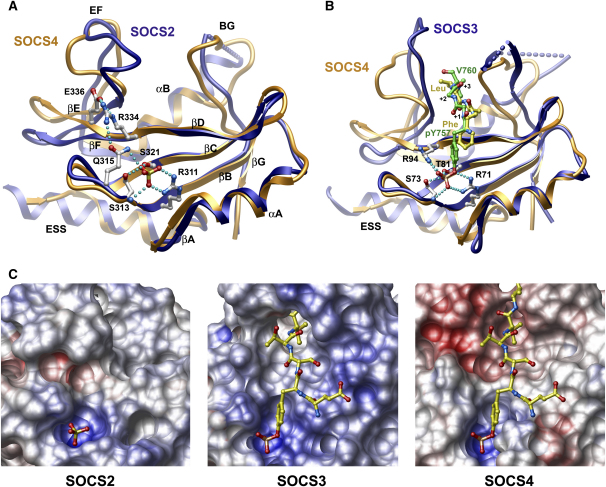
Figure 2.
Structural Comparison of the SH2 Substrate Pockets in SOCS2, SOCS3, and SOCS4
(A) Overlay of the crystal structures of the SOCS4 SH2 (orange) and the SOCS2 SH2 (blue, PDB code: 2C9W). The binding site of the phosphotyrosine moiety in SOCS2 is indicated by the presence of a bound sulfate ion. SOCS4 residues at this site are shown in ball-and-stick representation with their potential hydrogen bonding.
(B) Overlay of the crystal structures of the SOCS4 SH2 (orange) and the murine SOCS3 gp130 complex (blue, PDB code: 2HMH). The phosphotyrosine and three C-terminal residues from the murine gp130-derived peptide are colored green. N-terminal tag residues from a crystallographic SOCS4 neighbor occupy the same SH2 pocket in the SOCS4 structure forming substrate mimetic interactions and are colored yellow.
(C) Surface representation of the SH2 substrate pocket in SOCS2, SOCS3, and SOCS4 colored by electrostatic potential. The bound sulfate ion identifies the phosphotyrosine binding site in SOCS2 (left). The gp130 peptide is shown in complex with SOCS3 (center) and docked onto the SOCS4 surface (right) by the overlay of the two structures (for SOCS4 only the gp130 residues corresponding to the pY−1 to pY+3 positions are shown).