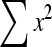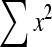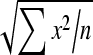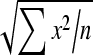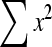Abstract
The hepatoprotective potential of earthworm extract (EE) (Lampito mauritii, Kinberg) was evaluated against paracetamol-induced liver injury in Wistar albino rat, in comparison with silymarin, the standard hepatoprotective drug. We observed a reduction in liver antioxidants, such as glutathione (GSH), superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT) and in serum total protein, and an increase in serum alkaline phosphatase (ALP), serum aspertate aminotranferase (AST), serum alanine aminotranferase (ALT), bilirubin and liver thiobarbituric acid reactive substances (TBARS) due to liver injury in the paracetamol-administered rats (2 g/kg). On the contrary, increased activities of liver GSH, SOD, GPx, CAT and serum total protein level, and decrease in the contents of serum ALP, AST, ALT, bilirubin and liver TBARS were observed in rats administered with different doses of EE (100, 200 and 300 mg/kg), which are similar to the activities of hepatoprotective drug silymarin (150 mg/kg). The mode of action of EE as evidenced by the above parameters may suggest that EE, on the one hand, prevents the formation of the reactive oxygen groups, or scavenges these groups, thereby preventing the damage on the hepatic cells, and, on the other hand, modulates the genes responsible for synthesis of antioxidant enzymes such as GPx, CAT and SOD in liver tissue and decreases the serum enzymatic activities such as ALP, AST and ALT.
Keywords: Earthworm extract, Hepatoprotective potential, Antioxidant, Paracetamol, Lipid peroxidation
INTRODUCTION
Reactive oxygen species have been implicated in numerous physiological and pathophysiological processes including vasorelaxation, aging and diseases. Initial research in this field was mostly focused on oxidative stress, a state in which oxidants overwhelm antioxidant defense mechanisms leading to elevation in the levels of oxidative damage markers. Hence antioxidants were mostly studied for the ability to prevent or minimize oxidative stress. But now it is known that oxidants, antioxidants and the cellular redox status can regulate gene expression; therefore, an exploration of antioxidants at the gene level is needed.
Cells have developed many kinds of protective mechanisms to cope with stress damage such as heat shock proteins (HSP), glucose-response protein (GRP), and growth arrest and DNA damage (GADD) gene families. It is now clear that cellular response to oxidative stress is a universal phenomenon. Many of the modulated genes by the oxidant stress in the bacterial systems were identified as genes coding antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT), alkyl hydroperoxidase reductase, glutathione reductase, and manganese superoxide dismutase (MnSOD). Shull et al.(1991) reported that MnSOD exhibited the greatest overall modulation of stress in tracheobronchial epithelia and the mRNA levels of CAT were elevated in response to H2O2.
The major functions of the liver are detoxification of bilirubin, epimerization of galactose to glucose as uridine-5-phosphate derivatives, synthesis of protein (albumin) and prothrombin, handling of enzymes, such as alkaline phosphatase (ALP), release of aspertate aminotranferase (AST) and alanine aminotranferase (ALT) (Sheila and Dooley, 1993). Most of the hepatotoxic chemicals damage liver cells mainly by lipid peroxidation and other oxidative damages. Liver disease is still a worldwide health problem. Unfortunately, conventional or synthetic drugs used in the treatment of liver diseases are not enough and mostly can cause serious side effects. This is one of the reasons for many people worldwide, including those in developed countries, to turn to complimentary or alternative medicine (CAM).
Extracting and using biologically active compounds from earthworms have traditionally been practiced by indigenous people throughout the world, more particularly in Asia, including India, Myanmar, China, Korea and Vietnam (Ranganathan, 2006). Earthworms have a dense nutritional content because of their soil-based origin. Previous studies on earthworm have shown its antipyretic, antispasmodic, detoxic, diuretic, antihypertensive, antiallergic, antiasthmatic, spermatocidal, antioxidative, antimicrobial, anticancer, antiulceral and anti-inflammatory activities (Ismail et al., 1992; Cooper, 2005; Prakash et al., 2007; Balamurugan et al., 2007). Earthworm Lampito mauritii, found throughout India, especially south India, has been earlier reported to have antimicrobial, anti-inflammatory, antioxidative and antiulceral properties (Balamurugan et al., 2007; Prakash et al., 2007). In the present study we investigate how the earthworm extract (EE) is involved in the hepatoprotective role, whether by preventing the damage by oxidative stress, by enhancing the antioxidant activity, by reducing lipid peroxidation, or by all these mechanisms, in rats whose livers were damaged by paracetamol.
