Abstract
Introduction. Blood loss and bile leakage are well-known risk factors for morbidity and mortality during liver resection. Bleeding usually occurs during parenchymal transection, and surgical technique should be considered an important factor in preventing intraoperative and postoperative complications. Objective. Many approaches and devices have been developed to limit bleeding and bile leakage. The aim of the present study was to determine whether a bipolar vessel sealing device allows a safe and careful liver transection without routine inflow occlusion, achieving a satisfactory hemostasis and bile stasis, thus reducing blood loss and bile leak and related complications. Patients and methods. A total of 50 consecutive patients (24 males, 26 females, with a mean age of 57 years) underwent major and minor hepatic resections using a bipolar vessel sealing device. A clamp crushing technique followed by energy application was used to perform the parenchymal transection. Inflow occlusion was used when necessary to control blood loss but not as a routine. No other devices were applied to achieve hemostasis. Results. The instrument was effective in 45 patients and failed to achieve hemostasis in 5 cases, all of whom had a cirrhotic liver. Median blood loss was 490 ml (range 100–2500 ml) and intraoperative blood transfusions were required in eight cases (16%). Mean operative time was 178 min (range 50–315 min). Inflow occlusion was necessary in 16 (32%) patients. The postoperative complication rate was 24%, with a postoperative hemorrhage in a cirrhotic patient. There was no clinical evidence of bile leak or procedure-related abdominal abscess. Conclusion. We conclude that the device is a useful tool in standard liver resection, achieving good hemostasis and bile stasis in patients with normal liver parenchyma, but its use should be avoided in cirrhotic patients.
Keywords: liver resection, bipolar vessel sealing device, bile leakage, blood loss
Introduction
Despite standardized techniques for liver resections, at high volume centers, partial hepatectomy has been reported to yield morbidity rates of 23–46%, and surgical death rates ranging from 4% to 5% 1,2,3. Intraoperative blood loss is a powerful determinant of operative outcome and remains a major concern for surgeons operating on the liver, with a reported median blood loss of 700–1200 ml. Bleeding represents the major intraoperative surgical complication, and together with bile leaks and hepatic failure, is also one of the major postoperative complications 4,5,6.
In fact a high intraoperative blood loss is associated with the extensive use of vessel occlusion techniques (directly related to higher risk of postoperative hepatic failure and ischemic/reperfusion injury of the liver) and with a high transfusion rate (cause of host immunosuppression with more perioperative infections and shorter long-term survival for early recurrence of malignancies in neoplastic patients) 7,8,9,10. Most blood loss during liver resection occurs during parenchymal transection. Multiple approaches have therefore evolved to reduce hemorrhage in this phase of the procedure. Four main types of strategies have been developed: a) limit blood flow through the liver by prophylactic vessel occlusion (improved by techniques of intermittent inflow occlusion and Clavien's preconditioning) 11,12,13; b) reduce central venous pressure during the transection phase of the intervention 14; c) perform anatomical limited resections based on the preoperative evaluation and on intraoperative ultrasonography (IOUS); and d) prevent blood loss along the plane of transection during parenchymal division. Several techniques have been developed for safe and careful dissection of parenchyma. In addition to blunt dissection using the ‘finger fracture’ and the clamp crushing techniques various device have been developed. On one hand there are those that dissect but are not able to achieve hemostasis such as CUSA and the water jet scalpel. On the other hand are various devices that can directly reduce blood loss: they are unipolar or bipolar cautery, harmonic scalpel, stapling devices, laser systems, microwave tissue coagulation, and the floating ball system developed by Tissuelink. With these techniques, resection has become quite safe and major blood loss is uncommon. Because none of these devices can achieve complete hemostasis during dissection, blood vessels and biliary tract branches need to be clipped or sutured. It would be desirable to have a quick and reliable mean of transecting the parenchyma without using clips or ligatures, avoiding the routine use of inflow occlusion. Moreover some of these instruments have shown a higher incidence of bile leakage compared with conventional clamp crushing techniques 15, and it is well known that biliary complications could lead to life-threatening events, such as sepsis and liver failure 16. Resection techniques and devices that minimize hemorrhage, bile leak, and the removal of functional parenchyma will prove most useful in the new era of surgical management of liver disease and there is still room for improvement.
