Abstract
When a discrete cue (a “sign”) is presented repeatedly in anticipation of a food reward the cue can become imbued with incentive salience, leading some animals to approach and engage it, a phenomenon known as “sign-tracking” (the animals are sign-trackers; STs). In contrast, other animals do not approach the cue, but upon cue presentation go to the location where food will be delivered (the goal). These animals are known as goal-trackers (GTs). It has been hypothesized that individuals who attribute excessive incentive salience to reward-related cues may be especially vulnerable to develop compulsive behavioral disorders, including addiction. We were interested, therefore, in whether individual differences in the propensity to sign-track are associated with differences in responsivity to cocaine. Using an autoshaping procedure in which lever (conditioned stimulus) presentation was immediately followed by the response-independent delivery of a food pellet (unconditioned stimulus), rats were first characterized as STs or GTs and subsequently studied for the acute psychomotor response to cocaine and the propensity for cocaine-induced psychomotor sensitization. We found that GTs were more sensitive than STs to the acute locomotor activating effects of cocaine, but STs showed a greater propensity for psychomotor sensitization upon repeated treatment. These data suggest that individual differences in the tendency to attribute incentive salience to a discrete reward-related cue, and to approach and engage it, are associated with susceptibility to a form of cocaine-induced plasticity that may contribute to the development of addiction.
Keywords: cocaine, sensitization, addiction, stereotypy, locomotion, sign-tracking, goal-tracking, individual differences, autoshaping, Pavlovian learning
INTRODUCTION
When a discrete cue (conditioned stimulus, CS) is repeatedly presented in anticipation of a food reward (unconditioned stimulus, US) many different conditioned responses (CR) emerge, including changes in emotional and motivational states that are manifested by complex changes in behavior. Zener (1937) first described individual differences in complex skeletal CRs in dogs trained using a classic Pavlovian conditioning procedure (i.e., a bell paired with food reward). He reported that some dogs responded to the CS with “an initial glance at the bell” followed by “a constant fixation…to the food-pan …”, whereas a few dogs exhibited a “small but definite movement of approach toward the conditioned stimulus … followed by a backing up later to a position to eat”, a sequence described by Zener as a “striking phenomenon” [56, pg. 391]. These two different CRs to a food-related CS were later characterized in rats by Boakes (1977), who called CS-elicited approach to the cue “sign-tracking” (note that the procedure is sometimes called “autoshaping”) and CS-elicited approach to the location where the food would be delivered “goal-tracking” [5]. In rats the sign-tracking response not only consists of approach, but often includes a repertoire of behaviors similar to those involved in consuming the US [16, 31]. For example, if presentation of a lever is followed by the response-independent delivery of a food pellet, animals not only begin to approach the lever but often grasp and gnaw the lever [30, 50]. Whether an individual develops a sign-tracking CR or a goal-tracking CR may reflect individual differences in the degree to which incentive salience is attributed to the reward-associated cue.
Incentive salience refers to a motivational component of reward, one that “transforms mere sensory information about rewards and their cues (sights, sounds, and smells) into attractive, desired, riveting incentives” [4]. That is, incentive stimuli become “motivational magnets” [3], eliciting approach towards them, as in the case of Pavlovian conditioned approach behavior towards rewards and their signals [12]. Reward-related cues in the environment not only guide and energize normal behavior, but can also lead to pathological and apparently compulsive behavior [23, 50]. Thus, the way individuals respond to signals associated with rewards may confer vulnerability to psychopathology, such as substance abuse.
We recently reported that sign-trackers (STs) and goal-trackers (GTs) show different experience-dependent changes in dopaminergic gene expression [27] and others have implicated the dopamine system in sign-tracking behavior. For example, Tomie et al. [51] reported increased levels of dopamine and DOPAC in the nucleus accumbens of STs and found a positive correlation between accumbens dopamine levels and the vigor with which rats engaged the cue. In addition, Dalley et al. [15] demonstrated that dopamine D1 receptors in the nucleus accumbens are necessary for the acquisition of a sign-tracking response and Phillips and colleagues [40], using electrical brain-stimulation as the US, reported disruption of sign-tracking following the administration of neuroleptic drugs. Finally, it is well known that the presentation of reward-related cues alters the activity of dopamine and striatal neurons [for review see 17]. Taken together, these studies highlight the involvement of the mesolimbic dopamine system in the emergence of Pavlovian conditioned approach behavior. We hypothesized, therefore, that individual differences in the propensity to sign-track may also be related to differences in responsivity to drugs that increase dopamine neurotransmission, such as cocaine. Thus, in the current study we investigated both the acute psychomotor response to cocaine and the ability of repeated injections of cocaine to induce psychomotor sensitization in STs and GTs.
METHODS
Subjects
Forty-two adult male Sprague-Dawley rats from Charles River (Wilmington, MA, USA) weighing 250-300 g upon arrival were used. Rats were housed in pairs and kept on a 12-hr light/dark cycle (lights on 0600 hours) with controlled temperature and humidity. Food and water were available ad libitum throughout the study. The experiments followed the “Principles of Laboratory Animal Care” (http://www.nap.edu/readingroom/books/labrats/) and the “Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research” (National Research Council 2003), and the procedures approved by the University Committee on the Use and Care of Animals.
Pavlovian Conditioned Approach
Conditioning Chambers
Fifteen MED Associates test chambers (21.6 × 17.8. cm floor area, 12.7 cm high; MED Associates, St. Albans, VT) were used for Pavlovian training. Each chamber was equipped with a food receptacle, which was located in the center of the 21.6-cm-wide wall, 3 cm above the stainless steel grid floor. An illuminated retractable lever (MED Associates, ST. Albans, VT) was located approximately 2.5 cm to the left or right of the food receptacle, 3 cm above the floor. The side of the lever with respect to the food receptacle was counter-balanced across boxes to eliminate any side bias. A red house light was located on the wall opposite the food receptacle and remained on throughout the training sessions. Two nose-poke ports were located approximately 3 cm above the grid floor on either side of the house light. Responses in the nose-poke ports were without consequence and served as an index of general exploratory behavior. A white LED was flush-mounted on the inside of the retractable lever and could be used to illuminate the slot through which the lever protruded. The lever required only a 10-gram force to operate, such that most contacts with the lever were recorded as a “lever press.” Operation of the pellet dispenser (Med Associates, St. Albans, VT) delivered 45-mg banana-flavored food pellet (Bio-Serv®, #F0059, Frenchtown, NJ) into the food receptacle. Head entry into the food receptacle was recorded each time a rat broke the infrared photobeam located inside the receptacle (approximately 1.5 cm above the base of the food cup). Each conditioning chamber was located in a sound-attenuating enclosure and white noise was supplied by a ventilating fan to mask outside noise.
