Abstract
Advanced liver cirrhosis is associated with hyperdynamic circulation consisting of systemic hypotension, decreased peripheral resistance, and cardiac dysfunction, termed cirrhotic cardiomyopathy. Previous studies have revealed the role of endocannabinoids and vascular CB1 receptors in the development of generalized hypotension and mesenteric vasodilation in animal models of liver cirrhosis, and CB1 receptors have also been implicated in the decreased β-adrenergic responsiveness of isolated heart tissue from cirrhotic rats. Here we document the cardiac contractile dysfunction in vivo in liver cirrhosis and explore the role of the endocannabinoid system in its development. Rats with CCl4-induced cirrhosis developed decreased cardiac contractility, as documented through the use of the Millar pressure-volume microcatheter system, low blood pressure, and tachycardia. Bolus intravenous injection of the CB1 antagonist AM251 (3 mg/kg) acutely increased mean blood pressure, as well as both load-dependent and -independent indexes of systolic function, whereas no such changes were elicited by AM251 in control rats. Furthermore, tissue levels of the endocannabinoid anandamide increased 2.7-fold in the heart of cirrhotic compared with control rats, without any change in 2-arachidonoylglycerol levels, whereas, in the cirrhotic liver, both 2-arachidonoylglycerol (6-fold) and anandamide (3.5-fold) were markedly increased. CB1-receptor expression in the heart was unaffected by cirrhosis, as verified by Western blotting. Activation of cardiac CB1 receptors by endogenous anandamide contributes to the reduced cardiac contractility in liver cirrhosis, and CB1-receptor antagonists may be used to improve contractile function in cirrhotic cardiomyopathy and, possibly, in other forms of heart failure.
Keywords: cirrhosis, cannabinoid CB1 receptors, endocannabinoids, cardiac contractility, pressure-volume loops
It has long been recognized that liver cirrhosis is associated with abnormal cardiovascular function: portal hypertension is associated with hyperdynamic circulation, manifested by increased heart rate (HR) and cardiac output and reduced splanchnic and systemic vascular resistance with decreased mean arterial pressure (MAP) (9, 14, 18). Experimental and clinical studies during the past decades have provided strong evidence for the existence of latent heart failure with impaired responsiveness to pharmacological or physiological stress, termed “cirrhotic cardiomyopathy” (9, 18, 20). Despite the elevated baseline cardiac output, cirrhotic cardiomyopathy is associated with decreased β-adrenergic responsiveness, and there are also conductance abnormalities and defective excitation-contraction coupling (9, 10). The peripheral vasodilation in cirrhosis triggers reflex tachycardia, and the resulting increase in cardiac output can mask the impaired cardiac function. However, when patients are challenged by physical or mental stress or pharmacological stimulation, the symptoms of cardiac dysfunction could become manifest, precipitating frank heart failure, which is difficult to treat due to the sluggish responsiveness of the cirrhotic heart to inotropic agents (9, 18, 20).
Recent studies have suggested that endocannabinoids and their receptors play an important role in the hypotension associated with various pathological states (32, 33, 34), including advanced liver cirrhosis (2, 19, 29). In cirrhotic rats, administration of the CB1 antagonist rimonabant increased arterial blood pressure and total peripheral resistance (2, 29) and decreased mesenteric blood flow (2), whereas cardiac output remained unaffected (19, 29). The involvement of CB1 receptors and their endogenous ligands was further indicated by the increased expression of CB1 receptors in vascular endothelial cells from cirrhotic human livers (2) and the increased relaxation of mesenteric arteries from cirrhotic vs. control rats in response to the endocannabinoid anandamide [arachidonoyl ethanolamide (AEA)] (7). These in vivo and in vitro studies highlighted the vascular mechanisms contributing to the endocannabinoid-mediated hypotension in cirrhosis. However, in these earlier studies, the potential direct cardiac effects were not evaluated. Recent detailed in vivo hemodynamic analyses clearly indicate that AEA-induced hypotension is of predominantly cardiac origin due to a CB1-mediated decrease in cardiac contractility (4, 22), which has also been documented in human isolated cardiac preparations (5). In a recent in vitro study in rat isolated papillary muscle, CB1 blockade was found to reverse the decreased β-adrenergic responsiveness observed in preparations from bile duct-ligated cirrhotic rats (10). This was the first indication that CB1 receptors are involved in some aspects of abnormal myocardial contractility in liver cirrhosis (10), although in vivo evidence for this and direct proof for activation of the endocannabinoid system have been lacking. In the present study, we characterized the hemodynamic profile of rats with carbon tetra-chloride (CCl4)-induced advanced liver cirrhosis and analyzed the effects of the CB1-receptor antagonist AM251 on cardiac contractile function under both load-dependent and -independent conditions. We also document the changes in tissue endocannabinoid levels associated with cirrhosis. The results indicate that cirrhosis activates the endocannabinoid system, both in the heart and liver, and extends earlier findings on the role of CB1 receptors in cardiac contractile dysfunction by demonstrating their contribution under in vivo conditions.
METHODS
Rat model of micronodular cirrhosis
All protocols were approved by the National Institute on Alcohol Abuse and Alcoholism Animal Care and Use Committee and were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male Sprague-Dawley rats (~200 g) received phenobarbital (35 mg/dl) through drinking water and were gavaged weekly with CCl4 in corn oil (1:1), or with corn oil only (controls), as described (2, 28). Body weight was monitored daily, and the dose of CCl4 was adjusted individually as described (2, 28). Systolic blood pressure was monitored daily using an automated tail-cuff procedure. As a result of the regular, repeated measurements, the animals had become well adapted to the procedure, resulting in reproducible, stable levels of arterial pressure. After 10–12 wk of treatment, CCl4-treated rats became hypotensive and, within 6–10 days, developed ascites. Cirrhosis was verified by postmortem microscopic examination of trichrome-stained sections of the liver.