The purpose of our study was to present our experience with a bipolar vessel sealing device (LigaSure Valleylab Inc., Boulder, CO, USA), which has been shown to permanently occlude blood vessels as large as 7 mm in diameter by fusing the collagen matrix in the vessel wall. After experimental and clinical experience 17 we applied this technique in 50 consecutive patients undergoing liver resection, and we evaluated the efficacy of hemostasis and bile stasis and analyzed biochemical and histological tissue damage effects.
Patients and methods
In this prospective study we gained experience with the bipolar vessel-sealing device (BVSD) during hepatic resection in 50 patients. The study was begun in January 2002 and completed in January 2005. The terminology for liver anatomy and resection used is the Brisbane 2000 terminology of the International Hepato-Pancreato-Biliary Association 18.
The initial steps of the procedure were performed in a standard fashion, according to the type of resection being performed. Isolation, occlusion, and transection of the portal pedicle was performed in patients undergoing right hepatectomy (segments 5–8) or right trisectionectomy (segments 4–8). In these cases the liver was also dissected off the inferior vena cava, and the right hepatic vein was isolated and ligated. In patients undergoing bisegmentomies (two Couinaud segments), segmentectomies (one Couinaud segment), or nonanatomical resections, no preliminary dissection of blood vessels was performed. Inflow occlusion was not routinely used, but was instituted in the course of transection if blood loss exceeded 500–750 ml, according to age, preoperative hematocrit values, and cardiac and hemodynamic status. Low central venous pressure was not used.
The cutting line was marked on the liver surface by electrocautery and standard electrocoagulation was applied to a depth of approximately 2 mm from the liver surface. The underlying liver tissue was then divided and the vessels up to 5–6 mm were sealed using the LigaSure (Figure 1); the vessels over 5–6 mm in diameter were sutured. Initially the LigaSure device was also used to achieve isolation of bilio-vascular pedicles from surrounding soft hepatic tissue. However, the dimension of the cross-sectional area of the blade's instrument is relatively large and blades are blunt, so bleeding caused by mechanical trauma to small vessels was quite frequent. Therefore a combination of clamp crushing method and LigaSure technique was adopted. A kelly clamp was used for isolation of bilio-vascular pedicles from surrounding soft hepatic tissue and for exposure of a portion of vessel compatible with the size of the BVSD's clamp; then the BVSD's blades were inserted carefully into the point of application and the power was applied. Complete isolation of the target vessel is very important in order to focus the radiofrequency power on the vessel wall that should be sealed and to minimize dispersion of power in surrounding parenchyma, resulting in poor hemostasis in the cutting surface. Then the device was released (although several times a second application of power was used to achieve a complete hemostasis), and the vessels were cut with scissors under direct vision. Once the clamp was removed care was taken to cut within the edges of the coagulated tissue, considering that the coagulated line is approximately 2–3 mm long and slightly curved. At the conclusion of the resection, the cut surface of the liver was explored for bile leaks, which were sutured if identified. Hemostasis was checked and eventually achieved if possible with the BVSD along the bare surface of the liver remnant, or alternatively with sutures or electrocautery. No other hemostatic agents or devices were employed.
Figure 1. .
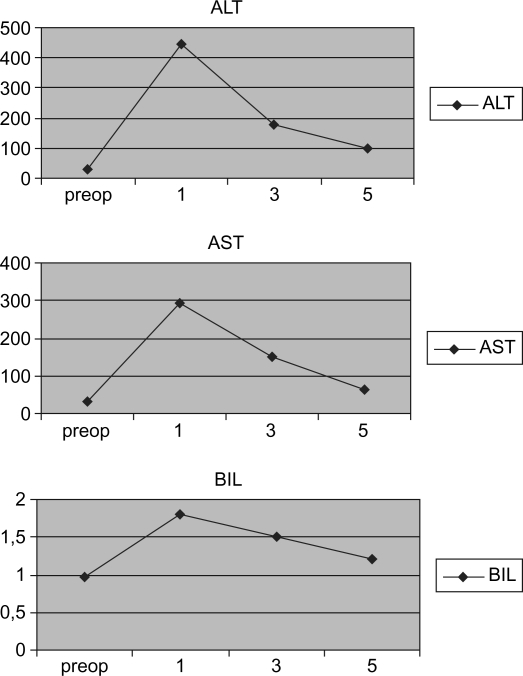
Serial changes in levels of serum aspartate aminotransaminase (AST), alanine aminotransaminase (ALT), and total bilirubin in the postoperative period.
