Abstract
Previous findings that reactive oxygen species (ROS) are involved in neuropathic pain, mainly through spinal mechanisms, suggest that ROS may be involved in central sensitization. To investigate the possible role of ROS in central sensitization, we examined in rats the effects of ROS scavengers on capsaicin-induced secondary hyperalgesia, which is known to be mediated by central sensitization. We used two different ROS scavengers: phenyl N-tert-butylnitrone (PBN) and 4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPOL). Intradermal capsaicin injection (20 μg in 20 μl olive oil) into the hind paw produced primary and secondary hyperalgesia. A systemic administration of PBN (100 mg/kg, i.p.) or TEMPOL (200 mg/kg, i.p.) alleviated capsaicin-induced secondary, but not primary, hyperalgesia. Intrathecal injection of PBN (1 mg in 50 μl saline) greatly reduced hyperalgesia, whereas intracerebroventricular or intradermal injection of PBN produced only a minor analgesic effect, suggesting that PBN takes effect mainly through the spinal cord. Electrophysiological recordings from wide dynamic range (WDR) neurons in the dorsal horn showed that intradermal capsaicin enhanced the evoked responses to peripheral stimuli; systemic PBN or TEMPOL restored the responses to normal levels. Removal of ROS thus restored the responsiveness of spinal WDR neurons to normal levels, indicating that ROS may be involved in central sensitization, at least in part by sensitizing WDR neurons.
Keywords: free radicals, inflammatory pain, central sensitization, ROS
1. Introduction
Neural tissue is sensitive to the cytotoxic effects of oxygen free radicals. Oxidative stress is considered a prominent factor in both acute and chronic neurodegenerative diseases and traumatic brain injuries (Jenner, 1994; Contestabile, 2001). Under normal physiological conditions, production of reactive oxygen species (ROS) is balanced by several cellular antioxidant mechanisms (Jenner, 1994; Contestabile, 2001). In certain conditions, however, levels of ROS rise to the point that may endanger the functional and structural integrity of cells, sometimes leading to irreversible damage (Jenner, 1994; Lewén et al., 2000; Contestabile, 2001). These dangerously high levels of ROS may be due to either greater production of ROS or a deficiency in defensive antioxidant mechanisms.
Recent studies indicate that ROS are also involved in persistent pain. For example, removal of excessive ROS by free radical scavengers, such as phenyl N-tert-butylnitrone (PBN) and 4-hydroxy-,2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPOL), produced significant analgesic effects in both neuropathic pain (Tal, 1996; Kim et al., 2004) and inflammatory pain (Thiemermann, 2003). Furthermore, increased production of ROS (Park et al., 2006) and enhanced antioxidant activity (Guedes et al., 2006) were observed in the spinal cord after a peripheral nerve injury. Increased levels of extracellular hydrogen peroxide were also observed in the spinal trigeminal nucleus after formalin injection into the lip of the rat, and this increase coincided with pain behaviors (Viggiano et al., 2005). These studies suggest that higher levels of ROS and increased antioxidant activity in the spinal cord and brainstem after peripheral nerve injury or tissue inflammation may be important factors in persistent pain.
While it is becoming clear that ROS are involved in persistent pain, the mechanisms by which they contribute to pain are still not clear. Capsaicin, a pungent agent found in hot peppers, produces acute inflammatory pain when injected into the skin. Capsaicin-induced pain has well-defined peripheral and central components (Willis, 2001). Thus, it is an excellent model for studying the mechanisms of ROS in persistent pain and for determining the effects of ROS on the central versus the peripheral components of pain. The present study examined the effects of PBN and TEMPOL on capsaicin-induced primary and secondary hyperalgesia. In addition, we examined the electrophysiological activities of dorsal horn neurons in rats after treatment with capsaicin alone or with capsaicin plus PBN or TEMPOL. The study suggests that ROS play a critical role in the development and the maintenance of capsaicin-induced hyperalgesia through central sensitization in the spinal cord. Parts of this study have been presented in abstract form (Lee et al., 2005).
2. Materials and methods
2.1. Experimental animals
Adult male Sprague-Dawley rats (Harlan Sprague-Dawley Co., Houston, TX) were used for behavioral studies (200-250 g body weight) and electrophysiological studies (300-350 g). Animals were housed in groups of two or three in plastic cages with soft bedding and free access to food and water under a 12/12 hour reversed light-dark cycle (dark cycle: 8:00 a.m.–8:00 p.m.). All animals were acclimated for 1 week before any experimental procedures. All experimental protocols were approved by the Animal Care and Use Committee of the University of Texas Medical Branch and are in accordance with the National Institute of Health’s Guide for the Care and Use of Laboratory Animals.
2.2. Capsaicin injection
For injection of capsaicin, each rat was anesthetized with halothane (3% for induction and 2% for maintenance) in a flow of O2 and was placed in a prone position. Capsaicin (20 μg in 20 μl of olive oil) was injected intradermally (i.d.) into the left hind paw using a 27-gauge needle attached to a Hamilton syringe. The needle was inserted at a site near the heel (marked X in Fig. 1A) and was advanced to the middle of the plantar surface (site I in Fig. 1A). A successful injection was noted by formation of a small bleb about 5 mm in diameter, which lasted for a couple of minutes. The needle insertion site was pressed for 1 minute after removal of the needle to prevent leakage of the solution. Anesthesia was discontinued, and the rats revived in about 5 minutes. They were then returned to their cages.
Fig. 1.