Hemodynamic measurements
Cirrhotic and control animals were anesthetized with 2% isoflurane and tracheotomized to facilitate breathing. The animals were placed on controlled heating pads, and core temperature measured via a rectal probe was maintained at 37°C. The femoral artery and vein were cannulated for monitoring of MAP and injecting drugs, respectively. A microtip pressure-volume catheter (2F; SPR-838; Millar Instruments, Houston, TX) was inserted into the right carotid artery and advanced into the left ventricle (LV) under pressure control, as described (4, 25, 27). After stabilization for 20 min, the signals were continuously recorded at a sampling rate of 1,000/s using an ARIA pressure-volume conductance system (Millar Instruments) coupled to a Powerlab/4SP analog-to-digital converter (AD Instruments, Mountain View, CA), and then stored and displayed on a computer. All pressure-volume loop data were analyzed using a cardiac pressure-volume analysis program (PVAN3.2; Millar Instruments), and the HR, maximal LV systolic pressure, LV end-diastolic pressure (LVEDP), MAP, maximum (dP/dtmax) and minimum slope of systolic pressure increment (dP/dtmin), cardiac index (calculated as the body weight-adjusted cardiac output), stroke work (SW), and relaxation time constant (τ) were computed. In six additional experiments, cardiac contractility parameters were determined under conditions of changing preload, elicited by transiently compressing the inferior vena cava, as described earlier (25, 27). These measures include the slope of the end-systolic (ESPVR) and end-diastolic pressure-volume relations (EDPVR) and the time-varying maximal cardiac elastance (Emax). They also include the preload-recruitable SW (PRSW) (13, 27), which represents the slope of the relation between SW and end-diastolic volume and is independent of chamber size and mass.
Western blot analyses
Frozen myocardial tissue from cirrhotic animals was homogenized in RIPA lysis buffer, containing protease inhibitor cocktail set III (EMD, San Diego, CA). One hundred micrograms of lysate protein were size-fractionated by 10% SDS-PAGE and transblotted to a nitrocellulose membrane. Western blotting, with rabbit CB1 polyclonal antibody (Cayman Chemicals, Ann Arbor, MI), rabbit fatty acid amide hydrolase (FAAH) antibody (Alpha Diagnostic, San Antonio, TX), or mouse anti-actin monoclonal antibody (Chemicon, Temecula, CA) was done as described previously (4). Immunoreactive bands were visualized with Supersignal West Pico chemiluminescent substrate kit (Pierce Biotechnology, Rockford, IL) and quantified by densitometry with background correction.
Measurement of tissue endocannabinoid content
For measuring tissue endocannabinoid levels, rats were euthanized, their livers and hearts removed, and the lipids extracted. Myocardial and hepatic levels of anandamide (AEA) and 2-arachidonoyl glycerol (2-AG) were quantified by liquid chromatography/in-line mass spectrometry, as previously described (35). Values are expressed as femtomoles or picomoles per milligram of wet tissue.
Drugs
AM251 was from Tocris (Baldwin, MO) and was emulsified in 10% DMSO, 10% Tween 80, and 80% saline.
Statistical analyses
Results are presented as means ± SE. One-way ANOVA followed by Newman-Keuls multiple-comparisons post hoc analysis or unpaired t-test for pairwise comparisons were used (GraphPad Prism, San Diego, CA). Significance was assumed if P < 0.05.
RESULTS
Tissue endocannabinoid levels of cirrhotic and control rats
Anandamide levels in both the liver and the heart are increased from cirrhotic animals (Fig. 1, left) compared with control rats. Interestingly, 2-AG levels were only elevated in the liver tissue, but not in the heart (Fig. 1, right).
Fig. 1.
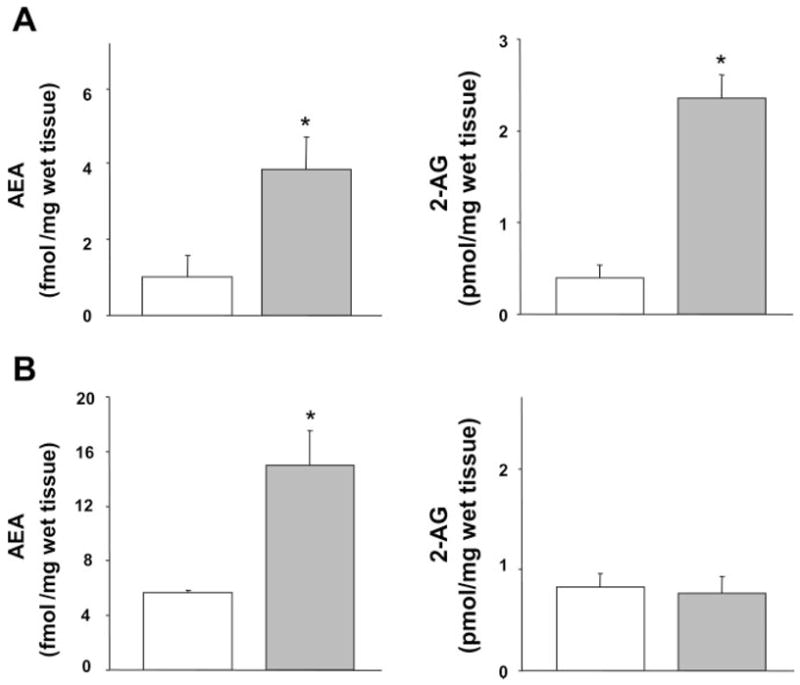
Endocannabinoid content of heart and liver from control and cirrhotic rats. Anandamide [arachidonoyl ethanolamide (AEA)] and 2-arachidonoyl glycerol (2-AG) levels in the liver (A) and heart (B) from control (open bars) and cirrhotic rats (shaded bars) are shown. Values are means ± SE; n = 6 in each group. *P < 0.05, compared with corresponding control value.