Results
The LigaSure vessel-sealing system has been used in 50 consecutive hepatic resections. Patients were 24 males and 26 females (median age 57.1 years, range 25–77 years). Indications were as follows: 19 metastatic colorectal cancers, 14 hepatocellular carcinomas (HCCs), 4 angiomas, 2 metastatic gastric carcinomas, 2 echinococcal cysts, 2 biliary cysts, and 1 each of metastatic ovarian tumor, metastatic melanoma, metastatic pancreatic carcinoma, metastatic spinocellular carcinoma, hepatic adenomas, hepatic aspergillosis, and gallbladder cancer. Patients’ characteristics, indications, and surgical procedures performed are summarized in Table I. The BVSD was effective in 46 of these procedures (92%), requiring transection through normal or near-normal hepatic parenchyma. In four cases of HCC associated with Child-Pugh B cirrhosis, the device was ineffective in achieving bloodless parenchymal transection, and the technique was abandoned because of high blood loss. Hemostasis was achieved with sutures and clips. Inflow occlusion (Pringle maneuver) was used in 16 (32%) of these patients; the occlusion never lasted longer than 40 min (30 min in cirrhotic patients). Intraoperative median blood loss was 490 ml (range 100–2500 ml), and intraoperative blood transfusions were required in 8 patients (16%). Five of these eight patients had a cirrhotic liver and four required a change of the technique of resection to control hemorrhage. Eleven patients were transfused in the perioperative period; altogether 16 patients (32%) were transfused, considering as one patient those transfused both intra- and perioperatively.
Table I. Patients’ characteristics and surgical procedures.
| Patients (n=50) | No. |
|---|---|
| Males/females | 24/26 |
| Median age (range) | 57.1 (25–77) |
| Indications | |
| Colorectal metastases | 19 |
| Hepatocellular carcinoma | 14 |
| associated with cirrhosis | 10 |
| associated with chronic hepatitis | 3 |
| Hepatic angioma | 4 |
| Echinococcal cysts | 2 |
| Metastatic gastric adenocarcinoma | 2 |
| Biliary cysts | 2 |
| Metastatic spinocellular tumor | 1 |
| Metastatic pancreatic adenocarcinoma | 1 |
| Metastatic melanoma | 1 |
| Metastatic ovarian carcinoma | 1 |
| Galbladder cancer | 1 |
| Adenoma | 1 |
| Hepatic aspergillosis | 1 |
| Surgical procedures | No. |
| Right trisectionectomy | 1 |
| Left trisectionectomy | 1 |
| Right hepatectomy | 5 |
| Left hepatectomy | 1 |
| Trisegmentectomy | 2 |
| Bisegmentectomy 2,3 | 6 |
| Other bisegmentectomy | 12 |
| Segmentectomy | 3 |
| Subsegmentectomy | 4 |
| Cystopericystectomy | 2 |
| Nonanatomical resection | 13 |
When BVSD was ineffective, persistent bleeding after parenchymal transection was treated using 4/0 polypropylene sutures. Transection of the liver tissue using the devices was quite rapid in all cases, with the exception of the cirrhotic livers where the LigaSure failed in achieving hemostasis. Mean operative time was 178 min (range 50–315 min). Results are shown in Table II.
