Abstract
By means of the specific immuno-recognition and ultra-sensitive mass detection, a quartz crystal microbalance (QCM) biosensor for Escherichia coli O157:H7 detection was developed in this work. As a suitable surfactant, 16-mercaptohexadecanoic acid (MHDA) was introduced onto the Au surface of QCM, and then self-assembled with N-hydroxysuccinimide (NHS) raster as a reactive intermediate to provide an active interface for the specific antibody immobilization. The binding of target bacteria with the immobilized antibodies decreased the sensor’s resonant frequency, and the frequency shift was correlated to the bacterial concentration. The stepwise assembly of the immunosensor was characterized by means of the electrochemical techniques. Using the immersion-dry-immersion procedure, this QCM biosensor could detect 2.0×102 colony forming units (CFU)/ml E. coli O157:H7. In order to reduce the fabrication time, a polyelectrolyte layer-by-layer self-assembly (LBL-SA) method was adopted for fast construction. Finally, the reproducibility of this biosensor was discussed.
Keywords: Biosensor, Escherichia coli O157:H7, Immunosensor, Layer-by-layer self-assembly (LBL-SA), Quartz crystal microbalance (QCM)
INTRODUCTION
Escherichia coli O157:H7 is one of the most dangerous food born pathogens, which may cause life threatening complications—hemorrhagic colitis and hemolytic uremic syndrome in humans (CDC, 2006; Rangel et al., 2005; Su and Li, 2005). Illness due to E. coli O157:H7 infection is often misdiagnosed and commonly requires invasive and expensive medical tests to make a correct diagnosis. The infective dose of this bacterium is possibly fewer than 100 organisms (Tuttle et al., 1999). Since the loss caused by E. coli O157:H7 is enormous in terms of medical cost and product recall, it is extremely urgent to develop some fast and sensitive methods to detect this kind of bacteria in food or water supplies.
Traditional methods for detection of E. coli O157:H7 involve plating and culturing, enumeration methods, biochemical testing, microscopy and flow cytometry. Some new methods have been developed, including immunoassays (Magliulo et al., 2007), immunomagnetic separations (Wang et al., 2007), nucleic acid probe-based methods based on hybridization and polymerase chain reaction (PCR) (Johnston et al., 2005), and the DNA microarrays (Jin et al., 2005). However, many of these methods are so time-consuming, expensive or complicated that they are not suitable for fast detection of E. coli O157:H7. As alternative to the conventional methods, biosensors have been explored for pathogenic bacteria detection in recent years (Berganza et al., 2007; Berkenpas et al., 2006; Varshney and Li, 2007). Advantages of this approach include its continuous data acquisition ability, target specificity, fast response, mass produce feasibility and sample preparation simplicity. Originally the design of most biosensors is based on the sandwich immunoassay, which forms an immuno-complex consisting of the immobilized primary antibodies, the captured target bacteria, and the second antibodies labeled with enzymes. The signal resulting from the enzymatic reaction is detected by optical absorbance (Demarco and Lim, 2002; Liu and Li, 2001), chemiluminescence (Liu et al., 2003), potentiometry (Tu et al., 2000), or electrochemical impedance (Ruan et al., 2002). Though these immunosensors can improve the detection sensitivity and reduce the assay time, they are label-dependent, so costly and complicated. As a label-free biosensor, the quartz crystal microbalance (QCM) possesses attractive advantages of cost effectiveness, speed and simplicity of operation for detection of E. coli O157:H7. As been well known, QCM is a very sensitive mass-measuring sensor, and the crystal resonance frequency decreases with the increase in mass on the QCM. The relationship between the frequency change and the mass loading is described by Sauerbrey equation:
| ∆F=CF2∆m/A, |
where, C is constant, ΔF is the frequency change, Δm is the mass change due to the surface deposition, F is the basic frequency of the QCM, and A is the area of the Au electrode. Based on the combination of highly specific immuno-recognition and sensitive mass detection, QCM offers such an opportunity to detect the bacteria directly. According to Sauerbrey equation, the frequency decrease is proportional to the mass change, which relates to the bacterial concentration (Su and Li, 2004).
Based on forementioned biosensors with different transmitting mechanism for bacteria detection, it is rewarding to develop such a QCM biosensor for E. coli O157:H7 detection based on direct immunoassay. That is to say, specific antibodies for target are anchored onto the modified Au substrate, and then the bacteria will be captured by the combination with antibodies. The additional mass loading from the specific binding can cause a shift in the QCM’s resonant frequency, which requires no labeled secondary antibodies or pre-separation. Therefore the direct immunoassay-based QCM biosensors excel those founded on sandwich immunoassays. Meanwhile, they exceed the PCR-based QCM sensors in the simplicity and rapidness. In the development of direct immunoassay-based QCM biosensors, antibody immobilization is a crucial step to capture the target bacteria successfully. There are many methods to improve the immobilization efficiency, such as polymer membrane (Wong et al., 2002), protein A (Babacan et al., 2000), and self-assembled monolayer (SAM) (Fung and Wong, 2001). Among these methods, the SAM technique has attracted more and more attention of scientists because it can provide a reproducible, ultrathin, and well-ordered functional layer for the late antibody immobilization. Aiming at the Au electrode substrate of QCM, the way to form an SAM of 16-mercaptohrxadecanoic acid (MHDA) with 1-ethyl-3-(3-dimethylaminoprppyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS) ester has been adopted in antibody immobilization popularly (Fung and Wong, 2001; Qian et al., 2002; Su and Li, 2004), which may improve the detection sensitivity, speed, and reproducibility.