Hemodynamic profile of cirrhotic rats
Following 10–12 wk of CCl4 treatment, the rats became hypotensive, with systolic pressure of 90.2 ± 6.5 mmHg (n = 6) vs. 130.9 ± 16.9 mmHg in the controls (n = 6, P < 0.05), as monitored in the unanesthetized animals using the tail-cuff technique. Following anesthesia, MAP of the cirrhotic animals was 57.8 ± 8.6 vs. 100.5 ± 5.6 mmHg in controls (P < 0.005), with the respective systolic values (82.5 ± 10.8 vs. 123 ± 8.2 mmHg) not being significantly different from those measured by the tail-cuff technique before anesthesia (P < 0.3 for both). Basal HR of the cirrhotic group was elevated compared with the controls (Fig. 2). In agreement with published observations (19, 29), cardiac index was increased in the cirrhotic animals (Fig. 2). Analysis of the LV function demonstrated that the LV systolic pressure, SW, and dP/dtmax were decreased, indicating a systolic dysfunction and impaired contractility in the cirrhotic animals (Fig. 3). The load-independent indexes of systolic contractile function (Emax, ESPVR, and PRSW) were also decreased in the cirrhotic animals, indicating an impairment of the intrinsic inotropic state of the heart (Fig. 4, A and B). LV end-diastolic pressure and τ, indicators of diastolic function, were increased, and dP/dtmin was decreased. However, the EDPVR, an index of LV stiffness (13, 26), was not significantly altered in the cirrhotic animals (Fig. 4C).
Fig. 2.
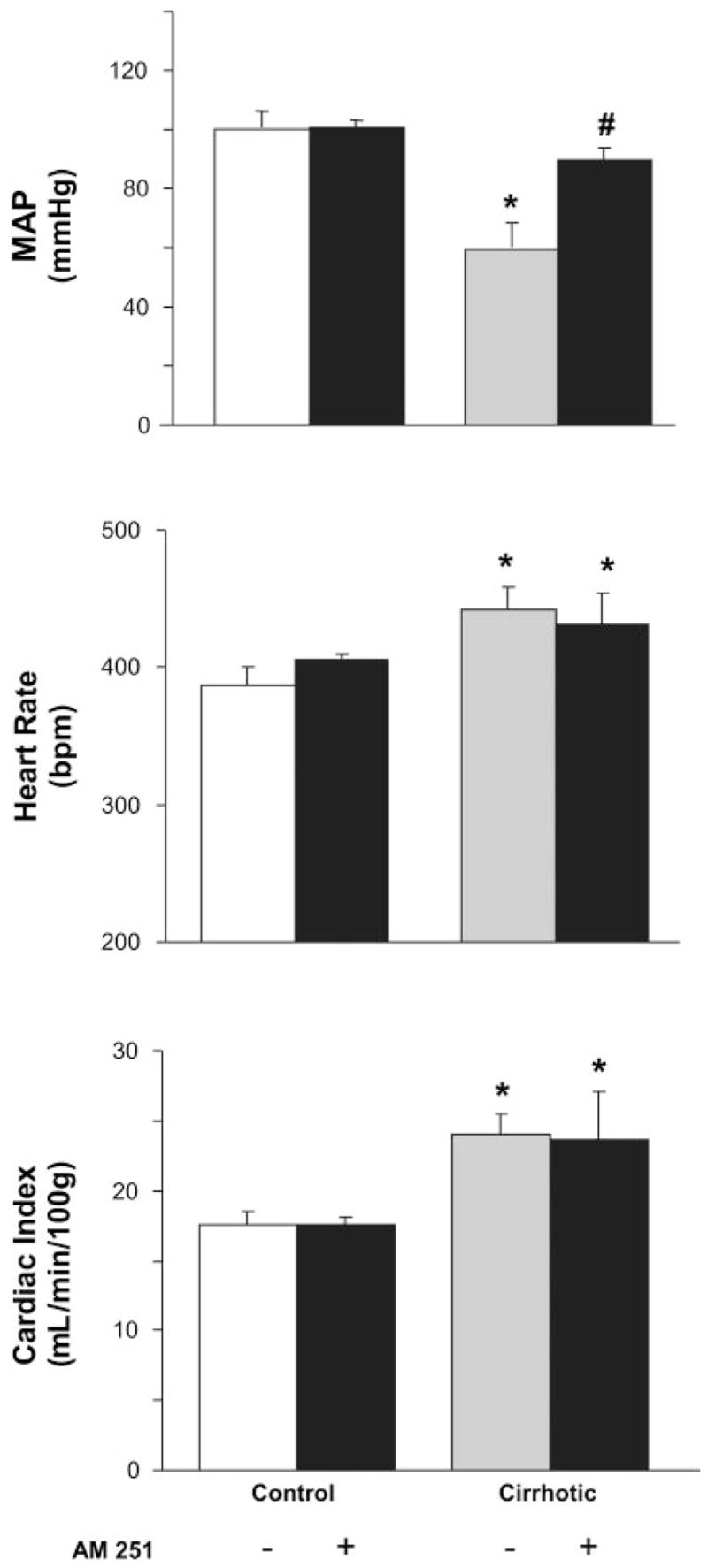
Effects of AM251 on hemodynamic parameters. Mean arterial pressure (MAP), heart rate, and cardiac index in control and cirrhotic rats treated with vehicle (open or shaded bars, respectively) or AM251 (3 mg/kg iv, solid bars) are shown. bpm, Beats/min. Values are means ± SE; n = 6–8 rats per group. P < 0.05, *control vs. cirrhotic or #vehicle vs. AM251 value.
