Summary
Background
Natural selection has resulted in a complex and fascinating repertoire of innate behaviors that are produced by insects. One puzzling example occurs in fruitfly larvae that have been subjected to a noxious mechanical or thermal sensory input. In response, the larvae “roll” using a motor pattern that is completely distinct from the style of locomotion that is used for foraging.
Results
We have precisely mapped the sensory neurons that are used by the Drosophila larvae to detect nociceptive stimuli. Using complementary optogenetic activation and targeted silencing of sensory neurons, we have demonstrated that a single class of neuron (Class IV multidendritic neuron) is sufficient and necessary for triggering the unusual rolling behavior. In addition, we find that larvae have an innately encoded directional preference in the directionality of rolling. Surprisingly, the initial direction of rolling locomotion is towards the side of the body that has been stimulated. We propose that directional rolling might provide a selective advantage in escape from parasitoid wasps that are ubiquitously present in the natural environment of Drosophila. Consistent with this hypothesis, we have documented that larvae can escape attack of Leptopilina boulardi parasitoid wasps by rolling, occasionally flipping the attacker onto its back.
Conclusions
The Class IV multidendritic neurons of Drosophila larvae are nociceptive. The nociception behavior of Drosophila melanagaster larvae includes an innately encoded directional preference. Nociception behavior is elicited by the ecologically relevant sensory stimulus of parasitoid wasp attack.
Introduction
Relatively little is known about the molecular and neuronal circuits that encode somatosensory information in Drosophila. The circuits used by the brain to encode information about external temperature, touch, and body position are virtually unknown. However, for other sensory systems, studies of Drosophila have led to an exquisitely detailed understanding, and to the realization that some of the basic principles that are used to encode sensory information are shared between mammalian and insect brains. For example, the glomerular architecture of the olfactory system is evolutionarily conserved [1–5]. Thus, by analogy, the study of somatosensation in Drosophila may provide significant insight into basic and evolutionarily conserved mechanisms of somatosensory processing.
To study this problem we previously developed a behavioral paradigm for the study of nociception (pain sensing) in the Drosophila larva[6]. This paradigm was based upon the simple observation that Drosophila larvae produce a stereotyped defensive behavior in response to noxious mechanical, chemical or thermal stimuli. The pattern of locomotion elicited by noxious stimulation causes the larvae to roll in a highly stereotyped corkscrew-like fashion [6]. This defensive motor output is completely distinct from the well-known rhythmic peristaltic locomotion that is used for other larval behaviors such as foraging. To our knowledge, the rolling pattern of locomotion occurs only following noxious stimulation (heat, mechanical or chemical). The behavior is therefore nocifensive (defensive behavior that is elicited by sensory stimuli that have the potential to cause injury).
Although the nocifensive behavior can be elicited readily in the laboratory, the relevance of the behavior to stimuli that might be encountered in the natural environment remains unclear. For this behavior to have evolved, and for it to be innately encoded in the genome, the behavior presumably provides a selective advantage. This advantage must exceed the energetic costs of maintaining the neuronal circuits that mediate the response.
Predation is one possible selective pressure that may have favored the evolution of the rolling escape behavior. For example, many small parasitoid wasps of the superfamilies Chalcidoidea and Ichneumonoidea attack Drosophila larvae [7]. The female wasp penetrates the Drosophila larval cuticle with a sharp ovipositor and lays its egg. The larval wasp then devours the Drosophila from within and an adult wasp eventually emerges rather than a fly. The cellular immune system of the Drosophila larvae has the ability to encapsulate and destroy the wasp egg so that all wasp infections do not result in the death of the larva [8]. An evolutionary arms-race causes the wasp to evolve its own strategies to disrupt the host immune system [9–11]. Wasp parasitism is believed to impose strong selective pressures on Drosophila. Studies of natural populations of Drosophila have found rates of infection that exceed sixty percent [12]. The ecological importance of wasp parasitism is not limited to Drosophila. Greater than 300,000 species of parasitic wasps exist in nature, and they in turn affect many more species of plants and insects [13].
Several previous lines of evidence suggest that multidendritic (md) sensory neurons of the larva might function as nociceptors. First, they morphologically resemble vertebrate nociceptive neurons since they have multiply-branched [14–21] naked nerve endings attached to epidermal cells. Second, larvae with genetically silenced md neurons are completely insensitive to noxious stimulation and fail to produce the nocifensive response [6, 22]. Third, the Painless TRP channel is required for nociception and is expressed in md neurons [6]. Based on these lines of evidence we have previously proposed that md neurons function as nociceptors.
However, the morphology of md neurons suggests that these neurons are not a uniform population of cells. Rather, at least four morphological subtypes have been identified. According to the complexity of the dendritic arbors and other morphological features, the neurons are termed Class I–IV. The Class I neurons have the simplest dendritic arbors while the Class IV are the most complex [23]. Central projections of each of the md neurons have been analyzed in detail [17, 24, 25]. The projections were consistent with the possibility that the neurons within a morphological class might provide similar informational content to the brain (i.e. suggesting a similar function). The central axonal projections were found to vary among classes of md neurons, suggesting functional specialization among the classes [24]. Class I neurons project to motor neuropile of the dorsal abdominal ganglion and have been proposed to provide feedback to motor neurons. However, Class II, Class III and Class IV neurons all project to ventral neuropile and, by analogy to other insects, are predicted to have somatosensory functions.
Several studies have suggested non-nociceptive functions for md neurons [26–28]. For example, deletion of pickpocket, which encodes a DEG/ENaC type ion channel that is expressed solely in the Class IV md neurons, results in an interesting larval locomotion phenotype. pickpocket mutant larvae move more rapidly than the wildtype larvae and turn less frequently [26]. More recently, Hughes and Thomas found that the synaptic output of Class I md neurons and the bipolar dendritic (dbd) neurons, were required for propagation of peristaltic muscle contraction that occurs during normal larval locomotion [27].
The various central projection patterns and dendritic morphologies of multidendritic neurons led us to ask: are all multidendritic neurons nociceptors or is nociception function restricted to a particular subtype? In addition, we have begun to investigate selective advantages that may have led to the evolution of rolling locomotion. We propose that selective pressures imposed by parasitoid wasps may have played a role in the evolution of this behavior.
Results
GAL4 drivers that target multidendritic neuron subtypes
The Drosophila GAL4/UAS system allows for targeting of gene expression to precise cells of the animal [29]. Several GAL4 drivers have been identified that allow for targeting of gene expression to arborizing md neurons. We first examined these drivers to determine if they would be useful for targeting specific subsets of md neurons in behavioral experiments. We planned to cross the drivers to UAS-tetanus toxin light chain (UAS-TnT-E) lines [30] and then to test animals that were trans-heterozygous for the GAL4 driver and UAS-TnT-E in nociception behavioral assays. Since the tetanus toxin light chain cleaves the v-snare synaptobrevin, it reduces evoked synaptic vesicle release in the neurons which express the GAL4 driver, effectively silencing them.
