Abstract
Arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) is an inherited heart muscle disease that occurs primarily in young and middle-age individuals. It is characterized by ventricular arrhythmias (VA), sudden death, and by heart failure occurring later in life (1–5). Ventricular electrical instability may occur at any time during the disease depending upon possible different pathophysiologic mechanisms including: a) Inflammation and apoptosis leading to ventricular fibrillation; or b) Fibro-fatty tissue repair leading to scar-related ventricular tachycardia (VT). Heart failure may occur later in life secondary to slow, progressive loss of right and left ventricular myocardium (1–8). The role of pharmacological therapy in controlling VA and preventing sudden death has been evaluated in single-center studies (9), and the results of catheter ablation have been described in small series of patients (10–15). The efficacy and safety of implantable cardioverter/defibrillator (ICD) have also been reported in small, single-center studies (16–19) and recently in larger single and multicenter studies (20–27). The main questions regarding the risk stratification and the therapeutic strategy in ARVC/D are: 1) differential diagnosis with idiopathic VT (right ventricular outflow tract VT) in an apparently normal heart. The prognosis of this latter condition is usually excellent with rare cases of sudden cardiac death; 2) prognostic and therapeutic significance of noninvasive and invasive investigations including electrophysiologic study (EPS); 3) efficacy of antiarrhythmic drugs (AAD) to prevent VT and sudden cardiac death and the adverse effects of these drugs in this population; 4) indications and results of catheter ablation; 5) identification of patients at high risk of sudden cardiac death who need ICD implantation as well as the complications of ICD in a diseased right ventricular myocardium. It is important to recognize that ARVC/D is a progressive disease and risk factors may change during follow-up requiring periodic revaluation of risk as well as of therapy.
With the identification of family members who carry a disease causing gene, the therapeutic dilemma has broadened to include risk stratification in genotype positive family members who may have occasional ventricular ectopy or have no clinical evidence of the disease.
Keywords: Right Ventricular Dysplasia, Cardiomyopathy, Automatic Implanted Defibrillator, Catheter Ablation, Risk Stratification
Natural history and mortality
The low incidence of the disease as well as the different underlying genetic defects and data collected from different populations of patients with ARVC/D primarily from tertiary centers do not provide uniform information on the natural history of the disease. There is a wide spectrum of clinical presentation including severely symptomatic patients as well as asymptomatic relatives of affected patients. Sudden death and heart failure are well known adverse events in ARVC/D. However, long-term favourable course of the disease has also been described. The mortality rate in published studies is shown in Table 1 (7, 8, 28–33). Recently, Hulot et al. (31), described the natural history of 130 patients with ARVC/D who were referred to a tertiary center and followed for 8.1±7.8 years. There were 21 deaths of cardiovascular cause with an annual mortality rate of 2, 3%. Progressive heart failure was the cause of death in 59% of patients, while sudden death occurred in 29%. In this series the high prevalence of death due to heart failure may be explained by a change in the cause of death because of aggressive therapeutic management (AAD, ablation, ICD, surgery) of tachyarrhythmias in a tertiary arrhythmia center that are referred patients with recurrent symptomatic ventricular tachyarrhythmias. In another tertiary center, Lemola et al. (32) followed 61 ARVC/D patients for a mean of 55±47 months. They reported 10 deaths secondary to presumed arrhythmic causes in 8 patients and to heart failure in the other two. Nava et al (33) reported long term follow-up in a totally different population. In a systematic study of 151 patients from 37 families in which one or more members were affected with the disease, they reported a low mortality rate of 0.08 patient/year. Recently, Hodgkinson et al (23) reported the results of a study from a large genetically homogeneous population with ARVC/D caused by a mutation on chromosome 3. They found a higher mortality rate compared with other familial forms of ARVC/D, and a significantly increased relative risk of sudden death in men as compared with women. Also the men died at a younger age. These data illustrate the wide spectrum of the natural history of the disease, the presence of subgroups of patients with variable degrees of risk and the need for international registries to better assess the natural course of the disease (34).
Table 1.
ARVC/D-Mortality.
| Patients N | Mean FU (yrs) | Death n (rate per 100 person-years) | |
|---|---|---|---|
| Blomstrom-Lunqvist (1987) (7) | 15 | 8.8 | 2(1.5) |
| Marcus (1989) (8) | 12 | 5 | 2(3.0) |
| Leclercq (1989) (28) | 39 | 8.8 | 1(0.3) |
| Canu (1993) (29) | 22 | 10.7 | 3(1.2) |
| Nava (2000) (33) | 151 | 8.5 | 1(0.07) |
| Hulot (2004) (31) | 130 | 8.1 | 21(2.0) |
| Lemola K (2005) (32) | 61 | 4.5 | 10 (3.6) |
Clinical diagnosis and risk stratification
Standardized diagnostic criteria are available (35) but lack quantitative assessment of right ventricular global and regional abnormalities. There is no diagnostic gold standard to verify the presence of the disease except for genetic identification. The diagnostic protocols are not uniform from one center to another. Non invasive diagnostic tests include a 12-lead electrocardiogram, ambulatory ECG monitoring, signal-averaged electrocardiogram, exercise stress test and two-dimensional echocardiography. The role of magnetic resonance imaging (MRI) is controversial. Invasive diagnostic tests include right ventricular angiography, electrophysiology study (EPS) and endomyocardial biopsy. The latter procedure is performed in selective centers. Ideally, the diagnosis should be based on the results of multiple non invasive/invasive tests utilizing the major and minor criteria stipulated by the international task force (35). Recently, electrocardiographic depolarization/repolarization abnormalities (36) and electroanatomic voltage mapping (37) have been reported to have a high diagnostic value.
It is important not to rely on any single criteria to arrive at a diagnosis of ARVC/D, particularly imaging studies. It is well documented that overinterpretation of wall motion abnormalities and intramyocardial fat is common by MRI (38–40). There are many implications of labelling individuals with the diagnosis of ARVC/D including prognosis, treatment, and genetic inheritance in family members. Therefore, all reasonable efforts should be made to firmly establish the diagnosis.