Fig. 3.
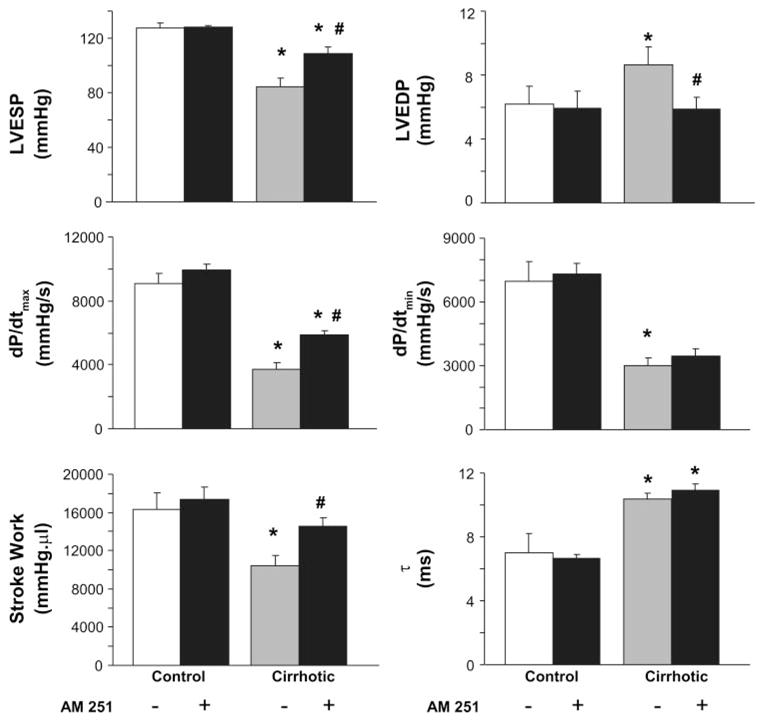
Effects of AM251 on cardiac contractile function. Effect of AM251 on parameters of systolic [left ventricular (LV) end-systolic pressure (LVESP), stroke work, and maximum slope of systolic pressure increment (dP/dtmax)] and diastolic function [LV end- diastolic pressure (LVEDP), minimum slope of systolic pressure increment (dP/dtmin), and relaxation time constant (τ)], measured at 30 min postinjection, is shown. Control and cirrhotic rats were treated with an intravenous bolus of vehicle (open and shaded bars, respectively) or AM251 (3 mg/kg, solid bars). Values are means ± SE; n = 6–8 rats per group. P < 0.05, *control vs. cirrhotic, or #vehicle vs. AM251 value.
Fig. 4.
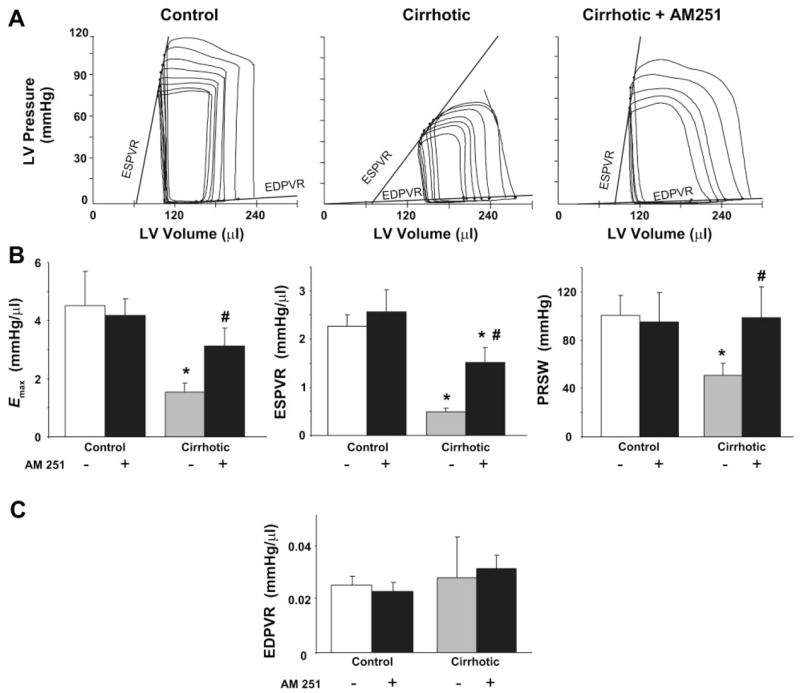
Effects of AM251 on load-independent contractile parameters. A: representative pressure-volume (PV) loops obtained with a P-V conductance catheter system at different preloads are shown. Note that the slope of the end-systolic P-V relation (ESPVR) is less steep in cirrhotic compared with control animals, indicating decreased contractile function, and is acutely increased following treatment of the cirrhotic rats with 3 mg/kg AM251. Effect of AM251 on load-independent contractile parameters measured as maximal cardiac elastance (Emax), ESPVR, preload-recruitable stroke work (PRSW) for systolic (B) and end-diastolic P-V relation (EDPVR) for diastolic function (C) (see METHODS) is shown. Control and cirrhotic animals were treated with vehicle (open and shaded bars, respectively) or 3 mg/kg AM251 (solid bars). Values are means ± SE; n = 6–8 rats per group. P < 0.05, *control vs. cirrhotic, or #vehicle vs. AM251 value.