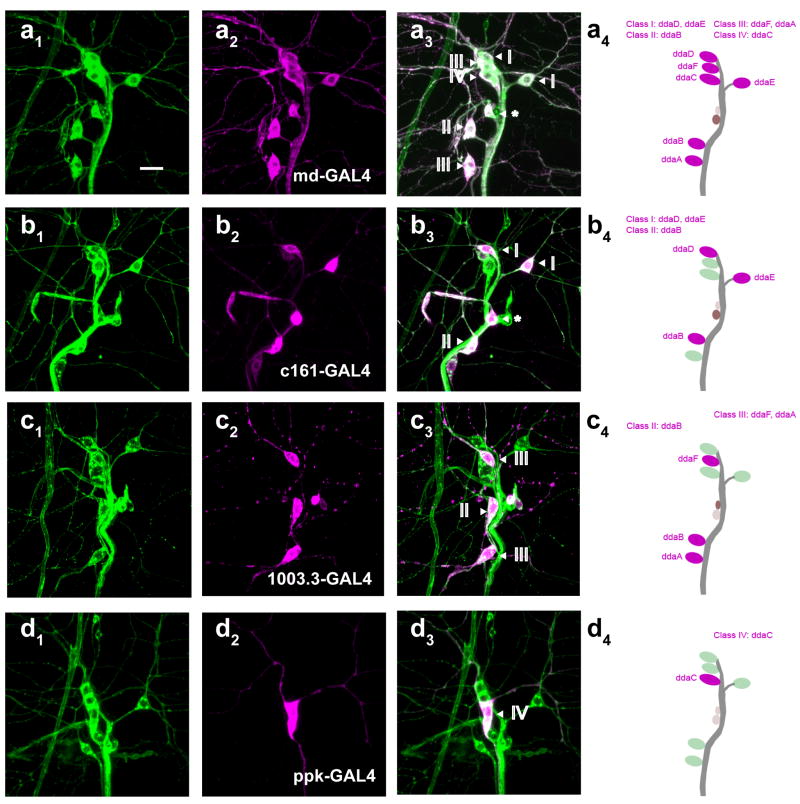
Four GAL4 drivers were identified that would be useful for our purposes. The GAL4109(2)80 driver [16](md-GAL4) was expressed in all four classes of md neurons (Figure 1A) whose projections decorated the entire epidermis.
Figure 1. GAL4 drivers which target distinct subsets of multidendritic neurons.
(A-D) Confocal microscope images of third instar larval multidendritic neurons (dorsal cluster labeled with UAS-MCD8-GFP). (A1-D1) Immunostaining with the pan-neuronal marker anti-HRP-FITC (green). (A2-D2) Co-immunostaining with anti-GFP (magenta). (A3-D3) Merge. (A4-D4) Schematic diagrams of a dorsal cluster of MD neurons labeled by class and name for each GAL4 driver. (A) Expression pattern of md-GAL4 (Class I-IV). (B) Expression pattern of c161-GAL4 (Class I-II). (C) Expression pattern of 1003.3-GAL4 (Class II-III). (D) Expression pattern of ppk1.9-GAL4 (Class IV). Roman numerals represent multidendritic neuron class. Asterisk indicates the dmdI neuron. Scale bar is 10 μm.
The class I and II neurons, whose relatively unbranched dendrites tile only a subset of the entire epidermis, were strongly targeted by the c161-GAL4 driver [31] (Figure 1B). As mentioned above, based on central projections, and on behavioral evidence, the class I neurons have been previously proposed to function as proprioceptors [24, 27]. The function of class II md neurons is not known.
The class III and IV md neurons possess more complex dendrites that tile the entire epidermis. There is no region that lacks endings from these cells, a feature that would be expected for nociceptive neurons. The Class III neurons (as well as Class II neurons) were targeted by the recently described 1003.3-GAL4 driver (Figure 1C) [27]. Finally, the pickpocket1.9-GAL4 (ppk-GAL4) [26] driver targets class IV md neurons (Figure 1D).
Silencing of class IV multidendritic neurons eliminates thermal nociception behavior
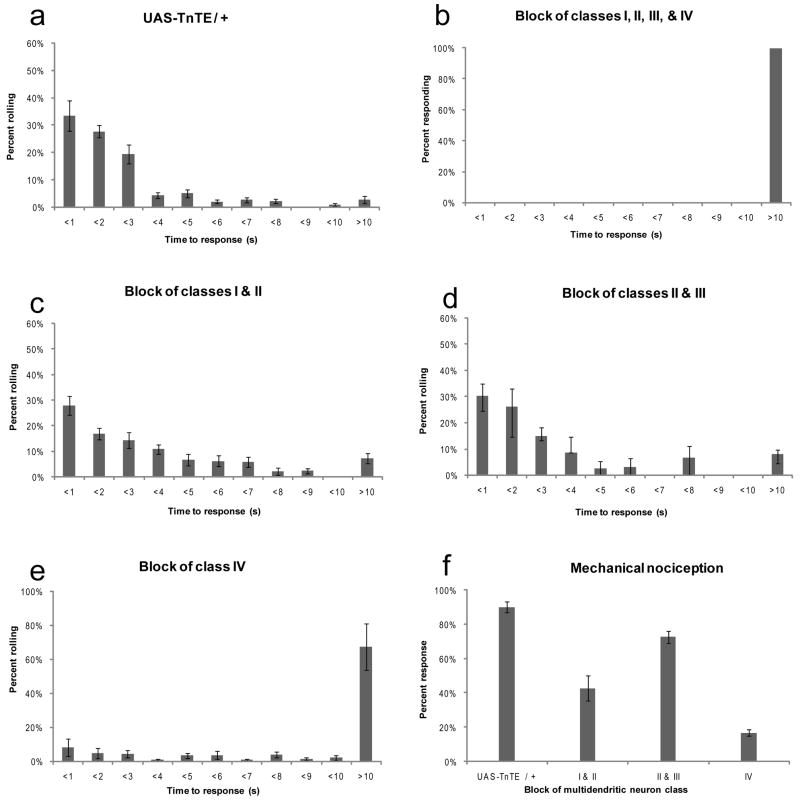
As shown previously, when md-GAL4 was used to drive the expression of UAS-TnT-E in all four classes of md neurons, the behavioral response to noxious heat was completely abrogated [6] (Figure 2B) compared to controls without a driver UAS-TnT-E/+ (Figure 2A).
Figure 2. Class IV multidendritic neurons are necessary for nocifensive behavior.
(A-F) The distribution of latencies of thermal nocifensive behavior in third instar larvae that were lightly touched with a 47°C probe. (A) Nocifensive response of the UAS-TnTE/+ larvae without a GAL4 driver (n=112). (B) Blocking the synaptic output of all four classes of multidendritic neurons completely blocked thermal nocifensive responses (n=27). (C) Blocking the output of class I and II md neurons using the C161-GAL4 driver slightly increases the latency of thermal nociception behavior (n=195). (D) Blocking the output of class II and III md neurons using the 1003.3-GAL4 driver did not affect thermal nociception behavior (n=63). (E) Silencing of class IV multidendritic neurons dramatically impaired thermal nociception behavior (n=139). (F) Effects of blocking different subsets of md neurons upon the frequency of mechanical nociception behavior (UAS-TnTE/+ n=179, c161-GAL4 n=79, 1003.3-GAL4 n=61, ppk-GAL4 n=65). In all panels, error bars indicate standard error of mean.
We next assayed the c161-GAL4 driver which targeted to all neurons of the Class I and II subtypes. Compared to the control (UAS-TnT-E/+), larvae expressing UAS-TnT-E under control of c161-GAL4 showed only a slight (although statistically significant, Wilcoxon Rank-Sum Test, p<0.05) delay in their initiation of nocifensive responses (Figure 2C) to the noxious heat probe which contrasts with the results seen with md-GAL4 (Figure 2B). However, the rolling behavioral output did not appear to be as coordinated as in the wild type and it took these larvae longer than control lines to achieve a complete roll. This result is consistent with previous studies that implicated the Class I md neurons in proprioceptive feedback that plays a role in peristaltic locomotion. Our results may indicate that the Class I (or possibly the Class II) md neurons also provide proprioceptive feedback necessary for completion of rolling behavior.