Table II. Surgical outcomes.
| Parameter | Value |
|---|---|
| Mean operative time (range), minutes | 178 (50–315) |
| Intraoperative median blood loss (range), ml | 490 (100–2500) |
| Postoperative morbidity (%) | 12 (24%) |
| Postoperative mortality (%) | 1 (2.2%) |
| Intraoperative blood transfusions | 8 (16%) |
| Postoperative blood transfusions | 11 (23.9%) |
| Total transfusion rate | 16 (32%) |
| Patients transfused with ≤2 units of packed red cells | 10 |
| Patients transfused with >2 units of packed red cells | 6 |
| Utilization of Pringle's maneuver | 16 (32%) |
Although this is not a comparative study, duration of surgery does not seem to have been longer than that reported in the literature for other techniques of transection 19,20. There was no clinical evidence of postoperative bile leaks. Twelve patients (24%) reported postoperative complications, as follows: three right pleural effusions (one requiring drainage) associated with pneumonia, three cases of transient postoperative hepatic failure, two cases of prolonged ascites at the drain site without evidencyeof hepatic insufficiency, one hemoperitoneum that required reintervention (a cirrhotic patient with HCC), one wound infection, one case of intra-abdominal abscess in a patient already operated for hepatic aspergillosis (the abscess was percutaneously drained and was a new localization of the aspergillus), and one pulmonary and cardiac insufficiency.
One patient died because of hemorrhagic intraoperative shock after iatrogenic lesion of the right hepatic vein; in the perioperative period she developed a thrombosis of the cava vein with a subsequent lethal hemorrhagic shock. The 30-day mortality was 2% in this series (1/50).
Postoperative serum levels of aspartate transaminase (AST), alanine transaminase (ALT), and total bilirubin were monitored as signs of hepatic trauma; postoperative levels are reported in Figure 1. Figure 2 shows a hematoxylin and eosin (H&E)-stained image of the transected liver using the BVSD, and demonstrates the effect on hepatic collagen, bile ducts, and vessels. From a histologic point of view, at the cut surface level the parenchyma presents coagulative necrosis with electrocautery mark for a mean of 1.3 mm in length (range 1–2 mm) with a gradual reduction of the thermic damage at about 2–3 mm from the central section. The portal areas, including the bile ductule and artery, demonstrated sclerotic occlusion.
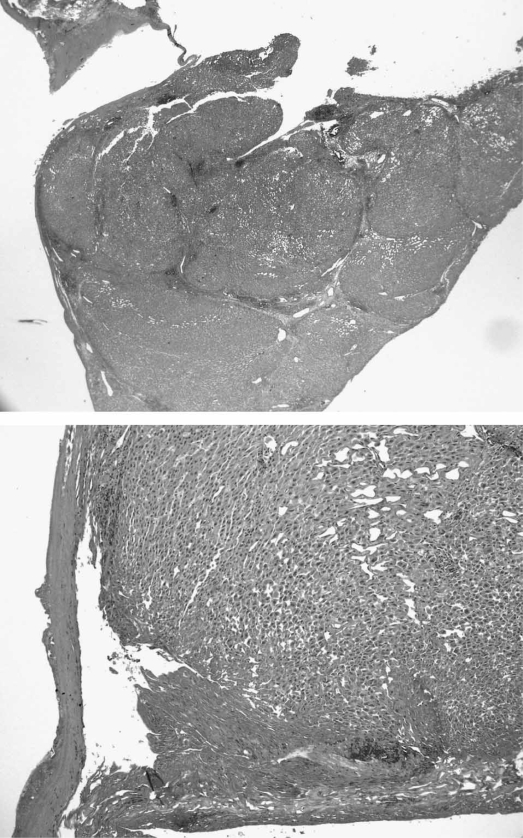
Figure 2. .
Specimen histopathology. Histologic images of resection edge margin demonstrate that the center of the sample is necrotic with almost total disappearance of parenchyma for about 2.3 mm in diameter. The parenchyma in immediate proximity presents coagulative necrosis with electrocautery mark for 1.2–1.6 mm in length; the portal areas present destruction of the interlobular vessels (hepatic artery, portal vein, and bile ductule) with sclerotic occlusion. We observed gradual reduction of the thermic damage at about 0.2–0.4 cm from the central section. H&E stain, ×250.