Pavlovian Conditioning Procedures
All training sessions were conducted between the hours of 1300 and 1700. Three waves of rats (14 animals per wave) were tested per day. For two days prior to the start of training 45-mg banana-flavored food pellets were placed into the rats' home cages to familiarize the animals with this food. After one week of acclimation to the colony room rats were placed into the testing chambers for pre-training sessions during which the red house-light remained on but the lever was retracted. Fifty food pellets were delivered on a variable interval (VI) 90-s schedule, and it was determined whether the rats were reliably retrieving the pellets from the food receptacle. The pre-training sessions lasted approximately 25 min. By the end of the 2nd pre-training session all of the rats consumed all of the food pellets. Thus, after 2 days of pretraining Pavlovian training commenced using procedures similar to those described previously [27]. During a Pavlovian training session each individual trial consisted of presentation of the illuminated lever (CS) into the chamber for 8 s, and immediately following retraction of the lever the pellet dispenser was activated and one 45-mg food pellet (US) was delivered into the food receptacle. The beginning of the next intertrial interval (ITI) commenced immediately after pellet delivery. The CS was presented on a random interval 90 s schedule (i.e., one presentation of the CS occurred on average every 90 s, but the actual time between CS presentations varied randomly between 30 and 150 s). Each Pavlovian training session consisted of 25 trials, resulting in a 35-40 min session, and training was conducted over 5 consecutive days. We recorded the following events: (1) the number of lever presses, (2) the latency to the first lever press, (3) the number of receptacle entries during presentation of the CS, (4) the latency to the first receptacle entry following CS presentation, (5) the number of receptacle entries during the ITI, and (6) the number of nose-pokes (as an index of general exploratory behavior). These data were recorded using Med Associates software (St. Albans, VT). The number of food pellets consumed was also recorded following each session.
Psychomotor Activating Effects of Cocaine
Activity Chambers and Video Recording Devices
In-house custom made activity chambers were used. Each chamber was made from expanded PVC (33.02 × 68.58 × 60.96 cm tall) with a stainless woven wire cloth grid floor (30.48 × 60.96, 7.62 × 7.62-cm squares), complete with a catch tray. Directly above each activity chamber was a camera (CCTV Specialty Bullet Cameras, Lake Worth, FL) used to record behavior during the drug testing sessions. A Pelco (Clovis, CA) DX9100 digital video recorder (DVR) was used to transfer the videos to a computer for automated analysis (see below).
Cocaine Treatment
Cocaine treatment began one day following the last day of Pavlovian training. Animals were transferred from the colony room and placed into the activity chambers on the 1st day of testing. Rats were allowed to habituate to the activity chambers for 45 min before they received a vehicle (0.9% saline) injection. After this, the animals received escalating doses of cocaine (7.5, 15, 30 mg/kg, i.p.). A 45-min period elapsed between injections, during which time behavior was video-recorded. After the first day of testing rats were returned to their home cages where they received 15 mg/kg cocaine once a day for 6 days. On day 7, the rats returned to the activity chambers where they again were allowed to habituate and then received escalating doses of cocaine (starting with vehicle, as on day 1). This procedure generates within-subjects dose-effect information, allowing us to compare the day 7 to the day 1 response to assess sensitization.
Automated Behavioral Analysis
Clever Sys, Inc. (Reston, VA, USA, www.cleversysinc.com) Drug Scan software was used to analyze the video-recorded behavior [25]. This software is designed to utilize information from the animals' full body as well as specific body parts (e.g. nose, base of tail, etc.) in order to identify behaviors of interest. For the purpose of this study we analyzed locomotor activity and repetitive head movements as two indices of the psychomotor activating effects of cocaine. Locomotor activity was measured as the number of crossovers from one end (e.g. far right) of the chamber to the other (e.g. far left) based on user-defined areas of the activity chamber. Repetitive head movements were defined as the number of lateral head movements made during each period the animal was “in place” (i.e. not locomoting) for a period of at least 2 sec. A lateral head movement was recorded each time the rat's head moved at least 10 degrees from the centerpoint of its body in either direction. The frequency of in place head movements was calculated by dividing the number of head movements by the time spent in place. This provides an index of the vigor of drug-induced head movements (movement/sec) when the animals are not engaged in other competing behaviors such as locomotion [24, 25, 46]. We have found that the frequency of head movements provides a very sensitive index of psychomotor activation following the administration of cocaine [24, 25].
Statistical Analysis
Pavlovian Conditioned Approach Behavior
Linear mixed-effects models [54] were used to assess longitudinal trends in Pavlovian conditioned approach behavior. The covariance structure was explored and modeled appropriately for each dependent variable (i.e. lever press, latency to lever press, receptacle entry, latency to receptacle entry, nosepokes, percent difference in approach and percent of trials with approach to lever and receptacle). Group differences in approach responses were examined using one sample t-tests (with hypothesized value of 0) to determine whether either group exhibited a preference for approach to the lever vs. approach to the food receptacle (see Fig. 2).
Figure 2.
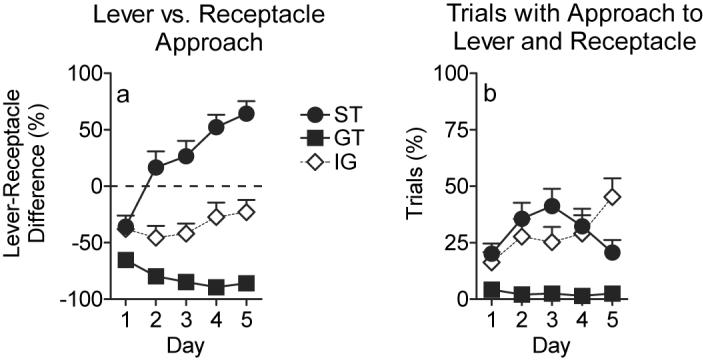
Representation of different patterns of approach behavior for STs (circles, n=14), GTs (squares, n=14) and intermediate animals (diamonds, n=12). a) Data are expressed as mean ± SEM percent of trials with approach to the lever minus the percent of trials with approach the food receptacle (i.e. lever-receptacle difference). A score of zero (indicated by the dashed line) suggests that neither approach to the lever nor approach to the receptacle was dominant. b) Mean ± SEM percent of trials with approach to both the lever and the food receptacle during the same CS period.
Psychomotor Activating Effects of Cocaine
Time course examination of the 45-min period following each cocaine injection suggested that the peak behavioral response fell between 10 and 35 min. Thus, the statistical analyses were conducted using cocaine-induced behavior collected during this 25-min peak period. Dose-effect data generated from locomotor activity (crossovers) and repetitive head movements were analyzed using linear mixed-effects models [54] with day and dose as the repeated variables. The effect of the vehicle injection was not included in the dose-effect analyses. When significant main effects or day × dose interactions were revealed, Bonferonni post-hoc comparisons were conducted.
Fisher's Exact Test and logistic regression models (SAS® software) were used to assess group differences in the number of animals that met criteria for “restricted” movement (see below and Fig. 5). Generalized estimating equations (GEE) were used to adjust for repeated measures within the same animal [1]. Because the general modeling procedure cannot be completed with values of zero, one ST (on day 1 at 15 mg/kg) was assigned a value which was arbitrarily weighted as 0.1. This allowed the analysis (i.e. algorithm) to be completed without significantly affecting the outcome of the results. For all analyses significance was set at P≤0.05.
Figure 5.