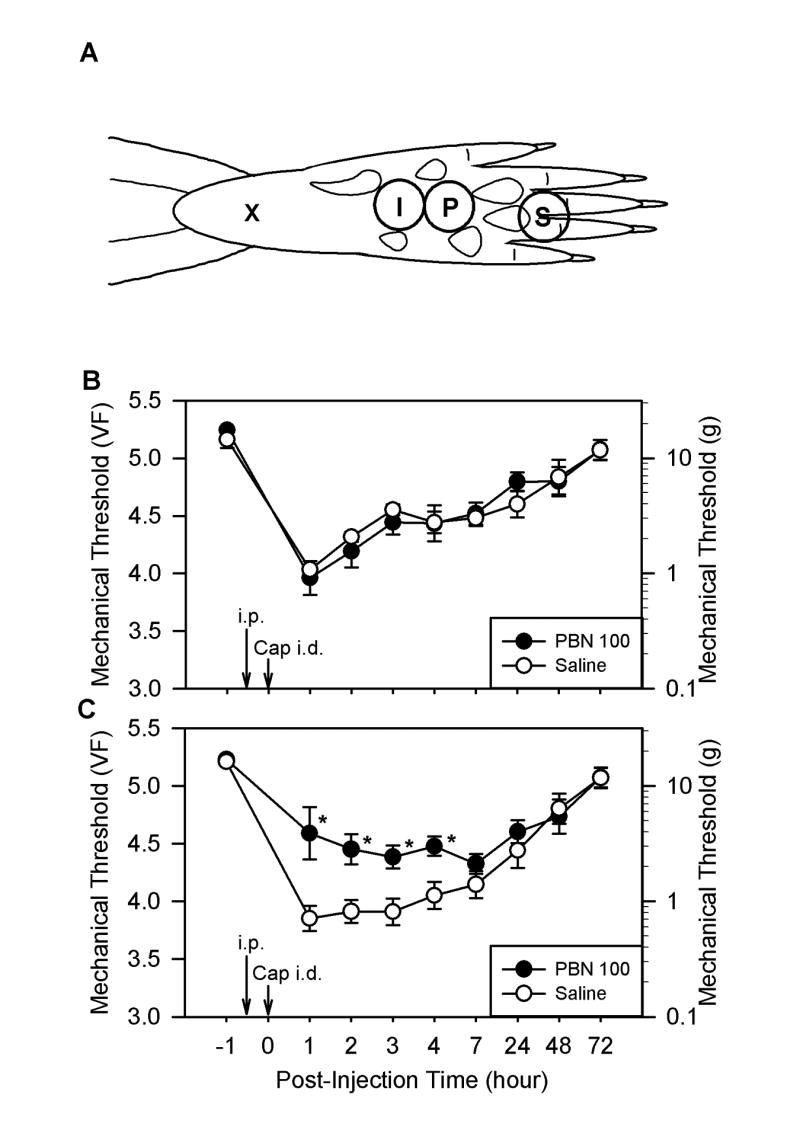
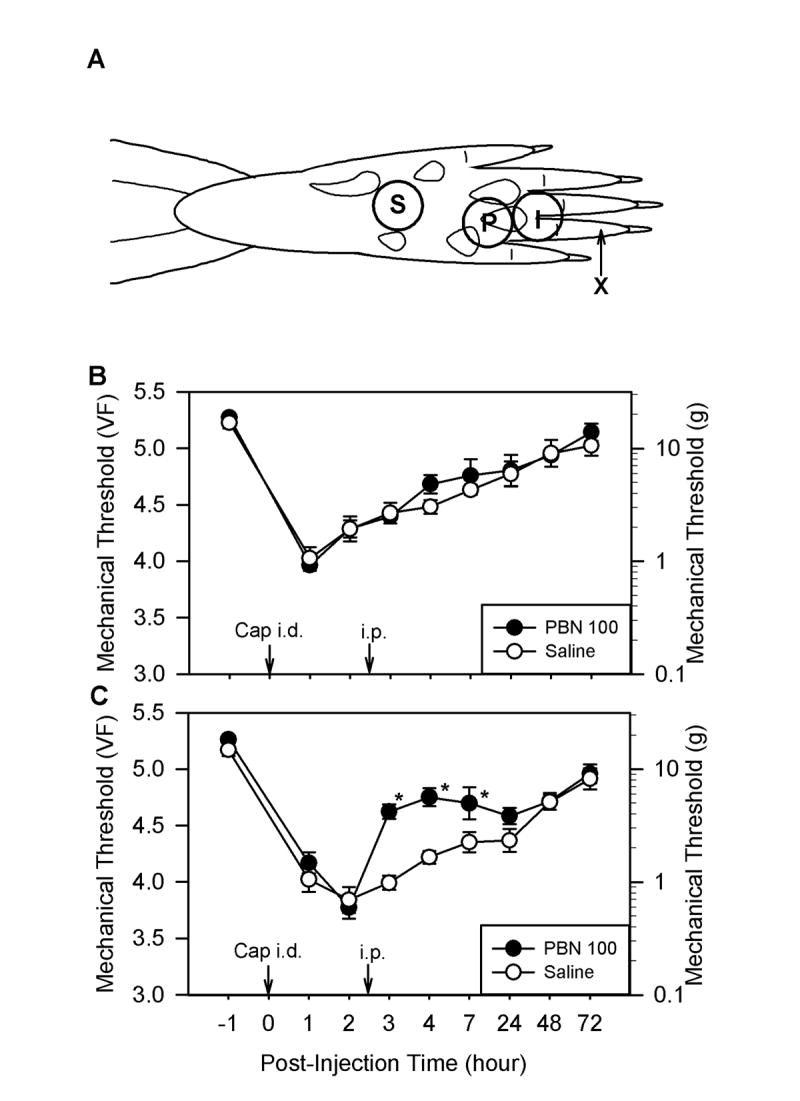
A: Drawing showing the sites of capsaicin injection and behavioral testing in the rat hind paw. For capsaicin injection, a 27-gauge hypodermic needle was inserted subcutaneously at the heel of the foot (X) and advanced to the injection site (I), and capsaicin (20 μg in 20 μl olive oil) was injected intradermally. Mechanical thresholds to von Frey filament stimuli were measured at site P for primary hyperalgesia and at site S for secondary hyperalgesia. B and C: The effect of systemic PBN pretreatment on capsaicin-induced hyperalgesia. Panels B and C show the effects of PBN on primary and secondary hyperalgesia, respectively. Mechanical thresholds shown in B and C were measured at sites labeled “P” and “S” in panel A, respectively. After measuring baseline mechanical threshold (at 1 hour before capsaicin treatment), PBN (100 mg/kg, i.p.) was injected, and then capsaicin was injected 0.5 hours later (Cap i.d., 20 μg in 20 μl of olive oil). The same volume of saline was injected instead of PBN for the control group. Data are expressed as mean ± SEM, and asterisks indicate values significant different from corresponding values in the saline group according to Duncan’s post hoc test after two-way repeated ANOVA. PBN 100: rats received 100 mg/kg PBN and capsaicin (n = 9); Saline: rats received saline and capsaicin (n = 8); i.p.: intraperitoneal injection of either PBN or saline; Cap i.d.: intradermal capsaicin injection.
2.3. Behavioral testing for assessment of mechanical thresholds
The 50% foot withdrawal thresholds in response to mechanical stimuli applied to the left hind paw were measured, and these mechanical thresholds were used as an indicator for pain. Mechanical thresholds were assessed before capsaicin injection and 1, 2, 3, 4, 7, 24, 48, and 72 hours after injection. For each test, the animal was placed in a plastic chamber (8.0 × 8.5 × 20 cm) on top of a mesh screen platform and habituated for at least 10 minutes. Thresholds were determined by the up-down method (Dixon, 1980; Chaplan et al., 1994) using a set of von Frey monofilaments (von Frey numbers: 3.65, 3.87, 4.10, 4.31, 4.52, 4.74, 4.92 and 5.16; equivalent to 0.45, 0.74, 1.26, 2.04, 3.31, 5.50, 8.32 and 14.45 g, respectively). The extent of primary and secondary hyperalgesia was measured by applying stimuli to the inflamed area (site P) and the surrounding area (the base and/or the proximal part of the third and fourth digits, site S), respectively (Fig. 1A). A von Frey filament was applied perpendicularly to the designated site with sufficient force to bend the filament slightly for 2–3 seconds. An abrupt withdrawal of the foot during stimulation or immediately after stimulus removal was considered to be a positive response. The first stimulus was always initiated with the 4.31 filament. If there was a positive response, the next lower filament was used, and if not, the next higher filament was applied. This testing pattern was continued until we had recorded responses to six von Frey stimuli from the first change of response (either higher or lower than the first stimulus, depending on whether the first response was negative or positive). The responses were then converted into a 50% threshold value using the formula: 50% threshold = 10(X + kd)/104, where X is the value of the final von Frey hair used in logarithmic units, k is the tabular value for positive/negative responses, and d is the mean difference between stimuli in logarithmic units (0.22) (Dixon, 1980). When positive or negative responses were still observed at the end of a stimulus session, values of 3.54 or 5.27 were assigned, respectively, by assuming a value of ± 0.5 for k in these cases. The behavioral data were plotted using a linear scale in von Frey values as well as in grams.