MATERIALS AND METHODS
Preparation of earthworm extract from Lampito mauritii
Earthworms, Lampito mauritii (Kinberg) were collected from the stock culture from the Division of Vermibiotechnology, Department of Zoology, Annamalai University, India. Five hundred adult clitellated worms (900 mg/worm) were kept in 0.65% NaCl at room temperature for 1~2 h until their digestive systems became clean with a few times of solution changes. Animals were kept out of the solution and minced with scissors. Three grams of earthworm tissue was homogenized in 40 ml of chloroform-methanol solution and left overnight at 4 °C. The following day, 16 ml of distilled water was added to the homogenate. Then the mixture was centrifuged at 2460×g for 10 min. Three clearly visible layers were obtained. The upper, water/methanol layer was pipetted out and evaporated on a rotavapor until no methanol was left. An opalescent fluid, pH 7, was obtained, freeze-dried and kept at 4 °C until use (Hrzenjak et al., 1992).
Allocation of groups and experimental procedure
Healthy and pure strain male albino rats (Rattus norvegicus), weighing 150~200 g, were procured from the Department of Experimental Science, Central Animal House, Rajah Muthiah Medical College, Annamalai University, Annamalai Nagar, India. The rats were maintained under standard conditions [(28±2) °C, 55%~60% RH] fed on a standard diet of rat, and given water ad libitum. The experiments were carried out according to the institutional regulations and national criteria for animal experimentation. The Institutional Animal Ethics Committee reviewed the entire animal protocols prior to conducting the experiments. The rats were divided into 6 groups comprising 6 animals in each group. Group I was maintained as the control (vehicle, 1 ml of 20% Tween 80). Group II received paracetamol (Sigma Chemical Co., USA) suspension (2 g/kg bw) once a day orally for 7 d. Group III received paracetamol (2 g/kg bw) followed by standard drug silymarin (150 mg/kg bw), the known hepatoprotective compound (Scott, 1998), once a day orally for 7 d. Groups IV, V and VI received paracetamol (2 g/kg bw) followed by EE (100, 200 and 300 mg/kg bw, respectively) once a day orally for 7 d.
Serum biochemical assay
The blood was obtained from the experimental and control rats by puncturing tetro-orbital plexus. The blood samples were allowed for clotting after the serum was separated by centrifugation at 2500 r/min for 15 min and used for the estimation of various biochemical parameters. Serum AST and ALT, serum ALP, total bilirubin and total protein were assayed according to the methods of Bergmeyer et al.(1978), King and Armstrong (1934), Malloy and Evelyn (1937) and Lowry et al.(1951), respectively.
Antioxidant assay
After collection of blood samples the rats were sacrificed, and the livers were excised, rinsed in ice cold normal saline followed by 0.15 mol/L Tris-HCl, dried, and weighed. The activities of non-enzymatic antioxidant such as reduced glutathione (GSH) and enzymatic antioxidants such as reduced glutathione peroxidase (GPx), SOD, CAT and thiobarbituric acid reactive substances (TBARS) were assayed according to the methods of Ellman (1959), Rotruck et al.(1973), Kakkar et al.(1984), Sinha (1972) and Niehans and Samuelsson (1968), respectively. The protein content in the tissue homogenate was estimated by following the protocol of Lowry et al.(1951).
Statistical analysis
The statistical significance of difference was tested at 0.05 levels using one-way analysis of variance (ANOVA).
