Abstract
We evaluated whether the association between low education and greater risk of dementia is explained by genetic influences, using three different types of analyses. The HARMONY study (Swedish for “health” (Hälsa), “genes” (ARv), “environment” (Miljö), “and” (Och), and “new” (NY)) includes members of the Swedish Twin Registry who were aged 65 and older and alive in 1998, and who were screened and clinically assessed for dementia. There were 394 cases with dementia and 7786 unrelated controls. Analyses included co-twin control, tests for association between education and a measured genotype, and bivariate twin modeling. Low education was a significant risk factor for dementia both in case-control analyses (odds ratio=1.77, 95% confidence interval 1.38 to 2.28) and co-twin control analyses with monozygotic twin pairs (odds ratio=3.17, 95% confidence interval 1.26 to 7.93). Apolipoprotein E genotype was not associated with education and did not account for the relationship between education and dementia. Bivariate twin modeling showed that the association between education and dementia was not mediated by genetic influences in common between education and dementia. The association was mediated by shared environmental influences that were related to both dementia and to education. Low education is confirmed as a risk factor for dementia. Findings from three different analytic approaches showed that genetic influences did not explain this association.
Keywords: Dementia, Education, Risk factors, Twin studies
1. Introduction
Dementia, defined as age-related progressive impairment of memory, language, visual processing, problem solving skills, and eventually ability to function independently, is a major public health problem, affecting up to 40% of adults aged 85 and older [1-2]. Especially among the oldest old, the majority of dementia is accounted for by Alzheimer disease, with the next most frequent type being vascular, although increasingly these subtypes are being viewed as less distinctive [3]. It is generally claimed that half of the explanation for Alzheimer’s disease is genetic and half is environmental, while identification of specific gene variants and specific environmental exposures that account for risk of dementia remains incomplete [4-5].
Low educational achievement is one of the few variables consistently reported to be related to higher risk of dementia, beyond age and family history [6-14]. Only a small number of studies have failed to confirm this association [15-17]. Summaries of the literature suggest that the strongest relationships between education and dementia occur in geographical locales where education is more likely based on intellectual potential, whereas weaker relationships characterize places where education is more likely made available based on position in society [18-19]. Further, it has been shown that the association observed between education and dementia cannot simply be explained as an artifact of differential case detection of dementia in individuals with less education [13].
Mechanisms to explain the association between dementia and education remain uncertain. The predominant interpretation, often referred to as “use it or lose it,” is that education indexes level of mental activity and that mental stimulation strengthens connectivity in the brain, e.g., increasing synaptic density [20]. This line of theorizing is supported by studies that have found associations between greater engagement in mentally stimulating leisure activities and lower risk of Alzheimer’s disease [21-23], and has led to recommendations that individuals might reduce their risk of dementia through increasing their level of cognitive activity—for instance, by learning a foreign language or working crossword puzzles [24-25].
An alternative possibility is that differences in education reflect genetic influences associated with individual differences in intelligence [26]. It is well established that individual differences in IQ scores in part reflect genetic differences [27-28]. In turn, children who score higher on intelligence tests are likely to progress further in school. Furthermore, Scottish data have shown that those who became demented had lower IQ test performance at age 11 compared to those who did not become demented [29].
We addressed these two contrasting explanations using a study of dementia in a population-based twin sample. We took three approaches to testing the extent to which the relationship between low education and risk of dementia was explained by genetic influences.
We evaluated low education as a risk factor for dementia using both an unrelated comparison group and monozygotic co-twin controls [30]. Analysis of unrelated controls is essentially a classic case–control study, comparing twins diagnosed as demented with other twins not related to the index probands. The co-twin control design, on the other hand, compares the demented twin to their non-demented monozygotic co-twin as control. The co-twin control design controls for potential confounding from genetic factors, as the cases and controls are genetically the same.
We tested the role of Apolipoprotein E ε4, the best established measured genetic risk factor for dementia[4] in explaining the association between education and dementia.
We employed bivariate twin modeling to test whether genetic influences in common between education and dementia explain the correlation between dementia and education.