Risk stratification
Various studies have found that risk factors for sudden cardiac death include a history of cardiac arrest, marked right ventricular enlargement, singly or in combination, left ventricular involvement (decreased left ventricular ejection fraction and/or enlargement), and a history of syncope or sudden cardiac death as well as young age. Peters et al (41) found that risk factors for arrhythmic events and unfavourable prognosis include severe right and left ventricular involvement and electrocardiographic depolarization/repolarization abnormalities (JT interval prolongation, T wave inversion beyond V3, increased right precordial QRS duration and precordial QRS dispersion). Some of these parameters reflect marked or diffuse involvement of the right ventricle. Hulot et al (31) reported that patients without sustained VT and heart failure had a very low risk of cardiac events, whereas the presence of heart failure and sustained VT identified a high-risk subgroup of patients. Likewise, in a multivariate analysis, Lemola et al. (32) found that history of congestive heart failure and the presence of left ventricular involvement were independent risk factors for an adverse outcome. Syncope was also considered to be a risk for recurrence of malignant ventricular arrhythmias. This was recently confirmed by a multicenter trial documenting that unexplained syncope correlated with subsequent ICD discharge for life threatening tachyarrhythmias (VF/VFlutter) at an annual rate of 8% per year in patients implanted with an ICD for primary and secondary prevention (20). In this trial, appropriate ICD therapy during follow-up was observed frequently in young patients considered to be at high risk with previous cardiac arrest, VT with hemodynamic compromise and left ventricular involvement (10% per year of follow-up). These events occurred despite treatment with beta-blockers and/or antiarrhythmic drugs including sotalol. In contrast, no VT/VF occurred in patients implanted because of a family history of sudden death and in only one patient with previously well-tolerated VT (very low risk subgroup) (20). Further evidence that left ventricular involvement and poorly-tolerated fast VT are strong predictors of VT recurrences was recently documented by Pezawas et al. (42). In addition, they confirmed that spontaneous well-tolerated VT with longer cycle length (326±37 ms) identified patients at low risk.
In a retrospective study, Turrini et al. studied three groups of patients with ARVC/D. There were 20 victims of sudden cardiac death, 20 patients with monomorphic VT, and 20 patients with premature ventricular beats, compared with 20 control subjects. In a multivariate analysis they found that the strongest predictor of sudden death was a QRS dispersion of ≥40 ms (43).
Nasir et al (36) confirmed the value of electrocardiographic abnormalities for diagnosis as well as for prognosis. They found that a prolonged S-wave upstroke in V1 through V3 was the most prevalent ECG finding (95%) and correlated with disease severity. This ECG finding was an independent predictor of VT induction. In addition, this feature best distinguished ARVC/D, diffuse and localized, from idiopathic RVOT tachycardia. Recently, Antoniades et al (44) evaluated clinical disease expression, non-invasive diagnosis, and prognosis in families with dominant versus recessive ARVC/D due to mutations in related desmosomal proteins plakophilin-2 (PKP2) and plakoglobin (JUP), respectively. T-wave inversion in V1–V3, right ventricular wall motion abnormalities, and frequent ventricular extrasystoles of left bundle branch block configuration were the most sensitive/specific markers for identification of mutation carriers. In this population, QRS dispersion (≥40 ms) was an independent predictor of syncope but not of sudden death.
Role of Electrophysiologic study
The prognostic role of EPS in ARVC/D patients is controversial. Programmed ventricular stimulation (PVS) is performed in some centers as part of standardized protocol. In other centers it is done only in selected patients for catheter ablation therapy, during serial drugs testing or to assess the hemodynamic consequences of VT and the susceptibility of VT to respond to antitachycardia pacing (ATP). EPS maintains a role primarily for diagnostic, but also to assess efficacy of antiarrhythmic drug therapy in patients with VT (9). EPS is also performed for risk stratification. In some centers, it is done for prognostic purposes in selected patients with a strong family history of sudden cardiac death or with unexplained syncope, nonsustained VT associated with right and left ventricular involvement. In a retrospective analysis of patients who had an implanted defibrillator, Corrado et al.(20) showed the limited value of the EPS in identifying patients at risk of lethal VA (VF/Vflutter) after ICD implantation, with a low predictive accuracy of PVS. There were approximately 50% of both false-positive and false-negative results. Data from a multicenter study showed that non-inducibility did not reliably predict a low likelihood of appropriate ICD therapy (26). In contrast, using multivariate analysis, Roguin et al (22) showed that VT induction during EPS was associated with increased risk for ICD therapy in ARVC/D patients implanted with an ICD. Different populations (multicenter vs single-center study), different types of interventions (shocks vs shocks and ATP), different VA (VF/sustained monomorphic VT vs VF/Vflutter) requiring ICD intervention, a relatively high ICD therapy in Roguin’s trial (probably due to clinical characteristics of the population), may explain the different results. Recently, Pezawas et al. (42) demonstrated that spontaneous VT cycle length, PVS-induced VT and VT during the follow-up correlate well indicating that a PVS-guided approach does not provide additional information. However, EPS may provide useful information if the spontaneous VT cycle length was not recorded.
In summary, the available data suggest that young age (<35 years old), prior cardiac arrest, fast and poorly-tolerated VT with different morphologies, syncope, severe right ventricular dysfunction, heart failure with left ventricular involvement, familial occurrence of juvenile sudden death, are the major determinants in predicting sudden death and worse outcome (4–8, 16–24, 31, 32). In patients with ARVC/D vigorous or competitive sport activities should be restricted to decrease the rate of structural and/or functional progression of the disease as well as the risk of arrhythmic death.
Pharmacological treatment
Drugs and dosage are often individualized and are based on the local experience of the different centers (9, 30) since there are no randomized controlled studies. Patients with palpitations due to premature ventricular beats (PVCs) or with well-tolerated VT are the best candidates for pharmacological therapy. Sotalol appears to be the most effective antiarrhythmic drug (AAD) to prevent inducible VT in patients with ARVC/D (9). Amiodarone alone or as combination with beta blocking drugs may be an alternative in nonresponders, but frequent side effects of amiodarone during long-term therapy in young patients limit this pharmacological strategy.