RESULTS
The levels of serum AST, ALT, ALP, bilirubin and total protein in the normal, paracetamol injured, silymarin treated and EE administered rats are shown in Tables 1 and 2. The levels of AST, ALT, ALP and bilirubin increased significantly in the paracetamol-treated rats, while the content of protein decreased significantly when compared to the control. Treatment with different doses of EE was found to reverse this effect, i.e., to reduce the activities of AST, ALT, ALP and bilirubin and increase the protein content. Especially, 300 mg/kg of EE was very effective, as the activities of those enzymes returned to normal.
Table 1.
Effects of earthworm extracts and silymarin on serum biochemical contents in liver damaged rats treated with paracetamol
| Group | AST (U/L) | ALT (U/L) | ALP (U/L) | Bilirubin (g/dl) | Protein (g/dl) |
| I | 51.42±0.02 | 70.40±2.28 | 190.43±0.76 | 0.95±0.01 | 7.3256±0.01 |
| II | 114.14±0.08 | 132.14±1.81 | 294.81±0.19 | 2.98±0.02 | 9.2546±0.02 |
| III | 51.47±0.01 | 72.48±1.28 | 212.58±0.09 | 0.91±0.01 | 7.1894±0.01 |
| IV | 81.47±0.01 | 100.94±2.88 | 270.14±0.16 | 1.85±0.01 | 9.8543±0.01 |
| V | 74.62±0.01 | 80.56±1.88 | 255.25±0.01 | 1.31±0.03 | 8.1936±0.02 |
| VI | 62.14±0.01 | 75.17±1.27 | 234.46±0.12 | 0.98±0.01 | 7.2981±0.01 |
Table 2.
ANOVA for effects of earthworm extracts and silymarin on serum biochemical contents in liver damaged rats treated with paracetamol (P<0.05)
| Between groups |
Within groups |
F-value | |||||
|
|
|
|
||||
| AST | 14062.865 | 2812.573 | 12.014 | 0.501 | 5618.815 | ||
| ALT | 14417.745 | 2883.549 | 12.173 | 0.507 | 5685.007 | ||
| ALP | 23698.560 | 4739.712 | 14.828 | 0.618 | 671.624 | ||
| Bilirubin | 5.838 | 1.168 | 8.054 | 0.336 | 3.480 | ||
| Protein | 80.977 | 16.195 | 12.015 | 0.501 | 32.351 | ||
Effects of administration of different doses of EE on the levels of liver TBARS, GSH, SOD, CAT and GPx in the paracetamol-treated rats in comparison with the normal and the silymarin-treated rats are shown in Tables 3 and 4. Level of GSH and activities of SOD, GPx and CAT in the paracetamol-treated rats (Group II) were found to be lower than the control. On the contrary, TBARS level of the paracetamol-treated rats increased. Group III, treated with silymarin, was found to have enhanced and restored almost normal activities of non-enzymatic antioxidant GSH and the enzymatic antioxidants SOD, GPx and CAT, which was comparable to control (Group I). In the silymarin-treated rats, the TBARS level was reduced. Also in the EE-treated rats, the TBARS level was reduced to nearly normal value. In the cases that rats were treated with different doses of EE (Groups IV, V and VI), the activities of the antioxidant enzymes were enhanced and restored to normal levels.
Table 3.
Effects of earthworm extract and silymarin on liver antioxidant activities in liver damaged rat treated with paracetamol
| Group | TBARS | GSH | SOD | CAT | GPx |
| I | 1.56±0.02 | 27.35±2.28 | 9.19±0.76 | 1.98±0.02 | 7.24±0.93 |
| II | 3.87±0.08 | 14.77±1.81 | 7.23±0.19 | 0.67±0.01 | 2.45±0.38 |
| III | 1.06±0.01 | 25.76±1.28 | 8.66±0.09 | 1.89±0.01 | 7.01±0.25 |
| IV | 2.83±0.01 | 23.14±2.88 | 7.34±0.16 | 1.11±0.01 | 5.67±0.15 |
| V | 2.71±0.01 | 24.13±1.88 | 7.87±0.01 | 1.31±0.03 | 6.14±0.12 |
| VI | 1.91±0.01 | 25.14±1.27 | 8.41±0.12 | 1.63±0.01 | 6.98±0.48 |
Units: TBARS (nmol/mg protein), GSH (µg/mg protein), SOD (µmol H2O2 consumed/(min·mg protein)), CAT (U/mg protein), CPx (µmol GSH consumed/(min·mg protein))
Table 4.