We also inactivated the Class II and Class III neurons using the 1003.3-GAL4 driver to drive UAS-TnT-E. These larvae appeared normal in their initiation of the behavioral response compared to the control UAS-TnT-E/+ (Wilcoxon Rank-Sum Test, p=0.32) (Figure 2D). The rolling response of larvae with silenced Class II and Class III neurons appeared coordinated and we did not observe any obvious defects in the quality of the behavior. This result also suggests that the coordination defects seen with the c161-GAL4 driver were unlikely to be due to inhibition of Class II neurons.
The data shown above indicate that blocking the output of Class I and Class II, or of Class II and Class III neurons did not strongly affect thermal nociception responses. This suggested that the Class IV neurons might be the relevant neurons involved in the thermal nociception response. To test this we were able to specifically inactivate the Class IV neurons using the ppk-GAL4 driver. Indeed, larvae of the ppk-GAL4/UAS-TnT-E genotype showed a dramatically impaired thermal nociception response compared to the control UAS-TnT-E/+ (Figure 2E, Wilcoxon Rank-Sum Test p<0.00001). The frequency of larvae that failed to perform nocifensive behavior even after 10 seconds of stimulation was significantly increased relative to control genotypes. The rare larvae of this genotype that did produce the rolling behavior appeared to be coordinated. Although blocking the Class IV neurons did not completely eliminate thermal nociception, the remaining response could be due to an incomplete block of the synaptic output in these cells by tetanus toxin light chain. Alternatively, parallel processing may occur through sensory neurons that we have yet to identify as nociceptive.
Our prior studies indicated that strong mechanical stimuli elicited the same nocifensive behavior that can be elicited by noxious heat. We thus tested which of the various subtypes of md neurons were required for mechanical responses (Figure 2F). Blocking the Class I and Class II neurons did impair the behavioral responses to mechanical stimuli. However, this result is difficult to interpret given that these larvae also appeared uncoordinated as described above. Blocking the output of classes II and III had a milder effect on mechanical nociception. Finally, as with thermal nociception, blocking Class IV neurons most strongly diminished the response to mechanical stimuli (Figure 2F) suggesting the possibility that these cells have polymodal nociceptive functions.
Combined, these results suggested that the synaptic output of Class IV md neurons plays a major role in initiating nocifensive behavioral responses to noxious heat or mechanical stimulation. However, there are caveats to this interpretation of the data. For example, although our data suggested that the Class IV md neurons are required for the nocifensive behavior they did not prove that these neurons are nociceptors. Instead, it was possible that these neurons were required for the central nervous system to efficiently control the behavior. For example, they could merely provide proprioceptive feedback.
Optogenetic activation of Class IV neurons elicits nocifensive behavior
Therefore, we next tested whether activation of md neurons would be sufficient to elicit nocifensive behaviors. If activating these neurons could be shown to trigger the nocifensive behavior, it would place them upstream of the rolling behavioral output in this neuronal pathway.
Targeted photo-activation of neurons has been achieved using Channelrhodopsin-2 [32–35] (ChR2) a light activated cation channel [36] from green algae. Importantly, ChR2 has been shown to be capable of causing light induced action potentials in Drosophila motor neurons [34]. Further, GAL4 lines were used to drive UAS-Channelrhodopsin-2 in dopaminergic or octopaminergic neurons. Remarkably, illumination with blue light resulted in specific associative learning effects in larvae that were dependent on feeding of larvae all trans-retinal (which forms the chromophore for Channelrhodopsin-2) [34].
We generated flies that express fluorescently tagged Channelrhodopsin-2 (ChR2-YFP or ChR2-mCherry) under control of the GAL4/UAS system (UAS-ChR2-YFP, UAS-ChR2-mCherry) [32, 33]. The fluorescent tags allowed us to identify a priori, lines that generated detectable expression levels of ChR2 in the md neurons (Figure 3A,B). Expression of identical ChR2-YFP and ChR2-mCherry proteins has been previously shown to render rat hippocampal neurons light sensitive [32, 33].
Figure 3. Optogenetic activation of Class IV multidendritic neurons is sufficient to elicit the nocifensive response.
(A1–3) Expression of Channelrhodpsin2-YFP under control of ppk-GAL4. (A1) pan-neuronal marker anti-HRP (green). (A2) anti-GFP (magenta). (A3) Merge of a1 and a2. (B) Expression levels of UAS-ChR2-YFP lines measured by pixel intensity of confocal images and normalized to staining intensity of line C. (C) Optogenetic activation of various subsets of multidendritic neurons triggers the “accordion” phenotype at high frequency. (D) Optogenetic activation of Class IV multidendritic neurons elicited nocifensive rolling behavior at high frequency. Rolling behavior with activation of classes I-IV was also elicited (15%). (E) ChR2-YFP expression levels are correlated with efficiency of nocifensive behavior. Sample sizes for c and d (Class I neuron driver 2–21-GAL4: atr+ n=54, atr- n=43), (Class I-IV neuron driver md-GAL4: atr+ n=103, atr- n=92 ), (Classes I & II driver c161-GAL4: atr+ n=117, atr- n=21), (Class II & III driver 1003.3-GAL4: atr+ n=46, atr- n= 52), (Class IV ppk-GAL4 atr+ n=181, atr- n=112). Sample sizes for e (Chop2 n=173, Line 1 n=20, Line 2 n=84, Line AB n=181, Line C n=80). UAS-Chop2 is an untagged Channelrhodopsin-2 line from the Fiala laboratory[34] with an insertion on the third chromosome. Error is standard error of the mean.
We selected several of the YFP tagged lines for behavioral analysis. The lines selected showed detectable fluorescence in peripheral sensory neurons when we crossed them to appropriate GAL4 drivers (Figure 3B). We chose a more strongly expressing line (AB) with UAS insertions on both the second and third chromosomes, for behavioral analysis and crossed it to the GAL4 strains described above. The larval progeny from these crosses were raised to third instar fed on yeast paste that either contained all-trans retinal (atr+) or on yeast paste that did not contain all-trans retinal (atr−); the latter contained the diluent alone (0.5% EtOH). Individual third instar larvae were then transferred to a small droplet of water in a Petri dish so that they could be viewed on a fluorescent stereomicroscope that was equipped with a video recorder (allowing the behavioral response to illumination of blue light to be recorded).
The atr+ yeast paste had no effect in control larvae that lacked a GAL4 driver (UAS-ChrR2-YFP/+). However, in the presence of GAL4 drivers we indeed observed behaviors to pulses of blue light (460–500nm) that were strongly dependent on feeding of all-trans retinal. Even in the presence of GAL4 drivers, ChR2-YFP expressing larvae that were fed the atr- yeast paste only rarely produced behaviors in response to blue light (Figure 3C, D, Figure 4A, Supplementary Information, Video 1).
Figure 4. Optogenetic activation of Class IV md neurons triggers nocifensive response.