In a large single-center experience, sotalol had to be withdrawn in 4 of 73 patients (5.5%) due to hypotension after hospital discharge in 1 patient and bradycardia in the other three (9). Amiodarone treatment was discontinued during long-term therapy in 5 of 17 patients (29.4%) because of serious side effects. Class I antiarrhythmic agents appear to be less effective (9). In patients with recurrence of VT during drug therapy, catheter ablation should be considered (10). In the subgroup of high-risk patients (prior cardiac arrest, VT with hemodynamic compromise) ICD implantation is life-saving and results in improved survival compared with pharmacological therapy (20–23) but AAD are often administered to reduce shocks or inappropriate interventions.
It seems reasonable to prescribe pharmacological treatment, including ace-inhibitors (or AT-1 receptor blockers), beta blockers, diuretics, aldosterone antagonists, and anticoagulant drugs in patients with severe right and left ventricular involvement associated with heart failure.
Catheter ablation
Progressive right ventricular atrophy and replacement of myocardial fibers by adipose tissue and fibrosis is the pathobiological substrate of ARVD/C (3). Islands of surviving myocardial fibers interspersed in fat and fibrosis result in areas of slow conduction that is a substrate for re-entrant tachycardias. Endocardial mapping during sinus rhythm or VT can identify these areas so that ablation can be performed between areas of scar and normal myocardium or between scar and anatomic boundaries. Catheter mapping techniques to identify the target sites for RF ablation are: pace-mapping, recording of fractionated electrograms or middiastolic potentials (during sinus rhythm), activation mapping (during VT), entrainment mapping/entrainment with concealed fusion, stimulus-QRS interval equal to endocavitary electrogram-QRS interval and post-pacing interval during VT (10). Recently, electroanatomic voltage mapping (CARTO) during sinus rhythm (11, 12) or non contact mapping (14) were used to identify the target sites for VT ablation. Ablation is typically performed with conventional deflectable ablation catheter (4 mm-tip electrode), but 8-mm-tip or irrigated-tip catheters are used to extend the lines or create new, deeper/larger lines. In some refractory cases, an epicardial approach may be considered (15).
Treatment with catheter ablation is indicated in patients with drug-refractory incessant VT, frequent VT after ICD implantation, single morphology of spontaneous and induced VT in patients with localized ARVC/D (10). Short- and long-term results of RF catheter ablation in the setting of ARVC/D have been reported in small series of patients. In 30 patients, Wichter et al (10) reported acute success in 22 (73%) with VT recurrences during follow-up of 52±32 months in 18 patients (60%). During a mean follow-up of 27±22 months, Marchlinski et al. (11) reported no VT recurrences in 17 of 21 patients (89%). More recently, in a series of 22 patients, Verma et al. (12) achieved short-term success in 18 patients (82%). However, during long-term observation, VT recurred after 1, 2, and 3 years’ follow-up in 23%, 27%, and 47% of patients respectively. Serious procedure-related complication (pericardial tamponade) was reported in 1 patient.
It is generally thought that the progressive nature of ARVC/D, multiple localizations (right and left ventricle) of the disease, multiple morphologies of VT indicate a palliative rather than a curative claim of ablation catheter for ARVC/D patients. However, recently reported series of patients treated with catheter ablation have shown improved long term success, possibly due to the use of computer based mapping and/or more extensive ablation across the scar or by linear anatomic ablation (14, 15).
Trials with ICD
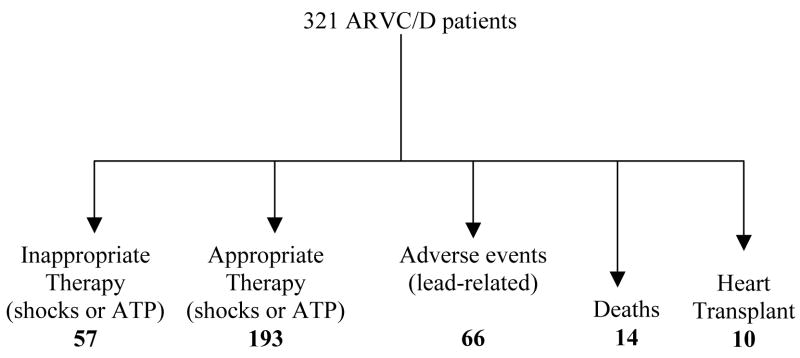
No prospective randomized trials have compared ICD therapy with AAD or catheter ablation in ARVC/D. Several studies regarding ICD implantation in ARVC/D are published (16–27), and the main data from some of them are summarized in Table 2 and Figure 1. There is a predominance of male gender with a mean age of nearly 40 years. It is difficult to define primary and secondary prevention because the definition is different from study to study. Particularly controversial is whether syncope should be included as primary or secondary prevention. In general, secondary prevention indicates ICD implantation after episodes of sustained VT, ventricular fibrillation, cardiac arrest, while primary prevention includes patients with syncope, nonsustained VT, family history of sudden death, inducibility of SVT/VF during EPS in the absence of spontaneous events. Two hundred-thirty-six of 321 patients (73.5%) received an ICD for secondary prevention; the remaining 85 had ICD implantation for primary prevention. Implantation was performed transvenously in 244 patients and by thoracotomy with epicardial lead systems in 9 patients. In 19 patients, additional subcutaneous (patch, array) or combination of epicardial/endocardial leads were required. A single-chamber and dual-chamber device was implanted in 190 and 53 patients, respectively. The data regarding the remaining patients were not fully reported.
Table 2.
Studies on ICD Therapy in ARVC/D (modified from 24)
| Author | Year | Pts N | Study Type | Males % | FU Mo | Primary Prev. % | Mortality Overall % | Appropriate ICD-Ther. % | Life-Saving ICD –Ther. % | Complications % |
|---|---|---|---|---|---|---|---|---|---|---|
| Breithardt | 1994 | 18 | SC | 72 | 17 | 0 | 0 | 50 | NR | NR |
| Link | 1997 | 12 | SC | 58 | 22 | 0 | 8 | 67 | 50 | 33 |
| Tavernier | 2001 | 9 | SC | 89 | 32 | 0 | 0 | 78 | 44 | NR |
| Corrado | 2003 | 132 | MC | 70 | 39 | 22 | 3 | 48 | 24 | 14 |
| Wichter | 2004 | 60 | SC | 82 | 80 | 7 | 13 | 68 | 40 | 45 |
| Rougin | 2004 | 42 | MC | 52 | 42 | 40 | 2 | 78 | NR | 14 |
| Hodgkinson | 2005 | 48 | MC | 63 | 31 | 73 | 0 | 70 | 30 | 6 |
NR= not reported; Prev.= prevention; Pts=patients; SC= single-center study; MC=multicenter study; Ther.=therapy; FU=follow-up; Mo=months.