ANOVA for effects of earthworm extract and silymarin on liver antioxidant activities in liver damaged rat treated with paracetamol (P<0.05)
| Between groups |
Within groups |
F-value | |||||
|
|
|
|
||||
| TBARS | 6.319 | 1.264 | 12.011 | 0.501 | 2.525 | ||
| GSH | 508.491 | 106.120 | 496.462 | 99.290 | 198.141 | ||
| SOD | 3.098 | 0.620 | 12.015 | 0.501 | 1.238 | ||
| CAT | 0.492 | 0.104 | 0.142 | 0.006 | 17.213 | ||
| GPx | 12.007 | 6.001 | 4.001 | 2.003 | 24.632 | ||
DISCUSSION
The extent of paracetamol induced hepatotoxic effect was assessed by the levels of released cytoplasmic enzymes such as ALP, AST and ALT in circulation. Bilirubin increased in the blood because of regurgitation of bile due to obstruction within the liver by the damage or inflammation caused by paracetamol (Stocker et al., 1987). And the regurgitation of bile resulted in the increase of ALP activity (Sallie et al., 1991). The observation that the levels of bilirubin and ALP were brought down to their normal levels in the paracetamol-treated rats indicates that the liver is restored to its normal activity by the hepatoprotective action of EE. Effective control of bilirubin content and ALP activity due to EE administration points towards an early improvement in the secretory mechanisms of the hepatic cell.
Elevated level of AST is known to indicate a liver damage due to viral hepatitis, cardiac infarction and muscle injury. ALT, a better bio-indicator of liver injury than AST, catalyses the conversion of alanine to pyruvate and glutamate, and is released in a similar manner (Willianson et al., 1996). Serum ALP and bilirubin levels conversely are related to the function of the hepatic cells. Elevated level of serum ALP is due to the increased synthesis in presence of increasing biliary pressure (Moss and Butterworth, 1974). Our study shows the increased activities of AST, ALT, ALP and bilirubin in the rats treated with paracetamol are due to extensive liver damage and cell necrosis. And treatments with EE as well as silymarin, on the other hand, decreased the serum AST, ALT, ALP and bilirubin and restored them to nearly normal condition, indicating the stabilization of plasma membrane as well as the repairing of the hepatic tissue damaged by paracetamol. This observation correlates with the findings of Tabassum et al.(2005) who had reported a significant increase of ALT due to paracetamol administration and a decrease of ALT due to the administration of hepatoprotective Liv52 in albino mice. Similarly, Sadasivan et al.(2006) had shown paracetamol overdose to cause damage in Wistar rats’ livers and to protect the liver by administration of methanolic extract of Hedyotis corymbosa as evidenced by decreased levels of serum ALP, AST, ALT and bilirubin. The above positive changes can be considered as an expression of the functional improvement of the hepatocytes, resulted from an acceleration of cell regeneration.
Any oxidative insult to a cell induces lipid peroxidation of cell membrane lipids. Lipid peroxidation has been postulated as the destructive process in liver injury due to paracetamol administration (Muriel et al., 1992). In the present study, the increase in the level of TBARS due to administration of paracetamol indicates enhanced lipid peroxidation leading to tissue damage and failure of antioxidant defense mechanisms in preventing the formation of excessive free radicals. The EE treatment had significantly reduced the level of TBARS, indicating its potential role to protect the hepatocytes by reducing lipid peroxidation in the liver.