2. Methods
2.1. Participants
Data for the present investigation come from the Swedish Twin Registry [30]. In 1961, 1963, and 1967 all like-sexed twin pairs born before 1926 were mailed a questionnaire. Nonresponders in 1967 were sent another questionnaire in 1970. A second cohort was added to the registry in the beginning of the 1970s, including all pairs born 1926 through 1958. Like-sexed twins in this cohort were sent a questionnaire in 1973. Compilation procedures covered 95% of all twin pairs. The questionnaire response rate was 91% [31]. Unlike-sexed pairs were recorded but not sent any mailings.
In 1998, all living members of the Swedish Twin Registry aged 65 and older were contacted for a study of dementia, called HARMONY. Like and unlike-sexed pairs were included. All individuals were invited for telephone screening, followed by clinical diagnostic evaluations for dementia in those who screened positive, their co-twins, and a sample of twins who had both screened negative. Participation rate in telephone screening was 71.5%. Of those who screened positive, dementia diagnoses were secured for 69.7%. A detailed discussion of the study design, including dropout analyses, can be found elsewhere [32].
Case-control analyses included all HARMONY participants who were members of like-sexed pairs. The sample included 394 cases with dementia (263 with Alzheimer’s disease, 87 with vascular dementia, 2 with frontal-temporal dementia, 2 with Lewy Body dementia, 15 with secondary dementia, and 25 with dementia not otherwise specified) and 7786 non-demented controls. Among the cases were 115 men and 279 women; average age when assessed was 82.7, range 65 to 103; average age of onset of dementia was 77.6, range 45 to 96. Among the non-demented controls were 3298 men and 4488 women; average age when assessed was 73.4, range 65 to 100. Among cases there were 152 monozygotic (MZ) and 242 dizygotic (DZ) twins; among controls, 2841 MZ and 4945 DZ. There were no differences in dementia prevalence for different zygosities.
For co-twin control analyses, there were 33 monozygotic twin pairs discordant for dementia. Among the index probands, 20 were diagnosed with Alzheimer’s disease, 6 with vascular dementia, 1 with frontal-temporal dementia, 3 with secondary dementia, and 3 with dementia of unspecified etiology. Of the 33 pairs, 13 were male and 20 were female. Average age when assessed was 78.9, range 67 to 92; average age of onset of dementia was 73.4, range 60 to 85.
Analyses of Apolipoprotein E (APOE) genotypes included all individuals in the case-control analyses who also had genotyping. Response rate for providing a blood sample during the clinical phase was 95%; APOE data were not available from those who died during the case ascertainment process, who were very demented and whose relatives did not agree to a physical examination, or who screened negative and were not personally visited. The available sample included 283 cases and 559 controls.
2.2. Measures
Updated information on educational attainment was taken from earlier Swedish Twin Registry questionnaires or from the recent telephone screening. Education was measured both as total years of education and as a dichotomous variable based on completion of any schooling past compulsory education. The tetrachoric correlation between dichotomous education variable from the 1963 or 1973 questionnaires and that collected from the telephone screening was .90.
Preliminary analyses tested whether individuals who refused to participate were more likely to have lower education. Participants at the screening phase had higher education than non-participants. Fewer years of education was a significant risk factor for non-participation, controlling for age, sex, and zygosity, OR = 1.18 (95% CI: 1.15, 1.20). However, amount of education did not differ between those who agreed to participate in the clinical phase and those who refused or were lost to follow-up. Controlling for age, sex, and zygosity, OR = 1.03 (95% CI: 0.98, 1.07).
2.3. Statistical analyses
The association between education and dementia was analyzed in the case-control design using alternating logistic regression (ALR). ALR provides an odds ratio (OR) and 95% confidence intervals for estimating the effects of low education as a risk factor for dementia. Sex and age were included as covariates. ALR allows for a dependence structure so that both members of twin pairs can be included in the model while allowing for different correlation structures for MZ and DZ twins [33-34]. Post hoc logistic regression analyses including unlike-sexed pairs and using zygosity as a covariate did not find results different from those presented here. Statistical analyses were performed in SAS 8.2 using GENMOD and LOGISTIC procedures (SAS Institute, Inc., Raleigh, NC).
Additional alternating logistic regression analyses tested whether APOE ε4 genotype was a risk factor for low education and whether ε4 accounted for the association between low education and dementia.