Discussion
Improved techniques for liver resection, better monitoring during anesthesia, and introduction of a number of technical devices have resulted in reduction in perioperative morbidity and mortality associated with blood loss, even though liver surgery still remains a challenging procedure 21. Moreover, intraoperative blood loss and the subsequent need for blood transfusion are considered significant risk factors for increased complication rates, poor postoperative outcome, and shorter disease-free survival 22,23. Also, intraoperative tissue damage associated with the technique of parenchymal dissection and the degree of postoperative injury due the use of inflow occlusion seem to affect patient outcome 24,25. Last but not least biliary leakage is a challenging complication, occurring in almost 10% of patients undergoing hepatectomy 26. For these reasons, technical improvements should be considered possible and desirable. Furthermore, efforts are currently being made to prevent the intraoperative blood loss so as to avoid ischemic damage of the parenchyma due to vascular occlusion 27. Our prospective series of hepatic resections demonstrates, as previously reported 28,29, that the LigaSure vessel-sealing system is a safe and effective instrument for transection of liver parenchyma employing radiofrequency (RF). It is able to permanently seal hepatic veins and arteries up to several millimeters in diameter, without additional devices or clips and knots. RF has already been demonstrated to be useful in liver disease. Percutaneous and intraoperative RF ablation are well known therapeutic approaches to primary and metastatic liver cancer and are historically considered alternative or complementary to surgery. The development of new techniques and devices has shown that RF-based surgery can be feasible and useful. In 2002 Habib's group 30 described a technique for liver resection using RF energy (monopolar electrodes) to coagulate liver resection margins before division of parenchyma with a surgical scalpel, allowing a quite complete bloodless liver resection without the use of sutures, clips, or glue. In 2001 Horgan described first the use of LigaSure System in liver resection 28 and in 2002 the monopolar floating ball by Tissuelink (TMFB) was introduced into clinical practice. Both TMFB and BVSD are able to seal vascular and biliary vessels by collagen fusion but TMFB is useful for preventive coagulation of the liver surface that guarantees fusion of vessels up to 2–3 mm in diameter, while BVSD can focus RF energy on the vessel enclosed between its clamps and is able to permanently seal hepatic veins, arteries, and biliary vessels up to 7 mm in diameter, preventing bleeding and providing a real and safe alternative to clips and sutures.
From a histologic point of view one of the advantages shown in our series is the modest tissue trauma that the BVSD produces and the controlled dissection of tissue that it allows. The lateral thermal spreading and conduction, which represents the area of coagulation outside the clamp, extend for a mean of 1.3 mm (range 1–2 mm) on either side and are significantly lower if compared with electrocoagulation, harmonic scalpel, and laser. Taking into account the known correlation of the extent of intraoperative tissue damage, healing complications, and postoperative septic complications, this can explain the low morbidity rate related to parenchymal damage in our results. Moreover the involvement of the biliocytes in the area of complete coagulative necrosis and the effective occlusion of bile ductules on histologic analysis demonstrate the rationale of the absence of postoperative bile leaks, as previously reported with this device 31. Intrahepatic biliary radicals smaller than segmental branches appeared to be well sealed by the BVSD, and bile leaks were recognized infrequently at the end of the transection on the cut surface of the liver. This is an important goal of the LigaSure sealing device because bile leaks represent a frequent and potentially life-threatening postoperative complication of liver resection, ranging from 4.5% to 11%, particularly considering that other devices such as the harmonic scalpel seem to be related to an increased incidence of postoperative bile leaks 32. This fact could be correlated with a higher capacity of RF in sealing biliary ducts if compared with ultrasonically activated energy. In fact, no bile leaks have been reported with the MTFB or Habib's technique 33,34, or in our experience. It has been reported that the microwave tissue coagulator method can induce bile leakage or infection due to necrotic detachment of the cut margin of the residual liver. Since the coagulation temperature with the LVSD is < 100°C, tissue damage is minimal. Indeed the necrotic changes at the cut margin of the residual liver following surgery with our technique were minimal, thus reducing complications such as postoperative bleeding, bile leakage, and abscess formation 35. In fact in our series we experienced only one intra-abdominal abscess, which was not related to the transection technique, but to the patient's disease (recurrence of aspergillosis). Moreover, in our experience the area of transection is under the direct visual control of the surgeon, allowing for the identification of intrahepatic structures. This is an advantage over some microwave coagulation techniques, which require an essentially blind tissue coagulation, obviating the opportunity to identify and coagulate key structures in a controlled setting, and contributing to the incidence of hemorrhage and bile leak. Postoperative serum levels of ALT, AST, and bilirubin were comparable to data reported in the literature after liver resection 35,36. This result of our series demonstrated that controlled use of RF on the target vessels of the BVSD reduces hepatic trauma and does not leave a large amount of necrotic tissue at the cut surface, thus not resulting in a major sacrifice of normal parenchyma, as happens with other techniques. The degeneration is limited to the transection surface and is well correlated with increase of AST/ALT levels and is different from the whole-liver ischemic damage caused by inflow occlusion.