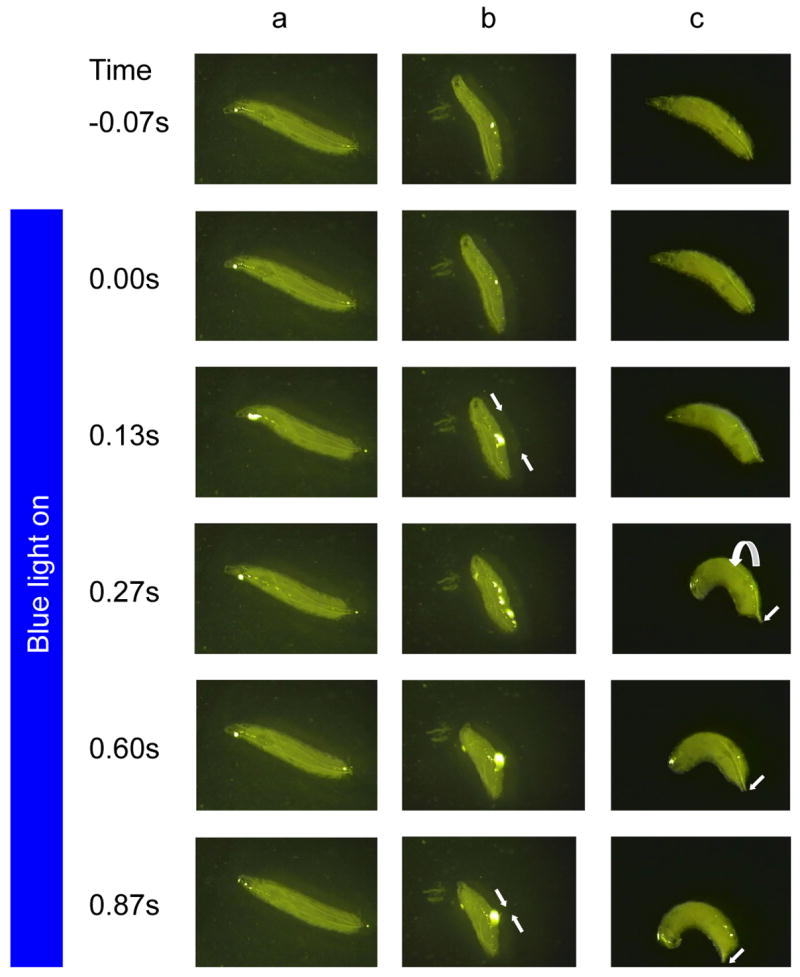
(A-C) Still images of third instar larvae and optogenetically activated behaviors extracted from videotaped responses. (A,C) ppk-GAL4 UAS ChR2-YFP larvae that been fed atr- yeast (A) or atr+ yeast and illuminated with blue light. (B) c161-GAL4 UAS ChR2-YFP larva fed ATR+ and illuminated with blue light. (A) A larva expressing ChR2YFP in Class IV md neurons showed little response to the blue light when fed yeast paste lacking all-trans retinal. (B) A larva expressing ChR2YFP in Class I and II md neurons simultaneously contracts muscles of every segment to produce the accordion like behavior (straight white arrows denote reduction in length of larva from contractions). (C) A larva fed atr+ yeast and also expressing ChR2YFP in the Class IV md neurons produced a rolling motor response (curved arrow) that was indistinguishable from nocifensive rolling behavior. Note the net lateral direction of movement (straight white arrows). The time point of the sequence is shown in the left with illumination occurring at time zero.
We first tested the effects of expressing ChR2-YFP using the Class I-IV (md-GAL4) driver. In response to blue light pulses we found that the larvae performed one of two distinct behavioral responses. In the most prevalent response, the blue light caused the larvae to simultaneously contract the muscles of all body segments and to scrunch like a compressed accordion (Figure 3C, similar to Supplementary Information Video 2).
In a more rarely occurring response, the larvae rolled using a motor pattern that appeared quite similar to the nocifensive rolling behavior (Figure 3D). However in these larvae, the light-induced rolling was eventually followed by the accordion like muscle contraction behavior. The latter behavior is never seen in response to noxious heat or mechanical stimuli.
We hypothesized that the accordion like behavior and the rolling behavior might reflect activation of distinct behavioral pathways, as a consequence of activating multiple neurons with distinct functions. For example, it seemed possible that some md-neurons might trigger segmental muscle contractions while others might trigger nocifensive rolling. If this were the case, then the behaviors described above could be the result of competition for these two pathways by light induced activation of the relevant triggering sensory inputs. We thus further tested whether activation of the two behavioral pathways could be separated, by more precise targeting of the ChR2-YFP to distinct md-neuron subsets.
Indeed, when we expressed ChR2-YFP in the Class I and II neurons (c161-GAL4), or in the Class II and III neurons (1003.3-GAL4) we never observed rolling behavior in response to illumination with blue light (Figure 3D). Instead, the blue light caused the larvae to perform the accordion response with high penetrance (Figure 3C, Figure 4B, Supplementary Information Video 2). That we did not observe rolling behavior in response to activation of the Class I, II, or III neurons is consistent with the inactivation studies described above since we did not find evidence that the synaptic output of these neuronal types was strongly required for the initial steps of thermal nociception behavior.
We hypothesize that the accordion phenotype reflects a role for the Class II and/or Class III md neurons in propagating the wave of muscle contraction during peristaltic locomotion. During normal locomotion, activation of the Class II or the Class III neurons may occur via muscle contraction within a segment. This may produce a signal coordinating the contraction of muscles in the next segment. The accordion phenotype likely represents a manifestation of this process, but in an abnormal situation where the signal is sent to all segments simultaneously via optogenetic activation.
In contrast, optogenetic activation of Class IV md neurons (ppk-GAL4/UAS-ChR2-YFP) caused robust nocifensive-like rolling behavior and never resulted in the accordion like behavior (Figure 3C,D, Figure 4C, Supplementary Information Video 3). The penetrance of the nocifensive response to the blue light pulse was impressive, with 87% of the larvae responding with rolling behavior in lines strongly expressing ChR2-YFP. With lines that expressed ChR2-YFP more weakly, the same behavior was observed, but with a reduced frequency (Figure 3E).
Qualitatively, this light induced nocifensive behavior appeared very similar to thermally and mechanically induced rolling behavior. However, the light induced behavior was initiated very rapidly (<100 ms after the light was turned on. This was more rapid than the rolling induced with our standard nociception stimulus of 47°C where the larvae often require thermal stimulation of several seconds to elicit the response. The rapid behavioral responses with ChR2-YFP likely reflect the extremely rapid kinetics of light activated channel relative to slower kinetics of thermal nociception at 47°C. Indeed, very rapid thermal nociception responses can also be observed (rolling that initiates <100ms following contact with the thermal probe) but these require a stronger thermal nociception stimulus (53°C probe) (W.D.T, R.Y.H, L.Z., unpublished).
Combined, these data conclusively demonstrate that activation of Class IV md neurons of the third instar Drosophila larva is sufficient to trigger a nocifensive-like motor output. In addition, we have found that the output of the Class IV neurons is necessary for triggering normal nocifensive behavior in thermal and mechanical nociception assays. We therefore propose that the class IV multidendritic neurons function as nociceptors.
Paradoxical directionality of rolling behavior
We have used a thermal stimulus as a convenient method for the identification of neurons and mutants that affect the nociception pathway. To perform the rolling escape response, the larva uses its muscles to move in a highly coordinated fashion that is distinct from peristaltic locomotion. This suggests the existence of multiple central pattern generators in the larval brain (Figure 7). Rolling locomotion causes larvae to move at a significantly higher velocity (3–5 mm/sec) (R.H., L.Z., unpublished) than typical peristaltic locomotion (1mm/sec). This increased velocity presumably provides a selective advantage in a rapid escape from a potentially damaging thermal insult. However, it is unlikely that the rolling behavior evolved solely for thermal nociception since strong mechanical stimuli also elicit the rolling response.
Figure 7.