FIG. 1.
Diagram summarizing the results of 321 ARVC/D patients with implantable cardioverter/defibrillator with a mean follow up of 37± 20mo.(16,17, 19–23).
Appropriate ICD interventions
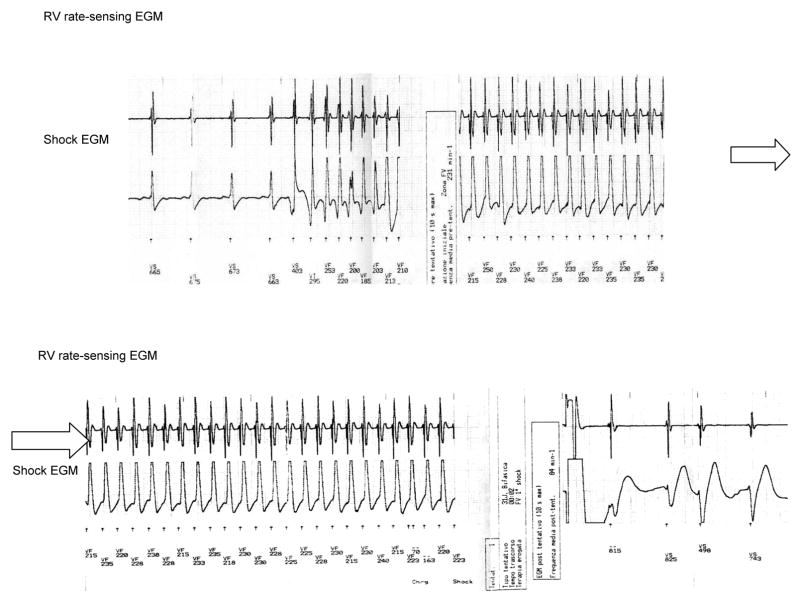
During a mean follow-up of 37±20 months (range 17–42 mo), appropriate therapy was recorded in 193 of 321 patients (60%), consisting either of shocks or ATP (Fig 1; Fig 2). The majority of interventions occurred during or after physical activity. More than one intervention was recorded in nearly 50% of patients who received therapy, and about 25% of them received several shocks or ATP. The interval between ICD implantation and the first delivered therapy ranged from 0.3 months to 8 years, and in few patients it was 4 years. Interestingly, “electrical storm” (defined as 3 or more clusters of multiple, consecutive VT episodes with frequent ICD therapies within 24 hours) occurred in about 33 patients (10 %) within a few days after the ICD implantation or during the follow-up. Passive or active new lead implantation in a diseased right ventricular myocardium can be a trigger for VT. Stress-related increase of sympathetic discharge could explain the VT storm occurring in the perioperative period. Progression of the disease or a “hot phase” secondary to transient inflammatory processes might be responsible for this phenomenon during follow-up.
FIG. 2.
A stored intracardiac electrogram of an ICD showing fast ventricular tachycardia with appropriate defibrillation
Multivariate analysis identified a history of cardiac arrest or poorly tolerated ventricular tachycardia, younger age, and extensive right ventricular dysfunction or decreased left ventricular ejection fraction as statistically significant predictors for potentially fatal events (VF/Vflutter) interrupted by ICD interventions. Unexplained syncope reached borderline statistical significance. In this subgroup of patients there was a high incidence of ventricular flutter/fibrillation (VF) that induced ICD therapy. Data from Piccini et al (25) as well as the recent study by Laidlaw et al (45) suggest that the frequency of ICD therapy for primary prevention (defined as no prior cardiac arrest or documented VT) is similar to that of patients when an ICD was implanted for secondary prevention.
The results of published trials strongly suggest an improvement in long-term prognosis by ICD therapy in high-risk patients with ARVC/D (Tab 2) (17–20, 23). The estimated benefit of ICD implantation in preventing potentially fatal events (i.e. VF/Vflutter) was 21%, 32%, 36%, and 35% after 1, 3, 5, and 7 years of follow-up, respectively (21). At a follow-up of 36 months, the actual patient survival rates were 96% compared with VF/Vflutter-free survival rates of 72% (20). In contrast, a low incidence of VF/Vflutter (1% per year of follow-up) was found in patients implanted because of well tolerated VT. On the basis of this data high risk and lower risk subgroup of patients may be identified.
Inappropriate ICD interventions
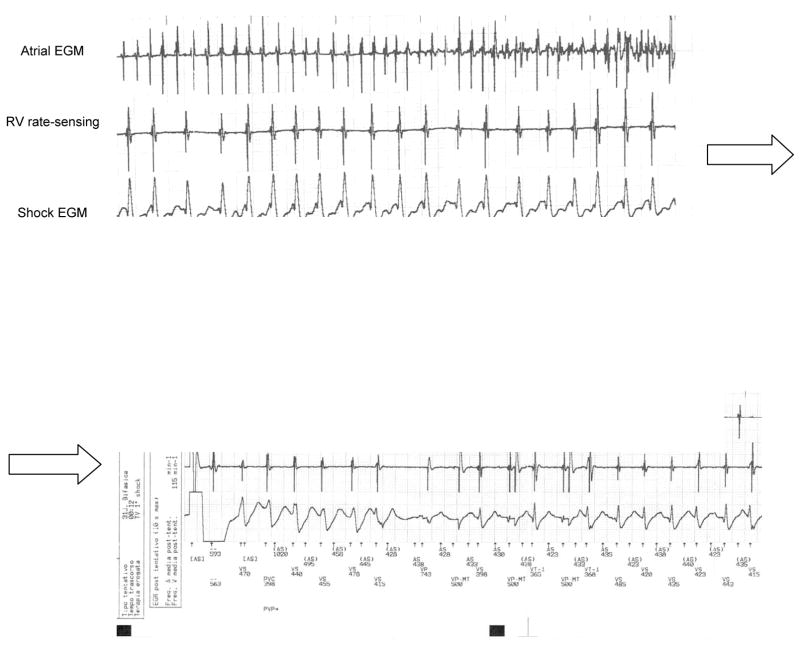
In 57 of 321 patients (18%) atrial tachyarrhythmias (AV node reentrant tachycardia, atrial fibrillation), exercise-related sinus tachycardia, and oversensing were the causes of inappropriate ICD therapy during the follow-up (Fig 1; Fig. 3). The frequency of therapy is likely to be increased in this population of younger, active patients. Furthermore, there may be an overlap between the rate of supraventricular tachyarrhythmias, exercise-induced sinus tachycardia and VT. This causes difficulty in programming specific algorithms such as sudden onset or rate stability to better discriminate supraventricular from ventricular arrhythmias. Other algorithms based on ventricular electrogram morphology analysis should enhance the ability for proper discrimination. Dual chamber detection algorithms with atrial and ventricular timing data improve discrimination of supraventricular from ventricular tachycardia and may therefore be of benefit in a young population with ARVC/D. However, because of the incidence of lead-related complications during long-term follow-up in young patients, dual-chamber ICDs should be selectively implanted in this population.