MATERIALS AND METHODS
Regent and materials
MHDA, EDC, NHS, poly ethylenimine (PEI) and poly acrylic acid (PAA) were all purchased from Sigma-Aldrich (USA). Affinity purified antibodies to E. coli O157:H7 were obtained from Chinese Center for Disease Control and Prevention (CDC, China). Bovine serum albumin (BSA) was supplied by Sino-American Biotechnology Inc. (Shanghai, China). All other chemicals such as NaOH, KCl, NaCl, Na2HPO4, H2SO4 (98%), H2O2, potassium ferrocyanide and potassium ferricyanide were of analytical pure grade or better quality. Ultrapure water (18.2 MΩ/cm) produced by a Milli-Q system (Bedford, MA, USA) was used throughout.
Bacteria and culture plating method
As the target bacteria, E. coli O157:H7 was presented by the Centre for Disease Control and Prevention in Zhejiang Province (Hangzhou, China) and E. coli K-12 was used for negative control.
E. coli O157:H7 or E. coli K-12 was cultured in nutrient broth at 37 °C for 12 h before use, and the bacterial concentration was decided by the surface plating-count method. The cultured bacteria should be killed in 100 °C water bath for 15 min, then be diluted to a series of desired concentrations with phosphate buffered saline (PBS, NaCl 8.00 g/L, KCl 0.20 g/L, Na2HPO4 1.38 g/L, KH2PO4 0.2 g/L, pH 7.4) for later use.
QCM and experimental apparatus
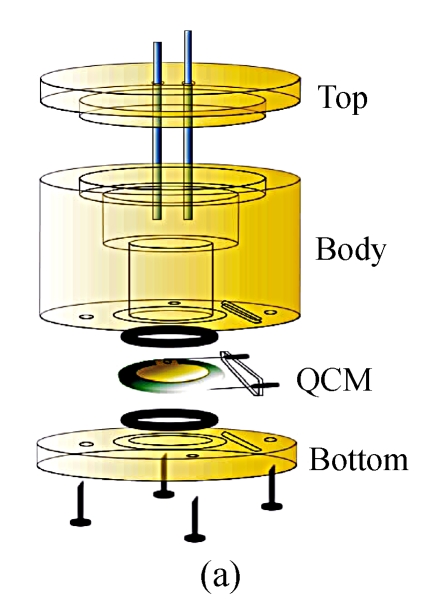
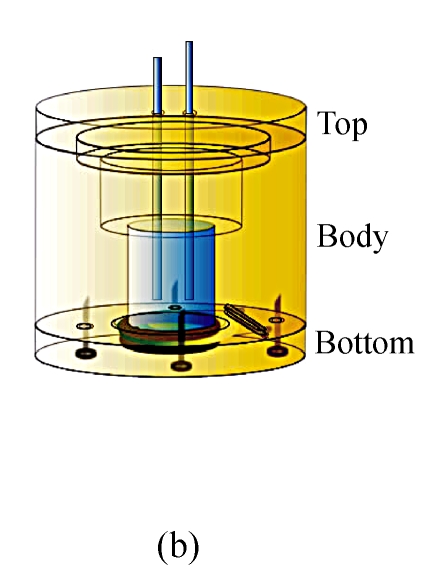
The immunosensors used in our work were developed on 8 MHz AT-cut quartz crystals (diameter=13.7 mm) sandwiched between two Au electrodes (diameter=5.1 mm, thickness=1 000 Å). During the course of fabrication and detection, the crystal was all through mounted in a detection cell composed of three round Teflon pieces (Fig.1). The top piece was the cell top to hold reference and counter electrodes used in the electrochemical measurement, the center piece was the cell body for solution, and the bottom piece was for mounting purpose. The seal was through two O-rings that were pressed together by four screws whose function was to tighten the bottom piece and center piece together.
Fig. 1.
The self-made detection cell. (a) Before tightening; (b) After tightening
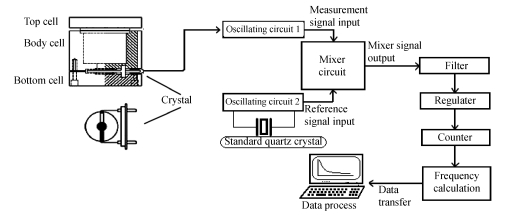
The frequency change conduced by mass alteration on the surface was acquired by an experimental Quartz Crystal Analyzer (QCA) which we ourselves designed, fabricated and developed as shown in Fig.2. The frequency counting was carried out with a frequency counter controlled by a computer. The frequency signal of the QCM was subtracted from a standard reference crystal with 8.20 MHz fundamental frequency and the variation of frequency measurement in the PBS buffer was less than 1 Hz.
Fig. 2.
The sketch of the whole detection system of experimental Quartz Crystal Analyzer
Biosensor fabrication and detection procedure
Before use, the crystal was cleaned with 1 mol/L NaOH for 20 min, 1 mol/L HCl for 5 min and Pirannha solution (30% H2O2:H2SO4=2:3, v/v) for 1 min, in sequence, to remove organic adsorbent impurities and obtain a clean Au surface. After cleaning pretreatment, the crystal was rinsed with ethanol and water successively, and dried in a stream of nitrogen.
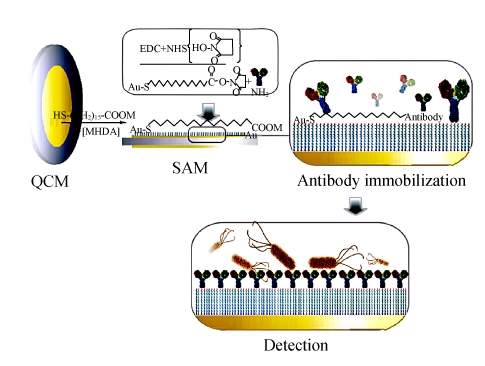
The cleaned crystal was fixed in the detection cell. One side of the Au surface was submersed in 200-μl ethanol solution of 10 mmol/L MHDA for 24 h in order to organize a well-ordered monolayer. After rinsed with ethanol and water successively, the MHDA-modified crystal was treated with the solution including 75 mmol/L EDC and 15 mmol/L NHS for 1 h to convert the terminal carboxylic group to an active NHS ester. After the co-addition of EDC and NHS, the following steps were PBS washing and nitrogen drying to prepare the crystal for the next treatment. Antibody immobilization was a vital part for the successful fabrication: 20 μl anti-E. coli O157:H7 antibodies were added and spread over the whole Au electrode, and then the electrode was stored at 37 °C for 1 h. After antibody immobilization, PBS buffer was used to remove the excess antibodies and the antibody-modified crystal was treated with 0.1% BSA-PBS for 1 h to block the untreated and nonspecific sites. The blocked crystal was rinsed by PBS and water, and then dried in nitrogen. Thus, the immunosensor was ready for use and should be stored at 4 °C. Every step of the biosensor fabrication would be detected by the experimental QCA to record the frequency shift of the sensor in 200 μl PBS until the baseline of PBS was stabilized.