We conducted co-twin control analysis using a Mantel-Haenszel test to assess education as a risk factor within twin pairs discordant for dementia. A co-twin control analysis by design controls for sex and age. The test provides an OR and 95% confidence intervals. In addition, we compared years of education for demented twins and their non-demented co-twins using a matched t-test. Only monozygotic twins (MZ) were included, as they share 100% of genes, thus fully controlling for unmeasured confounding by genetic effects. Results presented in this paper are based on total dementia cases. Case-control and co-twin control analyses limited to Alzheimer’s disease showed similar results.
Finally, we employed bivariate twin modeling using a Cholesky decomposition model and the Mx software program [35] with raw data from MZ and like-sexed DZ twins to test whether genetic influences in common between education and dementia explain the correlation between dementia and education.
3. Results
In the case-control analyses, two patterns are apparent. Years of education were lower among those born earlier and higher among those born more recently. In addition, average years of education was lower for demented than for non-demented across all birth cohorts (Fig. 1). Alternating logistic regression analysis results provided in Table 1 confirmed that low education was a significant risk factor for dementia, controlling for age and sex.
Fig. 1.
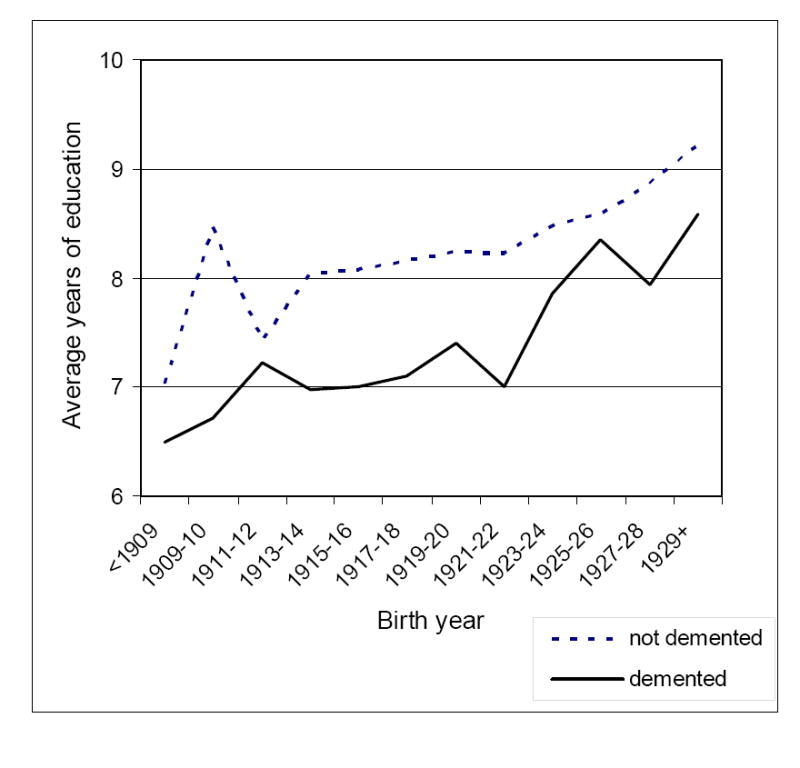
Years of education for dementia cases and non-demented members of the study population, by birth year
Table 1.
Case control and co-twin control analyses predicting risk for dementia
| Case Control | |||||
|---|---|---|---|---|---|
| EDUCATION | Cases
(N = 394) |
Unrelated Controls
(N = 7786) |
OR a | 95% CI | |
| Compulsory | 305 | 4435 | |||
| Higher | 89 | 3351 | 1.77 | 1.38, 2.28 | |
|
| |||||
| Co-Twin Control (N = 33 monozygotic pairs) | |||||
|
| |||||
| Co-twin
|
OR | 95% CI | |||
| EDUCATION | Compulsory | Higher | |||
|
| |||||
| Case | Compulsory | 6 | 19 | 3.17 | 1.26, 7.93 |
| Higher | 6 | 2 | |||
odds ratios controlling for age and sex
In the co-twin control sample of MZ twins, the non-demented twin had on average 0.85 more years of education than their demented twin partner, t = 2.19, p < .05. As shown in Table 1, risk of dementia was significantly associated with low education. The lack of attenuation of the odds ratio in the co-twin control analysis compared to the case-control analysis suggests that genetic associations with education are unlikely to account for the relationship of low education to dementia.
In the case-control sample, having at least one copy of the ε4 allele was a significant risk factor for dementia (Table 2, left), but ε4 genotype was not significantly related to level of education, odds ratio =1.21, 95% confidence interval 0.93 to 1.58. Controlling for APOE did not affect the association between education and dementia (Table 2, right).