Effect of CB1 blockade on cardiac function
Intravenous injection of AM251 had no effect on hemodynamic functions in normal rats. In contrast, in rats with advanced cirrhosis, intravenous injection of 3 mg/kg AM251 resulted in a gradual increase in MAP, reaching a plateau at ~30 min postinjection, which was maintained for over 1 h. The elevated basal HR and cardiac index were unaffected by AM251 (Fig. 2). In cirrhotic rats, AM251 treatment resulted in significant improvements of all measured parameters of LV systolic function (Fig. 3), including the load-independent indexes of contractility (Emax, ESPVR, and PRSW; Fig. 4B). The effect of AM251 was less clear for parameters of diastolic function. Although the increased end-diastolic pressure of cirrhotic rats was normalized following AM251 treatment, this may have resulted from a reduction in venous return due to blockade of endocannabinoid-mediated venodilation. On the other hand, diastolic chamber stiffness, as indicated by EDPVR, which was unaffected in cirrhosis, remained unchanged by AM251 treatment.
CB1-receptor and FAAH expression in the heart
The level of CB1-receptor expression in the myocardium, as detected by Western blotting, was similar in control and cirrhotic rats (Fig. 5). To test whether the elevated anandamide content of the cirrhotic heart (see Fig. 1) is due to its reduced in vivo degradation, we also quantified the myocardial expression of FAAH, the enzyme responsible for the metabolism of anandamide (6). FAAH protein levels were similar in control and cirrhotic hearts (Fig. 5).
Fig. 5.
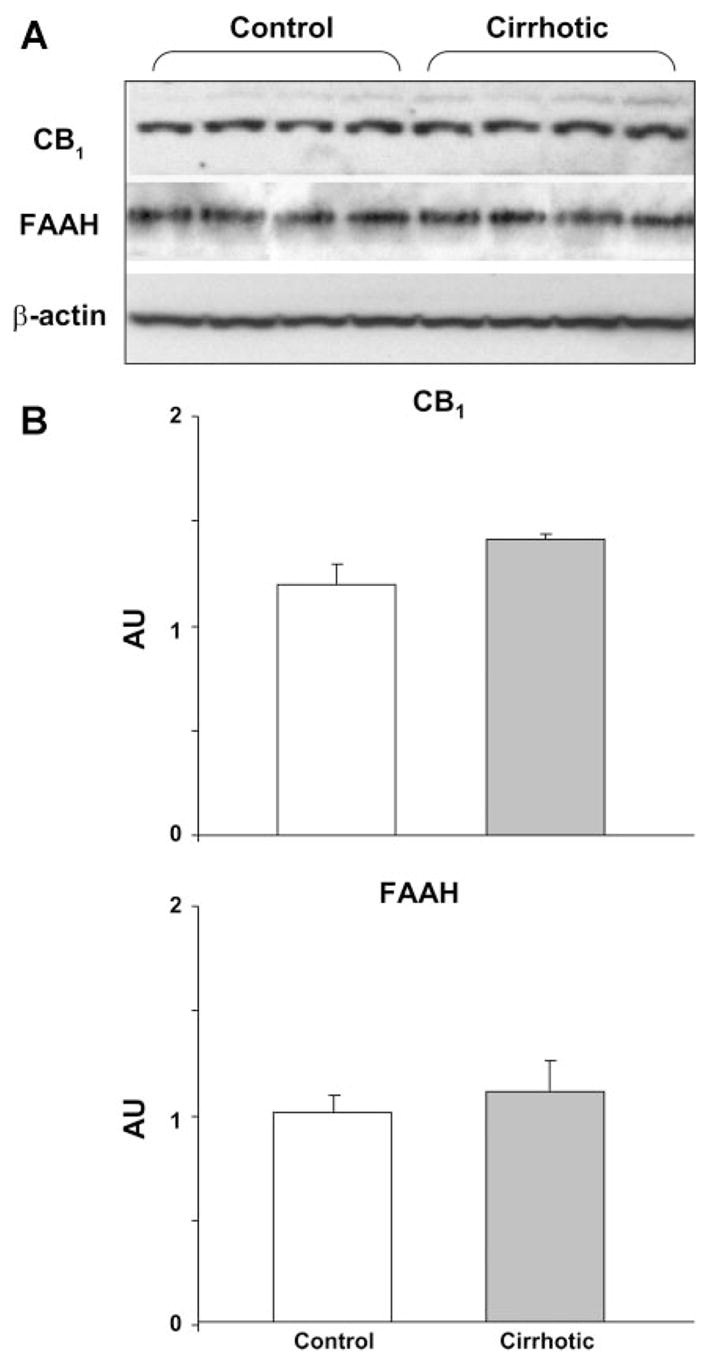
Cardiac CB1-receptor and fatty acid amide hydrolase (FAAH) expressions are unchanged in cirrhosis. Expression of CB1 receptors and FAAH in the heart of control (n = 4) and cirrhotic rats (n = 4), analyzed by Western blotting (A) and quantified by densitometry (B) is shown. β-Actin was used as loading control. Values are means ± SE. AU, arbitrary unit.
DISCUSSION
The present findings document, for the first time, the cardiac contractile dysfunction in vivo in rats with CCl4-induced advanced micronodular cirrhosis, as reflected by marked changes in both load-dependent and load-independent indexes of myocardial contractility. They provide evidence that increased activity of the endocannabinoid/CB1-receptor system is largely responsible for the impaired cardiac contractility in the intact animal. They further demonstrate that CB1 blockade can correct the contractile dysfunction in cirrhosis, which highlights the therapeutic potential of CB1 antagonists in this condition.