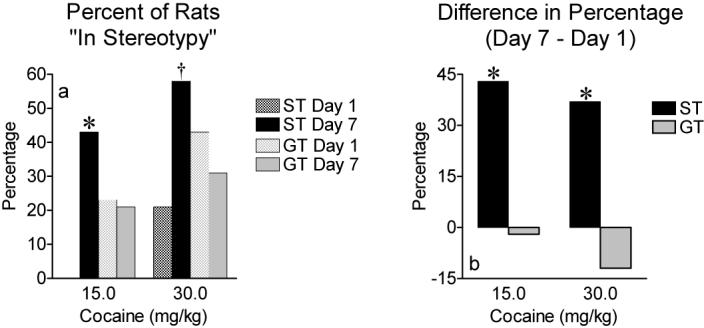
a) The percentage of rats that met criterion for restricted movement (i.e. frequency of head movement ≥ 1.0 movement/sec) at 15 and 30 mg/kg cocaine on day 1 and day 7. None of the STs met criterion for stereotypy at 15 mg/kg on day 1, thus the bar is not visible. A greater percentage of STs were in stereotypy on day 7 relative to day 1 at 15 mg/kg (*P=0.02) and 30 mg/kg (†P=0.11). b) The difference (day 7 – day 1) in the percentage of rats that met criterion for restricted movement at 15 and 30 mg/kg cocaine. A greater percentage of ST relative to GT were in stereotypy on day 7 relative to day 1 at both doses (*P<0.05).
RESULTS
Pavlovian Conditioned Approach Behavior
Previous studies have established that there is considerable individual variation in the development of sign-tracking behavior (especially in rats relative to pigeons), and only a sub-set of rats develop robust sign-tracking [5, 27, 51]. We were interested in directly comparing rats that sign-track with those that goal-track, and so we used the following criteria to subdivide the total sample of rats into distinct groups. Rats were categorized into 3 groups based on the number of lever presses averaged across the 5 days of training, because we previously found that this single variable is highly predictive of the sign-tracking phenotype, at least when food is used as the US [27]. Thus, the 3 groups consisted of: 1) the 14 rats with the highest number of lever presses (range 36-115, 35% of the sample); 2) the 14 with the lowest number of lever presses (0-3.5, 35% of the sample); and 3) the remaining 12 animals with an intermediate number of lever presses (4.2-35.4, 30% of the sample). Note, however, there is a strong correlation between the 5-day average lever press score and the number of lever presses on day 1 (r2 = 0.45; P<0.0001) and day 5 (r2 = 0.87; P<0.0001). The animals in Group 1 above will be referred to as sign-trackers (STs) and animals in Group 2 as goal-trackers (GTs), because an examination of the entire data set (see below) indicates that this best describes the behavior of the respective groups. Group 3 is referred to as the “Intermediate Group” (IG), because they showed a mixture of sign-tracking and goal-tracking behavior. For the purpose of the cocaine study only the ST and GT groups were used because we were interested in a comparison of animals that showed one phenotype or the other, and the Intermediate Group consisted of animals that showed a mixture of sign-tracking and goal-tracking behavior (see below).
Defining the groups based on the number of lever presses differentiated the three groups on all of the measures recorded during CS presentation. Figure 1a shows that STs exhibited a greater number of lever presses on each day of training relative to the other two groups (effect of group, (F(2,37) = 66.43; P<0.0001; effect of day, F(4,37) = 23.47; P<0.0001; group × day interaction, F(8,37) = 17.20; P<0.0001). There was also a significant increase in the number of lever presses across training days for STs (effect of day for STs, F(4,37) = 57.97; P<0.0001), but not for GTs (effect of day for GTs, F(3,37) = 0.03; p=0.99) or the intermediate group (effect of day for IG, F(3,37) = 1.41; P=0.25). Figure 1b further illustrates group differences in sign-tracking behavior as reflected in the latency to lever press. STs showed a large decrease in the latency to lever press across training days (effect of day for STs, F(4,95) = 50.45; P<0.0001), but there was no change for GTs (effect of day for GTs, (F(4,95) = 0.11; P=0.98). The IG also showed a significant decrease in the latency to lever press across training days (effect of day for IG, F(4,95) = 4.34; P=0.003), but this effect was less pronounced relative to STs. Indeed, STs approached the lever much more rapidly than GTs and the intermediate group on the last 4 days of training (overall effect of group, F(2,41) = 95.8; P<0.0001; effect of day, F(4,99) = 23.8; P<0.0001; group × day interaction, F(8, 99) = 15.2; P<0.0001).
Figure 1.
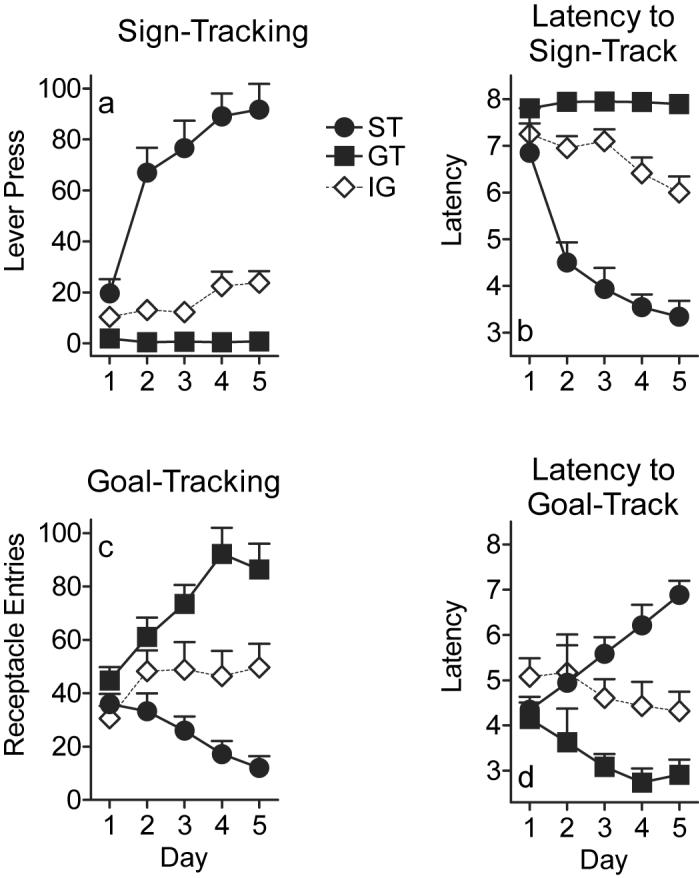
Lever press behavior and food receptacle entries during CS presentation. Squares represent goal-trackers (GTs; n=14), circles represent sign-trackers (STs; n=14), and open diamonds represent the intermediate group (IG; n=12). a) Mean lever press ± SEM on each of the 5 days of training. b) Mean latency to lever press ± SEM (in seconds), with 8 being the maximum latency. c) Mean number of receptacle entries during CS presentation ± SEM on each of the 5 days of training. d) Mean latency to receptacle entry during CS presentation ± SEM (in seconds).