Blood transfusions were infrequent in our series with the BVSD, with 16% of patients transfused intraoperatively, a lower rate than in major series reported in the literature. DeMatteo et al. reported a large series in which 38% of patients received intraoperative transfusions 37, and Nuzzo et al. reported a 41% rate of blood transfusions 38 during resections with inflow occlusion. Many of our patients did not have underlying cirrhosis, which may explain the minimal need for blood replacement during hepatic resection; nevertheless our results seem favorable when compared to literature reports. One concern about this instrument remains. In our experience, the BVSD fails to achieve a satisfactory hemostasis during transection of cirrhotic parenchyma. Use of this device was abandoned during liver resection in four cirrhotic patients, because of uncontrollable blood loss. We believe that the hard liver parenchyma, as in cirrhotic patients, made the crushing technique difficult, and the hepatic tissue between the blades of the devices may have dispersed the power applied, causing vessels to bleed. LigaSure works particularly poorly in cirrhotic patients and seems to be unable to achieve a correct hemostasis, requiring the use of alternative techniques. We believe that the limits of the BVSD are not related to the energy used (RF), but to the shape of the instrument that in the case of hard parenchyma is not able to ensure good functioning. In fact several studies report that the TMFB and the RF techniques are useful in cirrhotic patients. Operative times do not differ from those usually reported in similar series. Moreover the surgeon can avoid the use of too many metal clips, which may slip off and may cause rebleeding during subsequent manipulation, and the policy of ‘leaving nothing behind is an attractive strategy. In conclusion the LigaSure vessel-sealing system seems to be a useful device for limiting blood loss and postoperative bile leaks in patients who underwent hepatic surgery. Inflow occlusion and blood transfusion are needed infrequently when using this tool and it does not appear to contribute to abscess formation. Otherwise it is cumbersome in cirrhotic livers.
Acknowledgements and disclosures
No disclosures.
References
- 1.Rees M, Plant G, Wells J, Bygrave S. One hundred and fifty hepatic resections: evolution of tecnique towards bloodless surgery. Br J Surg. 1996;83:1526–9. doi: 10.1002/bjs.1800831110. [DOI] [PubMed] [Google Scholar]
- 2.Doci R, Gennari L, Bignami P, Montalto F, Morabito A, Bozzetti F, et al. Morbidity and mortality after hepatic resection of metastases from colorectal cancer. Br J Surg. 1995;82:377–81. doi: 10.1002/bjs.1800820332. [DOI] [PubMed] [Google Scholar]
- 3.Belghiti J, Hiramatsu K, Benoist S, Benoist S, Massault P, Sauvanet A. Seven hundred hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38–46. doi: 10.1016/s1072-7515(00)00261-1. [DOI] [PubMed] [Google Scholar]
- 4.Gozzetti G, Mazziotti A, Grazi LG, Jovine E, Gallucci A, Gruttadauria S, et al. Liver resection without blood transfusion. Br J Surg. 1995;82:1105–10. doi: 10.1002/bjs.1800820833. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham JD, Fong Y, Shriver C, Melendez J, Marx WL, Blumgart LH. One hundred consecutive hepatic resections: blood loss, transfusion and operative technique. Arch Surg. 1994;129:1050–6. doi: 10.1001/archsurg.1994.01420340064011. [DOI] [PubMed] [Google Scholar]