2.4. Treatment with ROS scavengers
To determine the effects of ROS scavengers on capsaicin-induced hyperalgesia, we used two different ROS scavengers: phenyl N-tert-butylnitrone (PBN; Sigma, St Louis, MO) and 4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPOL; Sigma, St Louis, MO). These agents were dissolved in saline and filtered using a filter with 0.2 μm pore size. One hundred milligrams of PBN (or 200 mg of TEMPOL) was dissolved in 5 ml of saline, and 1 ml of this solution was injected intraperitoneally per 200 g body weight (100 mg/kg for PBN and 200 mg/kg for TEMPOL). The same volume of saline was used as a control. The ROS scavenger treatment was administered either 0.5 hours before capsaicin treatment (pre-treatment) or 2.5 hours after (post-treatment). The tested dose of 100 mg/kg of i.p. PBN was the lowest, most effective dose without side effects, based on our previous study (Kim et al., 2004). On the other hand, the tested dose of 200 mg/kg of i.p. TEMPOL is based on preliminary studies done with several different doses (50, 100, 200 and 300 mg/kg). The 200 mg/kg i.p. TEMPOL was the lowest, most effective dose without side effects. To examine the dose response of a ROS scavenger, one of three doses of PBN (20, 50, or 100 mg/kg) or saline was injected intraperitoneally (i.p.) 2.5 hours after intradermal injection of capsaicin, and secondary hyperalgesia was measured as above.
To locate the main site of ROS action, the effect of a ROS scavenger on the secondary hyperalgesia was determined after injecting PBN via three different routes: intradermal (i.d.), intrathecal (i.t.), and intracerebroventricular (i.c.v.). For all three routes of administration, 1 mg PBN dissolved in 50 μl of saline was injected at 0.5 hours before or 2.5 hours after capsaicin treatment under halothane anesthesia (3% for induction and 2% for maintenance in a flow of O2). The same volume of saline (vehicle) was used as a control. The 1 mg of i.t. PBN is the lowest, most effective analgesic dose based on our preliminary studies as well as previous studies where several different doses of PBN between 0.1 to 2.5 mg were tested for analgesic effect in neuropathic pain (Kim et al., 2004). For intradermal injection, PBN was injected into the left hind paw at the same location where capsaicin was injected and via the same route. For intrathecal injection, the rat was anesthetized and the hair was clipped from its lower back. We used the modified method of direct transcutaneous intrathecal injection (Mestre et al., 1994; Lee et al., 2006). In brief, the lumbar vertebrae just cranial to the both iliac crests were held by the thumb and middle finger, and the sixth lumbar (L6) spinous process was located by palpating the highest spinous process with the index finger. A 27-gauge hypodermic needle (1.25 inch) connected to a 100-μl Hamilton syringe was inserted from the caudal end and immediately lateral to the L6 spinous process with a 45° angle to the vertebral column and was pushed slowly toward the cranioventral direction. When a sudden lateral tail movement occurred after penetration of the ligamentum flavum (felt as a pop), PBN or saline was injected slowly for 30 seconds. The syringe was held for 10 more seconds before removal to prevent outflow of the drug. The i.t. injection volume of 50 μl was sufficient enough to spread consistently to caudal thoracic vertebrae, which contain the lumbar enlargement of the spinal cord, as determined with i.t. injection of methylene blue dye in the preliminary study (Lee et al., 2006). For i.c.v. injection, the rat was anesthetized and placed on a stereotaxic apparatus. After a skin incision was made on the rat’s cranium, a small hole was drilled into the skull, and a 30-gauge needle was advanced into the right lateral ventricle (AP: −0.8 mm from the bregma; L: 1.6 mm from the midline; depth: 4 mm below the skull surface). The needle was connected to a Hamilton syringe, and PBN or saline was slowly delivered for 11 minutes by an infusion pump. The needle was held in the place for 5 more minutes and removed. The wound was then closed, and the rat was returned to its cage when it revived. Based on the preliminary studies done on 6 rats, the i.c.v. injection of the same volume (50 μl) of methylene blue dye was consistently distributed from the right ventricle caudally to the C3-T4 levels.
2.5. Extracellular recordings from wide dynamic range (WDR) neurons
The rat was anesthetized with urethane (1.5 g/kg, i.p.) for surgery and recordings. The trachea was cannulated to provide unobstructed ventilation, and a catheter was inserted into the left external jugular vein for administration of drugs. A laminectomy was performed to expose the spinal cord at the T13–L2 vertebral level. The rat was placed in a stereotaxic apparatus, and the spinal cord was bathed in a pool of warm (37°C) mineral oil. Core body temperature was maintained at 37°C by a heating blanket. The animal was paralyzed with an initial bolus of pancuronium bromide (1 mg/kg), and it was ventilated artificially to maintain an end-tidal CO2 between 3.5% and 4.5%. The level of pancuronium was maintained by continuous infusion (0.4–0.6 mg/kg/hour).
Extracellular recordings were made from the dorsal horn WDR neurons that responded to both innocuous and noxious mechanical stimuli. Cells were searched at the L4 and L5 segments of the spinal cord using low-impedance (0.4–0.8 MΩ) carbon filament electrode (Kation Scientific, Minneapolis, MN) mounted on an electronic micromanipulator. Brush stimuli were used to search for dorsal horn neurons. WDR neurons with receptive fields located on the plantar surface of the left hind paw were recorded extracellularly. Recordings were made only from single neurons whose spike amplitude could be easily discriminated from other neurons (at least twice the height). Electrophysiological activity was amplified and displayed on an oscilloscope, and then fed into a data analysis system (CED 1401, PC) with Spike-2 software. Throughout the experiment, spike size and configuration were continuously monitored with the use of Spike-2 software to ensure that the same WDR neuron was recorded and that the relationship of the recording electrode to the neuron remained constant.
After the receptive field of the identified neuron was mapped, graded mechanical stimuli were applied. The stimuli consisted of brushing the skin with a soft brush and application of von Frey filaments with three bending forces (1, 2, and 20 g) in a stereotyped manner (a series of 10 applications within a 10-second time period with a 10-second interval between each stimulation series, which was repeated three times). Baseline activities were recorded three times before capsaicin application. Capsaicin (300 μg in 100 μl olive oil) was injected within the receptive field, and mechanical stimuli were applied approximately 1.0–1.5 cm away from the capsaicin injection site. The responses of WDR neurons to the same mechanical stimuli were recorded 0.5 and 1 hour after capsaicin injection. Immediately after the second recording, PBN was injected either intraperitoneally (i.p., 100 mg/kg) or intravenously (i.v., 50 mg/kg) in bolus, and TEMPOL (200 mg/kg) was injected intraperitoneally. The responses of the same neurons to the same mechanical stimuli were recorded again 0.5 hours after PBN or TEMPOL administration. Responses to mechanical stimuli were measured as the number of discharges per second during stimulation, and measurements were performed three times.
2.6. Statistical analysis
Data are presented as mean plus or minus standard error of the mean (SEM) and were analyzed using the statistical program SigmaStat (Version 3., Systat Software, CA). Statistical analyses were performed using one-way and/or two-way analysis of variance (ANOVA) with one repeated factor (time), followed by Duncan’s post hoc test.
3. Results
3.1. Intradermal capsaicin injection induces primary and secondary hyperalgesia
The average mechanical threshold of the rat hind paw was the von Frey value of 5.27, which is equivalent to 18.71 g, in normal rats. One hour after the intradermal injection of capsaicin, mechanical thresholds decreased dramatically to 4.03 ± 0.07 (mean ± SEM, n = 8) and 3.85 ± 0.11 (n = 8), in the area where inflammation developed due to capsaicin (primary hyperalgesia, area P in Fig. 1A) and in the neighboring surrounding non-inflamed region (secondary hyperalgesia, area S in Fig. 1A), respectively (Fig. 1B and 1C). These reduced mechanical thresholds gradually and steadily recovered to pretreatment levels by 72 hours after capsaicin treatment for both primary and secondary hyperalgesia.