The approach behavior exhibited by GTs was directed towards the goal, rather than the sign that predicted the goal. Figure 1c shows that GTs entered the food receptacle during CS presentation more than both STs and the IG (overall effect of group, F(2,39) = 16.06; P<0.0001; effect of day, F(4,92) = 5.14; P=0.001; group × day interaction, F(8,92) = 7.76; P<0.0001) and this behavior significantly increased across training days for GTs (effect of day for GTs, F(4,111) = 13.44; P<0.0001). The IG showed a slight but significant increase in the number of receptacle entries during CS presentation throughout training (effect of day for IG, F(4,111) = 3.96; P=0.005), whereas STs showed a significant decrease on this measure across training days (effect of day for STs, F(4,111) = 3.10; P=0.02). Figure 1d nicely complements these data, illustrating a significant decrease in the latency to goal-track for GTs (effect of day for GTs, F(4,37) = 2.96; P=0.03) and a significant increase in latency for STs (effect of day for STs, F(4,37) = 11.83; P<0.0001) across training days. All three groups differed from one another on the latency to enter the food receptacle (overall effect of group, F(2,37) = 15.22; P<0.0001; group × day interaction F(8,37) = 7.29; P<0.0001) but the IG did not show a significant change on this measure over the course of training (effect of day for IG, F(4,37) = 0.91; P=0.47).
To further examine individual differences in sign-tracking and goal-tracking behavior we compared the percentage of individual CS periods in which a lever press was recorded to the percentage of CS periods a head entry response into the food receptacle was recorded [5]. Note, an animal could both contact the lever and enter the food receptacle during a single 8 sec CS period (see below). Thus, these responses are not mutually exclusive. If an animal came into contact with the lever on all 25 trials in a session and never made an entry into the food receptacle it would receive a score of +100%. In contrast, one that on all trials made only receptacle entries would score −100%. The “lever-receptacle difference” for each group on all 5 training days is illustrated in Figure 2a. All 3 groups differed significantly from one another on this measure (overall effect of group, F(2,37) = 56.17; P<0.0001; effect of day, F(4,37) = 6.66; P<0.0001; group × day interaction, F(8,37) = 10.90; P<0.0001). On the first day of training all of the groups made more responses directed at the food receptacle than the lever. This is not surprising because all of the rats were “pre-trained” to retrieve food pellets from the food receptacle. However, eventually STs exhibited a preference for the lever over the food receptacle (effect of day for STs, F(4,37) = 26.04; P<0.0001) and the lever press response was the dominant response for these animals by the 4th day of training (score>0 for STs on days 4 and 5, P<0.0001). Neither GTs (effect of day for GTs, F(4,37) = 1.84; P=0.14) nor the IG (effect of day for IG, F(4,37) = 0.98; P=0.43) significantly changed their preference over time, and both groups continued to display a dominant response for the food receptacle relative to the lever (score<0 for GTs on all 5 days; score<0 for IG on days 1,2,3; P<0.0001).
The percent of trials on which an animal contacted both the lever and entered the food receptacle during the same CS period was also examined. Figure 2b shows that STs and the intermediate animals had a tendency to approach both the cue and the goal on a given trial and this behavior changed differentially for the two groups over time (effect of group, F(2,43) = 14.9; P<0.0001; effect of day, F(4,96) = 3.6; P=0.009; group × day interaction, F(8,96) = 4.33; P<0.0001). STs exhibited a decrease in the percentage of trials on which they approached both the sign and the goal by the last training day (effect of day for STs, F(4,103) = 5.5; P<0.0001); whereas the IG showed an increase in this behavior (effect of day for IG, F(4,103) = 6.43; P<0.0001). Thus, in agreement with the data presented above, intermediate animals did not show a strong preference for either response and increased their tendency to vacillate between the cue and the goal over the course of training. STs also had a tendency to vacillate between the lever and the food receptacle on a given trial, but by the end of 5 days of training this behavior was seen only on about 20% of the trials, as their preference for the lever increased. In contrast to STs and the IG, GTs rarely approached both locations on a given trial.
Taken together, these data demonstrate that the behavior of both STs and GTs changed over time as a consequence of experience, but the nature of the conditioned response that emerged in the two groups was very different. STs quickly learned to approach and manipulate the cue (lever) signaling impending reward delivery, whereas GTs learned to approach the location where the reward would be delivered. Thus, over time STs exhibited an increased preference for the cue (Fig. 2), a decreased latency to approach the cue (Fig. 1b) and an increase in the vigor with which they engaged the cue (Fig. 1a). GTs, however, continued to preferentially approach the food receptacle throughout the training period (Fig. 2). Nevertheless, the acquisition of the goal-tracking response for GTs was evidenced by an increase in the probability of approach (data not shown), a decrease in the latency to approach the food source (Fig. 1d) and an increase in the vigor of the response, as indicated by the number of head entries (Fig. 1c).
Behavior during the inter-trial interval (ITI)
Behavior directed towards the food receptacle was also examined during the ITI (i.e., not during CS presentation). STs and GTs did not differ significantly on this measure, but the IG exhibited fewer receptacle entries during the ITI relative to STs (overall effect of group, F(2,42) = 3.6; P=0.04; post-hoc comparison IG vs. STs, P=0.05). Interestingly, the behavior the intermediate animals exhibited towards the food receptacle during the ITI was consistent during the 5 days of training (effect of day for IG, F(4,31) = 2.46; P=0.07), whereas both STs (effect of day for STs, F(4,32) = 4.23; P=0.007) and GTs F(4,52) = 4.02; P=0.006) showed a significant decrease in this behavior across time (overall effect of day, F(4,97) = 5.93; P<0.0001). Thus, both STs and GTs seemed to learn that there was no benefit in repeatedly entering the receptacle once they obtained the food pellet that was delivered during the previous trial.
General exploratory behavior
The number of (inconsequential) nosepokes each group made across the 5 days of training was also examined. There were no significant group differences on this measure (data not shown).
Response to Cocaine
Acute drug response
As noted above, only the ST and GT groups were used to examine the effects of acute and repeated cocaine treatment, and we obtained two measures of psychomotor activation: locomotion (“crossovers”) and the frequency of head movements. Figure 3a shows that both STs and GTs showed a dose-related increase in locomotion, but GTs were more sensitive to the acute locomotor activating effects of cocaine than STs (effect of group, F(1,77) = 9.84; P=0.002) although this was significant only at the 30 mg/kg dose (group × dose; F(2, 77) = 4.76; P=0.01; post hoc comparison, P<0.0001). Figure 3b shows that both GTs and STs also showed a marked dose-related increase in head movements, but there were no significant group differences on this measure on day 1 (effect of group; F(1,77) = 3.24; P=0.08).
Figure 3.