FIG. 3.
Stored electrogram of an atrial fibrillation episode inappropriately detected as ventricular tachycardia resulting in shock therapy delivery
Intraoperative testing
Compared with patients with other diseases, it can be difficult to achieve an adequate sensing threshold from the tip of the right ventricle lead because of fat and fibrous tissue that is present at the apex. In addition, the R-wave amplitude and pacing threshold are lower than in patients with other diseases (Table 3).
Table 3.
| Patients N | RV Lead Positions N | R-Wave mV | Pacing threshold V at 0,5 ms | DFT joules |
|---|---|---|---|---|
| 114 (ARVC/D) | 3.1±3.1 | 9.6±3.7 | 0.77±0.45 | 11±4.9 |
| (Coronary 713 artery disease) | 2.3±2.2 | 12.3±5.1 | 0.62±0.47 | 10±4.5 |
Identification of best candidates for ICD implantation
Accepted indications for ICD implantation in ARVC/D include secondary prevention after prior cardiac arrest and VT with hemodynamic compromise (41, 46). As previously reported, unexplained syncope is also considered a predictor of sudden death and appears to be an indication for ICD implantation (20). Recently, Laidlaw et al (45) could not identify baseline characteristics that distinguished patients with or without VT/VF requiring ICD therapy. Likewise, in patients with heart failure and poor right and left ventricular function in the absence of major arrhythmic events, the indications for ICD implantation have not been well established. The role of ICD implantation for primary prevention in the general ARVC/D population, particularly in asymptomatic ARVC/D patients and in relatives with high risk familial or genetic profile, remains to be defined. Concomitant conditions that would shorten life such as cancer deserve a careful evaluation before ICD implantation. Finally, the psychological effect of ICD implantation in young people should be considered because anxiety, depression, and fears are found in nearly half of the patients with ICD (47). Psychiatric consultation should be a part of the treatment for these patients before implantation and during the follow-up (48, 49).
Concomitant pharmacological treatment
After the ICD implantation, 205/321 patients (64%) also received AAD, including amiodarone, sotalol, class 1 AAD, or β-blockers. The drugs more frequently prescribed were sotalol, beta-blockers alone or a combination of β-blockers and amiodarone. The indication for concomitant pharmacological and ICD therapy was the occurrence of frequent VT or supraventricular tachycardia, including exercise-related sinus tachycardia or atrial fibrillation/flutter with inappropriate ICD discharge.
Deaths/Heart Transplantation
Fourteen of 321 patients (4.3%) died during the follow-up (Fig 1). The causes of the fatal events were: sudden death due to repeated VT/VF (“electrical storm”) that was appropriately recognized and cardioverted by the device but caused depletion of the ICD battery (4 pts) or due to VF after device explantation for pocket infection (1 pt); intractable heart failure (3 pts); infective endocarditis (1 pt) or noncardiac causes (5 pts). Ten of 321 patients (3.1%) underwent heart transplantation for intractable heart failure associated with VT/VF (9 pts) or marked increase of intracardiac defibrillation threshold in 1 patient in the presence of repeated episodes of VT that was not associated with progression of ventricular dysfunction.
Adverse events of ICD in ARVC/D
Major concerns have been expressed about potential risks and complications of lead implantation in a diseased thin walled right ventricular myocardium (Fig 1; Table 2).
No lead perforation has been reported. Link et al. (14) found sensing problems secondary to defects in the insulation of the sensing lead (2 endocardial and 1 epicardial) requiring a new lead implantation in 3/12 patients with inappropriate ICD discharges.
In a large multicenter study, nonfatal complications were described in 19/132 patients (14%) (20). Five patients required an additional septal lead because of undersensing or pacing failure secondary to lead fracture or migration. Increased pacing (1 pt) or defibrillation threshold requiring additional patches and high-energy devices (4 pts) occurred in 5 patients.
In a single-center experience with long-term follow-up, 37 of 60 patients (62%) had a total of 53 and 38 serious adverse events, respectively (21). Ten adverse events occurred during the perioperative phase and 43 during the follow-up. After discharge there were 31 lead-related adverse events in 21 patients (35%); insulation failure/oversensing in 10 pts, undersensing in 8, lead fracture in 5, lead dislodgment in 2, lead thrombosis in 2, and subcutaneous lead fracture in 1 pt. Surgical revision or implantation of an additional pace/sense lead were required in 26 of 31 lead-related complications (84%). Bleeding/pocket hematoma and infection requiring removal of ICD occurred in 7 and 5 patients, respectively (20, 21). More recent studies reported a lower number of lead fractures or insulation damage (22, 23).
Contraindications to ICD implantation
Incessant ventricular tachycardia is rarely reported in ARVC/D patients and is a relative contraindication to ICD implantation, while catheter ablation is the appropriate therapy for this tachyarrhythmia usually associated with concomitant ICD implantation. Psychiatric problems before implantation should be considered a relative contraindication to ICD therapy. Concomitant conditions that would expect to shorten life (≤6 months) are also contraindications to ICD implantation.