- 6.Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406. doi: 10.1097/01.SLA.0000029003.66466.B3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kooby DA, Stockman J, Ben-Porat L, Gonen M, Jarnagin WR, DeMatteo RP, et al. Influence of transfusion on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg. 2003;237:860–70. doi: 10.1097/01.SLA.0000072371.95588.DA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asahara T, Katayama K, Itamoto T, Yano M, Hino H, Okamoto Y, et al. Perioperative blood transfusion as a prognostic indicator in patients with hepatocellular carcinoma. World J Surg. 1999;23:676–80. doi: 10.1007/pl00012367. [DOI] [PubMed] [Google Scholar]
- 9.Kwon AH, Matsui Y, Kamiyama Y. Perioperative blood transfusion in hepatocellular carcinomas: influence of immunologic profile and recurrence free survival. Cancer. 2001;91:771–8. [PubMed] [Google Scholar]
- 10.Fujimoto J, Okamoto E, Yamanaka N, Tanaka T, Tanaka W. Adverse effect of perioperative blood transfusion on survival after hepatic resection for hepatocellular carcinoma. Hepatogastroenterology. 1997;44:1390–6. [PubMed] [Google Scholar]
- 11.Torzilli G, Makuuchi M, Midorikawa Y, Sano K, Inoue K, Takayama T, et al. Liver resection without total vascular exclusion: hazardous or beneficial? An analysis of our experience. Ann Surg. 2001;233:167–75. doi: 10.1097/00000658-200102000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smyrniotis V, Farantos C, Kostopanagiotu G, Arkadopulos N. Vascular control during hepatectomy: review of methods and results. World J Surg. 2005;29:1384–96. doi: 10.1007/s00268-005-0025-x. [DOI] [PubMed] [Google Scholar]
- 13.Clavien PA, Yadav S, Sindram D, Bentley RC. Protective effect of ischemic preconditioning for liver resection performed under inflow occlusion in humans. Ann Surg. 2000;232:155–62. doi: 10.1097/00000658-200008000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melendez JA, Arslan V, Fischer ME, Wuest D, Jarnagin WR, Fong Y, et al. Perioperative outcomes of major hepatic resection under low central venous pressure anesthesia: blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg. 1998;187:620–5. doi: 10.1016/s1072-7515(98)00240-3. [DOI] [PubMed] [Google Scholar]
- 15.Kim J, Ahamad SA, Lowy AM, Buell JF, Pennington LJ, Soldano DA, et al. Increased biliary fistulas after liver resection with the Harmonic Scalpel. Am Surg. 2003;69:815–19. [PubMed] [Google Scholar]
- 16.Lam CM, Lo CM, Liu CM, Fan ST. Biliary complication during liver resection. World J Surg. 2001;25:1273–6. doi: 10.1007/s00268-001-0109-1. [DOI] [PubMed] [Google Scholar]
- 17.Romano F, Caprotti R, Franciosi C, De Fina S, Colombo G, Uggeri F, et al. Laparoscopy splenectomy using LigaSure. A preliminary experience. Surg Endosc. 2002;16:1608–11. doi: 10.1007/s00464-001-9145-z. [DOI] [PubMed] [Google Scholar]
- 18.Terminology Committee of the International Hepato-Pancreato-Biliary Association. IHPBA Brisbane 2000 terminology of liver anatomy and resections. HPB Surg 2000;2:333–9. [Google Scholar]
- 19.Takenaka K, Kawahara N, Yamamoto K, Kajiyama K, Maeda T, Itasaka H, et al. Results of 280 liver resections for hepatocellular carcinoma. Arch Surg. 1996;131:71–6. doi: 10.1001/archsurg.1996.01430130073014. [DOI] [PubMed] [Google Scholar]
- 20.Allen PJ, Jarnagin WR. Current status of hepatic resection. Adv Surg. 2003;37:29–49. [PubMed] [Google Scholar]
- 21.Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 1999;229:322–30. doi: 10.1097/00000658-199903000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–18. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosen CB, Nagorney DM, Taswell HF, Helgeson SL, Ilstrup DM, van Heerden JA, et al. Perioperative blood transfusion and determinants of survival after liver resection for metastatic colorectal carcinoma. Ann Surg. 1992;216:493–504. doi: 10.1097/00000658-199210000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serracino-Inglott F, Habib NA, Mathie RT. Hepatic ischemia reperfusion injury. Am J Surg. 2001;181:160–6. doi: 10.1016/s0002-9610(00)00573-0. [DOI] [PubMed] [Google Scholar]