3.2. ROS scavengers reduce capsaicin-induced secondary hyperalgesia in a dose-dependent manner
Systemic injection of the ROS scavenger PBN (100 mg/kg, i.p.) 0.5 hours before capsaicin treatment did not have any effect on capsaicin-induced primary hyperalgesia (Fig. 1B, n = 9). PBN pretreatment, however, significantly reduced the capsaicin-induced secondary hyperalgesia to a von Frey value of 4.59 ± 0.23 (n = 9) at 1 hour after capsaicin treatment compared to 3.85 ± 0.11 with saline pretreatment (Fig. 1C). This antihyperalgesic effect of PBN on secondary hyperalgesia lasted approximately 4 hours. Similarly, PBN treatment (100 mg/kg, i.p.) 2.5 hours after capsaicin had no effect on primary hyperalgesia (Fig. 2A, n = 6) but significantly reduced secondary hyperalgesia for at least 3 hours (Fig. 2B). In the dose-response study (Fig. 2C), only 100 mg/kg of PBN (n = 9) significantly reduced secondary hyperalgesia, while 50 mg/kg PBN (n = 7) showed a small and statistically insignificant reduction from the saline controls. The 20 mg/kg PBN treatment (n = 6; Fig. 2C) did not show any difference from the saline controls. Another type of ROS scavenger, TEMPOL (200 mg/kg, i.p., n = 6), also showed a similar antihyperalgesic effect on capsaicin-induced secondary hyperalgesia but not on primary hyperalgesia (Fig. 3A and 3B).
Fig. 2.
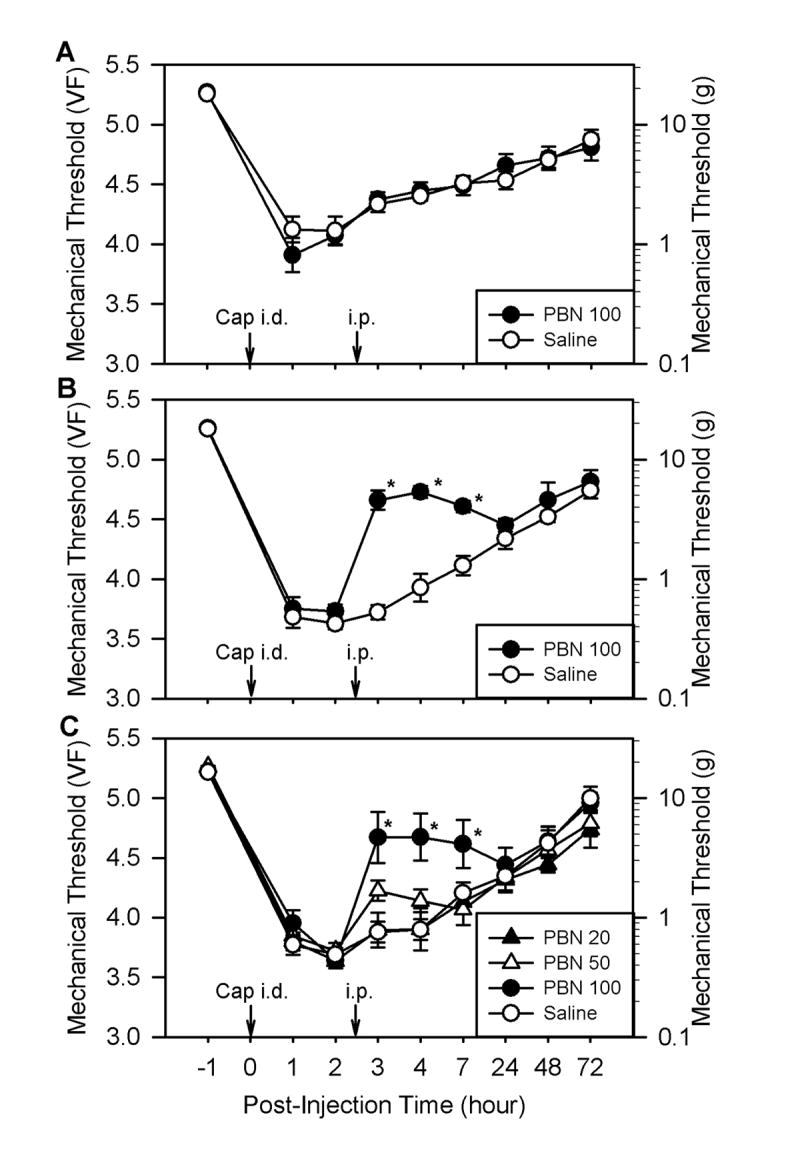
The effect of systemic PBN post-treatment on capsaicin-induced hyperalgesia. Panels A and B show the effects of PBN on primary and secondary hyperalgesia, respectively. Mechanical thresholds shown in panels A (n = 6 for both groups) and B (n = 6) were measured at sites labeled “P” and “S” in panel A of Fig. 1, respectively. Immediately after measuring the baseline mechanical threshold, we injected capsaicin intradermally at time 0 (Cap i.d., 20 μg in 20 μl of olive oil) and then injected PBN (100 mg/kg, i.p.) 2.5 hours later. The same volume of saline was injected instead of PBN for the control group. Data in panel C (n = 6 for PBN 20 mg/kg, n = 7 for PBN 50 mg/kg, n = 9 for PBN 100 mg/kg, and n = 10 for the saline control group) are multiple groups of graded doses of PBN. Data are expressed as mean ± SEM, and asterisks indicate values significantly different from the corresponding values in the saline group according to Duncan’s post hoc test after two-way repeated ANOVA.
Fig. 3.
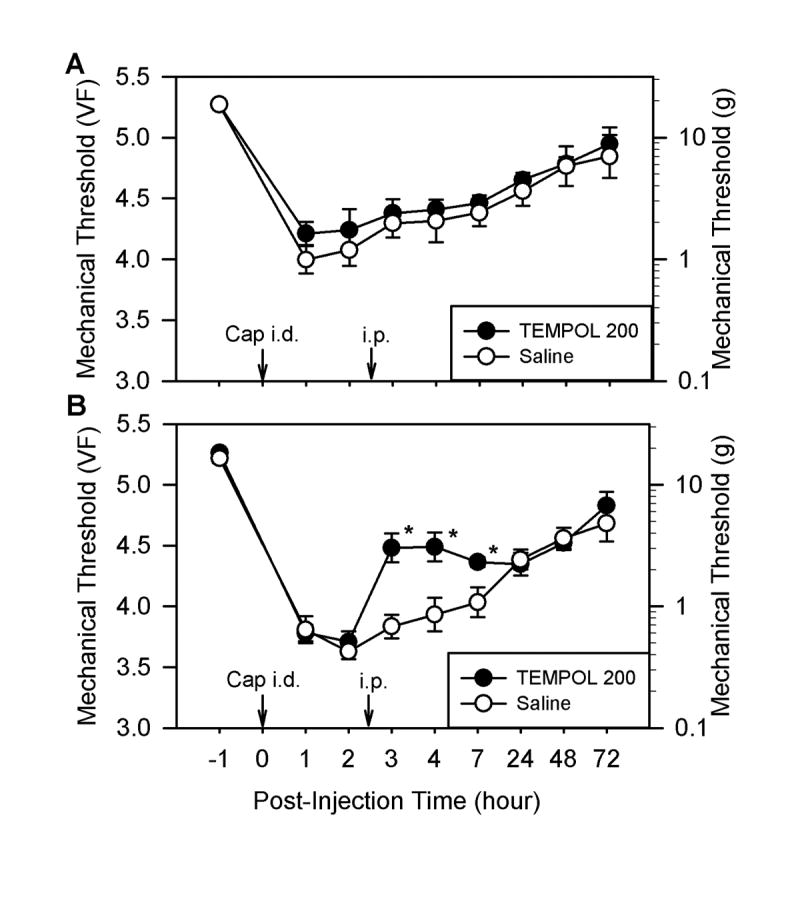
The effect of systemic TEMPOL post-treatment on capsaicin-induced hyperalgesia. Panels A and B show effects of TEMPOL on primary and secondary hyperalgesia, respectively. Mechanical thresholds shown in panels A (n = 6 for both groups) and B (n = 6) were measured at sites labeled “P” and “S” in panel A of Fig. 1, respectively. Immediately after measuring the baseline mechanical threshold, we injected capsaicin intradermally at time 0 (Cap i.d., 20 μg in 20 μl of olive oil) and then injected TEMPOL (200 mg/kg, i.p.) 2.5 hours later. The same volume of saline was injected instead of TEMPOL for the control group. Data are expressed as mean ± SEM, and asterisks indicate values significantly different from the corresponding values in the saline group according to Duncan’s post hoc test after two-way repeated ANOVA.