Table 2.
Case control analyses of APOE genotype, education and dementia
| UNIVARIATE TESTS | MULTIVARIATE TEST | ||||||
|---|---|---|---|---|---|---|---|
| RISK FACTOR | Cases
(N = 283) |
Controls
(N = 559) |
OR a | 95% CI | OR b | 95% CI | |
|
| |||||||
| Education | |||||||
| Compulsory | 219 | 340 | 1.89 | 1.35, 2.65 | 1.80 | 1.28, 2.53 | |
| Higher | 64 | 219 | |||||
|
| |||||||
| APOE | |||||||
| ε4+ | 399 | 137 | 2.70 | 1.96, 3.72 | 2.53 | 1.83, 3.50 | |
| ε4- | 160 | 146 | |||||
odds ratios controlling for age and sex
odds ratios controlling for age, sex, and other risk factor
Tetrachoric correlations between twins are shown in Table 3 both within and across traits. Within trait correlations indicate that twins were similar to one another on both education and dementia, with MZ twins more similar to one another than DZ twins. The cross-twin cross-trait correlations were not significantly different for MZ compared to DZ twins, suggesting that the association between education and dementia was not explained by genetic influences. Complete heritability analyses for dementia and for Alzheimer’s disease have been presented elsewhere [36]. Here our focus was the relationship between education and dementia. In the bivariate quantitative genetic analysis shown in Fig. 2, the genetic mediation path was 0.00, suggesting that the genetic influences on education and dementia were independent of each other. The mediation path for environmental factors unique to each individual was not significantly different from 0.00. The correlation between education and dementia largely reflected environmental factors in common to members of twin pairs, where the mediation path was significantly different from 0.00 (difference chi square = 5.18, degrees of freedom = 1, p < .02).
Table 3.
Tetrachoric correlations with 95% confidence intervals within twin pairs for each trait and cross-trait cross-twin correlations for monozygotic and dizygotic twins
| Monozygotic | Dizygotic | |
|---|---|---|
| Tetrachoric correlations within twin pair | ||
| Dementia | 0.80 (0.78, 0.87) | 0.68 (0.58, 0.77) |
| Education | 0.77 (0.72, 0.81) | 0.60 (0.55, 0.64) |
| Dementia and education | 0.22 (0.13, 0.31) | 0.26 (0.19, 0.34) |
|
| ||
| Cross-twin cross-trait correlations | ||
| Monozygotic | Dizygotic | |
| Dementia and education | 0.16 (0.09, 0.26) | 0.29 (0.23, 33) |
Note: Cross-twin cross-trait correlations show the correlation between one twin on dementia and the co-twin on education.
Fig. 2.
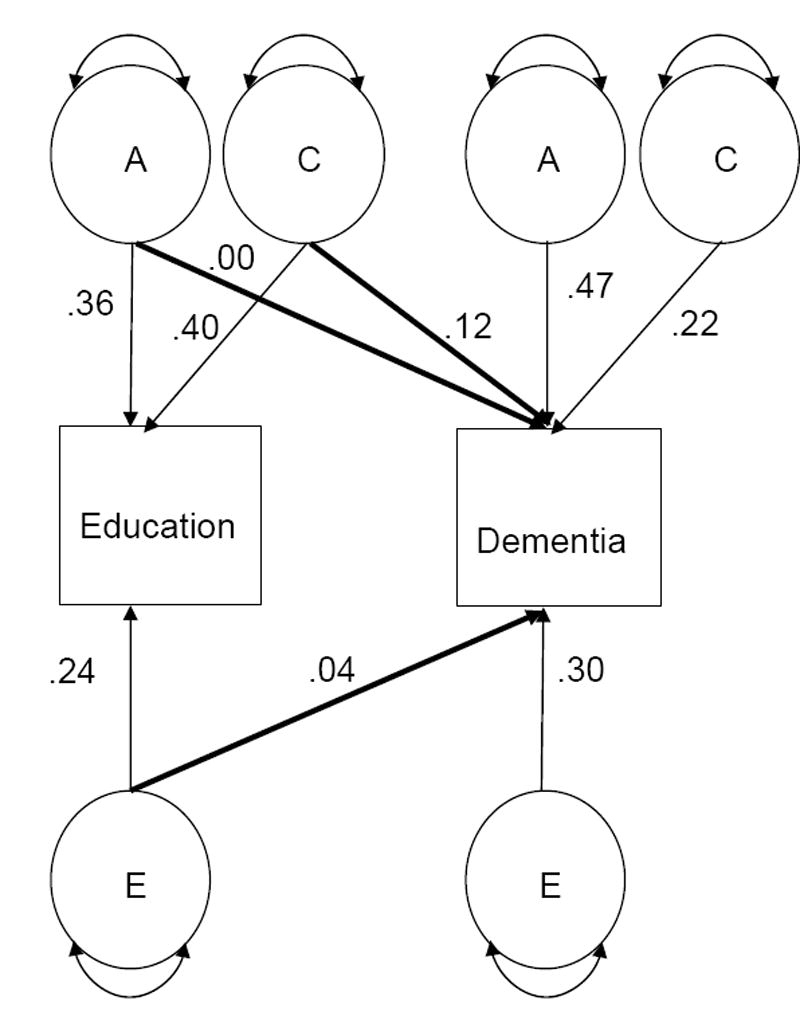
Bivariate model representing the relationship between education and dementia, with both genetic and environmental mediation. Notes. Only one member of the twin pair is represented. A= additive genetic variance; C= shared environmental variance; E = unique environmental variance. A1 indicates additive genetic variance for the first trait; A2 indicates additive genetic variance for the second trait, with analogous numbering for the other components of variance. Parameters are standardized path coefficients for the full model, with mediational paths bolded.
4. Discussion
The present study is the first to control for genetic effects on the association of education with dementia diagnosis through both twin designs and Apolipoprotein E ε4 genotypes. The results confirm the significant role of level of education in the expression of dementia in late life and show that this association is independent of genetic influences. Rather, the manner in which education influences dementia risk appears to reflect environmentally mediated influences, in particular those in common to both members of twin pairs.