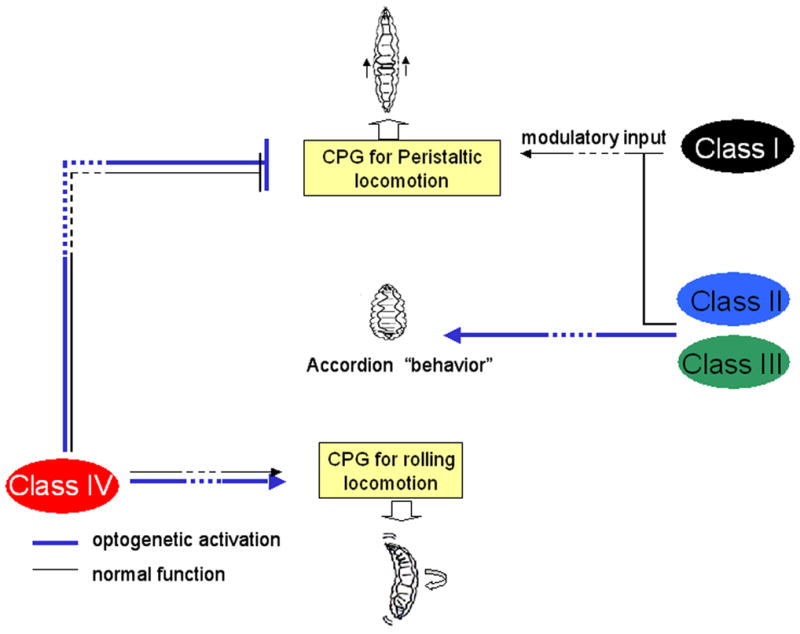
Model for sensory control of alternate patterns of larval locomotion. The Class IV neurons are both necessary and sufficient to trigger rolling escape behavior. Other Classes of md-neurons modulate peristaltic locomotion through a distinct Central Pattern Generator. When inappropriately activated via Channelrhodopsin the accordion phenotype is manifested.
Indeed, the following observations suggest that thermal nociception is unlikely to be the only selective pressure that drove the evolution of this behavior. Surprisingly, we have found that Drosophila larvae have a genetically encoded tendency to initially roll towards the noxious heat probe rather than to roll away from it. When animals were stimulated on the right side of the body, the preferred initial direction of rolling was to the right (ie. rolling clockwise) (Figure 5A). When stimulated on the left the preferred direction of rolling was towards the left (rolling counter-clockwise) (Figure 5A). The result was that the animals tended to initially roll in a direction that pushed them against the thermal probe rather than in a direction that carried them away. After failing to escape following several revolutions, the larvae did reverse direction of rolling. The choice of roll direction was not random, indicating that the larval brain was capable of sensing the direction from which the stimulus was coming. Yet, paradoxically, the circuitry that controlled rolling was strongly biased to produce the initial direction of motor output towards the side of the body that had been stimulated.
Figure 5. Paradoxical directionality of rolling behavior.
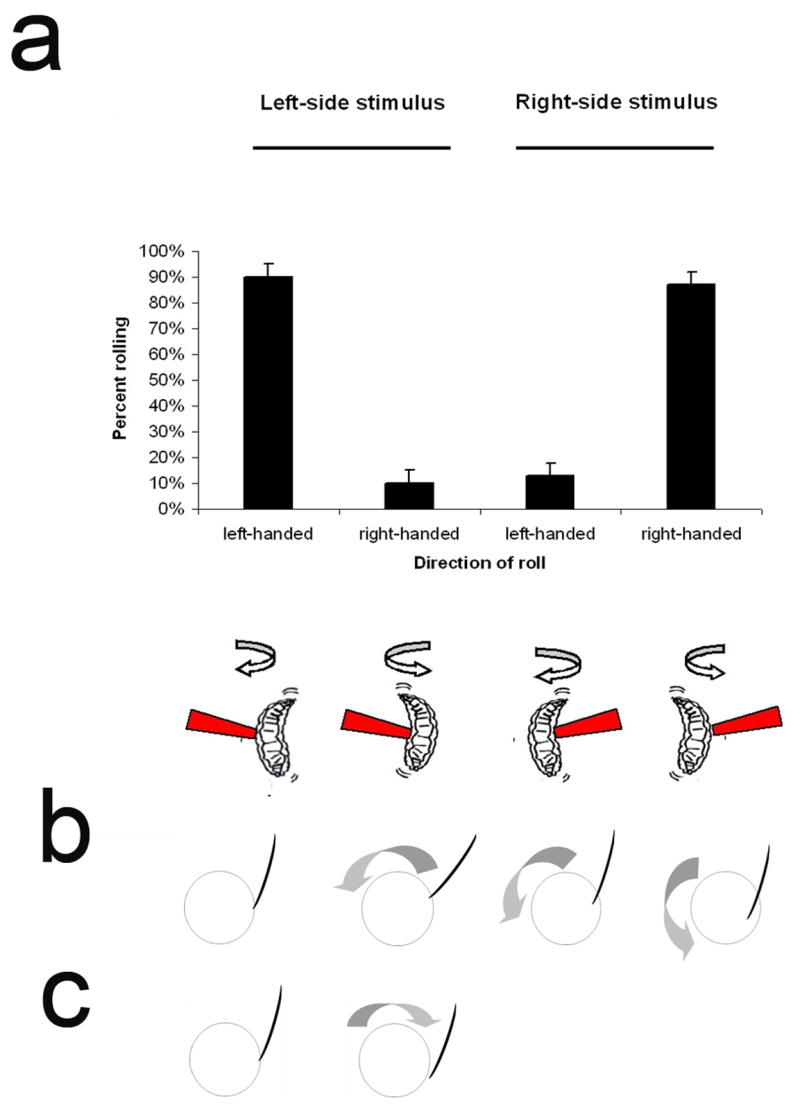
(A.) Directionality of larval rolling is biased. Top: Larvae had a strong tendency to roll towards the heat stimulus. When stimulated on the right (n=114), they predominantly rolled to the right. When stimulated on the left (n=102) they had a strong tendency to roll to the left. Roll direction was determined according to the first complete (ie. 360°) roll. Bottom: schematic representation of roll direction. (B) Hypothetical effects of rolling away from a wasp attack causes increased penetration. (C) Hypothetical effects of rolling towards the side of the body where attacked. In B and C the cross section of the larva is depicted as a circle and the ovipositor of the wasp attacking from above is depicted as a curved line.
In natural populations of Drosophila then, there must be a strong selective advantage in directional rolling towards the side of the body in which the nociceptors have been activated. It is not obvious to us how this would be the case if noxious heat were the sole selective pressure driving the evolution of this behavior.
Third instar larvae escape from wasp attacks by rolling
We sought an explanation for this paradox in the natural ecology of Drosophila. Diverse species of parasitoid wasps require a Drosophila host in order to complete their life cycle. For example, the figitid wasp, Leptopilina boulardi is an obligate and ubiquitous parasitoid of Drosophila melanogaster [37]. Female Leptopilina lay their eggs within the Drosophila by penetrating the larval cuticle and epidermis using a sharp ovipositor. It has been reported that larval hosts of sufficient size (third instar) can behaviourally defend from wasp attack with vigorous movements [38]. Inexperienced wasps have been reported to be more susceptible to larval defenses while experienced wasps eventually learn to attack smaller, more defenceless, animals [38]. Thus, we wished to observe larval behavioural responses to wasp attack in order to determine whether the defensive behaviours triggered by wasps was similar to the responses that were triggered by activation of Class IV neurons via Channelrhodopsin-2YFP.
We reasoned that the very fine and highly branched dendrites of the Class IV multidendritic neurons might be capable of detecting attacks from the wasp ovipositor. If this were the case, wasp attack should elicit the rolling response. We further reasoned that the bias in rolling direction that we observed experimentally might be of benefit in evading a wasp attack. Rolling in the direction counter to the side of the attack might actually assist the wasp in its attempts to penetrate the cuticle (Figure 5B). In contrast, rolling towards the direction of wasp attack might deflect the ability of the wasp ovipositor to penetrate the cuticle and allow the larva to escape (Figure 5C). We hypothesized that such a mechanism would favour the evolution of neuronal circuits that encoded a behavioral tendency to roll towards the side of the body in which nociceptors had been activated, rather than away.