3.3. The main site of ROS action is the spinal cord
To locate the major site of action of ROS, the effect of PBN on capsaicin-induced secondary hyperalgesia was examined by injecting PBN to three different sites: the periphery, the spinal intrathecal space, and the lateral ventricle. As for the periphery, PBN (1 mg in 50 μl saline, i.d., n = 11) was injected into the same location as the capsaicin injection (peripheral site I in Fig. 1A), 2.5 hours after capsaicin treatment. There was a small but significant increase in mechanical thresholds only at 1.5 hours after PBN treatment compared to saline treatment (Fig. 4A). Intradermal pretreatment with the same dose of PBN (0.5 hours before capsaicin treatment) did not have any effect on capsaicin-induced secondary hyperalgesia (data not shown).
Fig. 4.
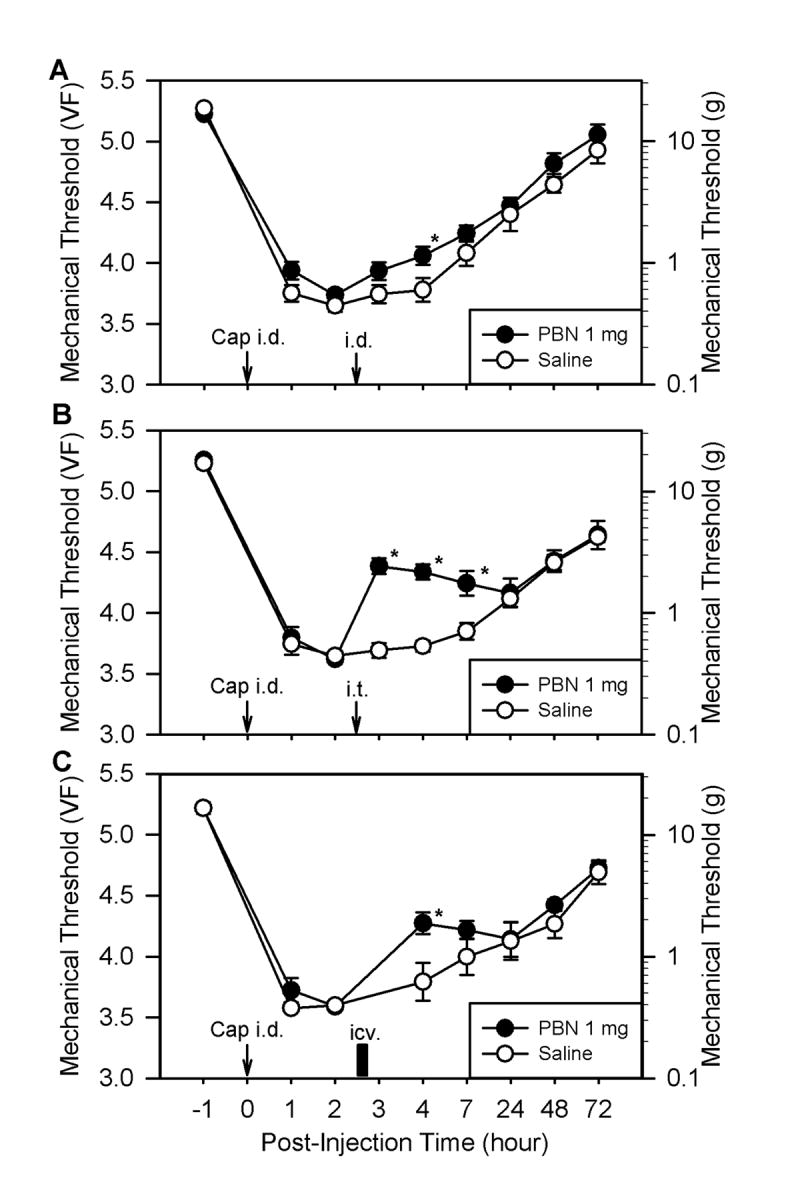
The effects of PBN injections given at three different locations on capsaicin-induced hyperalgesia. All data were collected by measuring the threshold at a site labeled “S” in panel A of Fig. 1 to examine secondary hyperalgesia. Panels A, B, and C show the effects of post-treatment with PBN (1 mg in 50 μl of saline) 2.5 hours after capsaicin injection. PBN was given intradermally at the peripheral injection site (i.d., n = 11), intrathecally to the lumbar spinal segments (i.t., n = 11), and intracerebroventricularly into the lateral ventricle (icv., n = 5), respectively. The same volume of saline was injected in the control group. Data are expressed as mean ± SEM, and asterisks indicate values significantly different from the corresponding values in the saline group according to Duncan’s post hoc test after two-way repeated ANOVA.
Intrathecal injection of PBN (1 mg in 50 μl saline, i.t., n = 11), on the other hand, significantly increased mechanical thresholds to 4.38 ± 0.06 (n = 9) 0.5 hours after PBN treatment compared to 3.69 ± 0.06 with saline treatment. This antihyperalgesic effect of PBN on secondary hyperalgesia lasted approximately 3 hours (Fig. 4B). A higher dose of PBN (2.5 mg in 50 μl saline, i.t.) did not show any greater increase in mechanical threshold, but extended the antihyperalgesic effect for up to 24 hours (data not shown). Intrathecal pretreatment (0.5 hours before capsaicin treatment) of the same dose of PBN (1 mg in 50 μl saline) produced significantly reduced secondary hyperalgesia (4.35 ± 0.08, 1 hour post-capsaicin) compared to saline pretreatment controls (data not shown).
The intracerebroventricular injection of PBN (1 mg in 50 μl saline, i.c.v., n = 5) produced a moderate but significant increase in mechanical thresholds compared to saline controls only at 1.5 hours after PBN injection (Fig. 4C).
The antihyperalgesic effect of PBN after intrathecal administration (average threshold was 4.38 ± 0.06 at 0.5 hours post-PBN) was only slightly smaller than after systemic administration (4.66 ± 0.08 at 0.5 hours post-PBN). These data, along with additional small effects from peripheral administration as well as from intracerebroventricular injections, suggest that the spinal cord is a major, but perhaps not exclusive, site for ROS action.