Cardiac index was increased in cirrhotic compared with normal rats and was unaffected by CB1 blockade (Fig. 2), which is in agreement with previous reports in rats with cirrhosis induced either by CCl4 treatment (28) or bile duct ligation (19). However, cardiac output is influenced by both preload and afterload, and the reduced cardiac contractile function in cirrhotic rats was likely offset by increased venous return and decreased peripheral resistance. Clearly, by relying on changes in cardiac output alone, one would have missed both the contractile dysfunction in cirrhosis and the role of endocannabinoids in mediating it. Although dP/dtmax has been widely used as an indicator of contractility, and it was markedly reduced in cirrhosis (Fig. 3), it too is load dependent, being particularly sensitive to changes in preload (13). The slope of the ESPVR and Emax have been proposed as load-insensitive indexes of contractility (13). ESPVR and Emax were markedly decreased in cirrhotic rats and increased toward control levels following CB1 blockade (Fig. 4B). However, ESPVR may be influenced not only by changes in inotropic state but also by differences in chamber geometry and other factors (13, 27). Therefore, we derived an additional contractile parameter, the PRSW, which sensitively reflects changes in systolic function and has been described as independent of chamber size and mass (13). The marked decrease in PRSW in cirrhotic rats and its return to near control levels following AM251 treatment thus clearly demonstrate that endocannabinoids induce systolic dysfunction in cirrhosis through directly affecting myocardial contractility.
End-diastolic pressure and τ were increased and dP/dtmin was decreased in cirrhosis, which could reflect impaired diastolic relaxation, an active process resulting from myocardial calcium sequestration. However, end-diastolic pressure may also be influenced by changes in preload, and its increase may have resulted from increased venous return related to the generalized vasodilated state in cirrhosis. The finding that the load-independent measure of stiffness, EDPVR, was unaffected in cirrhosis (Fig. 4C) suggests the absence of significant fibrosis, and thus the contractile dysfunction is related to impaired inotropy.
Endocannabinoids are highly lipophilic and remain largely cell associated upon their release (23). This and their rapid enzymatic degradation prevent sufficient amounts to reach the circulation, and thus they are believed to act locally as auto-crine/paracrine mediators (23). Increased plasma anandamide levels have been reported in cirrhosis (8); the relevance of this to cardiovascular function is unclear. Our finding of a marked elevation of anandamide, but not 2-AG, in the cirrhotic heart implicates anandamide as the endogenous agonist involved in the contractile dysfunction associated with liver cirrhosis. A possible mechanism for the increase in hepatic and cardiac endocannabinoid content may involve bacterial endotoxin (lipopolysaccharide), since advanced cirrhosis is known to be associated with endotoxemia (16), and lipopolysaccharide increases anandamide synthesis in macrophages (17), in isolated primary hepatocytes (4), and possibly other cell types/tissues. Endotoxin increases oxidative and nitrosative stress in various tissues (24, 15), which may also contribute to increased generation of endocannabinoids (3). Circulating macrophages, which are activated and have elevated anandamide content in cirrhosis (2), may also contribute to decreased cardiac contractility (30). The alternative possibility that the increase in the cardiac levels of anandamide is due to its decreased degradation is unlikely due to the unchanged expression of FAAH in the cirrhotic myocardium (Fig. 5).
The hyperdynamic circulation in cirrhosis has been related to either increased preload due to plasma volume expansion or decreased afterload resulting from arterial vasodilation. However, the use of load-independent measures of cardiac contractility in the present experiments has allowed us to unequivocally establish myocardial contractile dysfunction as the direct result of altered inotropic state.
Although the underlying cellular mechanisms have not yet been explored, CB1-receptor activation is known to inhibit L-type calcium channels (11) and to reduce cAMP levels (12), both of which may contribute to a negative inotropic effect. A physiological role of such a cardiodepressor mechanism could be to counteract inappropriate increases in cardiac contractility, such as in the early stages of hypertension. Indeed, CB1-receptor blockade was reported to further increase blood pressure and cardiac contractility in rats with different forms of hypertension (4, 22).
It is noteworthy that, whereas AM251 did not affect hemodynamic variables in normal control rats, improvements in contractile indicators similar to those described here in cirrhotic rats have been recently documented with AM281 and rimonabant in mice with doxorubicin-induced heart failure (21). In that study, doxorubicin also increased endocannabinoid production in the myocardium (21), presumably by oxidative/nitrosative stress-related mechanisms, which are pivotal in the cardiotoxicity of this chemotherapeutic agent (25). In addition, CB1 antagonist exerted potent cytoprotective effects against doxorubicin-induced cell death, both in vitro and in vivo (21). Together, these observations suggest a novel pathogenic role of endocannabinoids in heart failure of various origins and also raise the potential value of CB1 antagonists in the treatment of heart failure.
In addition to increased anandamide levels in the cirrhotic heart, we have also found upregulation of the endocannabinoid content of the cirrhotic liver, with both 2-AG and anandamide being markedly increased over levels measured in controls. It has been recently demonstrated that CB1-receptor knockout mice are resistant to liver fibrosis induced by different stimuli, and the progress in hepatic fibrosis in control mice can be delayed by chronic treatment with a CB1-receptor antagonist (31). Unlike the unchanged expression of CB1 receptors in the cirrhotic myocardium found in the present experiments, fibrogenic stimuli were reported to increase the expression of CB1 receptors in hepatic stellate cells (31). Thus the hepatic fibrogenic action of endocannabinoids may be increased in cirrhosis as the result of both increased end-organ sensitivity and increased concentrations of the endogenous ligands. Regardless of the underlying mechanism, however, the present findings suggest a unique therapeutic potential for CB1-receptor antagonists in cirrhosis, as they may not only slow the progress of the fibrotic process, but could also improve cardiac contractile performance and correct the associated hemodynamic abnormalities, including the systemic and mesenteric vasodilation. This, in turn, may reduce the risk for preterminal or life-threatening complications, such as the development of ascites or the rupture of varicose veins (1), helping patients to survive until a transplant becomes available.