During the detection process of E. coli O157:H7, 3 ml of 100~108 colony forming units (CFU)/ml bacterial suspension was added into the detection cell for 1 h. Then the sensor was rinsed with PBS and water, while the frequency shift caused by the combination was collected by the QCA in 200 μl PBS until the curve reached a plateau. The schematic diagram of sensor fabrication and detection process is presented in Fig.3.
Fig. 3.
Illustration for the fabrication and detection procedure of the QCM immunosensor
The whole analytical course including the biosensor fabrication and detection was named immersion-dry-immersion procedure, because in each fabricating and detecting step, one side of the crystal was always exposed to the reagents or bacterial suspension in a static cell, and the resonant frequency of the sensor was measured in liquid phase before and after immersion. During the immunosensor fabrication, the sensor should sometimes be dried with nitrogen stream.
Investigation for rapid construction and immunosensor regeneration
To better fabricate the biosensor, the cleaned QCM was fixed and treated by PEI (1 mg/ml, pH 10.0) for 15 min, then by PAA (3 mg/ml, pH 3.0) for 15 min. The following steps for NHS activation and antibody immobilization were the same as those for biosensor fabrication abovementioned.
Finally, in order to investigate and optimize the biosensor regeneration, 0.1 mol/L glycine-NaOH (pH 11.0) elution buffer was adopted to regain the blocked crystal, which could be used to detect the bacteria again. After soaking in 106 CFU/ml of E. coli O157:H7, the crystal was treated by the glycine-NaOH buffer for 5 min, and then rinsed by PBS to record the frequency shift, which should approximately return to the quondam blocking level.
Electrochemical characterization of the QCM electrodes
Electrochemical cyclic voltammetric (CV) experiments and impedance spectroscopy measurements were processed by VMP2 Multichannel Potentiostat (EG & G Inc., USA). Pt wire electrode was used as the counter electrode and Ag/AgCl (in saturated KCl) electrode as the reference electrode. The impedance spectra were recorded in the frequency range from 0.1 Hz to 100 kHz at the formal potential of the [Fe(CN)6]3−/4− redox couple, and the amplitude of the alternating voltage was 5 mV.
RESULTS AND DISCUSSION
Immunosensor fabrication
1. Detailed fabrication and analytical performances of the biosensor
The specific antibodies could be immobilized onto the Au surface of QCM in many ways in order to fabricate an immunosensor. In this study, a compact self-assembly membrane was used for the protein-linkage interface. MHDA is a long-chain carboxylic acid-terminating alkanethiol, which was proved to be more stable than other shorter chain ones (Mirsky et al., 1997), and an oriented monolayer of MHDA on the Au electrode of QCM was shaped through the strong Au-thiolate bond. The mechanism of Au-thiolate bond is denoted as follows:
| R-SH+Au=RS-Au+e−+H+. |
The co-addition of EDC and NHS could improve the stability of the linker compounds by activating the MHDA monolayer (Fung and Wong, 2001), and antibodies were immobilized by replacing the active NHS esters through the amide bonds.
The stepwise assembly of fabrication and the corresponding response performance of the immunosensor were investigated using the immersion-dry-immersion procedure. After each treatment during the fabrication, one face of the crystal was immersed in the background solution, i.e., PBS, until it reached equilibrium, generally within 15 min.
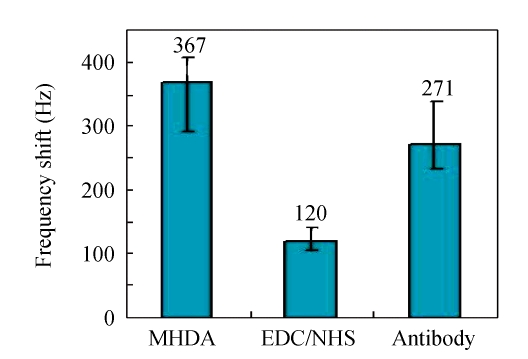
Fig.4 shows the frequency increase due to each step of the construction obtained with static liquid-phase measurement by the QCA. In the statistical histogram, it is shown that the mass increases of MHDA-SAM, activated NHS esters and immobilized antibodies induced the frequency shifts of (367±39), (120±21) and (271±67) Hz, respectively, with the frequency fluctuation of ±10.6%, ±17.5% and ±24.7%. It is well understood that the response frequency of piezoelectric sensor is always instable in liquid phase, because of its more complicated behavior in liquid phase than that in gas phase. So when the QCM is detected in liquid phase, it should be guaranteed that one side electrode of the crystal must be kept in gas phase which has the lower damping than that in liquid. The error of each step would be amplified in series due to the mutual influences of the conjoint steps. Some explanations indicated that, besides the specific and nonspecific adsorption, the variation of surface viscoelasticity, crystal interior stress, solution density and viscosity could also influence the response frequency of the QCM (Martin et al., 1991). Actually, the response frequency shift ΔF of the crystal measured in liquid can be depicted by the equation as follows:
| ΔF=ΔFm+ΔFη+ΔFρ. |
Thereinto, ΔFm and ΔFη are the frequency shifts induced by the mass increase on the electrode surface and solution character, respectively; ΔFρ is the frequency shift caused by crystal interior stress. However, the interior stress cannot be avoided when the QCM is mounted on the detection cell. So the ΔFρ comprises two parts, i.e.,
| ΔFρ=ΔFs+ΔFd, |
where, ΔF s represents the frequency shift from the initial static stress and ΔF d represents the frequency shift from the dynamic stress induced by the small perturbation of solution in cell. Owing to the presence of ΔF s, the repeatability for the initial frequency of the QCM is very poor. After the installation has been finished, the ΔF s remains constant, and the ΔF d becomes a variable value and cannot be ignored. Probably due to these complicated factors, the liquid-phase based method has showed poor reproducibility. However, this liquid-phase based procedure is actually the most appropriate method for revealing the biomolecular interaction in the solution (Morita et al., 2006). In this work, the static liquid-phase measurement was adopted to keep the background buffer in an immobile state, so the ΔFρ has been minimized and almost has no influence on the ΔF. Compared with the injection and flow system (IFS), the static liquid-phase measurement has fewer interferential factors, so the ΔF gained by static liquid-phase procedure is more reasonable and accurate.