GSH, one of the major tripeptide non-enzymatic biological antioxidants present in the liver, is concerned with the removal of free radicals and maintenance of membrane protein and thiols, and a substrate for GPx (Jollow, 1980). Deficiency of GSH within the living organisms can lead to tissue damage and injury (Leeuwenburgh and Ji, 1995). Paracetamol has been shown to be hepatotoxic, decrease GSH and increase lipid peroxidation level of liver tissue in chicks (Bhar et al., 2005). They have, however, also shown that GSH level could be elevated in chicks pretreated with hepatoprotective Enliv®. Similarly, in our present study the liver GSH content reduced in the paracetamol-treated rats was significantly enhanced after EE treatment, confirming its capacity of removing free radicals and reducing peroxidation.
The enzymic antioxidant defense systems are natural protective barriers against lipid peroxidation. SOD, CAT and GPx are important scavengers of superoxide ion and hydrogen peroxide. These enzymes prevent the generation of hydroxyl radicals and protect the cellular constituents from oxidative damage (Halliwell and Gutteridge, 1984). Gupta et al.(2004) reported the methanolic extract of stem bark of Bauhinia racemosa Lam administered in the rats with liver damage caused by paracetamol had increased the activities of SOD, CAT, GSH and GPx. In our present study, it was observed that administration of EE caused a significant increase in activities of antioxidant enzymes SOD, CAT and GPx in the paracetamol damaged liver of rats, which scavenged and prevented the reactive free radicals, thus lessening the oxidative damage to the tissues. The liver cell’s innate ability to arouse and maintain defense against oxidants by secreting more antioxidants is overpowered by the onslaught of the oxidative stress or damage caused by paracetamol. EE overpowers this onslaught by suppressing this formation of reactive oxygen species and protecting the antioxidant machinery.
Numerous genes have been now identified, whose steady-state mRNA levels are modulated by oxidant stress agents such as superoxide, hydroperoxide, nitric oxide, redox active quinines, hyperbaric oxygen, singlet oxygen, diethyl maleate, glutathione depletion and others (Crawford, 1999). Many of the modulated genes in bacterial systems are identified as antioxidant enzymes such as SOD, CAT, alkyl hydroperoxidase reductase, glutathione reductase and manganese superoxide dismutase. MnSOD exhibits the greatest overall modulation. In tracheobronchial epithelia, CAT mRNA was shown to be elevated in response to H2O2 (Shull et al., 1991).
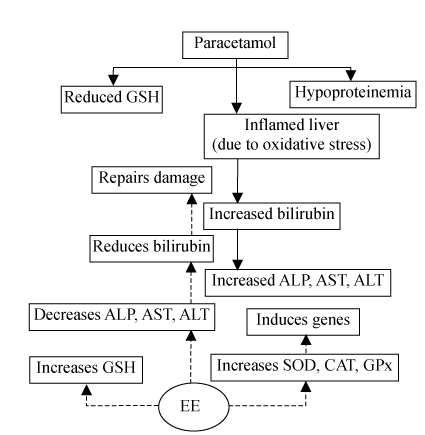
Similarly, the induction of the heme oxygenase-1 gene (HO-1) has been proposed to be a beneficial adaptive response to oxidative stress (Applegate et al., 1991). Further, Alum et al.(1995) have shown HO-1 gene to be induced by reactive oxygen species like H2O2 and singlet oxygen (1O2) which set the reduction reaction leading to enhanced bilirubin content. It is known that both biliverdin and bilirubin exhibit antioxidant properties. In the light of these observations, our findings suggest that the reactive oxygen species released from the damaged liver due to paracetamol induce HO-1 gene, and the HO-1 gene released catabolizes the heme to bilirubin, which in turn leads to increased activities of ALT, AST and ALP (Fig.1).
Fig. 1.
Mode of action of EE in the paracetamol damaged livers of rats
Simultaneously, the other cellular mechanism to prevent the oxidative stress damage is the modulation of the genes of antioxidant enzymes, such as SOD, CAT and GPx, as shown by Crawford (1999) and Shull et al.(1991).