The case-control and co-twin control analyses both resulted in odds ratios for education that were in the same range. If the odds ratio for the co-twin control analyses had been non-significant, it would have suggested that genetic factors accounted for the significant odds ratio in the case-control analyses where cases and controls were unrelated. However, our results provided no evidence that genetic factors accounted for the association between education and dementia. Although the point estimates for the odds ratios give the impression of stronger risk for the co-twin control design, the confidence intervals are much greater, and it would not be correct to infer any difference between the two odds ratios. Further, the average difference in years of education between demented twins and their non-demented co-twins was slightly less than the average difference in years of education within age groups for cases and unrelated controls.
Level of education was similar in members of twin pairs, as indicated by the tetrachoric correlation for level of education. Thus, the finding that among MZ twin pairs the member of the pair without dementia had the higher education was especially notable.
In the present study, Apolipoprotein E ε4 genotype was not significantly associated with educational attainment. One previous study [37] based on a population sample of non-demented individuals found that older ε4 carriers had significantly lower educational attainment. However, other studies in large representative samples have failed to confirm this finding [38-39]. Thus, while APOE genotype was associated with dementia, APOE genotype did not explain the association between education and dementia.
Finally, the results from the bivariate quantitative genetic analyses buttress the conclusion that the association between education and dementia is not mediated by genetic factors. Taken together, these results illustrate three applications of genetically informed designs—two using twin methods and one using a measured genotype—to the same research problem, with a convergence of findings from all three approaches.
We previously examined education as a risk factor for dementia comparing case-control to co-twin control results based on two selected subsamples of twins from the Swedish Twin Registry, where we combined MZ and DZ twin pairs. In that report, we also found that odds ratios resulting from co-twin control and case control analyses were similar [18]. The present analyses replicate the previous findings with a larger number of MZ pairs, more uniform case ascertainment, and cases drawn from the entire Swedish Twin Registry rather than particular subsamples.
Strengths of our study include the unique data set, including a co-twin design. However, APOE genotyping data were not available for all participants, especially not for older cases due to death or for a large number of controls who were not evaluated in person. Nonetheless, the results for education were similar in the subgroup with APOE genotyping and in the entire case-control sample. Despite the large number of dementia cases, the co-twin control sample was small, due to high concordance for dementia and to requiring that both members of the pair be assessed in person. Within the co-twin control sample, there may be pairs in which the nondemented twin will later become demented. However, the average number of years between age of onset of dementia in the demented twin and assessment of the nondemented twin was 5.6.