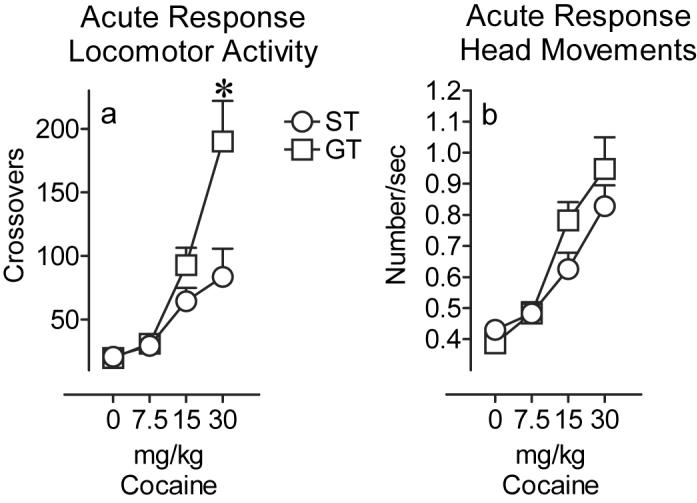
Acute (day 1) drug response for STs (n=14; open circles) and GTs (n=14; open squares) illustrated at each dose as the mean ± SEM for a) the number of crossovers and b) frequency of head movements. (*P<0.05, effect of group at specified dose)
Repeated cocaine administration
Figure 4 illustrates the effects of repeated cocaine treatment on STs and GTs by comparing the day 1 to the day 7 response for each group separately. As reported above, on day 1 cocaine produced a dose-related increase in locomotor activation in both groups. On the 7th day of treatment cocaine produced an inverted-U shaped dose-effect function in both groups (Fig. 4a and b). There was, however, no significant effect of day for either STs or GTs on this measure (effect of day for STs, F(1,38) = 1.9; P=0.2; GTs, F(1,38) = 0.5; P=0.47). There was a significant day × dose interaction for GTs (F(2,37) = 9.9; P<0.0001) and this was primarily because there was a significant decrease in locomotor activity following 30 mg/kg on day 7 relative to day 1 (Fig. 4b; post hoc comparison, P<0.0001). In summary, analysis of locomotor activity did not provide clear evidence of sensitization in either group.
Figure 4.
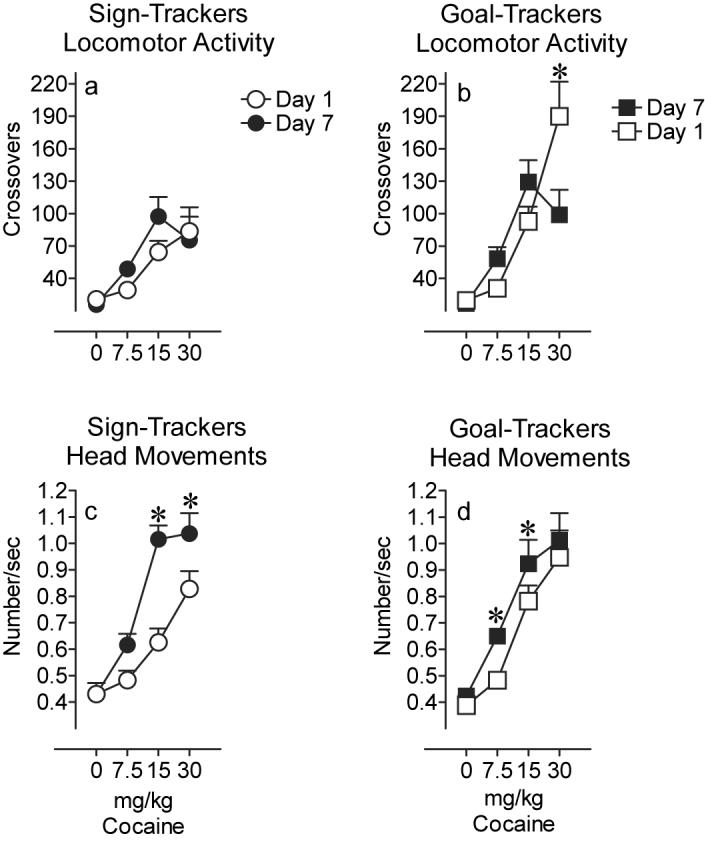
Locomotor activity illustrated as the mean ± SEM number of crossovers at each dose on day 1 (open symbols) and day 7 (closed symbols) for a) STs (n=14) and b) GTs (n=14). The mean ± SEM frequency of head movements at each dose on day 1 and day 7 for c) STs and d) GTs. (*P<0.05, effect of day at specified dose)
Analysis of the effects of repeated cocaine treatment on repetitive head movements did reveal clear evidence of sensitization (Fig. 4c and 4d). Cocaine produced a dose-related increase in the frequency of head movements on both the 1st and 7th day of treatment, but the dose-effect function was shifted significantly leftward in both groups as a function of repeated treatment (effect of day: STs, F(1,39) =35.10; P<0.0001; GTs, F(1,37) = 8.24; P=0.01; effect of dose: STs, F(2,39) = 21.675; P<0.001; GTs, F(2,38) = 9.64; P<0.001). However, the magnitude of the shift in the dose-effect function was greater in the STs than the GTs, as indicated by a significant group × day interaction (F(1,75) = 4.3; P=0.04).
To further assess the ‘degree’ of sensitization, we calculated for each rat the percent change in the frequency of head movements between day 1 and day 7 of treatment. There were no group differences in the percent increase in head movements in response to the lowest dose of the drug. However, the percent increase in head movements elicited by the middle and high doses was significantly greater in STs than in GTs (effect of group, F(1,24) = 5.8; P=0.02; data not shown).
When rats are given high doses of psychomotor stimulant drugs, or develop robust sensitization, they often show periods of reduced locomotion and fast repetitive head movements. This behavior is typically scored using rating scales as “stereotyped head movements with restricted locomotion” [21]. To quantify the proportion of animals in each group showing high frequency stereotyped head movements we set a criterion of greater than or equal to 1.0 head movement/second, based on video observation of the animals. Animals that made more than 1 head movement/sec were those that would be scored as making fast stereotyped head movements. Figure 5a illustrates the percentage of rats that met this criterion on day 1 and day 7 at 15 and 30 mg/kg. Based on this criterion, the groups did not differ significantly on day 1, but on day 7 a greater percentage of STs showed high frequency head movements relative to GTs and this reached a trend level of significance (effect of group across doses, P=0.06). Figure 5b illustrates the difference in the number of rats meeting this criterion between the first and last day of cocaine treatment. There was a large increase in the percent of STs that met this criterion between the first and last injection of 15 and 30 mg/kg (P<0.05), and no change in the GT group. These data further support the idea STs sensitized to a greater extent than GTs.
DISCUSSION
Cues in the environment that are reliably associated with the presentation of rewards come to powerfully control behavior through the process of Pavlovian learning [11, 22, 39, 52], in part because reward-associated cues acquire incentive value; i.e., they are attributed with incentive salience [3, 4, 45]. It has been hypothesized that if pathological levels of incentive salience are attributed to reward-related cues this can result in compulsive behavioral disorders, including addiction [35, 36, 45, 50]. There are large individual differences in the propensity to develop compulsive behavioral disorders such as addiction [2, 13, 18, 19, 41], but it is not known whether these individual differences are related to the extent to which reward-related cues come to control behavior. As a first step in exploring this issue we asked whether individual differences in learning about a food-associated cue were associated with differences in responsivity to either the acute psychomotor response to cocaine, or the ability of repeated cocaine to produce a form of neurobehavioral plasticity hypothesized to play a role in addiction – sensitization.
We first studied the influence of repeatedly presenting a cue (CS; lever extension) for 8 sec prior to the delivery of a food pellet into a nearby receptacle in a sample of 42 rats. We found that the rats could be divided into three non-overlapping groups based on how their behavior changed with experience. One group of rats (sign-trackers, STs) initially approached the food receptacle (where they had previously found food), but over 5 days of training they came to preferentially approach and vigorously engage the cue (the lever). Only after the lever was retracted did they go to the food receptacle and retrieve the food. This ‘sign-tracking’ behavior occurred even though it had no consequence: food was delivered no matter what the animal did.