It is concluded that the earthworm extract from Lampito mauritii is hepatoprotective as it enhances the activities of liver function such as the enhancement in the levels and activities of GSH, SOD, GPx and CAT, and decreases the levels and activities of serum ALP, AST, ALT, bilirubin and liver TBARS. The mode of action of earthworm extract prevents the formation of the reactive oxygen groups or scavenges these groups, and simultaneously modulates the genes responsible for synthesis of antioxidant enzymes such as GPx, CAT and SOD in liver tissue.
Acknowledgments
We thank the authorities of Annamalai University and Prof. K. Muralidhar, Head of Department of Pharmacology, Raja Muthia Medical College, Annamalai University, India, for providing facilities.
References
- 1.Alum J, Camhi S, Choi AM. Identification of a second region upstream of the mouse heme oxygenase-1 gene that functions as a basal level and inducer dependent transcription enhancer. J Biol Chem. 1995;270(20):11977–11984. doi: 10.1074/jbc.270.20.11977. [DOI] [PubMed] [Google Scholar]
- 2.Applegate LA, Luscher P, Tyrrell RM. Induction of heme oxygenase: A general response to oxidant stress in cultured mammalian cells. Cancer Res. 1991;51:974–978. [PubMed] [Google Scholar]
- 3.Balamurugan M, Parthasarathi K, Cooper EL, Ranganathan LS. Earthworm paste (Lampito mauritii, Kinberg) alters inflammatory, oxidative, haematological and serum biochemical indices of inflamed rat. European Review for Medical and Pharmacological Sciences. 2007;11(1):77–90. [PubMed] [Google Scholar]
- 4.Bergmeyer HU, Scelibe P, Wahlefeld AW. Optimization of methods for aspirate aminotransferase and alanine aminotransferase. Clin Chem. 1978;24:58–61. [PubMed] [Google Scholar]
- 5.Bhar MK, Das SK, Charkraborty AK, Mandal TK, Roy S. Hepatoprotective effect of Enliv on paracetamol-induced liver damage in broiler chicken. Indian J Pharmacol. 2005;37(4):257–258. [Google Scholar]
- 6.Cooper EL. CAM, eCAM, bioprospecting: The 21st century pyramid. Evidence-based Complementary and Alternative Medicine. 2005;2(2):125–127. doi: 10.1093/ecam/neh094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crawford DR. Regulation of Mammalian Gene Expression by Reactive Oxygen Spicies. In: Gilbert D, Cotton C, editors. Reactive Oxygen Species in Biological System. New York: Plenum; 1999. pp. 155–171. [Google Scholar]
- 8.Ellman GC. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 9.Gupta M, Mazumder UK, Sivakumar T, Gomathi P, Sampathkumar R. Antioxidant and hepatoprotective effects of Bauhinia racemosa against paracetamol and carbon tetrachloride induced liver damage in rats. IJPT. 2004;3:12–20. [Google Scholar]
- 10.Halliwell B, Gutteridge JM. Lipid peroxidation, oxygen radicals, cell damage, and antioxidant therapy. Lancet. 1984;323(8391):1396–1397. doi: 10.1016/S0140-6736(84)91886-5. [DOI] [PubMed] [Google Scholar]
- 11.Hrzenjak T, Hrzenjak M, Kasuba V, Efenberger-Marinculic P, Levanat S. A new source of active compounds—earthworm tissue (Eisenia foetida, Lumbricus rubellus) Comp Biochem Physiol Part A Physiol. 1992;102(3):441–447. doi: 10.1016/0300-9629(92)90191-R. [DOI] [PubMed] [Google Scholar]
- 12.Ismail SA, Pulandiran K, Yegnanarayan R. Anti-inflammatory activity of earthworm extracts. Soil Biol Biochem. 1992;24(12):1253–1254. doi: 10.1016/0038-0717(92)90102-4. [DOI] [Google Scholar]
- 13.Jollow DJ. Glutathione thresholds in reactive metabolite toxicity. Arch Toxicol Suppl. 1980;3:95–110. doi: 10.1007/978-3-642-67389-4_8. [DOI] [PubMed] [Google Scholar]