3.4. ROS scavengers reverse the capsaicin-induced enhancement of spinal WDR neuron activities
The responses of a total of twelve dorsal horn WDR neurons to mechanical stimuli were recorded after treatment with capsaicin and then with the ROS scavenger PBN. All recorded WDR neurons responded to all four stimuli (brush and three von Frey filaments with different bending forces) applied to the receptive fields. Two examples are shown in Fig. 5A and B. After intradermal capsaicin treatment, the rate of discharge of WDR neurons in response to the same mechanical stimuli was moderately increased at 30 minutes and further increased at 1 hour. In all recorded WDR neurons, systemic PBN treatment either by intraperitoneal (100 mg/kg, n = 7) or by intravenous (50 mg/kg, n = 5) injection reversed the capsaicin-induced enhanced discharges almost to the pre-capsaicin baseline levels within 30 minutes after PBN treatment (Fig. 5C). Systemic administration of TEMPOL (200 mg/kg, i.p.) had a similar effect (data not shown).
Fig. 5.
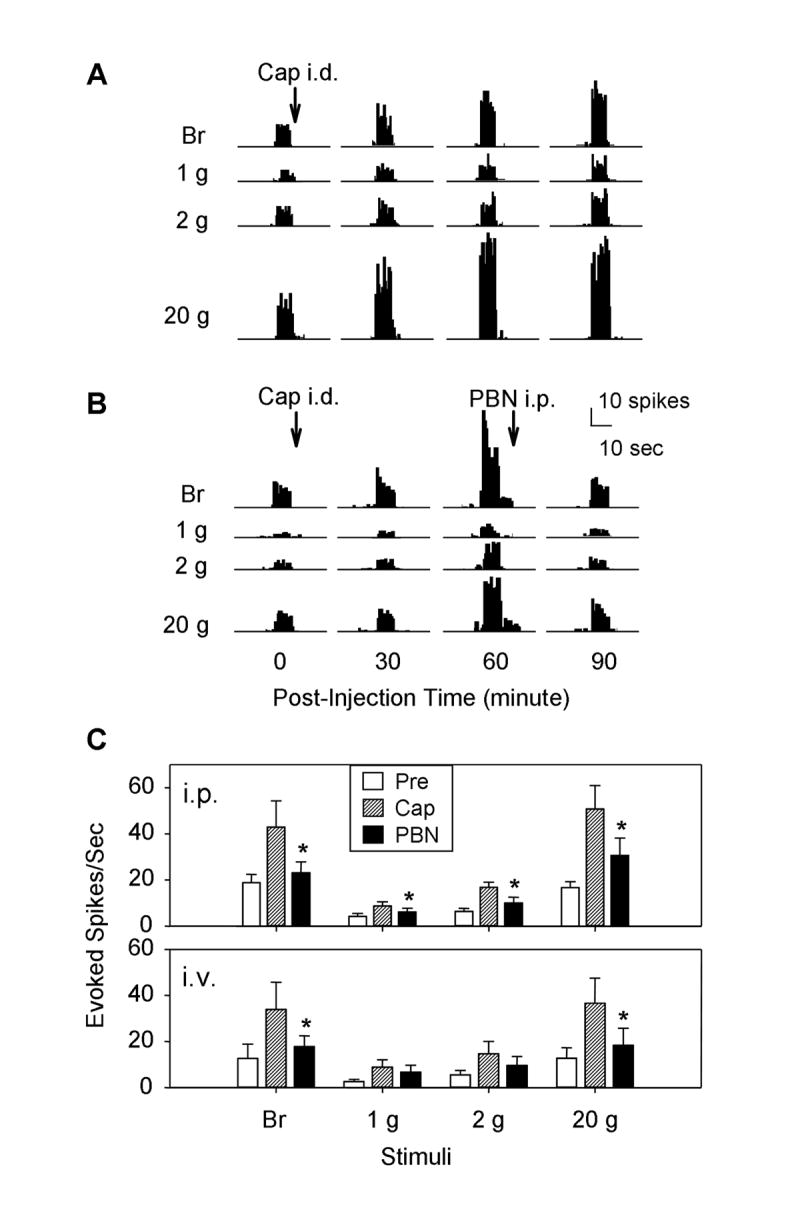
The responses of the spinal dorsal horn WDR neurons to mechanical stimuli in normal, after capsaicin and then PBN treatments. Panels A and B show examples of extracellular recordings of spinal WDR neuron responses to peripheral mechanical stimuli (brush and 1, 2, and 20 g von Frey filaments). In panel A, activities were recorded before and after capsaicin (300 μg in 100 μl olive oil, i.d.; time of injection indicated by “Cap i.d.”). The evoked responses were greatly enhanced by 60 and 90 minutes after the injection. In panel B, activities were recorded before and after capsaicin treatment and then again after PBN treatment (100 mg/kg, i.p.).The enhanced evoked responses induced by capsaicin injection were reduced by systemic injection of PBN. Panel C shows group data of all recorded WDR neurons after intraperitoneal (i.p., n = 7) and intravenous (i.v., n=5) injection of PBN. Data are expressed as mean ± SEM, and asterisks indicate values significantly different from the values after capsaicin treatment of the same group according to Duncan’s post hoc test after one-way repeated ANOVA.
3.5. Capsaicin-induced hyperalgesia is not due to capsaicin affecting the plantar nerve in passage
In our experimental design, capsaicin injection was administered proximal to the behavioral testing site, and thus there is a possibility that capsaicin might have affected the plantar nerve trunk innervating the receptive fields. To test this possibility, the capsaicin injection site was moved distal to the behavioral testing site, and then capsaicin effects were evaluated. As shown in Fig. 6A, capsaicin (20 μg in 20 μl olive oil, i.d.) was injected into the base of third and fourth digits (site I) by inserting the needle near the tip of the fourth digit (marked X). Levels of primary and secondary hyperalgesia were tested at sites P and S in Fig. 6A, respectively. The effect of a ROS scavenger was tested by systemic injection of PBN (100 mg/kg, i.p.) or saline 2.5 hours after capsaicin treatment.
Fig. 6.
The effect of systemic PBN on hyperalgesia induced by capsaicin injection at a distal site of the plantar surface. Panel A shows the needle insertion (X) and capsaicin injection site (I) and behavioral testing locations for primary (P) and secondary (S) hyperalgesia. Panels B and C show the effects of PBN on primary and secondary hyperalgesia, respectively. Mechanical thresholds shown in panels B (n = 6 for both groups) and C (n = 6) were measured at sites labeled “P” and “S” in panel A, respectively. Immediately after measuring the baseline mechanical threshold, we injected capsaicin intradermally at time 0 (Cap i.d., 20 μg in 20 μl of olive oil) and then injected PBN (100 mg/kg, i.p.) 2.5 hours later. We injected the same volume of saline instead of PBN for the control group. Data are expressed as mean ± SEM, and asterisks indicate values significantly different from the corresponding values in the saline group according to Duncan’s post hoc test after two-way repeated ANOVA.
When capsaicin was injected to the proximal part of the third and fourth digits, primary and secondary hyperalgesia developed at sites P and S, respectively, and both behavioral testing sites were proximal to the injection site (Fig. 6). The magnitude and duration of primary and secondary hyperalgesia were similar to those recorded with the capsaicin injection at the proximal location, as seen in Figs. 1 and 2. A systemic PBN treatment (100 mg/kg, i.p., n = 6) 2.5 hours after capsaicin injection did not affect primary hyperalgesia (Fig. 6B), but reduced secondary hyperalgesia significantly for 3 hours (Fig. 6C). These data suggest that capsaicin-induced secondary hyperalgesia is due to central sensitization and not due to sensitization of the axons in passage.
