 Å3 Da−1) or three (V M = 2.36 Å3 Da−1) protein molecules per asymmetric unit. A self-rotation function revealed no obvious NCS twofold or threefold axes. The structures of the three domains (PX, SH3 and PB1 domains) of p40phox have already been reported. Therefore, phase determination by the molecular-replacement method using these structures is now in progress.
Table 1. Data-collection statistics.
Values in parentheses refer to the highest resolution shell (3.11–3.00 Å).
| Resolution range (Å) | 50.0–3.00 |
| Observed reflections | 161611 |
| Unique reflections | 22612 |
| Completeness (%) | 99.5 (98.5) |
| Rmerge(I)† | 0.071 (0.479) |
| I/σ(I) | 20.3 (3.7) |
R
merge(I) = 
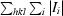 , where I
i is the intensity of the ith observation and 〈I〉 is the mean intensity.
, where I
i is the intensity of the ith observation and 〈I〉 is the mean intensity.
Acknowledgments
We thank the staff of beamline PF-AR NW12 for data-collection support. This work was supported by Grant-in-Aids for Scientific Research and National Project on Protein Structural and Functional Analyses from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- Ago, T., Takeya, R., Hiroaki, H., Kuribayashi, F., Ito, T., Kohda, D. & Sumimoto, H. (2001). Biochem. Biophys. Res. Commun.287, 733–738. [DOI] [PubMed] [Google Scholar]
- Babior, B. M. (1999). Blood, 93, 1464–1476. [PubMed] [Google Scholar]
- Babior, B. M., Lambeth, J. D. & Nauseef, W. (2002). Arch. Biochem. Biophys.397, 342–344. [DOI] [PubMed] [Google Scholar]
- Bergfors, T. (2001). The Pictorial Library of Crystallization Drop Phenomena. http://xray.bmc.uu.se/~terese/crystallization/library.html.
- Bravo, J., Karathanassis, D., Pacold, C. M., Pacold, M. E., Ellson, C. D., Anderson, K. E., Butler, P. J., Lavenir, I., Perisic, O., Hawkins, P. T., Stephens, L. & Williams, R. L. (2001). Mol. Cell, 8, 829–839. [DOI] [PubMed] [Google Scholar]
- Hashida, S., Yuzawa, S., Suzuki, N. N., Fujioka, Y., Takikawa, T., Sumimoto, H., Inagaki, F. & Fujii, H. (2004). J. Biol. Chem.279, 26378–26386. [DOI] [PubMed] [Google Scholar]
- Ito, T., Matsui, Y., Ago, T., Ota, K. & Sumimoto, H. (2001). EMBO J.20, 3938–3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuribayashi, F., Nunoi, H., Wakamatsu, K., Tsunawaki, S., Sato, K., Ito, T. & Sumimoto, H. (2002). EMBO J.21, 6312–6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massenet, C., Chenavas, S., Cohen-Addad, C., Dagher, M. C., Brandolin, G., Pebay-Peyroula, E. & Fieschi, F. (2005). J. Biol. Chem.280, 13752–13761. [DOI] [PubMed] [Google Scholar]
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed] [Google Scholar]
- Nakamura, R., Sumimoto, H., Mizuki, K., Hata, K., Ago, T., Kitajima, S., Takeshige, K., Sakaki, Y. & Ito, T. (1998). Eur. J. Biochem.251, 583–589. [DOI] [PubMed] [Google Scholar]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol.276, 307–326. [DOI] [PubMed]
- Ponting, C. P. (1996). Protein Sci.5, 2353–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumimoto, H., Miyano, K. & Takeya, R. (2005). Biochem. Biophys. Res. Commun.338, 677–686. [DOI] [PubMed] [Google Scholar]
- Vignais, P. V. (2002). Cell. Mol. Life Sci.59, 1428–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wientjes, F. B., Hsuan, J. J., Totty, N. F. & Segal, A. W. (1993). Biochem. J.296, 557–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, M. I., Gill, D. J., Perisic, O., Quinn, M. T. & Williams, R. L. (2003). Mol. Cell, 12, 39–50. [DOI] [PubMed] [Google Scholar]
- Yasukawa, T., Kanei-Ishii, C., Maekawa, T., Fujimoto, J., Yamamoto, T. & Ishii, S. (1995). J. Biol. Chem.270, 25328–25331. [DOI] [PubMed] [Google Scholar]