As we had predicted, we found that third instar Drosophila larvae did respond to attack by Leptopilina boulardi by producing the rolling response (Figure 6, Supplementary Video 4). The wasp triggered rolling resembled both mechanical and optogenetically triggered rolling. Also, as expected, we observed larvae that rolled towards the side of the body from which the wasp attack had come. However, the overall outcome of the larval responses was somewhat unexpected to us. Surprisingly, as a consequence of the larva rolling towards the wasp, the thin threadlike ovipositor of the wasp became wrapped around the larva (Figure 6, Supplementary Video 4). The rolling behavior of the larva thus acted like a spool winding a thread. After the larva had performed several revolutions, the wasp ovipositor eventually reached the limits of its length. The larva continued to roll and finally carried the wasp through the air and onto its back. At this point the wasp, clearly in danger of getting stuck in the medium, appeared to prematurely break off its attack. The larva then quickly left the area as the female wasp retracted its ovipositor
Figure 6.
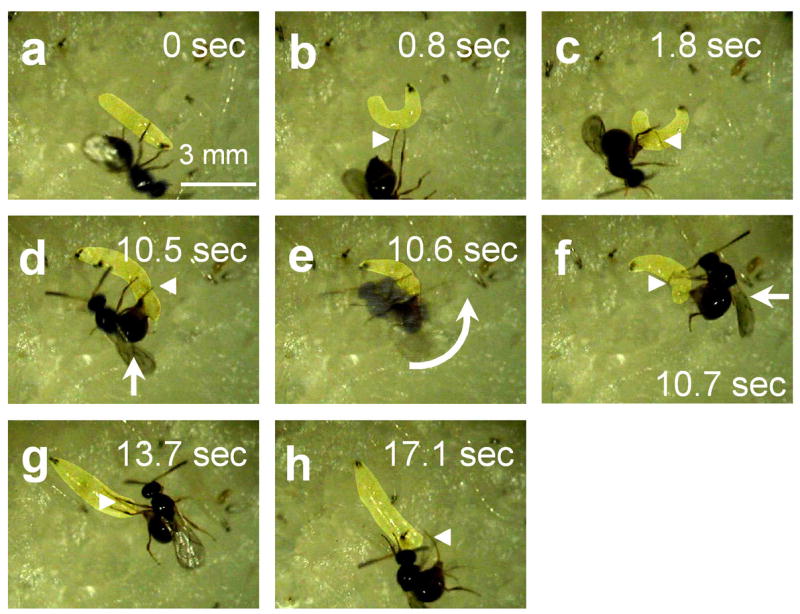
Rolling behavior allows Drosophila larvae to escape from parasitoid wasp attack. Leptopilina boulardi female and an early third instar Drosophila melanogaster larva. (a) Wasp ovipositor penetrates larval cuticle and epidermis; (b) Nocifensive rolling behavior is triggered. The long threadlike ovipositor of the wasp is clearly visible (arrowhead). (c) Nocifensive rolling results in the ovipositor being wrapped around the larva. (d-f) Rolling of larva flips parasitoid wasp onto her side; (note that the image of the wasp in panel e is blurred due to its rapid movement during the exposure). (g) larva moves quickly away from parasitoid wasp; (h) larva is freed from ovipositor. Note: Images have been digitally adjusted to increase the contrast of the larva relative to the background (so that it could be easily seen). The original data can be seen in Supplementary Video 4.
Discussion
In this study, we have provided strong evidence that at least one of the classes of Drosophila multidendritic neuron is nociceptive. These are the Class IV neurons which express the pickpocket gene. First, we have shown that blocking the synaptic output of class IV md neurons significantly impairs thermal and mechanical nociception behavior. Second, we have shown that optogenetic activation of these neurons is sufficient to trigger the stereotyped rolling response.
It is important to note that our data do not rule out the possibility that the Class IV neurons might have polymodal functions. It is possible for example, that the firing rate triggered by nociceptive stimuli exceeds a critical threshold that triggers rolling while lower firing rates could be used to regulate turning or rate of locomotion. The latter possibility would be consistent with prior studies which proposed that the Class IV md neurons function as proprioceptors. This hypothesis was based on the fact that larvae mutant for pickpocket move rapidly and turn less frequently than wild type larvae and by the fact that pickpocket is solely expressed in Class IV neurons [26]. It will be interesting to further investigate the pickpocket mutant phenotypes in light of the nociceptive function for the Class IV neurons that we have presented here.
The differences in the central projections of distinct types of md neurons are notable in light of the functions that we have observed. Of the four Classes of multidendritic neuron, only the Class IV md neurons have projections which cross the midline to innervate contralateral post-synaptic targets [24]. This has similarity to pain processing in vertebrate brains in which ascending tracts of the contralateral spinal chord carry painful sensory information to higher order neurons of the brain [39]. Future studies will allow us to determine whether the brain of the larva is involved in perception of the noxious stimulus or whether lower level processing in the abdominal or thoracic ganglion plays a role.
Perhaps the most interesting question related to this issue is whether input from the Class IV neurons of larvae, or of adult flies, results in a negative associative valence. In larvae, this might allow avoidance of regions of fruits whose odor is associated with the presences of wasps. Interestingly, the Class IV neurons persist through metamorphosis and are present in adult flies [21] where they are unlikely to activate a rolling central pattern generator. Furthermore, noxious heat is known to be an effective unconditioned stimulus in adult Drosophila operant learning paradigms [40–43]. Can optogenetic activation of the Class IV md neurons be used as a substitute for an unconditioned stimulus in operant negative associative learning paradigms? Does electric shock used in olfactory learning paradigms activate overlapping neuronal circuits?
Our unambiguous identification of the Class IV md neurons as nociceptors opens the door to further analysis of these interesting questions and will allow us to dissect this neuronal circuit, from the molecule to the behavior. In addition, our identification of parasitoid wasp attack as an ecologically relevant stimulus that elicits rolling demonstrates an evolutionarily important adaptive function for this fascinating behavior.
Experimental Procedures
Molecular cloning
To generate UAS-Channelrhodopsin2-EYFP the ChR2-YFP fusion gene was PCR amplified from the pLECYT template using the forward primer (5′CACCATGGATTATGGAGGCGGCCTGAGT3′) and the reverse primer (5′CTATTACTTGTACAGCTCGTC3′). To generate UAS-Channelrhodopsin2-mCherry the humanized ChR2-mCherry fusion gene was PCR amplified from the pFCK-hChR2-mCherry-W template using the forward primer (5′CACCATGGACTATGGCGGCGCTTTGTC3′) and the reverse primer (5′TTACTTGTACAGCTCGTCCATGCCGAG3′). The PCR products were then cloned into pENTR/D-TOPO (Invitrogen) and then into the Drosophila pUASt-W Gateway destination vector [44] using Clonase II (Invitrogen). The UAS-ChR2-EYFP and UAS-Chr2-mCherry constructs were used to generate transgenic animals by transposase mediated transformation of w1118.
Fly strains
yw; GAL4109(2)80 (md-GAL4), w; c161-GAL4, w;1003.3-GAL4, ppk-GAL4, w; 2-21-GAL4, w;UAS-mCD8::GFP, w; UAS-Channelrhodopsin-2::YFP The UASChR2YFP inserts of Line C and Line1 are on chromosome II. The insertion of Line2 is found on chromosome II. In Line AB two insertions are present: one on chromosome II and the other on chromsome III. The w; UAS-Chop2 line [34] was a third chromsome insertion. Drosophila stocks were raised on standard cornmeal molasses fly food medium at 25°C. Where possible balancers containing the Tb marker were used to follow inheritance of the UAS insertion(s). Alternatively, YFP fluorescence provided a means to follow UAS-ChR2YFP inheritance.