- 14.Kakkar P, Das B, Viswanathan PN. A modified spectroscopic assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–132. [PubMed] [Google Scholar]
- 15.King EJ, Armstrong AR. Determination of serum and bile phosphatase activity. J Can Med Assoc. 1934;31:376–379. [PMC free article] [PubMed] [Google Scholar]
- 16.Leeuwenburgh C, Ji LL. Glutathione depletion in rested and exercised mice: Biochemical consequence and adaptation. Arch Biochem Biophys. 1995;316(2):941–949. doi: 10.1006/abbi.1995.1125. [DOI] [PubMed] [Google Scholar]
- 17.Lowry OH, Rose Brough MJ, Farr AL, Randall RJ. Protein measurement with Folin-phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 18.Malloy HJ, Evelyn KA. The determination of bilirubin with the photoelectric colorimeter. J Biol Chem. 1937;119:481–490. [Google Scholar]
- 19.Moss DW, Butterworth PJ. Enzymology and Medicine. London: Pitman Medical; 1974. p. 139. [Google Scholar]
- 20.Muriel P, Garciapina T, Perez-Alvarez V, Murelle M. Silymarin protect against paracetamol-induced lipid peroxidation and liver damage. Drug Chem Toxicol. 1992;1:163–171. doi: 10.1002/jat.2550120613. [DOI] [PubMed] [Google Scholar]
- 21.Niehans WG, Samuelsson D. Formation of malondialdehyde from phospholipids arrachidonate during microsomal lipid peroxidation. Eur J Biochem. 1968;6(1):126–130. doi: 10.1111/j.1432-1033.1968.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 22.Prakash M, Balamurugan M, Parthasarathi K, Gunasekaran G, Cooper EL, Ranganathan LS. Anti-ulceral and anti-oxidative properties of “earthworm paste” of Lampito mauritii (Kinberg) on Rattus Norvegicus. European Review for Medical and Pharmacological Sciences. 2007;11(1):9–15. [PubMed] [Google Scholar]
- 23.Ranganathan LS. Vermibiotechnology—from Soil Health to Human Health. Jodhpur, India: Agrobios; 2006. [Google Scholar]
- 24.Rotruck JT, Rope AL, Ganther HF, Swason AB. Selenium: Biochemical role as a component of glutathione peroxide. Science. 1973;179(4073):588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 25.Sadasivan S, Latha PG, Sasikumar JM, Rajashekaran S, Shyamal S, Shine VJ. Hepatoprotective studies on Hedyotis corymbosa (L.) Lam. J Ethnopharmacol. 2006;106(2):245–249. doi: 10.1016/j.jep.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Sallie R, Tredgeri JM, Willion R. Drugs and the liver. Biopharmaceutics and Drug Disposition. 1991;12(4):251–259. doi: 10.1002/bdd.2510120403. [DOI] [PubMed] [Google Scholar]
- 27.Scott LND. A review of plants used in the treatment of liver disease: Part I. Altern Med Rev. 1998;3(6):410–421. [PubMed] [Google Scholar]
- 28.Sheila S, Dooley J. Diseases of the Liver and Biliary System. 9th Ed. Osney Mead, Oxford OX2 OEL: Backwell Scientific Publications; 1993. pp. 1–16. [Google Scholar]
- 29.Shull S, Heintz NH, Periasamy M, Manohor M, Jansseny M, Marsh JP, Mossman BT. Differential regulation of antioxidant enzymes in response to oxidants. J Biol Chem. 1991;266:24398–24403. [PubMed] [Google Scholar]
- 30.Sinha KA. Colorimetric assay of catalase. Ann Biochem. 1972;47(2):389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 31.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiologic importance. Science. 1987;235(4792):1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 32.Tabassum N, Chattervedi S, Aggrawal S, Ahmed N. Hepatoprotective studies on Phyllanthus niruri paracetamol induced liver cell damage in albino mice. Experimental Medicine. 2005;12(4):211–212. [Google Scholar]
- 33.Willianson EM, Okpako DT, Evans FJ. Selection, Preparation and Pharmacological Evaluation of Plant Material. England: John Wiley; 1996. [Google Scholar]