Another strength is that identification of cases of dementia was based on an extensive diagnostic process. However, a weakness is that 28.5% of the population refused to participate in screening, and 29.8% of those selected for in-person diagnostic evaluation refused to be visited by the assessment team. A dropout analysis found little evidence of systematic bias by demographic factors and that underestimation of cases was most likely for cases whose onset was under age 85 [32].
Another important limitation is that these results are specific to one country and to individuals born within a particular historical time. Especially for those born before 1926, variability in level of education was small. Those who received more education were likely to be from more privileged households.
5. Concluding remarks
The findings of these analyses do not support the hypothesis that genetic influences explain the association between dementia and low education. Rather, it appears that environmental influences in common between members of twin pairs account for the association. Examples might include early environmental influences on brain development that would be similar within a family, such as nutrition, parents encouraging their children’s active engagement in intellectual pursuits, or other social factors in childhood [40]; or factors in midlife such as occupational status, in which twins are more similar than unrelated individuals. The study also illustrates the use of various methods to address a research question, taking advantage of twin designs and of measured genotypes.
Acknowledgments
The authors thank Paul Dickman for providing statistical advice with respect to modelling twin data with generalized estimating equations. Supported by grants from the National Institute on Aging (R01-AG08724) and the Alzheimer’s Association (ZEN-02-3895).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berr C, Wancata J, Ritchie K. Prevalence of dementia in the elderly in Europe. Eur Neuropsychopharmacol. 2005;15:463–471. doi: 10.1016/j.euroneuro.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Fratiglioni L, De Ronchi D, Aguero-Torres H. Worldwide prevalence and incidence of dementia. Drugs Aging. 1999;15:365–375. doi: 10.2165/00002512-199915050-00004. [DOI] [PubMed] [Google Scholar]
- 3.Launer LJ. Demonstrating the case that AD is a vascular disease: epidemiologic evidence. Ageing Res Rev. 2002;1:61–77. doi: 10.1016/s0047-6374(01)00364-5. [DOI] [PubMed] [Google Scholar]
- 4.Ashford JW, Mortimer JA. Non-familial Alzheimer’s disease is mainly due to genetic factors. J Alzheimers Dis. 2002;4:169–177. doi: 10.3233/jad-2002-4307. [DOI] [PubMed] [Google Scholar]
- 5.Bird TD. Genetic factors in Alzheimer’s disease. NEJM. 2005;352:862–864. doi: 10.1056/NEJMp058027. [DOI] [PubMed] [Google Scholar]
- 6.Canadian Study of Health and Aging. Risk factors for Alzheimer’s disease in Canada. Neurology. 1994;44:2073–2080. doi: 10.1212/wnl.44.11.2073. [DOI] [PubMed] [Google Scholar]
- 7.Evans DA, Hebert LE, Beckett LA, Scherr PA, Albert MS, Chown MJ, Pilgrim DM, Taylor JO. Education and other measures of socioeconomic status and risk of incident Alzheimer disease in a defined population of older persons. Arch Neurol. 1997;54:1399–1405. doi: 10.1001/archneur.1997.00550230066019. [DOI] [PubMed] [Google Scholar]
- 8.Fratiglioni L, Grut M, Forsell Y, Viitanen M, Grafstrom M, Holmen K, Ericsson K, Bäckman L, Ahlbom A, Winblad B. Prevalence of Alzheimer’s disease and other dementias in an elderly urban population: Relationship with age, sex, and education. Neurology. 1991;41:1886–1892. doi: 10.1212/wnl.41.12.1886. [DOI] [PubMed] [Google Scholar]