Acknowledgments
GRANTS
This study was supported by the Intramural Research Program of National Institutes of Health/National Institute on Alcohol Abuse and Alcoholism.
References
- 1.Bataller R, Arroyo V, Gines P. Management of ascites in cirrhosis. J Gastroenterol Hepatol. 1997;12:723–733. doi: 10.1111/j.1440-1746.1997.tb00360.x. [DOI] [PubMed] [Google Scholar]
- 2.Bátkai S, Járai Z, Wagner JA, Goparaju SK, Varga K, Liu J, Wang L, Mirshahi F, Khanolkar AD, Makriyannis A, Urbaschek R, Garcia N, Jr, Sanyal AJ, Kunos G. Endocannabinoids acting at vascular CB1 receptors mediate the vasodilated state in advanced liver cirrhosis. Nat Med. 2001;7:827–832. doi: 10.1038/89953. [DOI] [PubMed] [Google Scholar]
- 3.Bátkai S, Osei-Hyiaman D, Pan H, El-Assal O, Rajesh M, Mukhopadhyay P, Hong F, Harvey-White J, Jafri A, Hasko G, Huffman JW, Gao B, Kunos G, Pacher P. Cannabinoid-2 receptor mediates protection against hepatic ischemia/reperfusion injury. FASEB J. 2007;21:1788–1800. doi: 10.1096/fj.06-7451com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bátkai S, Pacher P, Osei-Hyiaman D, Radaeva S, Liu J, Harvey-White J, Offertáler L, Mackie K, Rudd MA, Bukoski RD, Kunos G. Endocannabinoids acting at cannabinoid-1 receptors regulate cardiovascular function in hypertension. Circulation. 2004;110:1996–2002. doi: 10.1161/01.CIR.0000143230.23252.D2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonz A, Laser M, Kullmer S, Kniesch S, Babin-Ebell J, Popp V, Ertl G, Wagner JA. Cannabinoids acting on CB1 receptors decrease contractile performance in human atrial muscle. J Cardiovasc Pharmacol. 2003;41:657–664. doi: 10.1097/00005344-200304000-00020. [DOI] [PubMed] [Google Scholar]
- 6.Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci USA. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Domenicali M, Ros J, Fernandez-Varo G, Cejudo-Martin P, Crespo M, Morales-Ruiz M, Briones AM, Campistol JM, Arroyo V, Vila E, Rodes J, Jimenez W. Increased anandamide induced relaxation in mesenteric arteries of cirrhotic rats: role of cannabinoid and vanilloid receptors. Gut. 2005;54:522–527. doi: 10.1136/gut.2004.051599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez-Rodriguez CM, Romero J, Petros TJ, Bradshaw H, Gasalla JM, Gutierrez ML, Lledo JL, Santander C, Fernandez TP, Tomas E, Cacho G, Walker JM. Circulating endogenous cannabinoid anandamide and portal, systemic and renal hemodynamics in cirrhosis. Liver Int. 2004;24:477–483. doi: 10.1111/j.1478-3231.2004.0945.x. [DOI] [PubMed] [Google Scholar]
- 9.Gaskari SA, Honar H, Lee SS. Therapy insight: cirrhotic cardiomyopathy. Nat Clin Pract Gastroenterol Hepatol. 2006;3:329–337. doi: 10.1038/ncpgasthep0498. [DOI] [PubMed] [Google Scholar]
- 10.Gaskari SA, Liu H, Moezi L, Li Y, Baik SK, Lee SS. Role of endocannabinoids in the pathogenesis of cirrhotic cardiomyopathy in bile duct-ligated rats. Br J Pharmacol. 2005;146:315–323. doi: 10.1038/sj.bjp.0706331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gebremedhin D, Lange AR, Campbell WB, Hillard CJ, Harder DR. Cannabinoid CB1 receptor of cat cerebral arterial muscle functions to inhibit L-type Ca2+ channel current. Am J Physiol Heart Circ Physiol. 1999;276:H2085–H2093. doi: 10.1152/ajpheart.1999.276.6.H2085. [DOI] [PubMed] [Google Scholar]
- 12.Howlett AC, Bidaut-Russell M, Devane WA, Melvin LS, Johnson MR, Herkenham M. The cannabinoid receptor: biochemical, anatomical and behavioral characterization. Trends Neurosci. 1990;13:420–423. doi: 10.1016/0166-2236(90)90124-s. [DOI] [PubMed] [Google Scholar]
- 13.Kass DA, Maughan WL, Guo ZM, Kono A, Sunagawa K, Sagawa K. Comparative influence of load versus inotropic states on indexes of ventricular contractility: experimental and theoreticaol analysis based on pressure-volume relationships. Circulation. 1987;76:1422–1436. doi: 10.1161/01.cir.76.6.1422. [DOI] [PubMed] [Google Scholar]
- 14.Kowalski HJ, Abelmann WH. The cardiac output at rest in Laennec’s cirrhosis. J Clin Invest. 1953;32:1025–1033. doi: 10.1172/JCI102813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liaudet L, Pacher P, Mabley JG, Virág L, Soriano FG, Haskó G, Szabó C. Activation of poly(ADP-ribose) polymerase-1 is a central mechanism of lipopolysaccharide-induced acute lung inflammation. Am J Respir Crit Care Med. 2002;165:372–377. doi: 10.1164/ajrccm.165.3.2106050. [DOI] [PubMed] [Google Scholar]