The phenomenon of sign-tracking is well known [8, 9, 30, 43, 52, 53], and there are many examples in which it has been associated with seemingly bizarre and arguably compulsive behavior. In their book, “The Misbehavior of Organisms”, the animal trainers Breland and Breland [6] describe many examples of animals developing what appear to be irrational patterns of behavior directed towards food-related cues; behaviors that ultimately delay and oftentimes prevent delivery of the reward. In a typical example raccoons were trained to deposit a wooden coin through a slot in order to obtain a food reward. The raccoons initially performed this task without hesitation, but with further training they began to experience problems. Eventually, the raccoons seemed unable to let go of the coin, spending several minutes handling it with their forepaws—chewing, licking, rubbing and washing the coin—as if they were trying to clean a morsel of food. Similar “misbehavior” has been described in squirrel monkeys, chickens, turkeys, otters, porpoises, and whales [6, 7]. Another interesting example was originally described by Hearst and Jenkins [5], who paired illumination of a key light at one end of a long box with subsequent delivery of a food reward at the other end. Although no response was required to receive the food, pigeons approached and repeatedly pecked the key even though it prevented them from retrieving the food, which was available for only a limited amount of time. There are many other examples where reward-related cues become so irresistibly attractive that they engender seemingly maladaptive behavior (i.e., they persist despite the loss of reinforcement) [50, 55]. Tomie [50] has argued that when such cues are embedded in the device that delivers a drug, such as specialized glassware used to consume alcohol, these cues can promote especially compulsive patterns of behavior.
In STs the cue became powerfully attractive, but in GTs the cue appeared to merely signal impending reward delivery, and upon cue presentation GTs immediately went to where the reward would be delivered. This form of behavior (i.e., goal-tracking) was originally described by Zener [56] in dogs, and later by Boakes [5] in rats, but we are not aware of any reports since that directly compare (under the same experimental conditions) animals that sign-track to those that goal-track. It is important to emphasize that like sign-tracking, goal-tracking is a learned (conditioned) response. Thus, although GTs showed a preference for the food receptacle relative to the lever on all 5 days of training, with experience they came to approach the food source more reliably, more quickly and more avidly during CS presentation (the latter indicated by an increase in the number of head entries); they also came to approach the food receptacle preferentially during presentation of the CS, and significantly less often during the inter-trial interval. Therefore, both STs and GTs learned a Pavlovian conditioned approach response, but in STs approach was directed toward the cue and in GTs it was directed toward the place where the reward would be delivered [5, 27].
In the present study the intermediate group of animals did not develop either a consistent sign-tracking or goal-tracking response. Even after 5 days of training they approached the lever on some trials and the food receptacle on others, and on 50% of trials they vacillated between the cue and the food receptacle, approaching both during the same CS period. This behavior is very similar to that originally described by Zener [56], who reported that after pairing a bell with food delivery many dogs repeatedly exhibited successive glances from the bell to the food-pan and back during a single trial. Although this intermediate group is interesting in its own right, we chose to focus on the extremes of the population for the purpose of the cocaine study because we were interested in comparing animals that expressed either the sign-tracking or goal-tracking phenotype, and the IG did neither reliably. We were especially interested in assessing the propensity for psychomotor sensitization in two groups that differed in the degree to which incentive salience was attributed to a reward-related cue, because this form of drug-induced plasticity is thought to contribute to the development of addiction [45].
We found that GTs were more sensitive than STs to the acute locomotor activating effects of cocaine, but STs showed greater propensity for psychomotor sensitization upon repeated treatment. Relative to STs, GTs exhibited an increased locomotor response (crossovers) to cocaine on the first day of treatment, and this effect was most pronounced in response to the highest dose of the drug. Locomotor activity did not, however, provide clear evidence of behavioral sensitization. Although both groups exhibited an inverted U-shaped dose-effect function for locomotor activity on day 7, there was no significant difference between the day 1 and the day 7 dose-response function for either group. There were no significant group differences in cocaine-induced head movements on day 1 or day 7, but analysis of this measure did reveal that repeated cocaine treatment induced behavioral sensitization, and the degree of sensitization was greater in STs than in GTs. First, with repeated treatment the magnitude of the shift to the left in the dose-effect function for cocaine-induced head movements was larger in STs than GTs. Second, there was a large increase in the number of STs that met criterion for high frequency stereotyped head movements on day 7 relative to day 1, but not in GTs. Moreover, when the effects of repeated cocaine treatment were compared between groups, a greater percentage of STs exhibited high frequency stereotyped head movements relative to GTs on day 7.
Taken together these findings suggest that acutely, GTs respond either similarly (e.g. repetitive head movements) or more sensitively (e.g. locomotion) to cocaine; however, the impact of sensitization is significantly lower for them relative to sign trackers. These data support previous reports that the acute response to psychostimulants and the propensity for sensitization are dissociable [29, 44, 47, 48] and raise the possibility that the relative degree of sensitization (i.e. change from day 1 to day 7) may be a critical component of neurobehavioral plasticity [24, 25]. These findings are also consistent with reports that locomotion is sometimes a relatively insensitive indicator of behavioral sensitization [24, 25].
It is not clear what differences in brain organization lead to sign-tracking in some animals and goal-tracking in others. An obvious candidate system is the nucleus accumbens and related neural circuits [12], [11, 22, 39, 52]. We have previously shown, following 1 day of autoshaping training, that STs show greater expression of dopamine D1 receptor mRNA relative to GTs [27]. However, after 5 days of training (like that used in the present study), STs exhibit lower levels of dopamine transporter and tyrosine hydroxylase mRNA in the ventral tegmental area and lower levels of dopamine D2 receptor mRNA in the nucleus accumbens relative to GTs [27]. While it is unclear how these differences in mRNA levels translate into dopaminergic activity or neurotransmission, we speculate that these experience-dependent changes in gene expression are contributing to the acute and sensitized response to cocaine in the present study. Moreover, we are currently utilizing a selectively bred line of animals (see below) that may allow us to predetermine the ST/GT phenotype, providing the opportunity to assess the “basal” brain state of these animals prior to training.
The core subdivision of the nucleus accumbens is thought to be especially important in the development of Pavlovian conditioned approach behavior [20, 37, 38] and has also been implicated in responsivity to reward-related cues [28] and in psychomotor sensitization [10, 34, 42], including the emergence of progressively stereotyped behavior following the repeated administration of cocaine [33]. In contrast, the shell of the accumbens is thought to be involved in mediating the acute psychomotor activating effects of psychostimulant drugs and the actions of primary rewards [33, 49]. It is possible, therefore, that individual differences in the organization of the core and/or shell of the accumbens contribute to individual differences in the propensity to sign-track or goal-track, and to develop psychomotor sensitization. Additional studies are in progress to further investigate the neurobiological correlates of these behaviors and to address the neurochemical and anatomical heterogeneity of the nucleus accumbens.