Therapeutic strategy
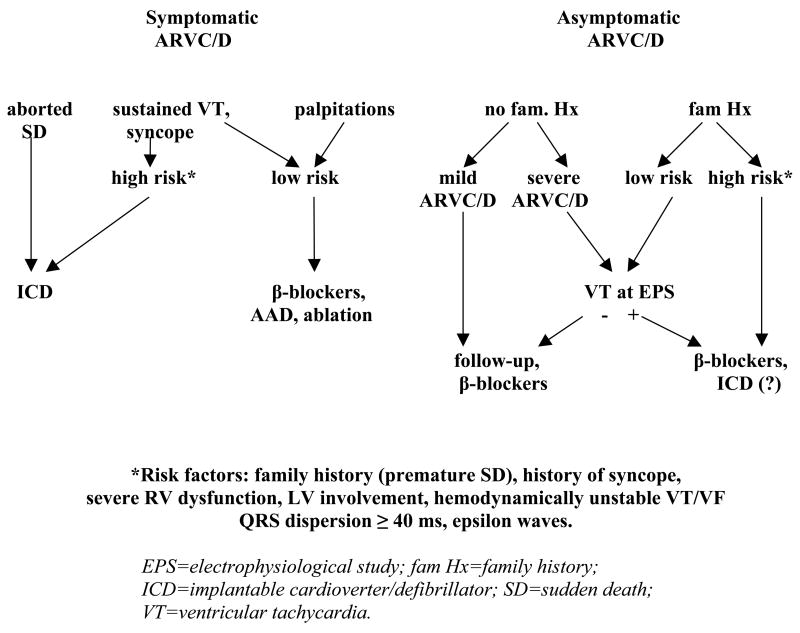
A decision tree for pharmacological or nonpharmacological therapy is proposed but needs to be validated by multicenter studies (Fig. 4) (50). At the present time, the decision to prescribe AAD, to perform catheter ablation or to implant an ICD in ARVC/D patients must be individualized based on risk assessment, physician judgement, and patient preference (50).
FIG. 4.
Algorithm for the antiarrhythmic management of ARVC/D
Conclusion
There is evidence that ICD therapy can effectively terminate malignant ventricular tachyarrhythmias in patients with ARVC/D leading to an improvement in long-term prognosis in a subgroup of high-risk patients. The best candidates for ICD implantation are patients with prior cardiac arrest, VT with hemodynamic compromise, syncope, and extensive right/left ventricular involvement. In contrast, patients with well-tolerated VT and localized right ventricular myocardial fibro-fatty substitution appear to have a good long-term prognosis. In this subgroup of patients, AAD and catheter ablation seems to be a reasonable first line therapy. The role of catheter ablation alone or in combination with antiarrhythmic drugs is being reevaluated in view of progress in mapping and different approaches to ablation. Progression of the disease can occur during follow-up and may require a change in therapeutic strategy. Lifestyle recommendations i.e. restriction of vigorous physical activity may improve the long term outcome.
ICD-lead implantation in young people with a diseased right ventricle requires careful placement and meticulous assessment of the sensing and pacing threshold to prevent adverse events and complications. After implantation, proper algorithms to better discriminate supraventricular from ventricular tachyarrhythmias are often difficult to program in these patients.
Data from larger series of patients collected in multicenter studies are needed to establish more definitive guidelines.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marcus FI, Fontaine GH, Guiraudon G, et al. Right ventricular dysplasia: a report of 24 adult cases. Circulation. 1982;65:384–98. doi: 10.1161/01.cir.65.2.384. [DOI] [PubMed] [Google Scholar]
- 2.Thiene G, Nava A, Corrado D, et al. Right ventricular cardiomyopathy and sudden death in young people. N Engl J Med. 1988;318:129–33. doi: 10.1056/NEJM198801213180301. [DOI] [PubMed] [Google Scholar]
- 3.Basso C, Thiene G, Corrado D, et al. Arrhythmogenic right ventricular cardiomyopathy: dysplasia, dystrophy, or myocarditis? Circulation. 1996;94:983–91. doi: 10.1161/01.cir.94.5.983. [DOI] [PubMed] [Google Scholar]
- 4.Corrado D, Basso C, Thiene G, et al. Spectrum of clinicopathologic manifestations of arrhythmogenic right ventricular cardiomyopathy/dysplasia: a multicenter study. J Am Coll Cardiol. 1997;30:1512–20. doi: 10.1016/s0735-1097(97)00332-x. [DOI] [PubMed] [Google Scholar]
- 5.Fontaine GH, Fontaliran F, Frank R. Arrhythmogenic right ventricular cardiomyopathy: clinical forms and main differential diagnoses. Circulation. 1998;97:1532–33. doi: 10.1161/01.cir.97.16.1532. [DOI] [PubMed] [Google Scholar]
- 6.Corrado D, Basso C, Thiene G. Sudden death. In: Nava A, Rossi L, Thiene G, editors. Arrhythmogenic right ventricular cardiomyopathy/dysplasia. Elsevier; Netherlands Amsterdam: 1997. pp. 36–45. [Google Scholar]
- 7.Blomstrom-Lunqvist C, Sabel CG, Olsson SB. Long term follow-up of 15 patients with arrhythmogenic right ventricular dysplasia. Br Heart J. 1987;58:477–488. doi: 10.1136/hrt.58.5.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcus FI, Fontaine GH, Frank R, et al. Long term follow-up in patients with arrhythmogenic right ventricular dysplasia. Eur Heart J. 1989;10 (suppl D):68–73. doi: 10.1093/eurheartj/10.suppl_d.68. [DOI] [PubMed] [Google Scholar]
- 9.Wichter T, Borggrefe M, Haverkamp W, Xu Chen, Breithardt G. Efficacy of antiarrhythmic drugs in patients with arrhythmogenic right ventricular disease. Results in patients with inducible and noninducible ventricular tachycardia. Circulation. 1992;86:29–37. doi: 10.1161/01.cir.86.1.29. [DOI] [PubMed] [Google Scholar]
- 10.Wichter T, Hindricks G, Kottkamp H, Breithardt G, Borggrefe M. Catheter ablation of ventricular tachycardia. In: Nava A, Rossi L, Thiene G, editors. Arrhythmogenic right ventricular cardiomyopathy/dysplasia. Elsevier; Netherlands Amsterdam: 1997. pp. 376–391. [Google Scholar]
- 11.Marchlinski F, Zado E, Dixit S, et al. Electroanatomic substrate and outcome of catheter ablative therapy for ventricular tachycardia in setting of right ventricular cardiomyopathy. Circulation. 2004;110:2293–98. doi: 10.1161/01.CIR.0000145154.02436.90. [DOI] [PubMed] [Google Scholar]
- 12.Verma A, Kilicaslan F, Schweikert RA, et al. Short- and long-term success of substrate-based mapping and ablation of ventricular tachycardia in arrhythmogenic right ventricular dysplasia. Circulation. 2005;111:3209–16. doi: 10.1161/CIRCULATIONAHA.104.510503. [DOI] [PubMed] [Google Scholar]