Fig. 4.
Frequency change for the individual coating on the quartz crystal
Error bars=SD (n=3~10)
According to Sauerbrey equation, the mass change on the surface can be deduced from the frequency shift, and then the amounts of molecules of each layer can be calculated according to the mass. Usually, Sauerbrey equation is depicted as follow:
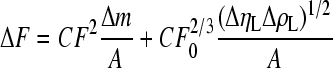 . .
|
(1) |
In the static liquid-phase measurement, the viscosity (η L) and density (ρ L) of the background buffer are constant in each detection, so the Δη L and Δρ L are all zero.
There, C=−2.26×10−6 cm2/(Hz∙g). Then this equation could be simplified as:
| ∆F=CF2∆m/A, | (2) |
thus,
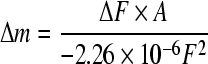 . .
|
(3) |
.
The molecule numbers N could be calculated by mass Δm, molecular weight M w and Avogadro’s constant A (6.02×1023 mol−1) as:
| N=∆m/Mw×6.02×1023, | (4) |
then,
| N=−2.664×1029×∆F×A/(Mw×F2) | (5) |
.
The results shown in Table 1 indicate that the fabricated immunosensor has the ability to adsorb the order of 1012 target antigens in an idealized state, but the real amount of adsorbed bacteria would be decided by many factors such as the antibody activity, temperature, the pH of buffer solution and so on.
Table 1.
Changes of mass and molecular numbers in each step
| Layer | MW | ΔF (Hz) | ΔM (ng) | Mol (nmol) | N |
| MHDA | 288.49 | 367.0 | 517.333 | 1.7932 | 1.0795×1015 |
| NHS | 115.09 | 120.3 | 169.615 | 1.4740 | 8.8735×1014 |
| Antibody | 150000 | 271.4 | 382.551 | 0.0026 | 1.5351×1012 |
The reaction efficiency of stepwise fabrication could be calculated from the data as follow:
| NNHS/NMHDA×100%=82.195%, |
| NAb/NNHS×100%=0.173%. |
It is shown that the activating efficiency of NHS is about 82.195%, namely almost 5 MHDA molecules could be activated by 4 NHS molecules. The activating efficiency can be satisfied, but the effect of antibody immobilization has declined so much, that is to say that about 500 activated MHDA can immobilize only one antibody. Analyzing the low immobilization efficiency, at least two possible reasons should be considered. One is that the volume of anti-E. coli O157:H7 antibody is more gigantic than that of MHDA, the other is that many active NHS esters would be hydrolyzed by H+ ions in the reagent during the course of immobilization reaction. It is very important to adjust the pH value of the antibody solution to the alkalescent level, so that the OH− ions could neutralize the H+ ions and inhibit the hydrolization of active NHS ester.
2. Preliminary exploration for rapid construction
The SAM technique employed in this assay is attached to the covalently combining method, and it has such an advantage that the assembly monolayer can provide diversified functional groups according to the experimental design in a uniform environment. But, some existing lacuna of the monolayer would result in the oxidation of the thiol groups, which could arouse the break of Au-S bond and the collapse or desquamation of assembly molecules, and further influence the immobilization efficiency. Besides the antibody immobilization effect, the preparation time is also a parameter to evaluate the performance of the biosensor. It has taken at least 24 h to form an MHDA-SAM in this work, so the desire to improve the procedure for more rapid construction of the immunosensor was raised. In order to enhance the stability of the deposited molecular layer and shorten the fabrication time, a polyelectrolyte layer-by-layer self-assembly (LBL-SA) method was explored for antibody immobilization. It is well known that the technique of polyelectrolyte multilayers based on electrostatic interaction is a general approach for the fabrication of multicomponent films on solid support in the field of the materials science (Zhang et al., 2007), but this is the first time the method is used in the immunosensor construction for bacteria detection. PEI and PAA are the materials used for deposition of cationic and anionic layers, which are rich in amido groups and carboxyl groups, respectively. The PEI, as a polycation, was used to form the sensing film of a toxic gas-detecting device (Kikuchi and Shiratori, 2005); the PAA, as a polyanion, was applied to shape a polymer film on the surface of surface plasmon resonance (SPR) to analyze amine compounds (Nishimura et al., 2002). In this report, a polymeric structure based on LBL-SA was formed by PEI+ and PAA− for improving the biosensor fabrication. The required time for the assembly of multilayer films was only 30 min in all, which was much less than that of MHDA-SAM. In succession, the carboxyl groups on PAA− membrane could be activated by NHS ester for 1 h, and thus the crystal surface was suitable for the antibody immobilization. The whole procedure of this LBL-SA based immunosensor could be accomplished within 3 h, which has the rapidity predominance compared with the MHDA-SAM based method. However, multilayer structures for the rapid construction of biosensor have exhibited a lower NHS activating effect and a lower antibody immobilization efficiency than those of the MHDA based approach, i.e., the amounts of NHS and antibodies immobilized onto the Au surface are 1.4743×1014 and 0.6923×1012 respectively, and both of them are fewer than those obtained with MHDA-SAM method. It is well known that there were many factors affecting the growth of polyelectrolyte multilayer, including the pH and character of the reagent, the concentration and molecular weight of the polyelectrolyte, and the processing time, and so on. How to improve the efficiency of antibody immobilization based on the LBL-SA method needs to be explored and discussed thoroughly in the further study, and the LBL-SA method would be a promising approach to rapidly construct the immunosensor, and could extend the application range of the biosensor greatly.