The individual differences reported here in the propensity to attribute incentive salience to a food-related cue may also be relevant to the study of addiction. The major clinical problem in addiction, of course, is the tendency to relapse even long after the discontinuation of drug use, and this is due in part to sensitivity to drug-associated stimuli. Interestingly, animal studies suggest that the core of the accumbens, which is critical for sign-tracking, is also part of a “final common path” in mediating reinstatement of drug-seeking and drug-taking behavior [32]. Although in the present study we characterized individual differences in the tendency to approach a food-related cue, Pavlovian conditioned approach to a cue associated with either cocaine [53] or alcohol [14] has also been reported. It will be important to determine, therefore, whether individual differences in the control over behavior by cues associated with food rewards, as described here, also applies to drug-associated cues, and the role this might play in the development of addiction and in the propensity for relapse. Consistent with this notion, we have found that rats bred for high responsivity to environmental novelty are almost exclusively STs and rats bred for low responsivity to environmental novelty are almost exclusively GTs. Importantly, in these animals (which allow us to predict the phenotype prior to any experimental manipulation), when cocaine is used as the US the STs all acquire sign-tracking to a cocaine-associated cue and none of the GTs do so [26]. Thus, the sign-tracking phenotype not only predicts how readily incentive salience is attributed to a food cue, but also a drug cue.
In conclusion, the present findings support a relationship between individual differences in the tendency to attribute incentive salience to reward-related cues and the ability of cocaine to induce one form of drug-induced plasticity that has been implicated in addiction - psychomotor sensitization. Although further studies are required we suggest that these different phenotypes may prove to be very informative in understanding the psychological and neurobiological mechanisms whereby Pavlovian learning contributes the development of compulsive behavioral disorders, which may include not only addiction, but compulsive eating, gambling, sex, etc. - disorders in which pathological levels in incentive salience may be attributed to reward-related cues.
Acknowledgments
This work was supported by grants from the National Institute of Drug Abuse to TER (R37 DA04294) and HA (R01 DA013386) and from the Office of Naval Research to HA and SJW (N00014-02-1-0879).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Allison PD. Logistic regression using the SAS system: Theory and application. 2001 [Google Scholar]
- 2.Anthony J, Warner L, Kessler R. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: Basic findings from the national comorbidity survey. Experimental and Clinical Psychopharmacology. 1994;2(3):244–268. [Google Scholar]
- 3.Berridge KC. Reward learning: reinforcement, incentives and expectations. In: Medin D, editor. Psychology of Learning and Motivation. Academic Press; 2001. pp. 223–278. [Google Scholar]
- 4.Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26(9):507–13. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- 5.Boakes R. Performance on learning to associate a stimulus with positive reinforcement. In: Davis H, Hurwitz H, editors. Operant-Pavlovian Interactions. Lawrence Erlbaum Associates; Hillsdale, NJ: 1977. pp. 67–97. [Google Scholar]
- 6.Breland K, Breland M. The Misbehavior of Organisms. Am Psychol. 1961;16:681–683. [Google Scholar]
- 7.Breland K, Breland M. Animal Behavior. Macmillan; New York: 1966. [Google Scholar]
- 8.Brown B, Hemmes N, Cabeza de Vaca S, Pagano C. Sign and goal tracking during delay and trace autoshaping in pigeons. Animal Learning and Behavior. 1993;21:360–368. [Google Scholar]
- 9.Burns M, Domjan M. Sign tracking versus goal tracking in the sexual conditioning of male Japanese quail (Coturnix Japonica) J Exp Psychol Anim Behav Process. 1996;22(3):297–306. doi: 10.1037//0097-7403.22.3.297. [DOI] [PubMed] [Google Scholar]
- 10.Cadoni C, Solinas M, Di Chiara G. Psychostimulant sensitization: differential changes in accumbal shell and core dopamine. Eur J Pharmacol. 2000;388(1):69–76. doi: 10.1016/s0014-2999(99)00824-9. [DOI] [PubMed] [Google Scholar]
- 11.Cardinal RN, Everitt BJ. Neural and psychological mechanisms underlying appetitive learning: links to drug addiction. Curr Opin Neurobiol. 2004;14(2):156–62. doi: 10.1016/j.conb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26(3):321–52. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 13.Crowley TJ, Mikulich SK, MacDonald M, Young SE, Zerbe GO. Substance-dependent, conduct-disordered adolescent males: severity of diagnosis predicts 2-year outcome. Drug Alcohol Depend. 1998;49(3):225–37. doi: 10.1016/s0376-8716(98)00016-7. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham CL, Patel P. Rapid induction of Pavlovian approach to an ethanol-paired visual cue in mice. Psychopharmacology (Berl) 2007;192(2):231–41. doi: 10.1007/s00213-007-0704-4. [DOI] [PubMed] [Google Scholar]
- 15.Dalley JW, Laane K, Theobald DE, Armstrong HC, Corlett PR, Chudasama Y, Robbins TW. Time-limited modulation of appetitive Pavlovian memory by D1 and NMDA receptors in the nucleus accumbens. Proc Natl Acad Sci U S A. 2005;102(17):6189–94. doi: 10.1073/pnas.0502080102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davey GC, Cleland GG, Oakley DA, Jacobs JL. The effect of early feeding experience on signal-directed response topography in the rat. Physiol Behav. 1984;32(1):11–5. doi: 10.1016/0031-9384(84)90062-3. [DOI] [PubMed] [Google Scholar]
- 17.Day JJ, Carelli RM. The nucleus accumbens and Pavlovian reward learning. Neuroscientist. 2007;13(2):148–59. doi: 10.1177/1073858406295854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Wit H, Uhlenhuth EH, Johanson CE. Individual differences in the reinforcing and subjective effects of amphetamine and diazepam. Drug Alcohol Depend. 1986;16(4):341–60. doi: 10.1016/0376-8716(86)90068-2. [DOI] [PubMed] [Google Scholar]
- 19.Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305(5686):1014–7. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- 20.Di Ciano P, Cardinal RN, Cowell RA, Little SJ, Everitt BJ. Differential involvement of NMDA, AMPA/kainate, and dopamine receptors in the nucleus accumbens core in the acquisition and performance of Pavlovian approach behavior. J Neurosci. 2001;21(23):9471–7. doi: 10.1523/JNEUROSCI.21-23-09471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellinwood EH, Jr., Balster RL. Rating the behavioral effects of amphetamine. Eur J Pharmacol. 1974;28(1):35–41. doi: 10.1016/0014-2999(74)90109-5. [DOI] [PubMed] [Google Scholar]
- 22.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481–9. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 23.Falk JL, Feingold DA. Environmental and cultural factors in the behavioral action of drugs. In: Meltzer HY, editor. Psychopharmacology: The Third Generation of Progress. Raven Press; New York, NY: 1987. pp. 1503–1510. [Google Scholar]