- 13.Satomi K, Kurita T, Suyama K, et al. Catheter ablation of stable and unstable ventricular tachycardias in patients with arrhythmogenic right ventricular dysplasia. J Cardiovasc Electrophysiol. 2006;17:1–8. doi: 10.1111/j.1540-8167.2006.00434.x. [DOI] [PubMed] [Google Scholar]
- 14.Yao Y, Zhang S, He DS, et al. Radiofrequency ablation of the ventricular tachycardia with arrhythmogenic right ventricular cardiomyopathy using non-contact mapping. PACE. 2007;30:526–533. doi: 10.1111/j.1540-8159.2007.00703.x. [DOI] [PubMed] [Google Scholar]
- 15.Reddy VY, D’Avila A, Aryana A, et al. Epicardial mapping and ablation of ARVC related ventricular tachycardia. Heart Rhythm. 2007;4 (Suppl):S329. [Google Scholar]
- 16.Breithardt G, Wichter T, Haverkamp W, et al. Implantable cardioverter defibrillator therapy in patients with arrhythmogenic right ventricular cardiomyopathy, long QT syndrome, or no structural heart disease. Am Heart J. 1994;127:1151–58. doi: 10.1016/0002-8703(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 17.Link M, Wang PJ, Haugh CJ, et al. Arrhythmogenic right ventricular dysplasia: clinical results with implantable cardioverter defibrillator. J Intervent Card Electrophysiol. 1997;1:41–8. doi: 10.1023/a:1009714718034. [DOI] [PubMed] [Google Scholar]
- 18.Wichter T, Bocker D, Borggrefe M, Hammel D, Breithardt G, Block M. Cardioverter-defibrillator therapy. In: Nava A, Rossi L, Thiene G, editors. Arrhythmogenic right ventricular cardiomyopathy/dysplasia. Elsevier; Netherlands Amsterdam: 1997. pp. 364–375. [Google Scholar]
- 19.Tavernier R, Gevaert S, de Sutter J, et al. Long term results of cardioverter defibrillator in patients with right ventricular dysplasia and malignant ventricular tachyarrhythmias. Heart. 2001;85:53–56. doi: 10.1136/heart.85.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corrado D, Leoni L, Link MS, et al. Implantable cardioverter-defibrillator therapy for prevention of sudden death in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2003;108:3084–91. doi: 10.1161/01.CIR.0000103130.33451.D2. [DOI] [PubMed] [Google Scholar]
- 21.Wichter T, Paul M, Wollmann C, et al. Implantable cardioverter/defibrillator therapy in arrhythmogenic right ventricular cardiomyopathy. Single-center experience of long-term follow-up and complications in 60 patients. Circulation. 2004;109:1503–8. doi: 10.1161/01.CIR.0000121738.88273.43. [DOI] [PubMed] [Google Scholar]
- 22.Rougin A, Bomma CS, Nasir K, et al. Implantable cardioverter-defibrillators in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Am Coll Cardiol. 2004;43:1843–53. doi: 10.1016/j.jacc.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 23.Hodgkinson KA, Parfrey PS, Basset AS, et al. The impact of implantable cardioverter-defibrillator therapy on survival in autosomal-dominant arrhythmogenic right ventricular cardiomyopathy (ARVD5) J Am Coll Cardiol. 2005;45:400–408. doi: 10.1016/j.jacc.2004.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wichter T, Breithardt G. Implantable cardioverter-defibrillator therapy in arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol. 2005;45:409–11. doi: 10.1016/j.jacc.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Piccini JP, Dalal D, Roguin A, et al. Predictors of appropriate implantable defibrillator therapy in patients with arrhythmogenic right ventricular dysplasia. Heart Rhythm. 2005;2:1188–1194. doi: 10.1016/j.hrthm.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 26.Budzikowski AS, Daubert JP, Sesselberg HW, et al. Non-inducibility of ventricular tachycardia does not prevent low likelihood of appropriate therapy in patients with ARVD. Heart Rhythm. 2007;4:S122. Abstract. [Google Scholar]
- 27.Laidlaw DW, Lucier D, Alsheikh-Ali A, et al. High incidence of appropriate defibrillator therapy in arrhythmogenic right ventricular dysplasia: Results from the multicenter ARVD registry. Heart Rhythm. 2007;4:S218–219. Abstract. [Google Scholar]
- 28.Leclercq JF, Coumel P. Characteristics, prognosis, and treatment of the ventricular arrhythmias of right ventricular dysplasia. Eur Heart J. 1989;10 (suppl D):61–7. doi: 10.1093/eurheartj/10.suppl_d.61. [DOI] [PubMed] [Google Scholar]
- 29.Canu G, Atallah G, Claudel JP, et al. Prognostic et évolution à long terme de la dysplasie arythmogène du ventricule droit. Arch Mal Cœur. 1993;86:41–58. [PubMed] [Google Scholar]
- 30.Nava A, Bauce B, Daliento L. Incidence and natural history. In: Nava A, Rossi L, Thiene G, editors. Arrhythmogenic right ventricular cardiomyopathy/dysplasia. Elsevier; Netherlands Amsterdam: 1997. pp. 17–23. [Google Scholar]
- 31.Hulot JS, Jouven X, Empana JP, Frank R, Fontaine GH. Natural history and risk stratification of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circulation. 2004;110:1879–1884. doi: 10.1161/01.CIR.0000143375.93288.82. [DOI] [PubMed] [Google Scholar]
- 32.Lemola K, Brunckhorst C, Helfenstein U, Oechslin E, Jenni R, Duru F. Predictors of adverse outcome in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy: long-term experience of a tertiary care center. Heart. 2005;91:1167–72. doi: 10.1136/hrt.2004.038620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nava A, Bauce B, Basso C, et al. Clinical profile and long-term follow-up of 37 families with arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol. 2000;36:2226–2233. doi: 10.1016/s0735-1097(00)00997-9. [DOI] [PubMed] [Google Scholar]