Immunosensor detection
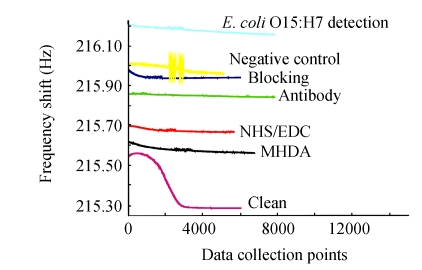
A typical sensor diagram for the stepwise sensor fabrication and detection is shown in Fig.5. Because the immersion-dry-immersion procedure had been used in the whole course, each temporal response curve of the fabrication and detection was indie and elevatory according as the ordinal treatment. The frequency shift was obtained in the static PBS buffer, so the curve would reach a plateau almost within 15 min. The change calculated from every two neighboring stable curves was regarded as the ΔF induced by the corresponding treatment. The immobilization of anti-E. coli O157:H7 antibodies caused more than 200 Hz increase of frequency, which confirmed the successful immobilization based on the MHDA-SAM and NHS ester activation. Consulting other pioneering works carried out by Mao et al. (2006), BSA was selected to effectively prevent the nonspecific adsorption of bacteria to the electrode. The 100 Hz frequency shift after BSA blocking suggested the adsorption of BSA to the surface of the QCM.
Fig. 5.
Temporal response curves of each step of the immunosensors fabrication and detection
Data collection points at 300 points/min
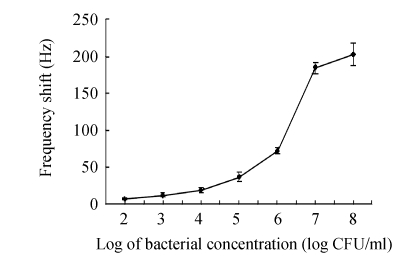
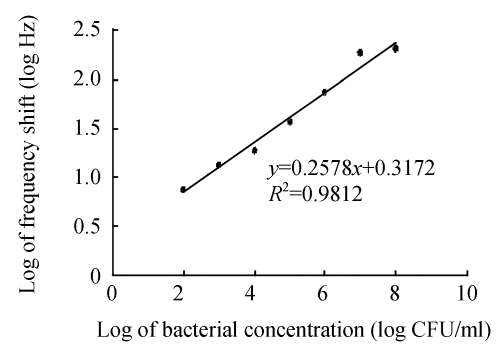
Immersed in different concentrations of E. coli O157:H7, the sensor’s resonant frequency increased accordingly due to the binding of bacteria onto the immobilized antibodies. The calibration graph of the frequency shift vs the logarithm of cell concentration is displayed in Fig.6. It can be seen that in the entire working range of 102~108 CFU/ml, the higher the concentration, the greater the sensor response, but the relationship is nonlinear and can be described by an approximate sigmoid modal. Moreover, it can provide some useful information about the kinetics of combination of target bacteria with immobilized antibodies. In Fig.7, the frequency shift and concentration are plotted in a double-logarithmic scale, and a well linear relationship is found between the logarithmic values of these two variables.
Fig. 6.
Frequency shift of the immunosensor as a function of bacterial concentration
Error bars=SD (n=3)
Fig. 7.
The logarithm graph for the frequency shift vs bacterial concentration
When the bacterial concentration was 102 CFU/ml, the frequency shift was about (7.0±1.5) Hz on average, whereas, when the bacterial concentration reached the 101 CFU/ml or the sample was the negative control, the temporal response curves could not be distinguished from the foresides, so this immunosensor has a detection range of 102~108 CFU/ml. It could be well understood that at the high bacterial concentration, abundant cells could saturate the immobilized antibodies on the surface, but at the low bacterial concentration, few cells only provided a small mass change, so the frequency shift would not change obviously at the lowest and highest concentrations.
Immunosensor is characterized by its high specificity, which depends upon the antibodies used. In our study, the affinity-purified antibodies were chosen to minimize the cross-reactivity to other E. coli strains, and the strict control to the procedure is important to ensure the correct tendency of frequency shift. The influence of several matrices including pure culture media and 0.1% BSA-PBS was also investigated. The immersion-dry-immersion procedure was subject to the interference of these matrices, and hence the electrode surface should be cleaned completely after each treatment, and the background buffer for test should be preserved with the same. Fortunately, no significant interference was found from these matrices with the immersion-dry-immersion method.
Electrochemical characterization
Electrochemical measurements which were useful in assessing the microscopic integrity and average behavior of such assemblies, have long been used to characterize modified electrodes.