- 24.Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE. Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry. 2005;58(9):751–9. doi: 10.1016/j.biopsych.2005.04.046. [DOI] [PubMed] [Google Scholar]
- 25.Flagel SB, Robinson TE. Quantifying the psychomotor activating effects of cocaine in the rat. Behav Pharmacol. 2007;18(4):297–302. doi: 10.1097/FBP.0b013e3281f522a4. [DOI] [PubMed] [Google Scholar]
- 26.Flagel SB, Watson SJ, Robinson TE, Akil H. An animal model of individual differences in “conditionability”: Relevance to psychopathology; Poster presented at American College of Neuropsychopharmacology Annual Meeting; Hollywood, Florida. 2006. [Google Scholar]
- 27.Flagel SB, Watson SJ, Robinson TE, Akil H. Individual differences in the propensity to approach signals vs. goals promote different adaptations in the dopamine system of rats. Psychopharmacology (Berl) 2007;191(3):599–607. doi: 10.1007/s00213-006-0535-8. [DOI] [PubMed] [Google Scholar]
- 28.Hall J, Parkinson JA, Connor TM, Dickinson A, Everitt BJ. Involvement of the central nucleus of the amygdala and nucleus accumbens core in mediating Pavlovian influences on instrumental behaviour. Eur J Neurosci. 2001;13(10):1984–92. doi: 10.1046/j.0953-816x.2001.01577.x. [DOI] [PubMed] [Google Scholar]
- 29.Harmer CJ, Phillips GD. Enhanced appetitive conditioning following repeated pretreatment with d-amphetamine. Behav Pharmacol. 1998;9(4):299–308. [PubMed] [Google Scholar]
- 30.Hearst E, Jenkins H. Monograph of the Psychonomic Society. Austin: 1974. Sign-tracking: the stimulus-reinforcer relation and directed action. [Google Scholar]
- 31.Jenkins HM, Moore BR. The form of the auto-shaped response with food or water reinforcers. J Exp Anal Behav. 1973;20(2):163–81. doi: 10.1901/jeab.1973.20-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162(8):1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 33.Lecca D, Cacciapaglia F, Valentini V, Acquas E, Di Chiara G. Differential neurochemical and behavioral adaptation to cocaine after response contingent and noncontingent exposure in the rat. Psychopharmacology (Berl) 2007;191(3):653–67. doi: 10.1007/s00213-006-0496-y. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Acerbo MJ, Robinson TE. The induction of behavioural sensitization is associated with cocaine-induced structural plasticity in the core (but not shell) of the nucleus accumbens. Eur J Neurosci. 2004;20(6):1647–54. doi: 10.1111/j.1460-9568.2004.03612.x. [DOI] [PubMed] [Google Scholar]
- 35.Newlin DB. A comparison of drug conditioning and craving for alcohol and cocaine. Recent Dev Alcohol. 1992;10:147–64. doi: 10.1007/978-1-4899-1648-8_8. [DOI] [PubMed] [Google Scholar]
- 36.Newlin DB. Evolutionary game theory and multiple chemical sensitivity. Toxicol Ind Health. 1999;15(34):313–22. doi: 10.1177/074823379901500305. [DOI] [PubMed] [Google Scholar]
- 37.Parkinson JA, Olmstead MC, Burns LH, Robbins TW, Everitt BJ. Dissociation in effects of lesions of the nucleus accumbens core and shell on appetitive Pavlovian approach behavior and the potentiation of conditioned reinforcement and locomotor activity by d-amphetamine. J Neurosci. 1999;19(6):2401–11. doi: 10.1523/JNEUROSCI.19-06-02401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parkinson JA, Willoughby PJ, Robbins TW, Everitt BJ. Disconnection of the anterior cingulate cortex and nucleus accumbens core impairs Pavlovian approach behavior: further evidence for limbic cortical-ventral striatopallidal systems. Behav Neurosci. 2000;114(1):42–63. [PubMed] [Google Scholar]
- 39.Pavlov IP. Conditioned Reflexes: An Investigation of the Physiological Activity of the Cerebral Cortex. Oxford University Press; London: 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phillips AG, McDonald AC, Wilkie DM. Disruption of autoshaped responding to a single of brain-stimulation reward by neuroleptic drugs. Pharmacol Biochem Behav. 1981;14(4):543–8. doi: 10.1016/0091-3057(81)90315-4. [DOI] [PubMed] [Google Scholar]
- 41.Piazza PV, Deroche-Gamonent V, Rouge-Pont F, Le Moal M. Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci. 2000;20(11):4226–32. doi: 10.1523/JNEUROSCI.20-11-04226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16(4):1550–60. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Purdy JE, Roberts AC, Garcia CA. Sign tracking in cuttlefish (Sepia Officinalis) J Comp Psychol. 1999;113(4):443–9. doi: 10.1037/0735-7036.113.4.443. [DOI] [PubMed] [Google Scholar]
- 44.Robinson TE. Stimulant drugs and stress: factors influencing individual differences in the susceptibility to sensitization. In: Kalivas PW, Barnes C, editors. Sensitization of the Nervous System. Telford; Caldwell, NJ: 1988. pp. 145–173. [Google Scholar]
- 45.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18(3):247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 46.Segal DS, Kuczenski R. Individual differences in responsiveness to single and repeated amphetamine administration: Behavioral characteristics and neurochemical correlates. J Pharmacol Exp Ther. 1987;242(3):917–26. [PubMed] [Google Scholar]
- 47.Short PH, Shuster L. Changes in brain norepinephrine associated with sensitization to d-amphetamine. Psychopharmacology (Berl) 1976;48(1):59–67. doi: 10.1007/BF00423307. [DOI] [PubMed] [Google Scholar]
- 48.Shuster L, Yu G, Bates A. Sensitization to cocaine stimulation in mice. Psychopharmacology (Berl) 1977;52(2):185–90. doi: 10.1007/BF00439108. [DOI] [PubMed] [Google Scholar]
- 49.Todtenkopf MS, Carreiras T, Melloni RH, Stellar JR. The dorsomedial shell of the nucleus accumbens facilitates cocaine-induced locomotor activity during the induction of behavioral sensitization. Behav Brain Res. 2002;131(12):9–16. doi: 10.1016/s0166-4328(01)00352-7. [DOI] [PubMed] [Google Scholar]
- 50.Tomie A. Locating reward cue at response manipulandum (cam) induces symptoms of drug abuse. Neurosci Biobehav Rev. 1996;20(3):505–35. doi: 10.1016/0149-7634(95)00023-2. [DOI] [PubMed] [Google Scholar]
- 51.Tomie A, Aguado AS, Pohorecky LA, Benjamin D. Individual differences in Pavlovian autoshaping of lever pressing in rats predict stress-induced corticosterone release and mesolimbic levels of monoamines. Pharmacol Biochem Behav. 2000;65(3):509–17. doi: 10.1016/s0091-3057(99)00241-5. [DOI] [PubMed] [Google Scholar]
- 52.Tomie A, Brooks W, Zito B. Sign-tracking: the search for reward. In: Klein S, Mowrer R, editors. Contemporary Learning Theories: Pavlovian Conditioning and the Status of Traditional Learning Theory. Lawrence Erlbaum Associates; Hillsdale, NJ: 1989. pp. 191–223. [Google Scholar]
- 53.Uslaner JM, Acerbo MJ, Jones SA, Robinson TE. The attribution of incentive salience to a stimulus that signals an intravenous injection of cocaine. Behav Brain Res. 2006;169(2):320–4. doi: 10.1016/j.bbr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 54.Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. Springer; New York: 2000. [Google Scholar]
- 55.Williams D, Williams H. Automaintenance in the pigeon: sustained pecking despite contingent non-reinforcement. Journal of the Experimental Analysis of Behavior. 1969;12:511–520. doi: 10.1901/jeab.1969.12-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zener K. The significance of behavior accompanying conditioned salivary secretion for theories of the conditioned response. American Journal of Psychology. 1937;(50):384–403. [Google Scholar]