- 34.Corrado D, Fontaine G, Marcus FI, et al. Arrhythmogenic right ventricular dysplasia/cardiomyopathy. Need for an international registry. Circulation. 2000;101:e101–e106. doi: 10.1161/01.cir.101.11.e101. [DOI] [PubMed] [Google Scholar]
- 35.McKenna WJ, Thiene G, Nava A, et al. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Br Heart J. 1994;71:215–18. doi: 10.1136/hrt.71.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nasir K, Bomma C, Tandri H, et al. Electrocardiographic features of arrhythmogenic right ventricular dysplasia/cardiomyopathy according to disease severity. A need to a broaden diagnostic criteria. Circulation. 2004;110:1527–34. doi: 10.1161/01.CIR.0000142293.60725.18. [DOI] [PubMed] [Google Scholar]
- 37.Corrado D, Basso C, Leoni L, et al. Three-dimensional electroanatomic voltage mapping increases accuracy of diagnosing arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2005;111:3042–50. doi: 10.1161/CIRCULATIONAHA.104.486977. [DOI] [PubMed] [Google Scholar]
- 38.Bluemke DA, Krupinski EA, Ovitt T, et al. MR Imaging of arrhythmogenic right ventricular cardiomyopathy: Morphologic findings and interobserver reliability. Cardiology. 2003;99:153–162. doi: 10.1159/000070672. [DOI] [PubMed] [Google Scholar]
- 39.Sievers B, Addo M, Franklin U, Trappe HJ. Right ventricular wall motion abnormalities found in healthy subjects by cardiovascular magnetic resonance imaging and characterized with a new segmental model. J Cardiovasc Magnetic Resonance. 2004;3:601–608. doi: 10.1081/jcmr-120038528. [DOI] [PubMed] [Google Scholar]
- 40.Bomma C, Rutberg J, Tandri H, et al. Misdiagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Cardiovascular Electrophysiology. 2004;15:300–306. doi: 10.1046/j.1540-8167.2004.03429.x. [DOI] [PubMed] [Google Scholar]
- 41.Peters S, Peters H, Thierfelder L. Risk stratification of sudden cardiac death and malignant ventricular arrhythmias in right ventricular dysplasia-cardiomyopathy. Int J Cardiol. 1999;71:243–50. doi: 10.1016/s0167-5273(99)00142-4. [DOI] [PubMed] [Google Scholar]
- 42.Pezawas T, Stix G, Kastner J, Schneider B, Wolzt M, Schmidinger H. Ventricular tachycardia in arrhythmogenic right ventricular dysplasia/cardiomyopathy: Clinical presentation, risk stratification and results of long-term follow-up. Int J Cardiol. 2006;107:360–68. doi: 10.1016/j.ijcard.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 43.Turrini P, Corrado D, Basso C, et al. Dispersion of ventricular depolarization-repolarization a non-invasive marker for risk stratification in arrhythmogenic right ventricular cardiomyopathy. Circulation. 2001;103:3075–80. doi: 10.1161/01.cir.103.25.3075. [DOI] [PubMed] [Google Scholar]
- 44.Antoniades L, Tsatsopoulou A, Anastasakis A, et al. Arrhythmogenic right ventricular cardiomyopathy caused by deletion in plakophilin-2 and plakoglobin (Naxos disease) in families from Greece and Cyprus: phenotype-genotype relations, diagnostic features and prognosis. Eur Heart J. 2006;27:2208–16. doi: 10.1093/eurheartj/ehl184. [DOI] [PubMed] [Google Scholar]
- 45.Laidlaw DW, Lucier D, Alsheikh-Ali A, et al. Clinical characteristics do not predict appropriate defibrillator therapy in patients with right ventricular dysplasia. Heart Rhythm. 2007;4 (Suppl):S30–31. [Google Scholar]
- 46.Priori SG, Aliot E, Blomstrom-Lunqvist C, et al. Task force on sudden cardiac death of the European Society of Cardiology. Eur Heart J. 2001;22:1374–1450. doi: 10.1053/euhj.2001.2824. [DOI] [PubMed] [Google Scholar]
- 47.Bilge AK, Ozben B, Demircan S, et al. Depression and anxiety status of patients with implantable cardioverter defibrillator and precipitating factors. PACE. 2006;29:619–626. doi: 10.1111/j.1540-8159.2006.00409.x. [DOI] [PubMed] [Google Scholar]
- 48.Kuhl EA, Dixit NK, Walker RL, et al. Measurements of patients fears about implantable cardioverter defibrillator shock: An initial evaluation of the Florida shock anxiety scale. PACE. 2006;29:614–618. doi: 10.1111/j.1540-8159.2006.00408.x. [DOI] [PubMed] [Google Scholar]
- 49.Sears SF, Vazquez S, Kuhl ES, et al. The ICD shock and stress management program: A randomized trial of psychological treatment to optimize quality of life in ICD patients. PACE. 2007;30:858–864. doi: 10.1111/j.1540-8159.2007.00773.x. [DOI] [PubMed] [Google Scholar]
- 50.Wichter T, Corrado D, Paul M. Risk stratification and antiarrhythmic drug therapy in arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) In: Marcus F, Nava A, Thiene G, editors. Arrhythmogenic right ventricular cardiomyopathy/dysplasia. Springer; 2007. pp. 171–179. [Google Scholar]
- 51.Fontaine GH, Zenati O, Tonet J, Hidden F, Himbert C, Frank R. The treatment of ventricular arrhythmias. In: Nava A, Rossi L, Thiene G, editors. Arrhythmogenic right ventricular cardiomyopathy/dysplasia. Elsevier; Netherlands Amsterdam: 1997. pp. 315–363. [Google Scholar]