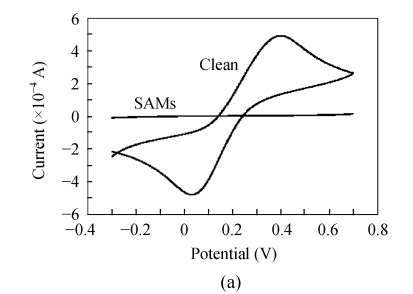
After each step, the immunosensor was monitored through cyclic voltammetry and electrochemical impedance spectroscopy using [Fe(CN)6]3−/4− as a redox couple. As shown in Fig.8a, the pretreated bare Au electrode gave a reversible cyclic voltammogram indicating the Au surface had been treated cleanly. However, the formation of MHDA monolayer on Au electrode resulted in a highly insulating surface and thus blocked the transfer of redox couple in a large scale (Fig.8b). So the CV curve of the SAM showed a conversed sigmoid, which indicated that the MHDA monolayer was so integrate and well ordered that there were no obvious pinholes on its surface. In fact, the presence of an n-alkanethiolate film of thickness sufficient to allow for close packing of the chains will provide up to a 99% electrochemical blocking effect, allowing only 1% of the current that could occur before SAM adsorption (Smith et al., 2004). The results shown in Fig.8 are consistent with this report.
Fig. 8.
Cyclic voltammograms of the same Au electrode in the presence of 10 mmol/L [Fe(CN)6]3−/4− for bare Au electrode (a) and MHDA-SAM (b)
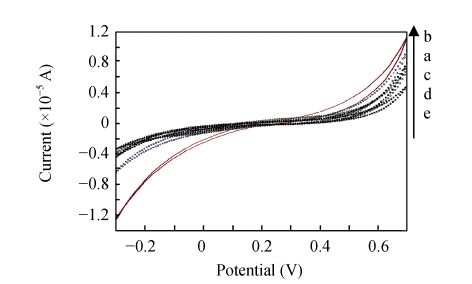
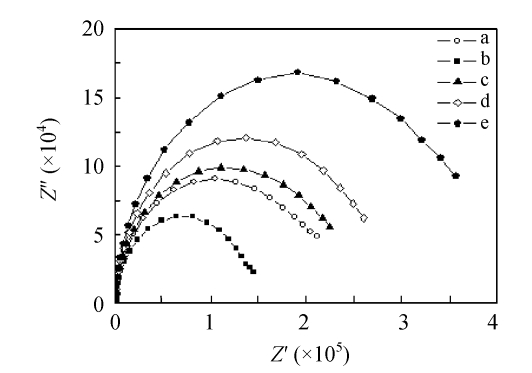
Fig.9 shows that the faradic current of the redox process decreased with the modification of Au electrode surface, and the impedance change of the electrode at each step could also be directly found in Fig.10. After activation, the MHDA monolayer became less insulating. Then an increased current response and decreased impedance were observed (curve b in Figs.9 and 10). The explain for this result was probably due to that when the terminal carboxylic group of MHDA was changed to EDC/NHS in pH 7.4, the former would take the negative charges which blocked the transfer of the negative redox ions [Fe(CN)6] 3−/4− to the QCM surface owing to the electrostatic repulsion, while the latter was positively charged and induced the opposite effect. Certainly, in some conditions such as the monolayer was activated in the organic solvent, the MHDA would deviate from the electrode surface which became less insulating, but the desorption of MHDA would be neglected in this study because the activation has taken place in the aqueous solution. Immobilization of antibodies reduced the penetration of the redox pair and decreased the current response (curve c in Fig.9). Insulation was further improved after BSA blocking (curve d in Figs.9 and 10) and the bacteria binding onto the specific antibodies (curve e in Figs.9 and 10).
Fig. 9.
Cyclic voltammograms of the same Au electrode in the presence of 10 mmol/L [Fe(CN)6]3−/4− for fabrication and detection. (a) MHDA-SAM; (b) EDC/NHS activation; (c) Antibody immobilization; (d) BSA blocking; (e) Bacteria binding
Fig. 10.
Electrochemical impedance spectroscopy for the biosensor fabrication and detection. (a) MHDA-SAM; (b) EDC/NHS activation; (c) Antibody immobilization; (d) BSA blocking; (e) Bacteria binding
Stability and repeatability of immunosensor
The QCM measurement was performed on the self-manufactured QCA system, so the stability of this equipment would directly influence the accuracy and sensitivity of the immunosensor. In this research, the stabilization of the QCA setup was so favorable that the frequencies could reach the same level when one crystal oscillated for three times (Fig.11).
Fig. 11.
The stability and repeatability of the Quartz Crystal Analyzer system
Data collection points at 300 points/min
Stability and reusability are also major factors in evaluating an immunosensor. It was found that a fabricated immunosensor based on MHDA-SAM could retain its activity for at least 1 week at 4 °C before its first use. How to regenerate a sensor for continuous use is a challenge for the majority of immunosensors, because the regeneration with extreme pH, temperature and chaotropic agents would decrease the activity significantly (Asanov et al., 1998). There were many methods proposed to settle on the solution to this problem, whose goal was to effectively dissociate most antibody-antigen binding interactions without permanently affecting protein structure, so the regenerated immunosensor could be used to detect the target bacteria again.
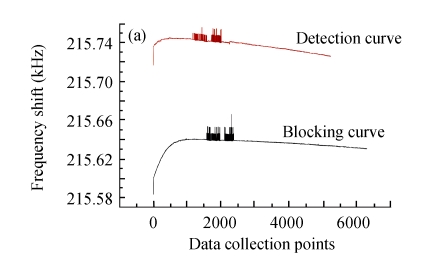
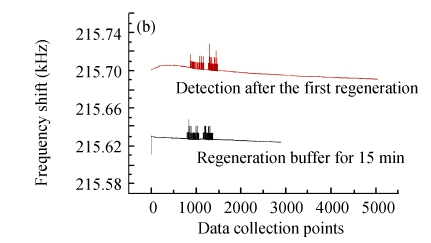
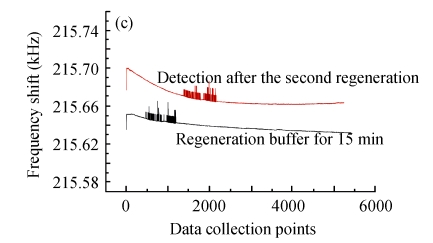
Fig.12 illustrates the variety of elution efficiency and detection efficiency after two rounds of regeneration by glycine-NaOH buffer (pH 11). It is shown that after each elution, the baseline would regress to the level of BSA blocking, but the baseline of antigens detection would decrease about 30% every time. The main reason for the stepwise declining curve of bacteria detection was possibly that the alkalescent solution would gradually denature the antibody conformation and weaken its activity. Therefore, this immunosensor based on MHDA-SAM could be used continuously for only 2~3 times, and the optimization of regeneration should be investigated in further research.