4. Discussion
The present study examined the effect of ROS scavengers, PBN and TEMPOL, on capsaicin-induced hyperalgesia and on the evoked responses of dorsal horn neurons. An intradermal injection of capsaicin induced primary and secondary hyperalgesia that developed within 1 hour and lasted for about 7 hours. A systemic injection of a ROS scavenger, either PBN or TEMPOL, transiently reduced secondary, but not primary, capsaicin-induced hyperalgesia in a dose-dependent manner. This antihyperalgesic effect was mediated mainly by spinal mechanisms. The evoked responses of the spinal dorsal horn WDR neurons were greatly enhanced by capsaicin treatment, and these enhanced responses were reversed to normal levels within 30 minutes after a systemic injection of PBN or TEMPOL. These data indicate that elevated levels of spinal ROS are involved in the enhancement of dorsal horn neuron responsiveness and secondary hyperalgesia induced by intradermal capsaicin injection, thus suggesting that ROS are involved in central sensitization.
In general, capsaicin-induced hyperalgesia is considered an acute inflammatory pain model because pain only lasts a few hours. However, it is well known that intradermal capsaicin induced pain have two components: primary and secondary hyperalgesia (Simone et al., 1991; Willis, 2001). The primary hyperalgesia is a result of sensitization of nociceptors by capsaicin as well as by inflammatory mediators, such as substance P and calcitonin gene-related peptide, released from the activated nociceptor terminals. The capsaicin-induced secondary hyperalgesia, on the other hand, is known to be mediated through central sensitization (Willis, 2001), the mechanism that underlies most persistent pain. A significant analgesic effect of ROS scavengers on the capsaicin-induced secondary hyperalgesia suggests that ROS may be involved in central sensitization. Further supporting evidence is that the most prominent analgesic effect of PBN is produced by intrathecal injection as compared to either peripheral or intracerebroventricular injection. In addition, the fact that the enhanced responses of the spinal dorsal horn WDR neurons after capsaicin treatment were almost completely reversed to normal levels by PBN further suggests ROS involvement in central sensitization. Not only is PBN treatment effective in reducing the hyperalgesia caused by capsaicin injection, but pretreatment with PBN can prevent the development of capsaicin-induced hyperalgesia. This finding suggests that ROS are involved not only in the maintenance but also the development of central sensitization in capsaicin-induced pain.
While it becomes clear that ROS are involved in central sensitization in capsaicin induced pain, the sources and types of ROS that are critical for central sensitization are not clearly identified. One possible cause of spinal ROS elevation is the increased production of nitric oxide (NO) in the spinal cord after tissue inflammation. The increased levels of nitric oxide synthase (NOS) and nitric oxide (NO) were observed in the spinal cord after tissue inflammation by capsaicin (Wu et al., 1998; 2001), and this increase is proposed to be a factor that maintains secondary hyperalgesia after capsaicin treatment (Meller and Gebhart, 1993; Wu et al., 1998; 2001). However, when specific and nonspecific NOS inhibitors were used to block pain, the results were inconsistent (Semos and Headley, 1994; Stanfa et al., 1996; Wu et al., 1998; 2001; Osborne and Coderre, 1999; Gühring et al., 2001). For example, two inducible NOS (iNOS) inhibitors, aminoguanidine (AG) and 2-amino-5,6-dihydro-methylthiazine (AMT), were effective in reducing carrageenan-induced thermal hyperalgesia (Osborne and Coderre, 1999), but another iNOS inhibitor, L-N6-[1-iminoethyl]lysine (L-NIL), was not effective (Gühring et al., 2001). In other studies, the nonselective NOS inhibitor L-NG-nitro-arginine methyl ester (L-NAME) produced a dose-dependent reduction of carrageenan-induced thermal hyperalgesia (Osborne and Coderre, 1999), but the same drug reduced mechanical hyperalgesia but not thermal hyperalgesia (Semos and Headley, 1994). Although there are discrepancies in the analgesic effect of various NOS inhibitors on different types of pain, it is generally accepted that increased NO production in the spinal cord plays an important role in inflammatory pain. A few studies, however, indicate that other ROS, besides NO, are also involved in persistent pain. In spinal nerve ligated neuropathic rats, it has been shown that the production of superoxide is increased in the dorsal horn neurons (Park et al., 2006) and ROS scavengers alleviate neuropathic pain in a reversible manner (Kim et al., 2004; Kim et al., 2006). The increased levels of extracellular hydrogen peroxide and decreased levels of superoxide dismutase (SOD) activity in the spinal trigeminal nucleus coincide with facial pain induced by a formalin injection into the lip (Viggiano et al., 2005). The treatment with SOD mimetics, such as TEMPOL and M40403, that remove superoxide radicals (Thiemermann, 2003), is effective in alleviating hyperalgesia in sciatic nerve constricted neuropathic rats (Tal, 1996) and in CFA treated rats (Wang et al 2004). Furthermore, superoxide mediated spinal SOD2 inactivation by nitration in the presence of NO is proposed as one mechanism in NMDA mediated hyperalgesia (Muscoli et al., 2004). Thus, data indicate that both an increased production of ROS and a decreased defense mechanism are involved in painful conditions. At this stage, it is clear that elevated levels of spinal ROS are involved in central sensitization, and thus hyperalgesia, after an intradermal capsaicin treatment. Further studies are warranted to investigate whether 1) elevated production of both superoxide and NO are required and/or 2) nitration of SOD2 are causing accumulation of superoxide and thus leading to central sensitization after capsaicin treatment.
It is evident that ROS scavengers produce analgesia and reverse the sensitization of spinal dorsal horn neurons induced by ROS. However, the exact mechanisms by which ROS produces such sensitization are not known. One possibility is that ROS are modifying intracellular signaling pathways rather than producing nonspecific damage to intracellular macromolecules. Recent studies have shown that ROS can modify various protein activities, such as protein phosphatases, protein kinases, and transcription factors, that are involved in cellular signaling pathways (Maher and Schubert, 2000; Guedes et al., 2006). Further studies are warranted to investigate whether PBN and TEMPOL produce their analgesic effect by modulating signaling pathways in dorsal horn neurons, and if so, to determine what signaling pathways are modified in central sensitization.
Perhaps capsaicin injection may sensitize afferent fibers of the nerve that passes through the injection site. Since peripheral nerve axons have functional capsaicin receptors (TRPV1) and can induce exocytosis of calcitonin gene-related peptide (Bernardini et al., 2004), capsaicin may induce direct stimulation of capsaicin receptors located on the axons of the medial and lateral plantar nerves. To check this possibility, the capsaicin injection site was moved distal to the behavioral testing sites in our last experiment. The development of primary and secondary hyperalgesia was similar to when capsaicin was injected proximally. The results thus suggest that sensitization of axons in passage either did not occur or did not contribute to capsaicin-induced hyperalgesia. Thus, the original model using capsaicin injection and behavioral testing is valid for measuring capsaicin-induced hyperalgesia and the effects of ROS scavengers.