- 16.Lin RS, Lee FY, Lee SD, Tsai YT, Lin HC, Lu RH, Hsu WC, Huang CC, Wang SS, Lo KJ. Endotoxemia in patients with chronic liver diseases: relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. J Hepatol. 1995;22:165–172. doi: 10.1016/0168-8278(95)80424-2. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Batkai S, Pacher P, Harvey-White J, Wagner JA, Cravatt BF, Gao B, Kunos G. Lipopolysaccharide induces anandamide synthesis in macrophages via CD14/MAPK/phosphoinositide 3-kinase/NF-kappaB independently of platelet-activating factor. J Biol Chem. 2003;278:45034–45039. doi: 10.1074/jbc.M306062200. [DOI] [PubMed] [Google Scholar]
- 18.Ma Z, Lee SS. Cirrhotic cardiomyopathy: getting to the heart of the matter. Hepatology. 1996;24:451–459. doi: 10.1002/hep.510240226. [DOI] [PubMed] [Google Scholar]
- 19.Moezi L, Gaskari SA, Liu H, Baik SK, Dehpour AR, Lee SS. Anandamide mediates hyperdynamic circulation in cirrhotic rats via CB(1) and VR(1) receptors. Br J Pharmacol. 2006;149:898–908. doi: 10.1038/sj.bjp.0706928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moller S, Henriksen JH. Cirrhotic cardiomyopathy: a pathophysiological review of circulatory dysfunction in liver disease. Heart. 2002;87:9–15. doi: 10.1136/heart.87.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukhopadhyay P, Batkai S, Rajesh M, Czifra N, Harvey-White J, Hasko G, Pacher P. Pharmacological inhibition of cannabinoid receptor-1 protects against doxorubicin-induced cardiotoxicity. J Am Coll Cardiol. doi: 10.1016/j.jacc.2007.03.057. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pacher P, Batkai S, Kunos G. Blood pressure regulation by endocannabinoids and their receptors. Neuropharmacology. 2005;48:1130–1138. doi: 10.1016/j.neuropharm.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pacher P, Bátkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pacher P, Liaudet L, Bai P, Mabley JG, Kaminski PM, Virag L, Deb A, Szabo E, Ungvari Z, Wolin MS, Groves JT, Szabo C. Potent metalloporphyrin peroxynitrite decomposition catalyst protects against the development of doxorubicin-induced cardiac dysfunction. Circulation. 2003;107:896–904. doi: 10.1161/01.cir.0000048192.52098.dd. [DOI] [PubMed] [Google Scholar]
- 26.Pacher P, Mabley JG, Liaudet L, Evgenov OV, Marton A, Hasko G, Kollai M, Szabo C. Left ventricular pressure-volume relationship in a rat model of advanced aging-associated heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H2132–H2137. doi: 10.1152/ajpheart.00405.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pacher P, Vaslin A, Benko R, Mabley JG, Liaudet L, Hasko G, Marton A, Batkai S, Kollai M, Szabo C. A new, potent poly(ADP-ribose) polymerase inhibitor improves cardiac and vascular dysfunction associated with advanced aging. J Pharmacol Exp Ther. 2004;311:485–491. doi: 10.1124/jpet.104.069658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Proctor E, Chatamra K. High yield micronodular cirrhosis in the rat. Gastroenterology. 1982;83:1183–1190. [PubMed] [Google Scholar]
- 29.Ros J, Claria J, To-Figueras J, Planaguma A, Cejudo-Martin P, Fernandez-Varo G, Martin-Ruiz R, Arroyo V, Rivera F, Rodes J, Jimenez W. Endogenous cannabinoids: a new system involved in the homeostasis of arterial pressure in experimental cirrhosis in the rat. Gastroenterology. 2002;122:85–93. doi: 10.1053/gast.2002.30305. [DOI] [PubMed] [Google Scholar]
- 30.Simms MG, Walley KR. Activated macrophages decrease rat cardiac myocyte contractility; importance of ICAM-1-dependent adhesion. Am J Physiol Heart Circ Physiol. 1999;277:H253–H260. doi: 10.1152/ajpheart.1999.277.1.H253. [DOI] [PubMed] [Google Scholar]
- 31.Teixeira-Clerc F, Julien B, Grenard P, Tran Van Nhieu J, Deveaux V, Hezode C, Mallat A, Lotersztajn S. CB1 cannabinoid receptor antagonism: a new strategy for the treatment of liver fibrosis. Nat Med. 2006;12:671–676. doi: 10.1038/nm1421. [DOI] [PubMed] [Google Scholar]
- 32.Varga K, Wagner JA, Bridgen DT, Kunos G. Macrophage- and platelet-derived endogenous cannabinoids are involved in endotoxin-induced hypotension. FASEB J. 1998;12:1035–1044. doi: 10.1096/fasebj.12.11.1035. [DOI] [PubMed] [Google Scholar]
- 33.Wagner JA, Hu K, Bauersachs J, Karcher J, Wiesler M, Goparaju SK, Kunos G, Ertl G. Endogenous cannabinoids mediate hypotension in cardiogenic shock after experimental myocardial infarction. J Am Coll Cardiol. 2001;38:2048–2054. doi: 10.1016/s0735-1097(01)01671-0. [DOI] [PubMed] [Google Scholar]
- 34.Wagner JA, Varga K, Ellis EF, Rzigalinski BA, Martin BR, Kunos G. Activation of peripheral CB1 cannabinoid receptors in haemorrhagic shock. Nature. 1997;390:518–521. doi: 10.1038/37371. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Liu J, Harvey-White J, Zimmer A, Kunos G. Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proc Natl Acad Sci USA. 2003;100:1393–1398. doi: 10.1073/pnas.0336351100. [DOI] [PMC free article] [PubMed] [Google Scholar]