- 25.Belghiti J, Noun R, Malafosse R, Jagot P, Sauvanet A, Pierangeli F, et al. Continuous versus intermittent portal triad clamping for liver resections. A controlled study. Ann Surg. 1999;229:369–75. doi: 10.1097/00000658-199903000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lo CM, Fan ST, Liu CL, Lai ECS, Wong J. Biliary complications after hepatic resection: risk factors, management and outcome. Arch Surg. 1998;133:156–61. doi: 10.1001/archsurg.133.2.156. [DOI] [PubMed] [Google Scholar]
- 27.Di Carlo I, Barbagallo F, Toro A, Sofia M, Guastella T, Latteri F, et al. Hepatic resection using a water-cooled, high-density, monopolar device: a new technology for safer surgery. J Gastrointestinal Surg. 2004;8:596–600. doi: 10.1016/j.gassur.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 28.Horgan P. A novel technique for parenchymal division during hepatectomy. Am J Surg. 2001;181:236–7. doi: 10.1016/s0002-9610(01)00556-6. [DOI] [PubMed] [Google Scholar]
- 29.Strasberg SM, Drebin JA, Linehan D. Use of bipolar vessel-sealing device for parenchymal transection during liver surgery. J Gastrointestinal Surg. 2002;6:569–74. doi: 10.1016/s1091-255x(02)00030-6. [DOI] [PubMed] [Google Scholar]
- 30.Weber JC, Navarra G, Jiao LR, Nicholls JP, Jensen SL, Habib NA. New technique for liver resection using heat coagulative necrosis. Ann Surg. 2002;236:560–3. doi: 10.1097/00000658-200211000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Constant DL, Slakey DP, Campeau RJ, Dunne JB. Laparoscopic nonanatomic hepatic resection employing the ligasure device. JSLS. 2005;9:35–8. [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidbauer S, Hallfeldt KK, Sitzmann G, Kantelhardt T, Trupka A. Experience with ultrasound scissors and blades (Ultracision) in open and laparoscopic liver resection. Ann Surg. 2002;235:27–30. doi: 10.1097/00000658-200201000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sugo H, Mikami Y, Matsumoto F, Tsumura H, Watanabe Y, Kojima K, et al. Hepatic resection using the harmonic scalpel. Surg Today. 2000;30:959–62. doi: 10.1007/s005950070055. [DOI] [PubMed] [Google Scholar]
- 34.Navarra G, Spalding D, Zacharoulis D, Nicholls JP, Kirby S, Costa I, et al. Bloodless hepatectomy technique. HPB Surg. 2002;4:95–7. doi: 10.1080/136518202760378470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakamoto Y, Yamamoto J, Kokudo N, Seki M, Kosuge T, Yamaguchi T, et al. Bloodless liver resection using the monopolar floating ball plus ligasure diathermy: preliminary results of 16 resections. World J Surg. 2004;28:166–72. doi: 10.1007/s00268-003-7167-5. [DOI] [PubMed] [Google Scholar]
- 36.Lesurtel M, Selzner M, Petrowsky H, McCormack L, Clavien PA. How should transection of the liver be performed? A prospective randomized study in 100 consecutive patients: comparing four different transection strategies. Ann Surg. 2005;242:814–23. doi: 10.1097/01.sla.0000189121.35617.d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Matteo RP, Fong Y, Jarnagin WR, Blumgart LH. Recent advances in hepatic resection. Semin Surg Oncol. 2000;19:200–7. doi: 10.1002/1098-2388(200009)19:2<200::aid-ssu11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 38.Nuzzo G, Giuliante F, Giovannini I, Tebala GD, de Cosmo G. Hepatic resection in normothermic ischemia. Surgery. 1996;120:852–8. doi: 10.1016/s0039-6060(96)80094-8. [DOI] [PubMed] [Google Scholar]