Wasp husbandry
Leptopilina boulardi-17 wasps were housed in plastic fly vials at 18°C and fed with several drops of 70% honey that was placed upon the plug of the vial. To propagate the stock, roughly 30 Drosophila were allowed to seed a vial of cornmeal medim for 24 hours at 25°C. The flies were then removed, and approximately ten wasps of mixed gender were added to the vial and allowed to infect the hatched first instar larvae for a period of 24 hours. The infected vials were then maintained at 25°C until the next generation of wasps emerged after 3–4 weeks . These protocols were kindly provided by Jorge Morales and Shubha Govind.
To document the behavioral response to wasp attack roughly 30 female Cantons flies were allowed to lay eggs for 4 hours on an apple juice agar plate with yeast paste. The larval progeny were then allowed to develop for an additional 72 hours until the first day of third instar. A single mated Leptopilina boulardi 17 female parasitoid wasp was then placed on the apple juice plate with the third instar larvae and the interactions with larvae were video-recorded through a stereomicroscope. The parasitoid wasps used in our experiments did not have prior experience infecting third instar larvae.
Confocal microscopy
For visualization of GAL4 driver patterns, larvae heterozygous for the indicated driver and UAS-mCD8-GFP were filleted, fixed in 4% paraformaldehyde, For immunostaining, mCD8-GFP was detected with rabbit anti-GFP (Invitrogen 1:1000) and the secondary anti-rabbit Alexa Fluor 568 (Molecular probes 1:1000) and the neurons were counterstained with FITC conjugated goat anti-HRP (Cappel). Similar methods were used for detection of YFP tagged ChR2. Microscopy was performed on a Zeiss LSM 5 Live Confocal System. Images shown are maximum intensity projections of confocal Z-stacks, brightness and contrast were adjusted using Adobe Photoshop.
Behavioral assays
The thermal nociception behavioral tests were performed as described previously [6] with slight modifications.
On the first day, crosses were established using 6 females and 3 male flies in each vial. For each genotype, 6 vials were established and maintained at 25°C, 75% humidity incubator.
On the sixth day of the experiment wandering third instar larvae from vials were rinsed out of the vial with distilled water and into a plastic 60mm petri dish. Excess water in the dish was aspirated such that the larvae remained moist, but not floating in the water that was remaining in the dish.
The temperature of the noxious heat probe (a soldering iron sharpened to a chisel tip shape of 0.6 mm wide) was controlled by adjusting the voltage. A digital thermocouple (Physitemp, BAT-12) welded to the tip of the probe was used to precisely measure its temperature. The stimulus was delivered by gently touching the larvae laterally, in abdominal segments 4, 5, or 6. Each larva was tested only once and discarded after the test. Analysis of videotaped behavior was performed offline. The response latency was measured as the time interval from the point at which the larva was first contacted by the probe, until it initiated the rolling movement (the beginning of the first, complete 360 degree roll).
In mechanical nociception assays wandering third instar larvae were collected as described for thermal nociception assays and then stimulated with a 100 mN calibrated Von Frey filament. Von Frey filaments were made from Omniflex monofilament fishing line Shakespeare™ (6 pound test, diameter 0.009 inch (0.23mm). Fibers were cut to a length of 14mm and attached to a glass pipette such that 4mm of the fiber protruded from the end and 10mm anchored the fiber. Noxious mechanical stimuli were delivered by rapidly depressing and releasing the fiber on the dorsal side of a larva. The stimulus was delivered to abdominal segments four, five, or six. A positive response was scored if at least one nocifensive roll occurred after the first or second mechanical stimulus.
Optogenetic activation
Four to six virgin female flies of the GAL4 driver strain were crossed to male flies of the UAS-ChR2YFP strain. Females were allowed to lay eggs for 24 hours on apple juice agar with a dollop of yeast paste (either atr+ (500 μM) or atr−). The larval progeny were allowed to develop and feed on the yeast paste for 72 additional hours. For behavioral analysis the larvae were transferred to 60 mm plastic Petri dishes containing 1–2 ml deionized H2O. Larvae were then stimulated with blue light (460–500nm) using the Hg light source of a Leica MZ16 FA stereomicroscope (145,000 lux). Blue light pulses were manually controlled and lasted for several seconds. Each larva was given three pulses of blue light. Behavioral responses were videotaped and analyzed off line. A positive nocifensive roll was scored if the larva completed at least 1 revolution (360 degrees) in response to any of the three blue light pulses. A positive accordion phenotype was scored if segmental muscle contractions occurred in response to the blue light.
Supplementary Material
Acknowledgments
Thanks to Nancy Stearns and other members of the Tracey lab for helpful suggestions on improving the manuscript. Yuh-Nung Jan for providing md-GAL4 . Wayne Johnson for ppk 1.9-GAL4. Cahir O’kane for providing UAS-TNTE and David Shephard for providing c161-GAL4. Cynthia Hughes kindly provided 1003.3-GAL4 and other drivers. We thank Dr. Shubha Govind for providing the wasp strain Leptopilina boulardi 17 and for instructions of their care. We also thank the Bloomington Drosophila Stock Center and the entire Drosophila community.
Author Information: The authors have no competing financial interests. KD is supported by the Culpeper, Coulter, Klingenstein, Whitehall, and McKnight Foundations, and by the NIH. Funding to WDT provided by NINDS R01-NS054899-01, the Whitehall Foundation and the Alfred P Sloan Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ressler KJ, Sullivan SL, Buck LB. Information coding in the olfactory system: evidence for a stereotyped and highly organized epitope map in the olfactory bulb. Cell. 1994;79:1245–1255. doi: 10.1016/0092-8674(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 2.Vassar R, Chao SK, Sitcheran R, Nunez JM, Vosshall LB, Axel R. Topographic organization of sensory projections to the olfactory bulb. Cell. 1994;79:981–991. doi: 10.1016/0092-8674(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 3.Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- 4.Gao Q, Yuan B, Chess A. Convergent projections of Drosophila olfactory neurons to specific glomeruli in the antennal lobe. Nat Neurosci. 2000;3:780–785. doi: 10.1038/77680. [DOI] [PubMed] [Google Scholar]
- 5.Vosshall LB, Wong AM, Axel R. An olfactory sensory map in the fly brain. Cell. 2000;102:147–159. doi: 10.1016/s0092-8674(00)00021-0. [DOI] [PubMed] [Google Scholar]
- 6.Tracey WD, Jr, Wilson RI, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- 7.Carton Y, Bouletreau M, van Alphen JJM, JC vL. The Drosophila parasitic wasps. In: Ashburner M, Carson HL, Thompson JN Jr, editors. The Genetics and Biology of Drosophla. 1986. pp. 347–394. [Google Scholar]
- 8.Rizki TM, Rizki RM, Carton Y. Leptopilina-Heterotoma and L-Boulardi - Strategies to Avoid Cellular Defense Responses of Drosophila-Melanogaster. Experimental Parasitology. 1990;70:466–475. doi: 10.1016/0014-4894(90)90131-u. [DOI] [PubMed] [Google Scholar]