- 9.Launer LJ, Andersen K, Dewey ME, Letenneur L, Ott A, Amaducci LA, Brayne C, Copeland JR, Dartigues JF, Kragh-Sorensen P, Lobo A, Martinez-Lage JM, Stijnen T, Hofman A. Rates and risk factors for dementia and Alzheimer’s disease: results from EURODEM pooled analyses. Neurology. 1999;52:78–84. doi: 10.1212/wnl.52.1.78. [DOI] [PubMed] [Google Scholar]
- 10.Letenneur L, Gilleron V, Commenges D, Helmer C, Orgogozo JM, Dartigues JF. Are sex and educational level independent predictors of dementia and Alzheimer’s disease? Incidence data from the PAQUID project. J Neurol Neurosurg Psychiatry. 1999;66:177–183. doi: 10.1136/jnnp.66.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mortimer JA, Snowdon DA, Markesbery WR. Head circumference, education and risk of dementia: findings from the Nun Study. J Clin Exp Neuropsychol. 2003;25:671–679. doi: 10.1076/jcen.25.5.671.14584. [DOI] [PubMed] [Google Scholar]
- 12.Ott A, Breteler MM, van Harskamp F, Claus JJ, van der Cammen TJ, Grobbee DE, Hofman A. Prevalence of Alzheimer’s disease and vascular dementia: association with education. The Rotterdam study. BMJ. 1995;310:970–973. doi: 10.1136/bmj.310.6985.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmand B, Smit J, Lindeboom J, Smits C, Hooijer C, Jonker C, Deelman B. Low education is a genuine risk factor for accelerated memory decline and dementia. J Clin Epidemiol. 1997;50:1025–1033. doi: 10.1016/s0895-4356(97)00121-2. [DOI] [PubMed] [Google Scholar]
- 14.Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA. 1994;271:1004–1010. [PubMed] [Google Scholar]
- 15.Beard CM, Kokmen E, Offord KP, Kurland LT. Lack of association between Alzheimer’s disease and education, occupation, marital status, or living arrangement. Neurology. 1992;42:2063–2068. doi: 10.1212/wnl.42.11.2063. [DOI] [PubMed] [Google Scholar]
- 16.Cobb JL, Wolf PA, Au R, White R, D’Agostino RB. The effect of education on the incidence of dementia and Alzheimer’s disease in the Framingham Study. Neurology. 1995;45:1707–1712. doi: 10.1212/wnl.45.9.1707. [DOI] [PubMed] [Google Scholar]
- 17.Graves AB, Larson EB, Edland SD, Bowen JD, McCormick WC, McCurry SM, Rice MM, Wenzlow A, Uomoto JM. Prevalence of dementia and its subtypes in the Japanese American population of King County, Washington state. The Kame Project. Am J Epidemiol. 1996;144:760–771. doi: 10.1093/oxfordjournals.aje.a009000. [DOI] [PubMed] [Google Scholar]
- 18.Gatz M, Svedberg P, Pedersen NL, Mortimer JA, Berg S, Johansson B. Education and the risk of Alzheimer’s disease: Findings from the Study of Dementia in Swedish Twins. J Gerontol Psychol Sci. 2001;56B:P292–P300. doi: 10.1093/geronb/56.5.p292. [DOI] [PubMed] [Google Scholar]
- 19.Ganguli M, Dodge HH, Chen P, Belle S, DeKosky S. Ten-year incidence of dementia in a rural elderly US population: The MoVIES Project. Neurology. 2000;54:1109–1116. doi: 10.1212/wnl.54.5.1109. [DOI] [PubMed] [Google Scholar]
- 20.Orrell M, Sahakian B. Education and dementia. BMJ. 1995;310:951–952. doi: 10.1136/bmj.310.6985.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson RS, Mendes de Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA, Bennett DA. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 2002;287:742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- 22.Fabrigoule C, Letenneur L, Dartigues JF, Zarrouk M, Commenges D, Barberger-Gateau P. Social and leisure activities and risk of dementia: A prospective longitudinal study. J Am Geriatr Soc. 1995;43:485–490. doi: 10.1111/j.1532-5415.1995.tb06093.x. [DOI] [PubMed] [Google Scholar]
- 23.Scarmeas N, Levy G, Tang M-X, Manly J, Stern Y. Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology. 2001;57:2236–2242. doi: 10.1212/wnl.57.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller KC. A 10-letter disease prevented by puzzles. The Los Angeles Times. 2005 March 12;:B19. [Google Scholar]