Fig. 12.
Two rounds of regeneration by glycine-NaOH buffer (pH 11) for the biosensor. (a) After detection of the bacteria; (b) The first round regeneration; (c) The second round regeneration
Data collection points at 300 points/min
CONCLUSION
The present study has detailedly introduced a successfully fabricated immunosensor with MHDASAM on Au surface, which could enable the subsequent immunobilization of anti-E. coli O157:H7 antibodies onto the monolayer. As a reaction intermediate, NHS ester would improve the stability of the linker compounds, and the frequency shifts obtained by QCM were (367±39), (120±21) and (271±67) Hz for the layers of MHDA, EDC/NHS and the antibodies, respectively. PEI and PAA were adopted as the polyelectrolytes in the LBL-SA method, and the results indicated that those two materials had the potential to shorten the fabrication time and extend the application range of the biosensor. The detection limit of the immunosensor based on MHDA-SAM was 102 CFU/ml, which was advantageous or comparable to the presently available immunosensors for bacteria detection. The immersion-dry-immersion procedure was very appropriate for our QCM detection system to reduce some interferential factors, and the detection of target bacteria could be completed within 30 min, which was very simple and convenient. Some attentions have been given to the exploration of biosensor reusability, but it should be studied in the further research.
There was no doubt that the immunosensor in our work was very sensitive and speedy, and provided a promising basis for developing a portable QCM biosensor apparatus for bacteria detection, and that even the possibility of a multi-channel detection system would be realized by forming a QCM array. Further melioration of the sensor’s sensitivity is needed through improving the fabrication and immobilization process in the future studies.
Footnotes
Project supported by the Talent Foundation of Zhejiang Province (No. R205502) and the Program of Education Department of Zhejiang Province (No. 20040197), China
References
- 1.Asanov AN, Wilson WW, Oldham PB. Regenerable biosensor platform: A total internal reflection fluorescence cell with electrochemical control. Anal Chem. 1998;70(6):1156–1163. doi: 10.1021/ac970805y. [DOI] [PubMed] [Google Scholar]
- 2.Babacan S, Pivarnik P, Letcher S, Rand AG. Evaluation of antibody immobilization methods for piezoelectric biosensor application. Biosens Bioelectron. 2000;15(11-12):615–621. doi: 10.1016/S0956-5663(00)00115-9. [DOI] [PubMed] [Google Scholar]
- 3.Berganza J, Olabarria G, Garcia R, Verdoy D, Rebollo A, Arana S. DNA microdevice for electrochemical detection of Escherichia coli O157:H7 molecular markers. Biosens Bioelectron. 2007;22(9-10):2132–2137. doi: 10.1016/j.bios.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 4.Berkenpas E, Millard P, Pereira da Cunha M. Detection of Escherichia coli O157:H7 with langasite pure shear horizontal surface acoustic wave sensors. Biosens Bioelectron. 2006;21(12):2255–2262. doi: 10.1016/j.bios.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 5.CDC (Centers for Disease Control and Prevention) Ongoing Multistate Outbreak of Escherichia coli Serotype O157:H7 Infections Associated with Consumption of Fresh Spinach. US: 2006. (Available from: Http://www.cdc.gov.mill1.sjlibrary.org/mmwr/preview/mmwrhtml/mm55d926a1.htm) [PubMed] [Google Scholar]
- 6.Demarco DR, Lim DV. Detection of Escherichia coli O157:H7 in 10- and 25-gram ground beef samples with evanescent-wave biosensor with silica and polystyrene waveguides. J Food Prot. 2002;65(4):596–602. doi: 10.4315/0362-028x-65.4.596. [DOI] [PubMed] [Google Scholar]
- 7.Fung YS, Wong YY. Self-assembled monolayers as the coating in a quartz piezoelectric crystal immunosensor to detect Salmonella in aqueous solution. Anal Chem. 2001;73(21):5302–5309. doi: 10.1021/ac010655y. [DOI] [PubMed] [Google Scholar]
- 8.Jin HY, Tao KH, Li YX, Li FQ, Li SQ. Microarray analysis of Escherichia coli O157:H7. World J Gastroenterol. 2005;11(37):5811–5815. doi: 10.3748/wjg.v11.i37.5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnston LM, Elhanafi D, Drake M, Jaykus LA. A simple method for the direct detection of Salmonella and Escherichia coli O157:H7 from raw alfalfa sprouts and spent irrigation water using PCR. J Food Prot. 2005;68(11):2256–2263. doi: 10.4315/0362-028x-68.11.2256. [DOI] [PubMed] [Google Scholar]
- 10.Kikuchi M, Shiratori S. Quartz crystal microbalance (QCM) sensor for CH3SH gas by using polyelectrolyte-coated sol-gel film. Sensors and Actuators B Chemical. 2005;108(1-2):564–571. doi: 10.1016/j.snb.2004.12.122. [DOI] [Google Scholar]
- 11.Liu Y, Li Y. An antibody-immobilized capillary column as a bioseparator/bioreactor for detection of E. coli O157:H7 with absorbance measurement. Anal Chem. 2001;73(21):5180–5183. doi: 10.1021/ac0104936. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Ye J, Li Y. Rapid detection of Escherichia coli O157:H7 in ground beef, chicken carcass, and lettuce samples using an immunomagnetic chemiluminescence fiber optic biosensor. J Food Prot. 2003;66(3):512–517. doi: 10.4315/0362-028x-66.3.512. [DOI] [PubMed] [Google Scholar]