- 9.Morales J, Chiu H, Oo T, Plaza R, Hoskins S, Govind S. Biogenesis, structure, and immune-suppressive effects of virus-like particles of a Drosophila parasitoid, Leptopilina victoriae. Journal of Insect Physiology. 2005;51:181–195. doi: 10.1016/j.jinsphys.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Russo J, Brehelin M, Carton Y. Haemocyte changes in resistant and susceptible strains of D. melanogaster caused by virulent and avirulent strains of the parasitic wasp leptopilina boulardi. Journal of Insect Physiology. 2001;47:167–172. doi: 10.1016/s0022-1910(00)00102-5. [DOI] [PubMed] [Google Scholar]
- 11.Rizki R, Rizki T. Selective destruction of a host blood cell type by a parasitoid wasp. PNAS. 1984;81:6154–6158. doi: 10.1073/pnas.81.19.6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powell JR. Progress and prospects in evolutionary biology : the Drosophila model. New York: Oxford University Press; 1997. [Google Scholar]
- 13.Quicke DLJ. Parasitic wasps. London ; New York: Chapman & Hall; 1997. [Google Scholar]
- 14.Anderson R, Ressegui M, Brenman J. Calcium/Calmodulin-Dependent protein kinase II alters structural plasticity and cytoskeletal dynamics in Drosophila. Journal of neuroscience. 2005;25:8878–8888. doi: 10.1523/JNEUROSCI.2005-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bodmer R, Carretto R, Jan YN. Neurogenesis of the peripheral nervous system in Drosophila embryos: DNA replication patterns and cell lineages. Neuron. 1989;3:21–32. doi: 10.1016/0896-6273(89)90112-8. [DOI] [PubMed] [Google Scholar]
- 16.Gao FB, Brenman JE, Jan LY, Jan YN. Genes regulating dendritic outgrowth, branching, and routing in Drosophila. Genes Dev. 1999;13:2549–2561. doi: 10.1101/gad.13.19.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merritt DJ, Whitington PM. Central projections of sensory neurons in the Drosophila embryo correlate with sensory modality, soma position, and proneural gene function. J Neurosci. 1995;15:1755–1767. doi: 10.1523/JNEUROSCI.15-03-01755.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore AW, Jan LY, Jan YN. hamlet, a binary genetic switch between single- and multiple- dendrite neuron morphology. Science. 2002;297:1355–1358. doi: 10.1126/science.1072387. [DOI] [PubMed] [Google Scholar]
- 19.Shima Y, Kawaguchi S, Kosaka K, Nakayama M, Hoshino M, Nabeshima Y, Hirano T, Uemura T. Opposing roles in neurite growth control by two seven-pass transmembrane cadherins. Nature Neuroscience. 2007;10:963–969. doi: 10.1038/nn1933. [DOI] [PubMed] [Google Scholar]
- 20.Williams DW, Shepherd D. Persistent larval sensory neurons in adult Drosophila melanogaster. J Neurobiol. 1999;39:275–286. [PubMed] [Google Scholar]
- 21.Williams DW, Truman JW. Cellular mechanisms of dendrite pruning in Drosophila: insights from in vivo time-lapse of remodeling dendritic arborizing sensory neurons. Development. 2005;132:3631–3642. doi: 10.1242/dev.01928. [DOI] [PubMed] [Google Scholar]
- 22.Rosenzweig M, Brennan KM, Tayler TD, Phelps PO, Patapoutian A, Garrity PA. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes Dev. 2005;19:419–424. doi: 10.1101/gad.1278205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grueber W, Jan L, Jan Y. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development. 2002;129:2867–2878. doi: 10.1242/dev.129.12.2867. [DOI] [PubMed] [Google Scholar]
- 24.Grueber WB, Ye B, Yang CH, Younger S, Borden K, Jan LY, Jan YN. Projections of Drosophila multidendritic neurons in the central nervous system: links with peripheral dendrite morphology. Development. 2007;134:55–64. doi: 10.1242/dev.02666. [DOI] [PubMed] [Google Scholar]
- 25.Schrader S, Merritt DJ. Central projections of Drosophila sensory neurons in the transition from embryo to larva. J Comp Neurol. 2000;425:34–44. [PubMed] [Google Scholar]
- 26.Ainsley JA, Pettus JM, Bosenko D, Gerstein CE, Zinkevich N, Anderson MG, Adams CM, Welsh MJ, Johnson WA. Enhanced locomotion caused by loss of the Drosophila DEG/ENaC protein Pickpocket1. Curr Biol. 2003;13:1557–1563. doi: 10.1016/s0960-9822(03)00596-7. [DOI] [PubMed] [Google Scholar]
- 27.Hughes CL, Thomas JB. A sensory feedback circuit coordinates muscle activity in Drosophila. Mol Cell Neurosci. 2007;35:383–396. doi: 10.1016/j.mcn.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song W, Onishi M, Jan LY, Jan YN. Peripheral multidendritic sensory neurons are necessary for rhythmic locomotion behavior in Drosophila larvae. Proc Natl Acad Sci U S A. 2007;104:5199–5204. doi: 10.1073/pnas.0700895104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 30.Sweeney S, Broadie K, Keane J, Niemann H, O’Kane C. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 31.Smith SA, Shepherd D. Central afferent projections of proprioceptive sensory neurons in Drosophila revealed with the enhancer-trap technique. J Comp Neurol. 1996;364:311–323. doi: 10.1002/(SICI)1096-9861(19960108)364:2<311::AID-CNE9>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 32.Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, Deisseroth K. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- 33.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 34.Schroll C, Riemensperger T, Bucher D, Ehmer J, Voller T, Erbguth K, Gerber B, Hendel T, Nagel G, Buchner E, Fiala A. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr Biol. 2006;16:1741–1747. doi: 10.1016/j.cub.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 35.Li X, Gutierrez DV, Hanson MG, Han J, Mark MD, Chiel H, Hegemann P, Landmesser LT, Herlitze S. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc Natl Acad Sci U S A. 2005;102:17816–17821. doi: 10.1073/pnas.0509030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci U S A. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carton Y. Experimental-Analysis of Interactions between Drosophila-Melanogaster and Parasitic Wasp - Leptopilina-Boulardi (Sympatry, Allopatry, Xenopatry) Genetics Selection Evolution. 1984;16:417–430. doi: 10.1186/1297-9686-16-4-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Lenteren JC, Isidoro N, Bin F. Functional anatomy of the ovipositor clip in the parasitoid Leptopilina heterotoma (Thompson) (Hymenoptera : Eucoilidae), a structure to grip escaping host larvae. International Journal of Insect Morphology & Embryology. 1998;27:263–268. [Google Scholar]
- 39.Purves D, Augustine GJ, Fitzpatrick D, Katz LC, LaMantia A, McNamara JO, Williams SM. Neuroscience. 2. Sunderland, MA: Sinauer Associates, Inc.; 2001. [Google Scholar]
- 40.Wolf R, Heisenberg M. Basic organization of operant behavior as revealed in Drosophila flight orientation. J Comp Physiol [A] 1991;169:699–705. doi: 10.1007/BF00194898. [DOI] [PubMed] [Google Scholar]
- 41.Liu L, Wolf R, Ernst R, Heisenberg M. Context generalization in Drosophila visual learning requires the mushroom bodies. Nature. 1999;400:753–756. doi: 10.1038/23456. [DOI] [PubMed] [Google Scholar]
- 42.Wustmann G, Rein K, Wolf R, Heisenberg M. A new paradigm for operant conditioning of Drosophila melanogaster. J Comp Physiol [A] 1996;179:429–436. doi: 10.1007/BF00194996. [DOI] [PubMed] [Google Scholar]
- 43.Zars T, Wolf R, Davis R, Heisenberg M. Tissue-specific expression of a type I adenylyl cyclase rescues the rutabaga mutant memory defect: in search of the engram. Learn Mem. 2000;7:18–31. doi: 10.1101/lm.7.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy T. The Drosophila Gateway Vector Collection. 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.