In conclusion, ROS scavengers, PBN and TEMPOL, significantly reduced secondary hyperalgesia and reversed the enhanced responses of spinal dorsal horn WDR neurons to almost normal levels in the capsaicin-treated rats. The data suggest that ROS are importantly involved in the development and maintenance of capsaicin-induced pain, particularly in the process of central sensitization in the spinal cord. Thus, removing elevated levels of ROS by administration of PBN or TEMPOL into the spinal cord may be an effective way to treat persistent pain.
Acknowledgments
This work was supported by NIH grants R01 NS31680, P01 NS11255, and R01 AT01474. We would like to express our gratitude to Ms. Denise Broker for her excellent assistance in editing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bernardini N, Neuhuber W, Reeh PW, Sauer SK. Morphological evidence for functional capsaicin receptor expression and calcitonin gene-related peptide exocytosis in isolated peripheral nerve axons of the mouse. Neuroscience. 2004;126:585–590. doi: 10.1016/j.neuroscience.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Contestabile A. Oxidative stress in neurodegeneration: mechanisms and therapeutic perspectives. Curr Top Med Chem. 2001;1:553–568. doi: 10.2174/1568026013394723. [DOI] [PubMed] [Google Scholar]
- Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- Guedes RP, Bosco LD, Teixeira CM, Araújo ASR, Llesuy S, Belló-Klein A, Ribeiro MFM, Partata WA. Neuropathic pain modifies antioxidant activity in rat spinal cord. Neurochem Res. 2006;31:603–609. doi: 10.1007/s11064-006-9058-2. [DOI] [PubMed] [Google Scholar]
- Gühring H, Tegeder I, Lötsch J, Pahl A, Werner U, Reeh PW, Rehse K, Brune K, Geisslinger G. Role of nitric oxide in zymosan induced paw inflammation and thermal hyperalgesia. Inflamm Res. 2001;50:83–88. doi: 10.1007/s000110050728. [DOI] [PubMed] [Google Scholar]
- Jenner P. Oxidative damage in neurodegenerative disease. Lancet. 1994;344:796–798. doi: 10.1016/s0140-6736(94)92347-7. [DOI] [PubMed] [Google Scholar]
- Kim HK, Park SK, Zhou J-L, Taglialatela G, Chung K, Coggeshall RE, Chung JM. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain. 2004;111:116–124. doi: 10.1016/j.pain.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Kim HK, Kim JH, Gao X, Zhou J-L, Lee IH, Chung K, Chung JM. Analgesic effect of vitamin E is mediated by reducing central sensitization in neuropathic pain. Pain. 2006;122:53–62. doi: 10.1016/j.pain.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Lee I, Kim HK, Kim JH, Chung K, Chung JM. The role of reactive oxygen species in capsaicin-induced hyperalgesia. Neurosci Abstr. 2005;51:5. [Google Scholar]
- Lee I, Park ES, Kim HK, Wang JG, Chung K, Chung JM. A modified direct lumbar puncture method in rats. Neurosci Abstr. 2006;835:20. [Google Scholar]
- Lewén A, Matz P, Chan PH. Free radical pathways in CNS injury. J Neurotrauma. 2000;17:871–890. doi: 10.1089/neu.2000.17.871. [DOI] [PubMed] [Google Scholar]
- Maher P, Schubert D. Signaling by reactive oxygen species in the nervous system. Cell Mol Life Sci. 2000;57:1287–1305. doi: 10.1007/PL00000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller ST, Gebhart GF. Nitric oxide (NO) and nociceptive processing in the spinal cord. Pain. 1993;52:127–136. doi: 10.1016/0304-3959(93)90124-8. [DOI] [PubMed] [Google Scholar]
- Mestre C, Pélissier T, Fialip J, Wilcox G, Eschalier A. A method to perform direct transcutaneous intrathecal injection in rats. J Pharmacol Toxicol Methods. 1994;32:197–200. doi: 10.1016/1056-8719(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Muscoli C, Mollace V, Wheatley J, Masini E, Ndengele M, Wang ZQ, Salvemini D. Superoxide-mediated nitration of spinal manganese superoxide dismutase: a novel pathway in N-methyl-D-aspartate-mediated hyperalgesia. Pain. 2004;111:96–103. doi: 10.1016/j.pain.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Osborne MG, Coderre TJ. Effects of intrathecal administration of nitric oxide synthase inhibitors on carrageenan-induced thermal hyperalgesia. Br J Pharmacol. 1999;126:1840–1846. doi: 10.1038/sj.bjp.0702508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park ES, Gao X, Chung JM, Chung K. Levels of mitochondrial reactive oxygen species increase in rat neuropathic spinal dorsal horn neurons. Neurosci Lett. 2006;391:108–111. doi: 10.1016/j.neulet.2005.08.055. [DOI] [PubMed] [Google Scholar]
- Semos ML, Headley PM. The role of nitric oxide in spinal nociceptive reflexes in rats with neurogenic and non-neurogenic peripheral inflammation. Neuropharmacology. 1994;33:1487–1497. doi: 10.1016/0028-3908(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Simone DA, Sorkin LS, Oh U, Chung JM, Owens C, LaMotte RH, Willis WD. Neurogenic hyperalgesia: central neural correlates in responses of spinothalamic tract neurons. J Neurophysiol. 1991;66:228–246. doi: 10.1152/jn.1991.66.1.228. [DOI] [PubMed] [Google Scholar]
- Stanfa LC, Misra C, Dickenson AH. Amplification of spinal nociceptive transmission depends on the generation of nitric oxide in normal and carrageenan rats. Brain Res. 1996;737:92–98. doi: 10.1016/0006-8993(96)00629-4. [DOI] [PubMed] [Google Scholar]
- Tal M. A novel antioxidant alleviates heat hyperalgesia in rats with an experimental painful peripheral neuropathy. NeuroReport. 1996;7:1382–1384. doi: 10.1097/00001756-199605310-00010. [DOI] [PubMed] [Google Scholar]
- Thiemermann C. Membrane-permeable radical scavengers (tempol) for shock, ischemia-reperfusion injury, and inflammation. Crit Care Med. 2003;31:S76–S84. doi: 10.1097/00003246-200301001-00011. [DOI] [PubMed] [Google Scholar]
- Viggiano A, Monda M, Viggiano A, Viggiano D, Viggiano E, Chiefari M, Aurilio C, De Luca B. Trigeminal pain transmission requires reactive oxygen species production. Brain Res. 2005;1050:72–78. doi: 10.1016/j.brainres.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Wang ZQ, Porreca F, Cuzzocrea S, Galen K, Lightfoot R, Masini E, Muscoli C, Mollace V, Ndengele M, Ischiropoulos H, Salvemini D. A newly identified role for superoxide in inflammatory pain. J Pharmacol Exp Ther. 2004;309:869–878. doi: 10.1124/jpet.103.064154. [DOI] [PubMed] [Google Scholar]
- Willis WD. Role of neurotransmitters in sensitization of pain responses. Ann N Y Acad Sci. 2001;933:142–156. doi: 10.1111/j.1749-6632.2001.tb05821.x. [DOI] [PubMed] [Google Scholar]
- Wu J, Fang L, Lin Q, Willis WD. Nitric oxide synthase in spinal cord central sensitization following intradermal injection of capsaicin. Pain. 2001;94:47–58. doi: 10.1016/S0304-3959(01)00340-2. [DOI] [PubMed] [Google Scholar]
- Wu J, Lin Q, McAdoo DJ, Willis WD. Nitric oxide contributes to central sensitization following intradermal injection of capsaicin. NeuroReport. 1998;9:589–592. doi: 10.1097/00001756-199803090-00005. [DOI] [PubMed] [Google Scholar]