- 13.Magliulo M, Simoni P, Guardigli M, Michelini E, Luciani M, Lelli R, Roda A. A rapid multiplexed chemiluminescent immunoassay for the detection of Escherichia coli O157:H7, Yersinia enterocolitica, Salmonella typhimurium, and Listeria monocytogenes pathogen bacteria. J Agric Food Chem. 2007;55(13):4933–4939. doi: 10.1021/jf063600b. [DOI] [PubMed] [Google Scholar]
- 14.Mao X, Yang L, Su XL, Li Y. A nanoparticle amplification based quartz crystal microbalance DNA sensor for detection of Escherichia coli O157:H7. Biosens Bioelectron. 2006;21(7):1178–1185. doi: 10.1016/j.bios.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 15.Martin SJ, Granstaff VE, Frye GC. Characterization of a quartz crystal microbalance with simultaneous mass and liquid loading. Anal Chem. 1991;63(20):2272–2281. doi: 10.1021/ac00020a015. [DOI] [Google Scholar]
- 16.Mirsky VM, Riepl M, Wolfbeis OS. Capacitive monitoring of protein immobilization and antigen-antibody reactions on monomolecular alkylthiol films on gold electrodes. Biosens Bioelectron. 1997;12(9-10):977–989. doi: 10.1016/S0956-5663(97)00053-5. [DOI] [PubMed] [Google Scholar]
- 17.Morita S, Nukui M, Kuboi R. Immobilization of liposomes onto quartz crystal microbalance to detect interaction between liposomes and proteins. J Colloid Interface Sci. 2006;298(2):672–678. doi: 10.1016/j.jcis.2005.12.043. [DOI] [PubMed] [Google Scholar]
- 18.Nishimura S, Yoshidome T, Tokuda T, Mitsushio M, Higo M. Application of a surface plasmon resonance sensor to analyses of amine compounds with the use of a polymer film and an acid-base reaction. Anal Sci. 2002;18(3):261–265. doi: 10.2116/analsci.18.261. [DOI] [PubMed] [Google Scholar]
- 19.Qian X, Metallo SJ, Choi LS, Wu H, Liang MN, Whitesides GM. Arrays of self-assembled monolayers for studying inhibition of bacterial adhesion. Anal Chem. 2002;74(8):1805–1810. doi: 10.1021/ac011042o. [DOI] [PubMed] [Google Scholar]
- 20.Rangel JM, Sparling PH, Crowe C, Griffin PM, Swerdlow DL. Epidemiology of Escherichia coli O157:H7 outbreaks, United States, 1982-2002. Emerg Infect Dis. 2005;11(4):603–609. doi: 10.3201/eid1104.040739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruan C, Yang L, Li Y. Immunosensor chips for detection of E. coli O157:H7 using electrochemical impedance spectroscopy. Anal Chem. 2002;74(18):4814–4820. doi: 10.1021/ac025647b. [DOI] [PubMed] [Google Scholar]
- 22.Smith RK, Lewis PA, Weiss PS. Patterning self-assembled monolayers. Progress in Surface Science. 2004;75(1-2):1–68. doi: 10.1016/j.progsurf.2003.12.001. [DOI] [Google Scholar]
- 23.Su XL, Li Y. A self-assembled monolayer-based piezoelectric immunosensor for rapid detection of Escherichia coli O157:H7. Biosens Bioelectron. 2004;19(6):563–574. doi: 10.1016/S0956-5663(03)00254-9. [DOI] [PubMed] [Google Scholar]
- 24.Su XL, Li Y. A QCM immunosensor for Salmonella detection with simultaneous measurements of resonant frequency and motional resistance. Biosens Bioelectron. 2005;21(6):840–848. doi: 10.1016/j.bios.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 25.Tu SI, Uknalis J, Irwin P, Yu LSL. The use of strepavidin coated magnetic beads for detecting pathogenic bacteria by light addressable potentionmetric sensor. J Rapid Meth Automat Microbiol. 2000;8(4):96–109. [Google Scholar]
- 26.Tuttle J, Gomez T, Doyle MP, Wells JG, Zhao T, Tauxe RV, Griffin PM. Lessons from a large outbreak of Escherichia coli O157:H7 infections: Insights into the infectious dose and method of widespread contamination of hamburger patties. Epidemiol Infect. 1999;122(2):185–192. doi: 10.1017/S0950268898001976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varshney M, Li Y. Interdigitated array microelectrode based impedance biosensor coupled with magnetic nanoparticle-antibody conjugates for detection of Escherichia coli O157:H7 in food samples. Biosens Bioelectron. 2007;22(11):2408–2414. doi: 10.1016/j.bios.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 28.Wang L, Li Y, Mustaphai A. Rapid and simultaneous quantitation of Escherichia coli O157:H7, Salmonella, and Shigella in ground beef by multiplex real-time PCR and immunomagnetic separation. J Food Prot. 2007;70(6):1366–1372. doi: 10.4315/0362-028x-70.6.1366. [DOI] [PubMed] [Google Scholar]
- 29.Wong YY, Ng SP, Ng MH, Si SH, Yao SZ, Fung YS. Immunosensor for the differentiation and detection of Salmonella species based on a quartz crystal microbalance. Biosens Bioelectron. 2002;17(8):676–684. doi: 10.1016/S0956-5663(02)00030-1. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Chen H, Zhang H. Layer-by-layer assembly: From conventional to unconventional methods. Chem Commun. 2007;14(14):1395–1405. doi: 10.1039/b615590a. [DOI] [PubMed] [Google Scholar]