- 25.Small G. The memory prescription: Dr. Gary Small’s 14-day plan to keep your brain and body young. New York: Hyperion; 2004. [Google Scholar]
- 26.Plassman BL, Welsh KA, Helms M, Brandt J, Page WF, Breitner JCS. Intelligence and education as predictors of cognitive state in late life: a 50-year follow-up. Neurology. 1995;45:1446–1450. doi: 10.1212/wnl.45.8.1446. [DOI] [PubMed] [Google Scholar]
- 27.Bouchard TJ, Jr, McGue M. Genetic and environmental influences on human psychological differences. J Neurobiol. 2003;54:44–45. doi: 10.1002/neu.10160. [DOI] [PubMed] [Google Scholar]
- 28.McClearn GE, Johansson B, Berg S, Pedersen NL, Ahern F, Petrill SA, Plomin R. Substantial genetic influence on cognitive abilities in twins 80 or more years old. Science. 1997;276:1560–1563. doi: 10.1126/science.276.5318.1560. [DOI] [PubMed] [Google Scholar]
- 29.Whalley LJ, Starr JM, Athawes R, Hunter D, Pattie A, Deary IJ. Childhood mental ability and dementia. Neurology. 2000;55:1455–1459. doi: 10.1212/wnl.55.10.1455. [DOI] [PubMed] [Google Scholar]
- 30.Lichtenstein P, de Faire U, Floderus B, Svartengren M, Svedberg P, Pedersen NL. The Swedish Twin Registry: a unique resource for clinical, epidemiological, and genetic studies. J Intern Med. 2002;252:184–205. doi: 10.1046/j.1365-2796.2002.01032.x. [DOI] [PubMed] [Google Scholar]
- 31.Cederlöf R, Friberg L, Lundman T. The interactions of smoking, environment, and heredity and their implications for disease etiology. Acta Med Scand. 1977;(Suppl No 612) [PubMed] [Google Scholar]
- 32.Gatz M, Fratiglioni L, Johansson B, Berg S, Mortimer JA, Reynolds CA, Fiske A, Pedersen NL. Complete ascertainment of dementia in the Swedish Twin Registry: the HARMONY study. Neurobiol Aging. 2005;26:439–447. doi: 10.1016/j.neurobiolaging.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Carey V, Zeger SL, Diggle P. Modelling multivariate binary data with alternating logistic regression. Biometrika. 1993;80:517–526. [Google Scholar]
- 34.Jansson M, Gatz M, Berg S, Johansson B, Malmberg B, McClearn GE, Schalling M, Pedersen NL. Association between depressed mood in the elderly and a 5-HTR2A gene variant. Am J Med Genet B Neuropsychiatr Genet. 2003;120:79–84. doi: 10.1002/ajmg.b.20016. [DOI] [PubMed] [Google Scholar]
- 35.Neale MC, Boker SM, Xie G, Maes HH. Mx: statistical modeling. 5. VCU Box 900126, Richmond, VA: Department of Psychiatry; 1999. [Google Scholar]
- 36.Gatz M, Reynolds CA, Fratiglioni L, Johansson B, Mortimer JA, Berg S, Fiske A, Pedersen NL. The role of genes and environments for explaining Alzheimer’s disease. Arch Gen Psychiatry. 2006;63:168–174. doi: 10.1001/archpsyc.63.2.168. [DOI] [PubMed] [Google Scholar]
- 37.Winnock M, Letenneur L, Jacqmin-Gadda H, Dallongeville J, Amouyel P, Dartigues JF. Longitudinal analysis of the effect of apolipoprotein E epsilon4 and education on cognitive performance in elderly subjects: the PAQUID study. J Neurol Neurosurg Psychiatry. 2002;72:794–797. doi: 10.1136/jnnp.72.6.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borenstein Graves A, Mortimer JA, Bowen JD, McCormick WC, McCurry SM, Schellenberg GD, Larson EB. Head circumference and incident Alzheimer’s disease: modification by apolipoprotein E. Neurology. 2001;57:1453–1460. doi: 10.1212/wnl.57.8.1453. [DOI] [PubMed] [Google Scholar]
- 39.Moceri VM, Kukull WA, Emanuel I, van Belle G, Larson EB. Early-life risk factors and the development of Alzheimer’s disease. Neurology. 2000;54:415–420. doi: 10.1212/wnl.54.2.415. [DOI] [PubMed] [Google Scholar]
- 40.Karp A, Kareholt I, Qiu C, Bellander T, Winblad B, Fratiglioni L. Relation of education and occupation-based socioeconomic status to incident Alzheimer’s disease. Am J Epidemiol. 2004;159:175–183. doi: 10.1093/aje/kwh018. [DOI] [PubMed] [Google Scholar]


